Abstract
Purpose of Review
The purpose of this review is to discuss novel studies in the last year that have examined the use of combinations of multiple markers to improve risk prediction in the setting of chronic kidney disease (CKD). We will focus on multi-marker panels to improve prediction of CKD onset; improve classification of CKD and risk-stratification of persons with CKD; and develop individual-level risk scores for progression to ESRD.
Recent Findings
One study reported that several novel circulation biomarkers may aid in predicting incident CKD and microalbuminuria. Second, our group has shown that the combination of creatinine, cystatin C and albuminuria improves detection and risk stratification for death, heart failure, cardiovascular events, and end stage renal disease compared with creatinine alone. Finally, a highly accurate individual risk score was developed to predict progression to ESRD using readily available clinical markers.
Summary
The combination of multiple markers improves detection and risk stratification in CKD. Future research is needed in understanding the use of a “renal panel” for detection, classification and risk stratification in kidney disease in diverse populations. The studies presented here represent the beginning of a paradigm shift to multi-marker panels in nephrology.
Keywords: Chronic kidney disease, albuminuria, creatinine, cystatin C, multi-marker
Introduction
Risk stratification in epidemiology, at its essence, refers to the use of available information to estimate future risk for adverse health outcomes. Perhaps the best known example is the Framingham Risk Score, a tool which has been fundamental in implementing strategies for prevention and treatment of cardiovascular disease.[1–2] Risk stratification models also exist for liver disease, death in hospitalized persons, among others.[3–4] A common feature of these models is the combination of multiple biomarkers with demographic and clinical characteristics to predict future health associated risks. Using multiple markers to predict adverse outcomes related to chronic kidney disease (CKD) has only recently gained traction, as epidemiological studies relied primarily on serum creatinine for decades. In this review, we discuss key developments in the last year that have examined the use of multiple biomarkers to: a) improve prediction of the onset of CKD, b) improve classification of CKD and risk-stratification of persons with CKD for death, cardiovascular events, and end stage renal disease (ESRD), and c) develop individual-level risk scores for progression from CKD to ESRD.
Several metrics can be used to investigate whether a new biomarker or set of biomarkers may be useful in risk prediction. A traditional approach is to investigate the association of a novel biomarker with adverse events using regression models and estimating relative risks, hazard ratios, etc. A second approach, which is the subject of this review, is to use metrics that evaluate whether a biomarker or set of biomarkers can improve prediction of risk beyond established factors. Some understanding of this methodology will be useful for the reader, but a thorough explanation is beyond the scope of this review. Briefly, two of the most commonly used methods include evaluation of improvement in the concordance statistic (C statistic) and estimation of net reclassification improvement (NRI). The C statistic is designed to quantify how well a model discriminates between persons who do and do not have an event, and it ranges from 0.5 (no better than chance) to 1.0 (perfect). The NRI is a tool that quantifies the improvement in risk prediction for individuals who are reclassified (moving into higher or lower risk groups) after addition of a new biomarker(s).[5] In-depth reviews useful for clinicians have been previously published.[5–6]
Traditional Use of Biomarkers in Nephrology
The standard biomarker for assessing kidney function in clinical practice has been serum creatinine. In the last two decades, equations to estimate glomerular filtration rate (eGFR) from serum creatinine have been developed and improved.[7–8] The level of estimated GFR has been shown to be independently associated with risk of death, cardiovascular disease and progression to ESRD.[9] Guidelines have adopted the use of two biomarkers to define CKD, namely as either an eGFR <60 mL/min/1.73m2 or an albumin-to-creatinine ratio (ACR) of ≥30 mg/g.[10] More recently, eGFR and ACR have been increasingly recognized as independent and complementary markers in the prediction of death, cardiovascular disease and progression to ESRD. The Chronic Kidney Disease Prognosis Consortium (CKDPC) recently showed that eGFR and ACR are both independent and additive risk factors for death.[11] The independent association of eGFR and proteinuria with adverse outcomes has also been shown in other studies.[12–14] Thus, upcoming Kidney Disease: Improving Global Outcomes (KDIGO) guidelines will suggest the use of both eGFR and ACR to diagnose and classify CKD. In this review, we will highlight recent studies that have explored novel panels of biomarkers beyond creatinine-based eGFR and ACR to improve risk prediction in CKD.
Multi-Marker Approach to Predict Incident CKD
Understanding risk factors for the onset of CKD has been an important development in CKD epidemiology. Factors such as age, hypertension and diabetes have been well established, but do not fully capture persons at risk for CKD.[15]. Fox et al. recently assessed whether serum biomarkers, selected based on their associations with cardiovascular disease, were associated with the development of CKD (defined as eGFR <60 mL/min/1.73m2) or microalbuminuria (MA, defined as urine ACR ≥25 (women) or 17 (men) mg/g on spot urine samples).[16] This study included 2,345 individuals without CKD and 1,822 without microalbuminuria from the Framingham Offspring Study who were followed over a mean of 9.5 years. Authors studied the association of the candidate biomarkers aldosterone, plasma renin concentration, BNP, C-reactive protein, plasminogen activator inhibitor-1, fibrinogen, and plasma total homocysteine, (and the entire biomarker panel) with incident CKD or incident MA. After adjustment for demographic, clinical factors and baseline eGFR (for incident CKD) or baseline log urine ACR (for incident MA), homocysteine and aldosterone were associated with incident CKD, whereas aldosterone, BNP, and homocysteine were associated with incident MA. Results are shown in Table 1, as reproduced from the original paper.[16] Next, they assessed the ability of these biomarkers to improve risk discrimination using the C statistic and the NRI. For incident CKD, the C statistic changed from 0.810 to 0.822 (P=0.0023 for difference) after addition of the biomarkers. For incident MA, the addition of biomarkers increased the C statistic from 0.732 to 0.748 (P=0.003 for difference). NRI’s were 6.9% (P=0.0004 for incident CKD and P=0.007 for incident MA) for both outcomes.
Table 2.
Association of the multi-maker panel and the individual biomarkers with incident CKD and MAa
| Biomarkers | P | Odds Ratio |
95% Confidence Interval |
|---|---|---|---|
| Incident CKD | |||
| entire panel specific markers |
0.0005 | ||
| homocysteine | <0.0001 | 1.41 | 1.20 to 1.65 |
| aldosterone | 0.047 | 1.17 | 1.002 to 1.36 |
| Incident microalbuminuria | |||
| entire panel specific markers |
0.003 | ||
| aldosterone | 0.017 | 1.23 | 1.04 to 1.46 |
| BNP | 0.0037 | 1.30 | 1.09 to 1.54 |
| homocysteine | 0.04 | 1.20 | 1.01 to 1.42 |
Markers that are selected after backward elimination; presented per SD unit increase.
We find these results intriguing, and they may generate hypotheses on the pathophysiology of renal disease. However, it is unclear how they may be useful in clinical practice. These biomarkers are not normally available, and there was only a modest improvement in the C statistic and in the NRI.[16] Some important methodological limitations should be noted, namely that the prevalence of MA was quite low at baseline and ACR did not enter the models for incident CKD though it is known to be one of the most important risk factors for incident stage 3 CKD.[17] The use of backward elimination does not completely address the issue of multiple testing. Future studies would be required to replicate these findings.
Multi-Marker Approach for CKD Classification, and Risk Stratification for Death, Heart Failure, Cardiovascular Disease and End Stage Renal Disease
Our group has evaluated whether adding cystatin C-based eGFR (eGFRcys) to creatinine-based eGFR (eGFRcreat) and ACR can improve classification and risk stratification for CKD.[18] Cystatin C is an alternative marker of kidney function, which has been shown to have stronger and more linear associations with death and cardiovascular events than creatinine.[19] It is thought to be less influenced by muscle mass, race, and age, compared with creatinine.[20] We hypothesized that using two endogenous filtration markers that may have different non-GFR determinants would improve classification and risk stratification in CKD.
In our first analyses,[18] we included 6,749 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) and 5,160 persons from the Cardiovascular Health Study (CHS). MESA is a racially diverse cohort study sponsored by the National Institutes of Health to investigate early cardiovascular disease. CHS is a community-based longitudinal study evaluating risk factors for the development and progression of cardiovascular disease. We estimated eGFRcreat and eGFRcys using equations from the CKD Epidemiology group [8,21] and then classified persons into four mutually exclusive categories: no CKD (eGFRcreat >60 and eGFRcys <60 mL/min/1.73m2), CKD by creatinine only (eGFRcreat <60 and eGFRcys >60 mL/min/1.73m2), CKD by cystatin C only (eGFRcreat >60 and eGFRcys <60 mL/min/1.73m2), and CKD by both (eGFRcreat <60 and eGFRcys <60 mL/min/1.73m2). We examined the association of each category with risk for all-cause mortality, cardiovascular events, heart failure, and kidney failure.[18] In our study, 79% were categorized as no CKD by both, 10% as CKD by creatinine only, 3% as CKD by cystatin C only, and 8% as CKD by both markers. Persons identified as CKD by both had the highest risk for all outcomes, followed by persons identified as CKD by cystatin C but not creatinine (CKD by cystatin C only). Interestingly, persons classified as CKD by creatinine but not confirmed by cystatin C were at similar risk for death, CVD, heart failure and slightly higher ESRD risk compared with persons with no CKD (Table 2).
Table 2.
Association of decreased GFR (<60 ml/min per 1.73 m2) by cystatin C and creatinine with adverse events in MESA and CHS
| MESA | CHS | |||||
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||||
| n | Demographic Adjusteda |
Fully Adjustedb | n | Demographic Adjusteda |
Fully Adjustedb | |
| All-cause mortalityc | ||||||
| GFR not decreased | 5759 | 1.00 (ref) | 1.00 (ref) | 3639 | 1.00 (ref) | 1.00 (ref) |
| decreased GFRcreat only | 614 | 0.76 (0.48, 1.20) | 0.80 (0.50, 1.26) | 605 | 1.10 (0.98, 1.22) | 1.09 (0.98, 1.21) |
| decreased GFRcys only | 107 | 3.43 (1.96, 5.98) | 3.23 (1.84, 5.67) | 227 | 1.94 (1.67, 2.25) | 1.78 (1.53, 2.08) |
| decreased GFR both | 269 | 1.97 (1.31, 2.96) | 1.93 (1.27, 2.92) | 689 | 1.96 (1.78, 2.16) | 1.74 (1.58, 1.93) |
| Cardiovascular diseased | ||||||
| GFR not decreased | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| decreased GFRcreat only | 1.18 (0.78, 1.78) | 1.22 (0.80, 1.85) | 1.13 (0.99, 1.29) | 1.05 (0.92, 1.20) | ||
| decreased GFRcys only | 2.22 (1.13, 4.39) | 1.92 (0.97, 3.82) | 1.83 (1.52, 2.21) | 1.52 (1.26, 1.84) | ||
| decreased GFR both | 2.07 (1.32, 3.24) | 1.67 (1.06, 2.63) | 1.86 (1.65, 2.09) | 1.46 (1.29, 1.65) | ||
| Heart failuree | ||||||
| GFR not decreased | 1.00 (ref) | 1.00 (ref) | ||||
| decreased GFRcreat only | 1.08 (0.91, 1.27) | 0.99 (0.84, 1.18) | ||||
| decreased GFRcys only | 2.12 (1.68, 2.66) | 1.69 (1.33, 2.13) | ||||
| decreased GFR both | 1.91 (1.64, 2.23) | 1.43 (1.22, 1.67) | ||||
| Kidney failuref | ||||||
| GFR not decreased | 1.00 (ref) | 1.00 (ref) | ||||
| decreased GFRcreat only | 2.67 (1.03, 6.90) | 2.60 (1.00, 6.75) | ||||
| decreased GFRcys only | 7.69 (2.78, 21.25) | 6.14 (2.18, 17.29) | ||||
| decreased GFR only | 30.95 (17.0, 56.34) | 23.82 (12.68, 44.76) | ||||
ref, referent group.
Adjusted for age, race, and gender.
Adjusted for age, race, gender, diabetes, hypertension, LDL, HDL, CRP, and prevalent CVD for CHS (persons with baseline CVD were excluded for incident CVD analyses).
223 deaths for MESA and 3345 deaths for CHS.
212 events for MESA and 2249 events for CHS.
1407 events for CHS.
84 events for CHS.
We concluded that eGFRcys can improve risk stratification for CKD by identifying persons at high risk among those labeled as CKD by creatinine. Moreover, cystatin C identified persons at high risk for adverse events that were missed by creatinine. In this study, we were limited by the lack of baseline measures of albuminuria in the CHS (where a large majority of the outcomes occurred). Though a sensitivity analysis using data from the year 7 CHS visit had similar results, this remains an important limitation. Furthermore, this study did not have measured GFR; however, this is not feasible in large, epidemiological studies.
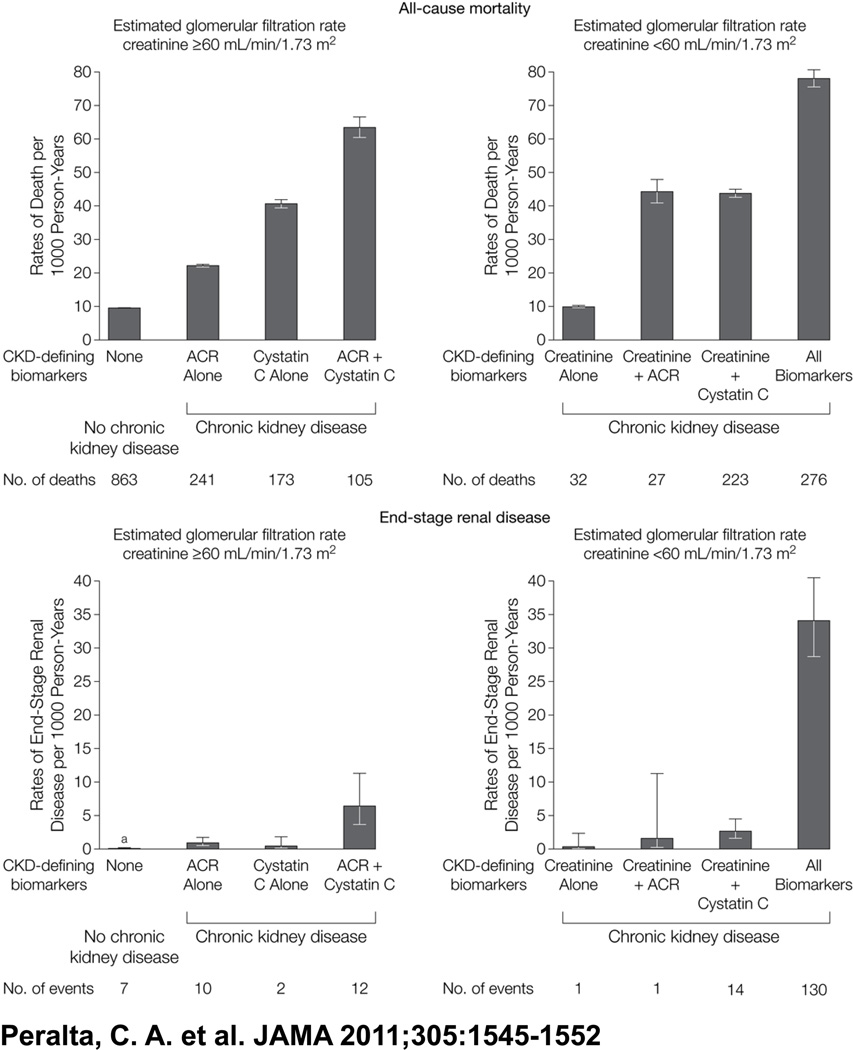
In a follow up study, our group investigated whether using a multi-marker panel of creatinine, cystatin C, and ACR to classify CKD can improve risk stratification for death and ESRD compared with creatinine alone among 26,643 participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study.[22] REGARDS is a large, population-based cohort study designed to identify factors contributing to excess stroke mortality in the “stroke belt” of the United States, as well as the excess stroke risk of black Americans.[23] For these analyses, we categorized persons into eight mutually exclusive CKD groups defined by eGFRcreat (≥ or <60 mL/min/1.73m2), eGFRcys (≥ or <60 mL/min/1.73m2) and ACR (≥ or <30 mg/g). Over a median follow-up of 4.6 years, 1,940 died and 177 developed ESRD. We found that participants with CKD by all three measures had the highest risk for death and ESRD. Among persons initially classified as having CKD by creatinine, persons who had their CKD confirmed by ACR or cystatin C were at elevated risk for death and ESRD. Among persons without CKD by creatinine, ACR and cystatin C identified different subgroups of persons who were also at increased risk. In fact, persons with CKD by cystatin C and ACR were the second highest risk group for ESRD (Table 3, Figure 1, originally Table 2 and Figure 2). We estimated the reclassification improvement after adding cystatin C and found that the NRI for mortality was 13% and 6.4% for ESRD.
Table 3.
Mortality Associated With Cystatin C, Estimated Glomerular Filtration Rate, and Albuminuria.
| HR (95% CI) | |||||
|---|---|---|---|---|---|
| No. of Patients |
Total No. of Deaths |
Adjusted Model 1a |
Adjusted Model 2b |
||
| Estimated GFR Creatinine ≥60 mL/min/1.73 m2 | |||||
| No CKD all | 19876 | 863 | 1 [Reference] | 1 [Reference] | |
| CKD defined by biomarker measuresc | |||||
| ACR alone | 2485 | 241 | 1.9 (1.6–2.2) | 1.7 (1.4–1.9) | |
| Cystatin C alone | 963 | 173 | 2.5 (2.1–3.0) | 2.2 (1.9–2.7) | |
| ACR + Cystatin C | 415 | 105 | 3.9 (3.1–4.7) | 3.0 (2.4–3.7) | |
| Estimated GFR Creatinine <60 mL/min/1.73 m2 | |||||
| CKD defined by biomarker measuresc | |||||
| Creatinine alone | 701 | 32 | 1 [Reference] | 1 [Reference] | |
| Creatine + ACR | 148 | 27 | 3.7 (2.2–6.2) | 3.3 (2.0–5.6) | |
| Creatinine + Cystatin C | 1172 | 223 | 3.5 (2.4–5.1) | 3.2 (2.2–4.7) | |
| All biomarkers | 883 | 276 | 6.6 (4.6–9.6) | 5.6 (3.9–8.2) | |
Abbreviation: ACR, albumin-to-creatinine ratio; CI, Confidence; CKD, chronic kidney disease; GFR, glomerular filtration rate; HR, hazard ratio.
Model 1 adjusts for age, race, sex, income, and educational attainment.
Model 2 adjusts for the above plus hypertension, diabetes, prevalent cardiovascular disease, smoking status, and body mass index.
see "Methods" section for definitions of biomarker measures.
Figure 1.
Association of Chronic Kidney Disease Definitions With All Stage Renal Disease All-Cause Mortality and End-Stage Renal Disease
We believe these findings support the notion that a multi-marker renal panel that includes ACR and cystatin C improves detection, classification and risk stratification for CKD compared with creatinine alone. Certain limitations need to be considered. We only had one measurement of the biomarkers, including albuminuria, which is known to have important variability in repeated measures, thus resulting in potential misclassification. Furthermore, the follow-up time for ESRD was short, limiting power for event-rates. As cystatin C has become easy and relatively inexpensive to measure, we believe its use should be considered in clinical practice to estimate GFR, particularly when creatinine-based measures may be most inaccurate (i.e. elderly, unpredictable muscle mass). Future studies are needed to understand the cost-effectiveness of a multi-marker approach for CKD confirmation and for CKD detection in clinical practice.
Use of Multiple Markers for Estimating Individual Risk for CKD Progression
Multi-marker approaches have also been used to predict individual risk for progression from CKD to ESRD. An individual risk score allows determination of each person’s risk of an event (which must be distinguished from reported population risks). Tangri et al. (2011) used demographic, clinical, and laboratory data from 2001–2008 in two Canadian CKD cohorts to develop and validate a prediction model for CKD progression to kidney failure (dialysis or transplantation).[24] The two cohorts were comprised of individuals with Stages 3–5 CKD who were referred to nephrology clinics. Cox proportional hazards regression was used to develop prediction models, testing candidate variables ascertained from the electronic medical records. Using the C statistic, NRI, and other methods, authors determined goodness of fit, discrimination, and calibration for the models. Of seven models, the most accurate model had the following variables: age, sex, baseline eGFR, ACR, serum calcium, phosphate, bicarbonate, and albumin. Using this model, the C statistic was 0.917 for the development cohort and 0.841 for the validation cohort, while the NRI for the validation cohort was 50.4% and 8.0% (compared to simpler models) for CKD stage 3.
We believe this study is particularly novel and has direct application to the clinical setting because the included markers are typically already available in a nephrology clinic. In fact, an online calculator for kidney failure risk can be found free online at http://jama.ama-assn.org/content/early/2011/04/05/jama.2011.451/suppl/DC2. The clinician can impute the variables and the model will estimate the person’s risk for kidney failure at 5 years. The study has the particular strength of having a large dataset and deriving a highly accurate model that is easily used. However, one must note that the model is still largely driven by age, baseline eGFR and ACR. Moreover, participants included were already referred to nephrology, and thus the model may not be applicable for general practice. The main limitation of this study, in our view, is the inability to estimate risk of death versus risk for kidney failure. Several studies have shown that persons with CKD are more likely to die than to develop ESRD,[9] so this information may be critical for dialysis planning. However, this study represents an important development in the use of multi-marker renal panels in nephrology.
Conclusions and Future Directions
The studies highlighted here have all made important gains in using novel approaches to CKD research and translation into clinical practice. We consider these four papers as seminal reports because they have pioneered a paradigm shift illustrating that a “renal panel” is likely to be more useful in the clinical setting than relying on serum creatinine alone (or even in combination with ACR). However, there is much to be done, as the “optimal” renal panel remains unknown. It is likely that the biomarkers included in the “optimal” panel will vary depending on the outcome of interest, whether the panel is used for screening versus prognosis in established/diagnosed CKD, or even the population in which it is used.
Therefore, many possible future directions remain to further develop this field. First, findings from the above studies need to be replicated in different populations, such as those of different ethnicities, ages, and eGFR ranges. Recent interest has been focused on the prediction of CKD. Novel biomarkers initially discovered to be elevated in the setting of acute kidney injury are now being studied as predictors for incident CKD.[25–26] Some of these biomarkers have also been studied as predictors of CKD progression.[27] Another avenue could be the addition of genetic markers to the panels, several of which have been recently identified.[28–30] Future studies should evaluate whether genetic markers can add to risk prediction, as has been done in recent cardiovascular research.[31] Research in this direction is promising for the development of multi-marker approaches to the detection of CKD, as well as for elucidating the associations of biomarkers with different pathways of kidney injury.
As discovery in this field advances, we must also consider the cost-utility and efficacy of different multi-marker approaches, and decide, as a community, where efforts are most effective to reduce CKD burden. We believe that the remaining “elephant in the room” will continue to be the paucity of data on screening for CKD. Thus, a priority for future directions should be to evaluate these biomarkers in targeted screening strategies, as universal screening is unlikely to be cost-effective. Although this is a relatively new field, we believe developments will result in improvements in multi-marker approaches for the prevention, detection and risk stratification of individuals at high risk for developing the disease and its complications.
Key Points.
Using combinations of multiple markers can improve classification and risk stratification in chronic kidney disease beyond serum creatinine and albumin-t-creatinine ratio.
A multi-marker approach to the detection and progression of disease is applicable for a wide variety of adverse outcomes.
Future research is needed on applying the concept of multi-marker panels in other groups, such as race/ethnic minorities, the very elderly, and a wider range of eGFR.
Acknowledgements
Sources of Funding
Funding Disclosures: C.P. is funded by the National Institutes of Diabetes and Digestive and Kidney Diseases (1K23SK082793-01) and a Robert Wood Johnson Harold Amos award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None declared.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
* • of special interest
* •• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p.).
- 1.Sullivan LM, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman LC, Norton EC. What's the Risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44:288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 4.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 5.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 6. Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. This article illustrates the principle of estimating and interpreting reclassification improvement methods.
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 10.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 11. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. This study represents the largest investigation showing that eGFR and albuminuria are independent and complementary predictors of death.
- 12.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 13.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, Neaton JD. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17:1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 14.Tonelli M, Jose P, Curhan G, Sacks F, Braunwald E, Pfeffer M. Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trial. Bmj. 2006;332:1426. doi: 10.1136/bmj.38814.566019.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 16. Fox CS, Gona P, Larson MG, Selhub J, Tofler G, Hwang SJ, Meigs JB, Levy D, Wang TJ, Jacques PF, et al. A multi-marker approach to predict incident CKD and microalbuminuria. J Am Soc Nephrol. 2010;21:2143–2149. doi: 10.1681/ASN.2010010085. This paper explores several novel circulation biomarkers that may aid in predicting incident CKD and microalbuminuria.
- 17.Shastri S, Katz R, Shlipak MG, Kestenbaum B, Peralta CA, Kramer H, Jacobs DR, Jr, de Boer IH, Cushman M, Siscovick D, et al. Cystatin C and albuminuria as risk factors for development of CKD stage 3: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2011;57:832–840. doi: 10.1053/j.ajkd.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peralta CA, Katz R, Sarnak MJ, Ix J, Fried LF, De Boer I, Palmas W, Siscovick D, Levey AS, Shlipak MG. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol. 2011;22:147–155. doi: 10.1681/ASN.2010050483. This paper assessed the use of cystatin C for identification of persons with CKD that were missed by creatinine, and who have high risk for adverse events. Among adults diagnosed with CKD using the creatinine-based CKD-EPI equation, the adverse prognosis is limited to the subset who also have CKD according to the cystatin C-based equation.
- 19.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 20.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 21.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, Safford MM, Zhang X, Muntner P, Warnock D. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. This paper evaluates using the combination of creatinine, cystatin C and albuminuria to improve detection and risk stratification for death, heart failure, cardiovascular events, and end stage renal disease compared with creatinine alone.
- 23.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 24. Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS. A predictive model for progression of chronic kidney disease to kidney failure. Jama. 2011;305:1553–1559. doi: 10.1001/jama.2011.451. The authors developed a highly accurate individual risk score to predict progression to ESRD using readily available clinical markers.
- 25.Astor BC, Kottgen A, Hwang SJ, Bhavsar N, Fox CS, Coresh J. Trefoil Factor 3 Predicts Incident Chronic Kidney Disease: A Case-Control Study Nested within the Atherosclerosis Risk in Communities (ARIC) Study. Am J Nephrol. 2011;34:291–297. doi: 10.1159/000330699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Seaghdha CM, Hwang SJ, Bhavsar NA, Kottgen A, Coresh J, Astor BC, Fox CS. Lower urinary connective tissue growth factor levels and incident CKD stage 3 in the general population. Am J Kidney Dis. 2011;57:841–849. doi: 10.1053/j.ajkd.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boger CA, Chen MH, Tin A, Olden M, Kottgen A, de Boer IH, Fuchsberger C, O'Seaghdha CM, Pattaro C, Teumer A, et al. CUBN is a gene locus for albuminuria. J Am Soc Nephrol. 2011;22:555–570. doi: 10.1681/ASN.2010060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu CT, Garnaas MK, Tin A, Kottgen A, Franceschini N, Peralta CA, de Boer IH, Lu X, Atkinson E, Ding J, et al. Genetic association for renal traits among participants of African ancestry reveals new loci for renal function. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002264. e1002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. This is a seminal article identifying a genetic variant strongly associated with kidney disease in African-Americans.
- 31.Paynter NP, Chasman DI, Buring JE, Shiffman D, Cook NR, Ridker PM. Cardiovascular disease risk prediction with and without knowledge of genetic variation at chromosome 9p21.3. Ann Intern Med. 2009;150:65–72. doi: 10.7326/0003-4819-150-2-200901200-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]