Abstract
Opioid analgesics are among the most effective agents for treatment of moderate to severe pain. However, the use of morphine after a spinal cord injury (SCI) can potentiate the development of paradoxical pain symptoms, and continuous administration can lead to dependence, tolerance, and addiction. Although some studies suggest that the addictive potential of morphine decreases when it is used to treat neuropathic pain, this has not been studied in a SCI model. Accordingly, the present studies investigated the addictive potential of morphine in a rodent model of SCI using conditioned place preference (CPP) and intravenous self-administration paradigms. A contusion injury significantly increased the expression of a CPP relative to sham and intact controls in the acute phase of injury. However, contused animals self-administered significantly less morphine than sham and intact controls, but this was dose-dependent; at a high concentration, injured rats exhibited an increase in drug-reinforced responses over time. Exposure to a high concentration of morphine impeded weight gain and locomotor recovery. We suggest that the increased preference observed in injured rats reflects a motivational effect linked in part to the drug's anti-nociceptive effect. Further, although injured rats exhibited a suppression of opiate self-administration, when given access to a high concentration, addictive-like behavior emerged and was associated with poor recovery.
Key words: behavioral assessments, locomotor function, spinal cord injury
Introduction
Moderate to severe pain, including neuropathic pain, is one of the most significant consequences of spinal cord injury (SCI), and is one of the primary symptoms that patients would like to have effectively treated (Anderson, 2004; Backonja and Stacey, 2004). Approximately 65% of individuals with SCI experience severe or excruciating pain (Budh and Lundeberg, 2005; Perry et al., 2009; Siddall et al., 2003). Unfortunately, typical pain relievers are often ineffective in treating this pain, and the pain tends to get worse with time rather than better (Budh and Lundeberg, 2005; Katz and Barkin, 2008; Zhao et al., 2004). Moreover, it has been demonstrated in both animal models and human studies that administration of analgesics such as morphine can potentiate the development of neuropathic pain, allodynia, and hyperalgesia (Chang et al., 2007; Hook et al., 2007; Liang et al., 2008; Parisod et al., 2003; Yu et al., 1997a,1997b). Considering the number of people affected by pain following SCI, it is important that it can be effectively treated.
Opioids are commonly used for the treatment of pain after SCI (Clark, 2002; Liang et al., 2008; O'Connor and Dworkin, 2009; Przewlocki and Przewlocki, 2005; Sindrup and Jensen, 1999; Widerstron-Noga and Turk 2003), and are considered to be among the most effective analgesics (Warms et al., 2002). However, the efficacy of opioid treatment following SCI has not been assessed using placebo-controlled trials (Attal et al., 2009). From a clinical perspective, the prescription of opioids is problematic because continuous administration can lead to dependence, tolerance, and addiction (Ballantyne and LaForge 2007; Ballantyne and Mao, 2003; O'Connor and Dworkin, 2009; Trescot et al., 2008). It is estimated that 18–45% of individuals using opioids for the management of chronic pain, or pain that lasts beyond the usual course of disease or healing, abuse the drug (Ballantyne and LaForge 2007; Compton and Volkow 2006; Contet et al., 2008; Heinemann et al., 1992; Morasco and Dobscha, 2008, Trescot et al., 2008). Inconsistencies between reports of the incidence of addiction stem from differences in definitions of abuse, methods of reporting, and populations being surveyed, along with a general lack of empirical research examining the efficacy of long-term opioid use and addictive potential (Bell and Salmon, 2009; Dersh et al., 2008; Hojsted and Sjogren, 2007; Morasco and Dobscha, 2008; Radnitz and Tirch, 1995). Clearly, the use of opioids for the management of pain must be further investigated.
The prevalence of opioid misuse in patients with chronic pain (Morasco and Dobscha, 2008; Ives et al., 2006; Michna et al., 2007) underscores the need to further examine the potential for addiction after SCI. Prior work suggests that the development of a morphine-induced conditioned place preference (CPP) may be reduced in rats experiencing neuropathic pain resulting from nerve ligation injury (Ozaki et al., 2002). Similarly, Lyness and associates (1989) found that arthritic rats self-administered significantly less morphine than their pain-free counterparts. These studies suggest that pain, regardless of type, may lower the addictive potential of morphine. Yet, survey studies of chronic pain in the human clinical population, as discussed above, do not support this hypothesis. In fact, studies have shown that opioid effects vary depending on the model, the pain assessment tool, and the route of analgesic administration (Yu et al., 1997a, 1997b). It is therefore important to examine self-administration in a SCI model. Heinemann and colleagues (1988) found that up to 62% of SCI patients had misused drugs or alcohol at the time of their injury. If prior behavior is indeed predictive of addictive potential, one might expect an increased incidence of addiction after SCI.
The current studies investigate the addictive potential of morphine in a rodent contusion model of SCI. The contusion model closely resembles the clinical condition of SCI (Hulsebosch, 2002), producing symptoms of chronic pain in approximately 80% of subjects (Mills et al., 2001). Clinically, morphine is used to treat pain in the acute and chronic phases of SCI. This study focused on the effects of morphine applied in the acute phase of injury. Understanding the effects of morphine applied in the acute phase is significant at two levels. First, the data address concerns of addiction to pain-relieving medications after SCI. Does morphine given for acute pain potentiate addictive behavior? Second, using a clinically-relevant self-administration paradigm, these studies examine the effects of repeated morphine administration on long-term recovery of function.
Our previous studies show that a single administration of morphine (IP or IT) given the day following SCI injury undermines long-term locomotor recovery (Hook et al., 2007, 2009, 2011). As opioid effects vary with the route of administration (Yu et al., 1997a, 1997b), it is important that we also examine the effects of IV administration. We found, commensurate with previous studies, that repeated administration of IV morphine also undermined locomotor recovery and weight gain in the chronic phase of injury. Pertaining to addiction, contused subjects displayed an increased CPP to morphine in the acute phase of SCI relative to sham and intact controls. However, using a self-administration paradigm, we found a dose-dependent effect of injury on the rate of morphine administration; at a moderate dose, contused subjects self-administered significantly less morphine in the acute phase of injury.
Methods
Subjects
Male Sprague-Dawley rats obtained from Harlan (Houston, TX) were used as subjects. The animals were 90–110 days old, weighed 350–400 g, and were individually housed in acrylic glass bins (length 45.70 cm, width 23.50 cm, and height 20.30 cm) with food and water available ad libitum. Their bladders were expressed manually in the morning (8–9:30 am) and evening (6–7:30 pm) until they regained bladder control, which was defined as 3 consecutive days with an empty bladder at the time of expression. The animals were maintained on a 12-h light-dark cycle, and all behavioral testing occurred during the light portion of the cycle.
All of the experiments were reviewed and approved by the institutional care committee at Texas A&M, and all National Institutes of Health guidelines for the care and use of animal subjects were followed.
Surgery
Contusion injury
Subjects were anesthetized with inhaled isoflurane (5% to induce anesthesia and 2–3% for maintenance), and an area approximately 4.5 cm above and below the injury site was shaved and disinfected with iodine. A 7-cm incision was made over the spinal cord, and two incisions extending 3 cm rostral and caudal to T12–T13 were made on either side of the vertebral column. The lamina of the T12–T13 vertebrae were removed, exposing spinal tissue. The vertebral column was then fixed within the MASCIS device (Constantini and Young, 1994; Gruner, 1992), and a moderate contusion injury was produced by allowing the 10-g impactor (outfitted with a 2.5-mm tip) to drop 12.5 mm. The wound was closed with Michel clips. Sham subjects received a laminectomy only (no weight drop), and intact subjects received anesthesia only.
For the first 24 h after surgery, the rats were housed in a recovery room maintained at 26.6°C. All subjects were treated (IP) with 100,000 U/kg penicillin G potassium immediately after surgery and again 2 days later. To help maintain hydration, the subjects were also given 3.0 mL of saline (IP injection) following surgery. The Michel clips were removed 14 days following surgery.
Jugular catheter surgery
In the self-administration experiments, a jugular catheter was inserted 5 days prior to the contusion injury. Rats were anesthetized using a combination of 80 mg/kg ketamine and 10 mg/kg xylazine (IP). While under anesthesia, a catheter consisting of 0.025-mm ID Silastic tubing was inserted into the jugular vein and sutured to the muscle tissue in the area of the vein. Using an 11-gauge stainless steel tube as a guide, the catheter was passed subcutaneously through the body of the animal so that it exited in the back between the scapulae. A back mount cannula pedestal (model 313-00BM-10-SPC; Plastics One Inc., Roanoke, VA) was implanted subcutaneously and connected to the catheter. The back mount exited the skin between the scapulae and allowed for the connection of a spring leash for drug delivery. All incisions were closed using cyanoacrylate glue.
For the first 24 h after surgery, the rats were housed in a recovery room maintained at 26.6°C. All subjects were treated with 100,000 U/kg penicillin G potassium immediately after surgery, and for 2 days following surgery. To help maintain hydration, the subjects were also given 3.0 mL of saline (IP) following surgery. During the 5-day recovery period following surgery, the catheters were flushed with heparinized saline (0.25 mL).
Assessment of motor and sensory recovery
Locomotor recovery
Locomotor behavior was assessed using the Basso-Beattie-Bresnahan (BBB) rating scale (Basso et al., 1995) in an open enclosure (99 cm diameter and 23 cm deep) on the day following the contusion injury. The subjects were acclimated to the apparatus for 5 min per day for 3 days prior to surgery. Twenty-four hours after surgery each subject was placed in the open field and observed for 4 min to assess locomotor function. All observers had high intra- and interobserver reliability (all r's >0.89), and were blind to the subjects' experimental treatment.
Locomotor scores were transformed to help assure that the data were amendable to parametric analyses (Ferguson et al., 2004a). This transformation pools BBB scores 2–4, removing a discontinuity in the scale. The transformation also pools scores from a region of the scale (14–21) that is seldom used for a moderate contusion injury. By pooling these scores, we obtain an ordered scale that is relatively continuous with units that have approximately equivalent interval spacing. Meeting these criteria allows us to apply metric operations (computation of mean performance across legs), improves the justification for parametric statistical analyses, and increases statistical power.
Thermal reactivity
Reactivity to a noxious thermal stimulus was assessed by applying radiant heat to the tail. A 375-W movie light was focused onto the subject's tail using a condenser lens positioned 8 cm below the light source. The subject's tail was positioned in a 0.5-cm deep groove cut into an aluminum block 4.7 cm below the condenser lens. The last 2.5 cm of the tail was taped to a wire hook and attached to an elastic band located 11 cm behind the aluminum block, exposing approximately 2 cm of the tail to the light source. The flexibility of the elastic band allowed for a tail flick response while maintaining the rat's tail under the heat source. The latency to vocalize was then assessed. After both movement and vocalization responses were detected, the heat was terminated. If a subject failed to respond, the test trial was automatically terminated after 8 sec of heat exposure to avoid tissue damage. The subjects were placed in the apparatus for 15 min prior to testing, and were assessed three times at 2-min intervals. The last two tests were averaged to derive a measure of reactivity.
Place preference procedure
CPP is designed to examine the motivational properties of a drug. In our experiments, morphine (the unconditioned stimulus; US) is paired with a neutral environmental context. With increased exposures to the drug in the context, the environmental stimuli gain motivational properties and can act as conditioned stimuli (CS), which elicit approach. In this paradigm, derivation of the ratio of time spent in the drug-paired context compared to the saline-paired context, provides an index of a preference for a morphine- or saline-induced state.
Apparatus
Acclimation to the training/testing environments took place in grey plywood boxes (length 41 cm, height 41 cm, width 38 cm) with smooth floors. Morphine place preference conditioning occurred in one of two distinct environments. One box (length 41 cm, height 41 cm, width 38 cm) was black with a smooth acrylic glass floor scented with 3% vinegar. The other was a white box and the floor was covered with pine chips. The boxes were cleaned with a disinfectant between subjects. Testing occurred in a box (length 91 cm, height 41 cm, width 38 cm) that was comprised of both of the training contexts separated by a neutral grey strip. The conditioning boxes and test box were illuminated in a manner that eliminated the natural preference for the black portion of the box, and were maintained in the same position for the duration of the experiment.
Acclimation
The subjects were brought into the room and were placed in the grey acclimation boxes (described previously) for 45 min. This was done to familiarize the rats with the handling, environment, and apparatus.
Training
As in previous designs (Ferguson et al., 2004b), training occurred in morning and afternoon (5 h later) sessions, allowing the animals to experience both the drug- and saline-paired context in the same day. The animals were given an injection of morphine sulfate (1.25 or 2.50 mg/kg [(C17H19NO3)2 H2SO4.5 H2O], equivalent to 0.94 or 1.88 mg/kg morphine base, respectively; Mallinckrodt, Hazelwood, MI) or 0.9% saline, and were placed in a training context (black or white) for 45 min before being returned to their home cage for 5 h. These doses of morphine are on the low end of those typically used for CPP (Tzschentke, 2007). In the afternoon session, subjects were injected with the other solution (rats that received saline with the first injection received morphine in the second). The rats were then placed in the other context for 45 min. Both the order of presentation and which context served as the drug-paired environment were counterbalanced across individuals.
Testing
On the day following the last training session, the rats were placed in the testing chamber and observed for 15 min to assess time spent in the drug-paired context, neutral area, and saline-paired context. Testing occurred in the middle of the day (12:00–14:00 h). Relative preference was determined using: (time spent in drug-paired context+1)/(time spent in vehicle-paired context+1). A “1” was added to both the numerator and denominator to avoid dividing by zero.
Self-administration procedure
Apparatus
Self-administration took place in operant chambers (model E10-10; Coulbourn, Allentown, PA) enclosed in sound-attenuating cubicles. Each chamber was equipped with two levers with a stimulus light positioned over each. Infusion pumps (Razel Scientific Instruments, Stanford, CT) controlled drug delivery to each of the boxes, and a 20-mL syringe delivered an IV infusion (160 μL) over a 6-sec time frame. The chambers were interfaced with two IBM computers, which controlled drug delivery and recorded lever depressions.
Self-administration procedure
All animals were implanted with a jugular catheter, and 5 days later received a contusion or a sham injury or remained intact. Twenty-four hours following the contusion or sham surgery, locomotor function was assessed and the animals were placed in the self-administration chambers (9:00 pm to 9:00 am). In the first self-administration experiment (12 subjects total, n=4), depression of the right lever (FR1) resulted in an IV infusion of 1.5 mg morphine (equivalent to 1.13 mg morphine base), with a maximum total dose of 30 mg morphine/day. This dose is similar to what is used clinically in humans. Patients receive up to 120 mg/kg morphine a day at the beginning of treatment (30 mg morphine is between 75–120 mg/kg/day; Chu et al., 2006), with doses increasing as needed for pain relief, often up to 180 mg morphine/day (Clark, 2002; Schneider and Kirsh, 2010). We recorded the amount of morphine administered for the first 7 days following injury.
When examining dose effects in the self-administration paradigm (32 subjects total, n=4), depression of the right lever resulted in a 160-μL IV infusion of morphine sulfate (0.75, 1.50, or 3.00 mg, equivalent to 0.56, 1.13, or 2.26 mg morphine base), and depression of the left lever was not reinforced. The number of responses on the reinforced lever were recorded, and used to determine the amount of morphine the animal administered each day. Animal weights and BBB locomotor scores were recorded.
Statistical analyses
The results were analyzed using analysis of variance (ANOVA). In experiments with a continuous independent variable, mixed-design ANOVAs were used. In cases where significant between-subject differences were obtained (main effect of a single variable), group means were compared using the Duncan's New Multiple Range Test (p<0.05). Trend analyses were also used to identify dose-dependent changes in behavior.
Results
The results of three experiments are presented below. We first assessed the addictive potential of morphine in the acute phase of injury using a CPP paradigm. The second and third experiments use a self-administration paradigm with more chronic administration of morphine. In the second experiment we examined whether sham, contused, and intact rats would self-administer morphine at one concentration/dose. In the third, we examined self-administration in sham and contused rats using three different doses of morphine, and monitored recovery for a 42-day period following injury.
SCI increases morphine CPP in the acute phase of injury
In this experiment, we examined whether contused subjects would develop a preference for a morphine-paired context using two doses of morphine.
Baseline locomotor assessment
Contused subjects had BBB scores indicative of a moderate contusion injury (3.19±0.61; 4.19±0.83 BBB converted and unconverted respectively in the 1.25 mg/kg group; 4.25±0.47; 4.89±0.63 BBB converted and unconverted respectively in the 2.50 mg/kg group). Sham subjects in both groups had converted BBB scores of 12.00±0.00. ANOVA confirmed that the groups differed [F(2,42)=405, p<0.05]. Post hoc analyses showed that the contused rats differed from the sham controls (p<0.05). No other differences were significant (p>0.05).
Assessment of place preference
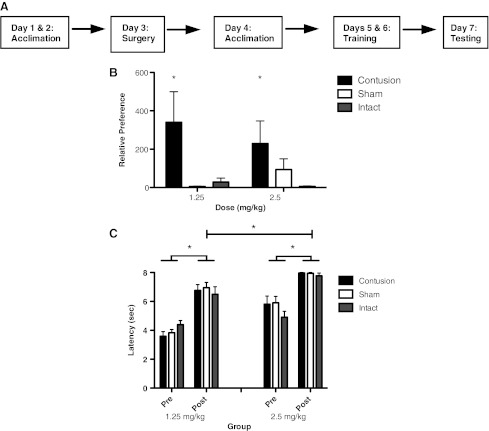
The animals were exposed to a total of two drug/context pairings over a period of 2 days, and place preference was assessed 24 h later (Fig. 1A). Preference for the morphine-paired context depended on both surgery and drug dose (Fig. 1B). At the doses tested, only contused rats exhibited a strong preference for the morphine-paired context, and this effect was most evident at the 1.25-mg/kg dose. Supporting this, an ANOVA yielded a significant main effect of surgery and a surgery×dose interaction (both Fs>6.46, p<0.05). The main effect of drug dose was not significant [F(1,36)=2.62, p>0.05].
FIG. 1.
For the conditioned place preference assessments, the subjects were trained according to the timeline depicted in A. They were exposed to a neutral context for 2 days prior to surgery, and again 1 day following surgery. Training took place over 2 consecutive days, and testing on a third day. As shown in B, contused subjects developed an increased preference for the morphine-paired context, relative to sham and intact controls, after only two morphine-context pairings. Relative preference was determined using: (time spent in drug-paired context+1)/(time spent in vehicle-paired context+1). Morphine administration produced analgesia in all groups, but was more robust in the 2.5-mg/kg group (C; *p<0.05).
Assessment of sensory reactivity after place preference training
To examine whether a contusion injury affects the anti-nociceptive impact of morphine, we tested the effect of IP morphine on tail withdrawal from radiant heat (tail flick test). Subjects first received an injection of saline, and baseline (Pre) tail flick latencies were assessed. Subjects were then administered the same dose of morphine that they had received during the conditioning phase, and tail flick latencies were re-assessed 30 min later. As shown in Figure 1C, subjects that previously received the high dose of morphine exhibited slightly higher baseline (Pre) tail flick latencies [F(1,42)=25.23, p>0.0001]. Baseline reactivity was not affected by surgery condition (both Fs<2.89, p>0.05). As expected, morphine produced robust anti-nociception [Post F(1,42)=185.87, p<0.0001], and subjects given the higher dose exhibited longer latencies [F(1,42)=38.97, p<0.0001]. More importantly, the impact of morphine treatment did not vary with surgery condition (all Fs<2.48, p>0.05). Morphine treatment also increased the latency to exhibit a vocalization response [F(1 42)=24.51, p<0.0001], and this was not affected by dose or surgery condition (all Fs<3.45, p>0.05).
SCI decreases morphine self-administration in the acute phase of injury
We found that a contusion injury does not affect the anti-nociceptive effect of morphine, but enhances the acquisition of a CPP. The latter suggests that a spinal injury may increase morphine-induced reward. If so, a contusion injury could enhance the drug's addictive potential in a self-administration paradigm. Sham, intact, and contused subjects were placed into self-administration chambers for 12 h for 7 nights. They received an IV infusion of 1.5 mg morphine (up to 30 mg) with each lever depression.
Response number
We first examined whether surgery condition affected the number of responses exhibited on the reinforced lever (Fig. 2A and B). Net responses on the reinforced lever were not affected by surgery condition (both F's <2.44, p>0.05).
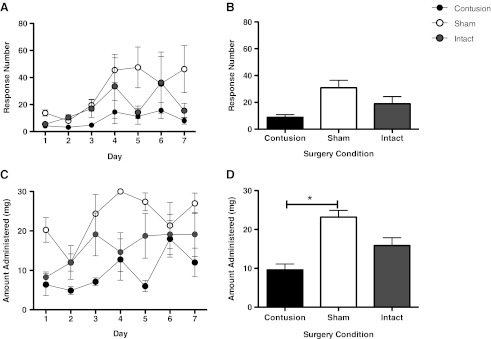
FIG. 2.
There were no group differences in the number of responses made on the reinforced lever (A and B). When examining the amount of morphine administered, however, (C and D), we found that contused animals administered significantly less morphine than sham controls (*p<0.05).
Morphine administered
Response number provides an index of the subject's propensity/capacity to bar press over the course of the session, but does not directly track the amount of drug that each subject received (because this dependent variable included responses performed after the maximum dose [30 mg] was received). For this reason, we also calculated the amount of morphine each subject self-administered. As shown in Figure 2C and D, subjects generally exhibited an increase in morphine administration over days [F(6,54)=3.52, p<0.05]. Contrary to our hypothesis, contused rats exhibited the lowest levels of morphine self-administration, yielding a main effect of surgery condition [F(2,9)=5.29, p<0.05]. The magnitude of this effect did not vary across days [F(12,54)=1.13, p<0.05]. Post-hoc comparisons showed that the contused rats self-administered less morphine than the sham controls (p<0.05). No other differences were significant (p>0.05).
Effects of injury on morphine self-administration are dose-dependent
Contrary to our hypothesis, contused rats self-administered less morphine. The present experiment examined this phenomenon over a range of doses. Sham and contused subjects were placed in self-administration chambers for 12 h for 7 days and received an IV injection of morphine (1.5 or 3.0 mg up to 30 mg/day) with each lever depression.
Response number
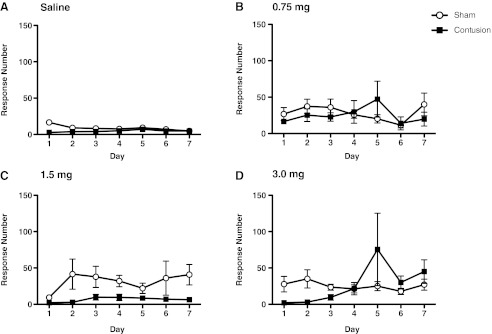
We first examined whether dose affected the number of responses exhibited on the reinforced lever (Fig. 3A–D). As expected, morphine treatment led to an increase in responses [F(3,24)=3.55, p<0.05). Net responses on the reinforced lever were not affected by surgery condition (both F's <2.49, p>0.05).
FIG. 3.
This figure depicts the number of responses made on the reinforced lever in the saline (A), 0.75- (B), 1.5- (C), and 3.0-mg (D) groups. Morphine treatment led to an increase in responses, but there was no effect of surgery condition.
Morphine administered
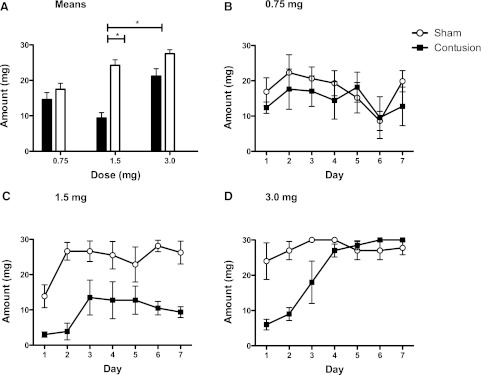
Net responses do not account for differences in morphine concentration across groups. As in the preceding experiment, we addressed this issue by examining the amount of morphine administered. Because the saline-treated subjects received no morphine, they were omitted from this analysis (Fig. 4A–D). As observed in the previous experiment, a contusion injury reduced self-administration at the intermediate dose (1.5 mg; Fig. 4C). A lower dose of morphine led to a moderate level of self-administration in both groups (Fig. 4B). At a high concentration (Fig. 4D), contused rats self-administered less morphine on days 1 and 2, but not days 4–7. ANOVA yielded a significant main effect of drug and surgery (both F's >5.59, p<0.05). In addition, the amount of morphine administered varied across days and the change observed depended upon both drug and surgery condition (all Fs >2.73, p<0.05). Post-hoc comparisons showed that contused rats given 1.5 mg of morphine administered less drug than sham-operated rats given the 1.5-mg dose and both of the 3.0-mg-treated groups (p<0.05). Contused and sham rats given 0.75 mg of morphine administered less than sham-operated animals given 3 mg (p<0.05). No other differences were significant (p>0.05).
FIG. 4.
Net responses do not account for differences in morphine administration across surgery groups. While there were no differences in morphine administration, for the sham and contused groups treated with the lowest dose (0.75 mg; B), a contusion injury reduced self-administration at the intermediate (1.5 mg; C) dose. At a high concentration (3.0 mg; D), contused rats also self-administered less morphine on days 1 and 2, but not on days 3–7. Additionally, contused animals in the 0.75- and 1.5-mg groups administered less morphine than contused subjects at the 3.0-mg dose (A); *p<0.05.
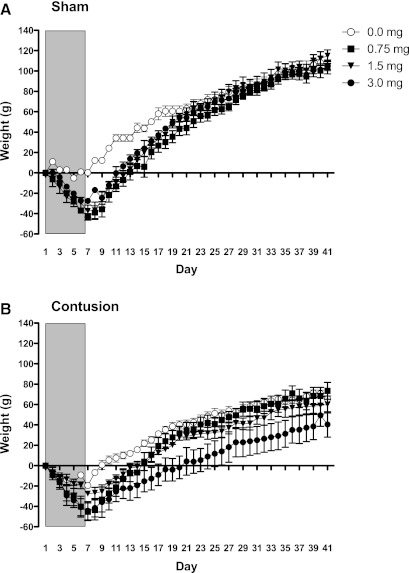
Weight gain
To assess the impact of morphine on recovery, we divided the data into two phases: during the period of self-administration (days 1–7), and during the period of recovery (days 8–42). Examining weight change during the period of self-administration, we found that morphine treatment led to weight loss over days independent of surgery condition. Supporting this, an ANOVA revealed main effects of drug treatment and day, as well as a drug×day interaction (both F's>6.51, p<0.05). No other terms were significant (all F's<3.16, p>0.05).
During the recovery period, the effect of morphine treatment on weight in the sham-operated rats waned across days (Fig. 5A). As observed in prior studies (Hook et al., 2007,2009,2011), contused rats gained less weight (Fig. 5B). While contused rats that received a low-to-moderate dose of morphine (0.75–1.5 mg) recovered to the same level as the saline-treated controls, subjects that had received the highest concentration (3.0 mg) exhibited poor recovery. An ANOVA confirmed that the main effects of drug, surgery, and day were significant (all F's>3.48, p<0.05). More importantly, the change in weight observed across days depended upon both drug and surgery treatment (all F's>1.90, p<0.0001). The drug×surgery interaction was not significant [F(3,24)=1.89, p>0.05].
FIG. 5.
Weights were monitored in two phases: during morphine administration (indicated by the grey shading), and during the recovery period. For the first 7 days, we found that morphine treatment led to weight loss independent of surgery condition (A and B). During the recovery period, the effect of morphine treatment on weight in the sham-operated rats waned across days (A). Contused rats treated with highest dose of morphine, however, gained significantly less weight than both sham-operated and saline-treated controls.
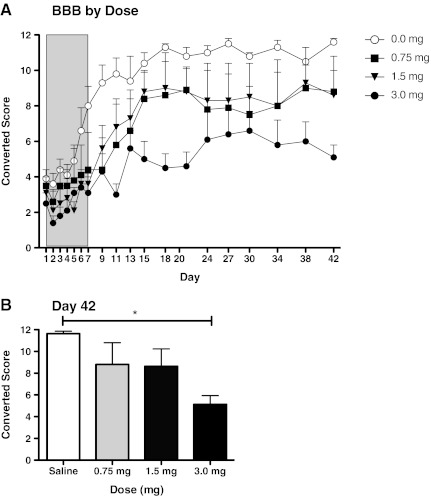
Locomotor recovery
BBB scores did not differ significantly on day 1 post-injury [F(3,12)<1.0, p>0.05]. As above, scores collected during and after the period of morphine self-administration were analyzed separately. During the period of self-administration, morphine treatment slowed recovery (Fig. 6). While the main effect of drug treatment was not significant [F(3,12)=2.40, p>0.05], trend analysis revealed a significant linear component (F=6.66, p<0.05]. Neither the quadratic nor cubic trends were significant (both F's<0.54, p>0.05).
FIG. 6.
Although Basso-Beattie-Bresnahan (BBB) scores did not differ significantly on day 1 post-injury, morphine administration significantly undermined locomotor recovery. Animals in the high-dose group (3.0 mg) received significantly lower BBB scores than animals administered saline or 1.5 mg morphine (A). Mean BBB scores at day 42 are shown in B (*p<0.05).
During the recovery period, morphine-treated rats continued to perform below the saline-treated controls. Again, while the main effect of drug treatment was not significant [F(3,12)=3.05, p>0.05], trend analyses yielded a significant linear component (F=7.72, p<0.05). Neither the quadratic nor cubic components were significant (both F's<0.43, p>0.05). Post-hoc comparisons of the group means showed that subjects who had received the highest concentration of morphine (3.0 mg) differed from the saline-treated controls (Fig. 6B) (p<0.05). No other differences were significant (p>0.05).
Discussion
In the acute phase of a spinal cord injury, contused subjects displayed an increased preference for a morphine-paired context relative to sham and uninjured controls. However, for the same phase of injury (days 1 and 2), contused subjects self-administered significantly less morphine than their sham counterparts. This decreased self-administration was time- and dose-dependent. After the acute phase of injury (days 3–7), contused subjects treated with the moderate dose (1.5 mg morphine) continued to administer significantly less morphine than the sham animals, whereas subjects treated with the high dose of morphine (3.0 mg) rapidly administered the total dose available at a rate commensurate with sham controls. Administration of this high dose of morphine undermined locomotor recovery and reduced weight gain after injury. These data concur with our previous studies (Hook et al., 2007, 2009, 2011), demonstrating that morphine has unwanted secondary consequences on recovery, regardless of the route of administration.
We chose to examine CPP and self-administration because these tasks depend on different forms of learning and can reveal divergent effects regarding the processes that underlie addictive behavior. The conditioning task relies on a form of pavlovian conditioning, wherein contextual cues (e.g., wall color, floor texture, and odor) serve as a kind of pavlovian conditioned stimulus (CS), and the drug-induced state acts as the unconditioned stimulus (US). Evidence suggests that CSs that have been paired with opiate administration acquire the capacity to act as “motivational magnets” that draw the subject to the opiate-paired context (Bardo and Neisewander, 1986). The CS is thought to elicit a kind of “wanting,” a conditioned motivation that can arise independent of whether human subjects report drug-induced pleasure (an emotional state that some characterize as “liking”; Berridge, 2009; Berridge et al., 2009).
Behavior in a self-administration paradigm depends on a distinct form of learning, instrumental conditioning, wherein subjects must perform a response (a bar-press) to obtain the reinforcer (an infusion of morphine). In this task the subject must work to obtain the goal, and for this reason, self-administration is thought to provide a better model of addictive behavior (Koob et al., 2009). The paradigm is also of clinical interest because it models patient-controlled delivery of analgesic agents (Martin et al., 2007; Sanchis-Segura and Spanagel, 2006). Here, behavior appears to be linked to the affective consequences of the opiate; in humans, drugs that bring pleasure and reduce pain are self-administered.
Prior work has shown that experimental manipulations can have divergent effects on wanting and liking (i.e., on motivation and pleasure). For example, destruction of dopaminergic pathways within the nucleus accumbens and striatum eliminate signs of drug wanting, but has little effect on behavioral signs of liking (Peciña et al., 2008). If spinal cord injury enhanced wanting, but diminished liking, it would have divergent effects on place preference and self-administration, enhancing the former while the latter is attenuated. We found divergent effects in these two paradigms, but only at moderate doses of morphine. A dose that induced a strong place preference in contused subjects, but not sham controls, led to decreased self-administration in contused subjects relative to sham animals.
Alternatively, the reduced administration may result from a decrease in the analgesic efficacy of morphine. Others have shown that SCI produces behavioral signs of neuropathic pain, and chronic pain is frequently reported after spinal injury in humans (Anderson, 2004; Perry et al., 2009; Siddall et al., 2003). Morphine administration would be expected to engage both neural systems associated with pleasure, and mechanisms that function to inhibit pain. We have shown that the dose of morphine used to induce a conditioned preference also produces robust anti-nociception. For this reason, injured rats may prefer the morphine-paired context because nociceptive input (pain signals) is inhibited. In the absence of pain, the anti-nociceptive effect of morphine may have little reward value (with the low doses used in the present study), and as a result, yield only a weak preference for the morphine-paired context. However, just as humans will self-administer an opiate to bring pain relief, we would expect injured rats to maintain a level of opiate infusion sufficient to diminish tonic pain. Indeed, injured rats given access to the low (0.75 mg) and moderate (1.5 mg) concentrations of morphine administered approximately 10 mg/kg of morphine over the 12-h training period, a dosage that would be sufficient to maintain anti-nociception (for approximately 6 h; the half-life of morphine is 2–3 h) if it were given in one administration. However, individual administrations (with each lever press) of the low (0.75 mg) and moderate (1.5 mg) doses may not provide sufficient analgesia with each administration for the animals to continue responding (thus allowing larger amounts of morphine to accumulate). This may explain the reduced administration seen in these groups, relative to sham controls treated with 1.5 mg of morphine, and both contused and sham rats treated with the 3-mg dose. Future studies will further delineate the roles of addiction versus analgesia in the spinal contusion model.
Although motivation variables remain to be determined, the behavior of the sham rats treated with the higher doses of morphine is suggestive of addiction. In a self-administration task, addictive behavior is evident from the near-maximal response to obtain the full amount of opiate available. Inspection of the amount of morphine taken by sham-operated rats (Fig. 4) indicates that an addictive tendency emerged at a moderate concentration (1.5 mg), and was fully evident at the high concentration (3.0 mg). Injured rats did not engage in the same level of addictive behavior, and even at the high concentration of morphine, this effect did not emerge until late in training. Sham-operated rats administered more morphine than intact rats (Fig. 2), possibly because the nociceptive pain from surgery potentiates the development of addiction. This pattern of results suggests that SCI reduces susceptibility to addiction, but does not eliminate it. Moreover, when subjects self-administered a high concentration of morphine, far more than that needed to control ongoing pain, the opiate adversely affected physiological signs of recovery (weight gain and locomotor function). These results are consistent with past studies that suggest that neuropathic pain can attenuate the development of addictive behavior (Martin et al., 2007; Ozaki et al., 2002; Lyness et al., 1989), and imply that at a low-to-moderate morphine concentration (and limited dosage), patient-controlled analgesia may have a minimal effect on long-term recovery. Care is warranted, however, because: (1) the dose-response function was not step-wise, but rather was linear; and (2) if addictive behavior does emerge, subjects may self-administer the drug at a dose that brings long-term harm.
Further work is needed to elucidate how spinal injury and opiate treatment interact, and to identify safe treatment regimens. A common link may involve injury-induced changes in immune function. Immediately following SCI, there is an increase in the number of lymphocytes, microglia, and astrocytes at the injury site (Ankeny and Popovich, 2009; Ankeny et al., 2006; Alexander and Popovich, 2009). Microglia expressing the non-classic opioid receptor TLR4 are activated within 72 h of SCI (Kigerl et al., 2007), and this activation is further increased by chronic morphine administration (Cao et al., 2010). Spinal cord injury, TLR4 activation, and morphine administration all increase mRNA and protein levels of the proinflammatory cytokine IL-1β in spinal cord tissue (Hutchinson et al., 2008; Liu et al., 2008; Lewis et al., 2010; Hook et al., 2011), which could contribute to the opiate-induced impairment in locomotor function. Supporting this, Hook and colleagues (2011) showed that pretreatment with an IL-1-receptor antagonist (IL-1ra) blocked the adverse effect of morphine exposure on locomotor recovery. In other paradigms, glial activation and cytokine release have been shown to be modulate opiate effectiveness (Hutchinson et al., 2008,2011), and this process could contribute to injury-induced changes in opiate self-administration.
Additional studies are also needed to disentangle how injury affects opiate mechanisms at different levels of the nervous system (e.g., brain versus spinal cord), and to identify effective analgesics that do not impede recovery. If, as hypothesized, the adverse effects of opiate treatment on recovery are linked to their action at non-classic receptors (e.g., TLR4; Watkins et al., 2009), drug cocktails that combine an opioid agonist with agents that reduce glial activation could have therapeutic benefits. Of course in the case of spinal injury, this presents a special challenge, given that alterations in immune function per se can have myriad effects on recovery (Ankeny et al., 2009; Gensel et al., 2011; Kigerl et al., 2009). For this reason, alternative therapeutic treatments that inhibit nociceptive signals (e.g., epidural lidocaine and hypothermia) may offer an attractive treatment alternative during the acute stage of injury.
Acknowledgments
These experiments were supported by NS041548, DA 031197, and HD058412 to James Grau and Michelle Hook, and by Mission Connect, a project of the TIRR foundation.
The authors would like to acknowledge Kevin C. Hoy Jr., Milly Lee, and Sandra Garraway for their comments on previous drafts.
A portion of the data from this study has been previously presented in abstract form.
Author Disclosure Statement
No competing financial interests exist.
References
- Alexander J.K. Popovich P.G. Neuroinflammation in spinal cord injury: Therapeutic targets for neuroprotection and regeneration. Prog. Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- Anderson K.D. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Ankeny D.P. Popovich P.G. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience. 2009;158:1112–1123. doi: 10.1016/j.neuroscience.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny D.P. Guan Z. Popovich P.G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny D.P. Lucin K.M. Sanders V.M. McGaugy V.M. Popovich P.G. Spinal cord injury triggers systemic autoimmunity: Evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- Attal N. Mazaltarine G. Perrouin-Verbe B. Albert T. Chronic neuropathic pain management in spinal cord injury patients What is the efficacy of pharmacological treatments with a general mode of administration? (oral, transdermal, intravenous) Ann. Phys. Rehab. Med. 2009;52:124–141. doi: 10.1016/j.rehab.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Backonja M.M. Stacey B. Neuropathic pain symptoms relative to overall pain rating. Pain. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Ballantyne J.C. LaForge K.S. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129:235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Ballantyne J.C. Mao J. Opioid therapy for chronic pain. N. Engl. J. Med. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- Bardo M.T. Neisewander J.L. Single-trial conditioned place preference using intravenous morphine. Pharmacol. Biochem. Behav. 1986;25:1101–1105. doi: 10.1016/0091-3057(86)90092-4. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beatie M.S. Breshnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bell K. Salmon A. Pain, physical dependence, and pseudoaddiction: Redefining addiction for ‘nice’ people? Intern. J. Drug Policy. 2009;20:170–178. doi: 10.1016/j.drugpo.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Berridge K.C. Robinson T.E. Aldridge J.W. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C. Wanting and liking: Observations from the neuroscience and psychology laboratory. Inquiry (Oslo) 2009;52:4. doi: 10.1080/00201740903087359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budh C.N. Lundeberg T. Use of analgesic drugs in individuals with spinal cord injury. J. Rehabil. Med. 2005;37:87–94. doi: 10.1080/16501970410020455. [DOI] [PubMed] [Google Scholar]
- Cao F. Gao F. Xu A.J. Chen Z.J. Chen S.S. Yang H. Yu H.H. Mei W. Liu X.J. Xiao X.P. Yang S.B. Tian X.B. Wang X.R. Tian Y.K. Regulation of spinal neuroimmune responses by prolonged morphine treatment in a rat model of cancer induced bone pain. Brain Res. 2010;1326:162–173. doi: 10.1016/j.brainres.2010.02.039. [DOI] [PubMed] [Google Scholar]
- Chang G. Chen L. Mao J. Opioid tolerance and hyperalgesia. Med. Clin. North Am. 2007;91:199–211. doi: 10.1016/j.mcna.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Chu L.F. Clark D.J. Angst M.S. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective pain study. J. Pain. 2006;7:44–48. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Clark J.D. Chronic pain prevalence and analgesic prescribing in a general medical population. J. Pain Symptom Manage. 2002;23:31–37. doi: 10.1016/s0885-3924(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Compton W.M. Volkow N.D. Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Constantini S. Young W. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J. Neurosurg. 1994;80:97–111. doi: 10.3171/jns.1994.80.1.0097. [DOI] [PubMed] [Google Scholar]
- Contet C. Filliol D. Matifas A. Kieffer B.L. Morphine-induced analgesic tolerance, locomotor sensitization and physical dependence do not require modification of mu opioid receptor, cdk5 and adenylate cyclase activity. Neuropharmacology. 2008;54:475–486. doi: 10.1016/j.neuropharm.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Dersh J. Mayer T.G. Gatchel R.J. Polatin P.B. Theodore B.R. Mayer E.A.K. Prescription opioid dependence is associated with poorer outcomes in disabling spinal disorders. Spine. 2008;33:2219–2227. doi: 10.1097/BRS.0b013e31818096d1. [DOI] [PubMed] [Google Scholar]
- Ferguson A.R. Hook M.A. Garcia G. Bresnahan J.C. Beattie M.S. Grau J.W. A simple transformation that improves the metric properties of the BBB scale. J. Neurotrauma. 2004a;21:1601–1613. doi: 10.1089/neu.2004.21.1601. [DOI] [PubMed] [Google Scholar]
- Ferguson A.R. Patton B.C. Bopp A.C. Meagher M.W. Grau J.W. Brief exposure to a mild stressor enhances morphine-conditioned place preference in male rats. Psychopharmacology. 2004b;175:47–52. doi: 10.1007/s00213-004-1780-3. [DOI] [PubMed] [Google Scholar]
- Gensel J.C. Donnelly D.J. Popovich P.G. Spinal cord injury therapies in humans: An overview of current clinical trials and their potential effects on intrinsic CNS macrophages. Expert Opin. Ther. Targets. 2011;15:505–518. doi: 10.1517/14728222.2011.553605. [DOI] [PubMed] [Google Scholar]
- Gruner J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- Heinemann A.W. McGraw T.E. Brandt M.J. Roth E. Dell'Oliver C. Prescription medication misuse among persons with spinal cord injuries. Int. J. Addict. 1992;27:301–316. doi: 10.3109/10826089209068744. [DOI] [PubMed] [Google Scholar]
- Heinemann A.W. Schnoll S. Brandt M. Maltz R. Keen M. Toxicology screening in acute spinal cord injury. Alcohol Clin. Exp. Res. 1988;12:815–819. doi: 10.1111/j.1530-0277.1988.tb01352.x. [DOI] [PubMed] [Google Scholar]
- Hojsted J. Sjogren P. Addiction to opioids in chronic pain patients: A literature review. Eur. J. Pain. 2007;11:490–518. doi: 10.1016/j.ejpain.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Hook M.A. Liu G.T. Washburn S.N. Ferguson A.R. Bopp A.C. Huie J.R. Grau J.W. The impact of morphine after a spinal cord injury. Behav. Brain Res. 2007;179:281–293. doi: 10.1016/j.bbr.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook M.A. Moreno G. Woller S. Puga D. Hoy K. Baldyn R. Grau J.W. Intrathecal morphine attenuates recovery of function after a spinal cord injury. J. Neurotrauma. 2009;26:741–752. doi: 10.1089/neu.2008.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook M.A. Washburn S.N. Moreno G.L. Woller S.A. Puga D.A. Lee K.H. Grau J.W. An IL-1 receptor antagonist blocks morphine-induced attenuation of locomotor recovery after spinal cord injury. Brain Behav. Immun. 2011;25:349–359. doi: 10.1016/j.bbi.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch C.E. Pharmacology of chronic pain after spinal cord injury: Novel acute and chronic intervention strategies. In: Yezierski R.P., editor; Burchiel K.J., editor. Spinal Cord Injury Pain: Assessment, Mechanisms, Management. Progress in Pain Research and Management. IASP Press; Seattle: 2002. pp. 189–204. [Google Scholar]
- Hutchinson M.R. Coats B.D. Lewis S.S. Zhang Y. Sprunger D.B. Rezvani N. Baker E.M. Jekich B.M. Wieseler J.L. Somogyi A.A. Martin D. Poole S. Judd C.M. Maier S.F. Watkins L.R. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav. Immun. 2008;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M.R. Shavit Y. Grace P.M. Rice K.C. Maier S.F. Watkins L.R. Exploring the neuroimmunopharmacology of opioids: An integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol. Rev. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives T.J. Chelminski P.R. Hammett-Stabler C.A. Malone R.M. Perhac J.S. Potisek N.M. Shilliday B.B. DeWalt D.A. Pignone M.P. Predictors of opioid misuse in patients with chronic pain: A prospective cohort study. BMC Health Serv. Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz W.A. Barkin R.L. Dilemmas in chronic/persistent pain management. Am. J. Therapeutics. 2008;15:256–264. doi: 10.1097/MJT.0b013e3181671c5a. [DOI] [PubMed] [Google Scholar]
- Kigerl K.A. Gensel J.C. Ankeny D.P. Alexander J.K. Donnelly D.J. Popovich P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl K.A. Lai W. Rivest S. Hart R.P. Satoskar A.R. Popovich P.G. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J. Neurochem. 2007;102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- Koob G.F. Lloyd G.K. Mason B.J. Development of pharmacotherapies for drug addiction: A Rosetta Stone approach. Nat. Rev. Drug. Discov. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S.S. Hutchinson M.R. Rezvani N. Loram L.C. Zhang Y. Maier S.F. Rich K.C. Watkins L.R. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165:569–583. doi: 10.1016/j.neuroscience.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D. Shi X. Qiao Y. Angst M. Yeomans D.C. Clark J.D. Chronic morphine administration enhances nociceptive sensitivity and local cytokine production after incision. Mol. Pain. 2008;4 doi: 10.1186/1744-8069-4-7. np. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Xu G.Y. Johnson K.M. Echetebu C. Ye Z.S. Hulsebosch C.E. McAdoo D.J. Regulation of interleukin-1beta by the interleukin-1 receptor antagonist in the glutamate-injured spinal cord: Endogenous neuroprotection. Brain Res. 2008;1231:63–74. doi: 10.1016/j.brainres.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Lyness W.H. Smith F.L. Heavner J.E. Iacono C.U. Garvin R.D. Morphine self-administration in the rat during adjuvant-induced arthritis. Life Sci. 1989;45:2217–2224. doi: 10.1016/0024-3205(89)90062-3. [DOI] [PubMed] [Google Scholar]
- Martin T.J. Kim S.A. Buechler N.L. Porreca F. Opioid self-administration in the nerve-injured rat: Relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology. 2007;106:312–322. doi: 10.1097/00000542-200702000-00020. [DOI] [PubMed] [Google Scholar]
- Michna E. Jamison R.N. Pham L.D. Ross E.L. Janfaza D. Nedeljkovic S.S. Narang S. Palombi D. Wasan A.D. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin. J. Pain. 2007;23:179–189. doi: 10.1097/AJP.0b013e31802b4f95. [DOI] [PubMed] [Google Scholar]
- Mills C.D. Johnson K.M. Hulsebosch C.E. Group I metabotropic glutamate receptors in spinal cord injury: Roles in neuroprotection and the development of chronic central pain. J. Neurotrauma. 2001;19:23–42. doi: 10.1089/089771502753460213. [DOI] [PubMed] [Google Scholar]
- Morasco B.J. Dobscha S.K. Prescription medication misuse and substance use disorder in VA primary care patients with chronic pain. Gen. Hosp. Psychiatry. 2008;30:93–99. doi: 10.1016/j.genhosppsych.2007.12.004. [DOI] [PubMed] [Google Scholar]
- O'Connor A.B. Dworkin R.H. Treatment of neuropathic pain: an overview of recent guidelines. Am. J. Med. 2009;122(10 Suppl.):S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Ozaki S. Narita M. Narita M. Iino M. Sugita J. Matsumura Y. Suzuki T. Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: Implication of the reduction in mu-opioid receptor functions in the ventral tegmental area. J. Neurochem. 2002;82:1192–1198. doi: 10.1046/j.1471-4159.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- Parisod E. Siddall P.J. Viney M. McCleland J.M. Cousins M.J. Allodynia after acute intrathecal morphine administration in a patient with neuropathic pain after spinal cord injury. Anesth. Analg. 2003;97:183–186. doi: 10.1213/01.ane.0000068482.67289.1a. [DOI] [PubMed] [Google Scholar]
- Peciña S. Opioid reward ‘liking’ and ‘wanting’ in the nucleus accumbens. Physiol. Behav. 2008;94:675–680. doi: 10.1016/j.physbeh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Perry K.N. Nicholas M.K. Middleon J. Spinal cord injury-related pain in rehabilitation: A cross-sectional study of relationships with cognitions, mood, and psychical function. Eur. J. Pain. 2009;13:5. doi: 10.1016/j.ejpain.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Przewlocki R. Przewlocki B. Opioids in neuropathic pain. Curr. Pharm. Des. 2005;11:3013–3025. doi: 10.2174/1381612054865055. [DOI] [PubMed] [Google Scholar]
- Radnitz C.L. Tirch D. Substance misuse in individuals with spinal cord injury. Int. J. Addict. 1995;30:1117–1140. doi: 10.3109/10826089509055831. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C. Spanagel R. Behavioral assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Siddall P.J. McClelland J.M. Rutkowski S.B. Cousins M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Sindrup S.H. Jensen T.S. Efficacy of pharmacological treatments of neuropathic pain: An update and effect related to mechanism of drug action. Pain. 1999;83:389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- Schneider J.P. Kirsh K.L. Defining clinical issues around tolerance, hyperalgesia, and addiction: a quantitative and qualitative outcome study of long-term opioid dosing in a chronic pain practice. J. Opioid Manag. 2010;6:385–395. doi: 10.5055/jom.2010.0036. [DOI] [PubMed] [Google Scholar]
- Trescot A.M. Helm S. Hansen H. Benyamin R. Glaser S.E. Aslaka R. Patel S. Manchikanti L. Opioids in the management of chronic non-cancer pain: An update of American Society of the Interventional Pain Physicians' (ASIPP) guidelines. Pain Physician. 2008;11:S5–S62. [PubMed] [Google Scholar]
- Tzschentke T.M. Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict. Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Warms C.A. Turner J.A. Marshall H.M. Cardenas D.D. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin. J. Pain. 2002;18:154–163. doi: 10.1097/00002508-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Watkins L.R. Hutchinson M.R. Rice K.C. Maier S.F. The “toll” of opioid-induced glial activation: Improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol. Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widerstron-Noga E.G. Turk D.C. Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: Influence of pain and psychosocial characteristics. Spinal Cord. 2003;41:600–609. doi: 10.1038/sj.sc.3101511. [DOI] [PubMed] [Google Scholar]
- Yu W. Hao J. Xu X. Wiesenfeld-Hallin Z. Comparison of the anti-allodynic and antinociceptive effects of systemic, intrathecal, and intracerebroventricular morphine in a rat model of central neuropathic pain. Eur. J. Pain. 1997a;1:17–29. doi: 10.1016/s1090-3801(97)90049-5. [DOI] [PubMed] [Google Scholar]
- Yu W. Hao J. Xu X. Wiesenfeld-Hallin Z. The development of morphine tolerance and dependence in rats with chronic pain. Brain Res. 1997b;756:141–146. doi: 10.1016/s0006-8993(97)00132-7. [DOI] [PubMed] [Google Scholar]
- Zhao C. Tall J.M. Meyer R.A. Raja S.N. Antiallodynic effects of systemic and intrathecal morphine in the spared nerve injury model of neuropathic pain in rats. Anesthesiology. 2004;100:905–911. doi: 10.1097/00000542-200404000-00021. [DOI] [PubMed] [Google Scholar]