Abstract
Although smallpox has been eradicated, other diseases caused by virulent orthopoxviruses such as monkeypox virus (MPV) remain endemic in remote areas of western and central sub-Saharan Africa, and represent a potential biothreat due to international travel and/or inadvertent exposure. Unfortunately, extensive antigenic cross-reactivity among orthopoxviruses presents a challenge to serological diagnosis. We previously reported a 20mer peptide-based ELISA that identified recent MPV infection with >90% sensitivity and >90% specificity. However, the sensitivity of this approach was not determined with samples obtained at later time points after antibody titers had declined from their peak levels. To improve assay sensitivity for detecting MPV-specific antibodies at later time points, we compared diagnostic 20mer peptides to 30mer peptides. In addition, optimal 30mer peptides were tested in combination or after conjugating selected peptides to a carrier protein (bovine serum albumin) to further improve assay performance. An optimized combination of four unconjugated 30mer peptides provided 100% sensitivity for detecting MPV infection at 2–6 months post-infection, 45% sensitivity for detecting MPV infection at >2 years post-infection, and 99% specificity. However, an optimized combination of two peptide conjugates provided 100% sensitivity for detecting MPV infection at 2–6 months post-infection, 90% sensitivity for detecting MPV infection at >2 years post-infection, and 97% specificity. Peptide-based ELISA tests provide a relatively simple approach for serological detection of MPV infection. Moreover, the systematic approach used here to optimize diagnostic peptide reagents is applicable to developing improved diagnostics to a broad range of other viruses, and may be particularly useful for distinguishing between closely-related viruses within the same genus or family.
Key Words: Antibody, Diagnostics, ELISA, Monkeypox, Peptide
Introduction
Monkeypox (MPV) is a member of the orthopoxvirus genus and represents a zoonotic disease endemic to remote regions of western and central Sub-Saharan Africa (Rimoin et al. 2010). The first recorded outbreak of MPV outside of Africa occurred in the United States in 2003 following importation of MPV-infected rodents (Reed et al. 2004). This demonstrates that in the era of global travel, rare or exotic pathogens can spread rapidly beyond their native geographical boundaries. Epidemiological surveillance of MPV has been hindered by a lack of simple diagnostic tools that provide a broad window for detection, and that are capable of differentiating between MPV immunity and pre-existing immunity resulting from vaccination against smallpox (i.e., vaccinia virus, a closely-related orthopoxvirus). Members of the orthopoxvirus genus, including variola, MPV, and vaccinia (Fenner et al. 1988), share many conserved surface antigens, resulting in extensive cross-reactivity in standard serological assays such as ELISA, radioimmunoassay, and immunofluorescence (Gispen et al. 1976; Marennikova et al. 1981; Karem et al. 2005; Jezek et al. 1987a). Thus, such assays can determine prior orthopoxvirus exposure, but are unable to reliably differentiate among infecting species. Diagnosis by standard clinical algorithms (Seward et al. 2004) may be difficult or unreliable (Klein et al. 2004), especially in adults who were previously vaccinated against smallpox and who exhibit mild symptoms (Sejvar et al. 2004; Karem et al. 2007), or are asymptomatic (Hammarlund et al. 2005). Non-classical presentation of disease during primary MPV infection, such as illness without exanthem, has also been reported (Jezek et al. 1986; Lewis et al. 2007). Similar to smallpox, MPV infection is often mistaken for chickenpox (i.e., varicella zoster virus) (Jezek et al. 1987b; Meyer et al. 2002; Rimoin et al. 2007), and this complicates diagnosis based on clinical symptoms alone (Sale et al. 2006; Rimoin et al. 2007). Bearing this in mind, it is important that simple, definitive assays for identification of virulent orthopoxvirus infections be developed for seroprevalence studies and potential use in outbreak situations.
Direct orthopoxvirus detection techniques such as PCR can be highly sensitive (Sofi Ibrahim et al. 2003; Kulesh et al. 2004; Olson et al. 2004). However, this approach relies strictly on the presence of virus during active infection, and likewise depends on adequate sample collection, storage, and processing, all of which may present unique challenges in the field. During the U.S. outbreak of MPV in 2003, 37 of >72 suspected or probable cases of MPV were confirmed by direct virological analysis (www.cdc.gov/od/oc/media/mpv/cases.htm) (Hammarlund et al. 2005). In contrast, immunodiagnostic tests are particularly well suited for epidemiological assessment of disease outbreaks as well as retrospective population studies because virus-specific IgG responses are long-lived (Crotty et al. 2003; Hammarlund et al. 2003; Putz et al. 2005; Amanna et al. 2007; Taub et al. 2008), and allow for a broad window of detection. Similarly to smallpox, monkeypox patients experience a 10- to 14-day incubation period, followed by 1–3 days of prodromal illness that may include fever and malaise prior to rash onset (Fenner et al. 1988). Orthopox-specific IgG has been detected as early as 2 days post-rash onset (Karem et al. 2005). Thus, the window of detection for IgG-based serological tests ranges from within days after onset of rash to several decades after resolving the infection.
A relatively large number of serological diagnostic approaches have been used to identify orthopoxvirus infection, including hemagluttination inhibition (Jezek et al. 1987a), gel precipitation (Gispen 1955; Gispen and Brand-Saathof 1974; Esposito et al. 1977), complement fixation (Esposito et al. 1977), cross-neutralization (Baxby 1982), immunofluorescence (Gispen et al. 1974, 1976), Western blot (Demkowicz et al. 1992; Jones-Trower et al. 2005; Dubois and Slifka 2008), radioimmunoassay/ELISA (Hutchinson et al. 1977; Marennikova et al. 1981; Jezek et al. 1987a; Karem et al. 2005; Hammarlund et al. 2005), or a modified post-adsorption ELISA (Dubois and Slifka 2008). Of these serological approaches, the ELISA technique is the least labor intensive and most likely to be amenable to high-throughput quantitative analysis. An IgM sandwich ELISA utilizing vaccinia antigen has also been described as sensitive and specific for detecting human MPV infection (Karem et al. 2005). An inherent limitation of IgM-based assays is the short time frame of IgM production, generally lasting only weeks to months after infection. In the case of MPV infection, the optimal detection window for IgM has been defined as >4 days and <77 days post-infection (Karem et al. 2005). While useful for diagnosing active infection, this relatively short diagnostic window reduces the opportunity to perform retrospective serological epidemiology. In contrast, virus-specific serum IgG can be detected for years after viral infection (Crotty et al. 2003; Hammarlund et al. 2003; Putz et al. 2005; Amanna et al. 2007; Taub et al. 2008).
Several studies have shown the utility of peptide-based diagnostics for identifying specific viral or bacterial infections. Peptide-based diagnostics have been particularly useful for detection of antibodies against human immunodeficiency virus (HIV). For instance, peptide reagents have been used to detect antibodies to HIV-1 and HIV-2 (Shin et al. 1997), distinguish between HIV-1 and HIV-2 (Gnann et al. 1987), or simultaneously identify HIV-1/2 in addition to herpes simplex virus (HSV) (Porstmann et al. 1993). Additionally, 96.7% sensitivity and 100% specificity have been reported for diagnosis of HSV-2, based on an ELISA using an 18-amino acid (AA) peptide from HSV-2 glycoprotein G (Oladepo et al. 2000). Peptide-based diagnostics are not just limited to viral pathogens, since a peptide-based ELISA utilizing a 26-AA peptide derived from the Borrelia burgdorferi VslE variant surface antigen has been shown to provide 100% sensitivity and 99% specificity for late-stage Lyme disease (Liang et al. 1999). Thus, peptide-based diagnostics play an ever-expanding role in serological identification of a variety of infectious diseases.
We previously reported a peptide-based ELISA that identified MPV peptide-specific IgG responses with >90% sensitivity and specificity during the first 2–4 months after MPV infection (Hammarlund et al. 2005). However, as antibody responses declined during the first 1–2 years after infection, assay sensitivity using 20mer peptides declined. To improve diagnostic sensitivity at later time points after antibody levels have declined but stabilized (e.g., 24–30 months after MPV infection), we developed a systematic approach to optimizing peptides that included the use of longer peptide sequences and combining different peptides to improve diagnostic sensitivity and specificity. An additional step involving conjugation of peptides to a carrier protein also resulted in marked improvement of detection of prior MPV infection in convalescent subjects. Together, these approaches to MPV diagnostic test development may be useful for retrospective field epidemiological studies or in the future development of rapid point-of-care diagnostic assays.
Methods
Subjects
Study subjects were selected from adults who presented with signs and symptoms of primary MPV infection following exposure to MPV-infected animals during the 2003 Wisconsin outbreak (Reed et al. 2004; Hammarlund et al. 2005). Cases were confirmed by virological (Reed et al. 2004) and/or immunological diagnostic tests (Hammarlund et al. 2005; Dubois and Slifka 2008). Samples from MPV patients with no previous smallpox vaccination history who were infected 2–6 months (n=10) or 24–30 months (>2 years, n=10) prior were assessed in this study. Eight of 10 subjects donated paired samples from 2–6 months post-infection, as well as 24–30 months post-infection that were represented in both groups. Control subjects included smallpox vaccinees between 2–4 months post-vaccination (n=10); revaccinated subjects between 2–4 months post-booster vaccination (n=10); smallpox vaccinees between 20 and 40 years post-vaccination (n=20); and orthopoxvirus-naive individuals (n=20). All study subjects provided informed written consent and completed a medical history questionnaire prior to participation in the study. Studies involving human subjects were reviewed and approved by the Institutional Review Board for Oregon Health and Science University.
Plasma and serum
Plasma and serum were isolated from whole blood as previously described (Hammarlund et al. 2005) and stored at −80°C. Preliminary studies indicated that plasma and serum could be used interchangeably in these assays (data not shown). Anti-smallpox International Serum Standard (Anderson and Skegg 1970) (1st British Standard, 63/024), was obtained from the National Institute for Biological Standards and Controls, Hertfordshire, U.K.
Peptides
MPV peptides were previously identified by screening a library of 20-AA peptides (overlapping by 10 AA) from MPV-Zaire B21R (accession no. NP_536609) (Hammarlund et al. 2005). Additional 20mer peptides were identified by screening a peptide library of the related variola major B22R (variola Bangladesh 1975, accession no. AAA60931) protein. The B22R peptide library was prepared using the PepScreen platform (Sigma-Genosys, St. Louis, MO). High-performance liquid chromatography-purified peptides of 30 AA (86–98% purity) and 40 AA (72% purity) in length were synthesized by Sigma-Genosys. An irrelevant 20mer peptide (peptide 90, AITAITGIIDTIKDIYYMFS, 90% purity) was used to establish nonspecific antibody binding in each experiment. Peptides were dissolved in DMSO (10 mg/mL). Working stocks were adjusted to 0.2 mg/mL in H2O and stored at −80°C.
Peptide sequences B21R.179/180, B21R.185/186, B22R.64/65, and B22R.82/83 were synthesized as 31mer peptides with an additional N-terminal cysteine residue (82–95% purity), and conjugated to bovine serum albumin (BSA) by m-maleimidobenzoyl-N-hydroxysuccinimide ester coupling (performed at Sigma-Genosys or Genscript, Inc., Piscataway, NJ). Peptide-conjugates were reconstituted to 2 mg/mL in H2O and stored at −80°C.
ELISA
Peptide-based ELISA was performed as previously described (Hammarlund et al. 2005) using 100 μL/well of peptide at 2 μg/mL with the following modifications: 4–8 control wells coated with peptide 90 were included for each subject on each plate to establish nonspecific background binding. For ELISA wells coated with multiple peptides, the final concentration was 2 μg/mL for each peptide in the mix. BSA-conjugated peptides were coated at an optimized concentration of 0.1 μg/mL (100 μL/well) diluted in PBS, and 4–6 wells were coated with BSA (0.1 μg/mL, fraction V; Sigma-Genosys) to provide nonspecific background adjustment for each subject. Subjects were considered seropositive if the OD490 of the test well was twice that observed in wells coated with control antigen. Confidence intervals (95% CI) were determined based on 3–5 independent experiments for unconjugated peptides, and 3 experiments for peptide-BSA conjugates. Sensitivity was defined as the number of true-positives/(true-positives + false-negatives), and specificity was calculated as 1 – [number of false-positives/(false-positives + true-negatives)]. Data from an individual experiment were removed if a technical failure occurred, or if a dataset could not be repeated. On rare occasions, if an OD490 >1.0 was observed in the irrelevant background antigen wells, then the results were deemed equivocal and the experiment was repeated.
Results
Peptide length alters diagnostic sensitivity and specificity
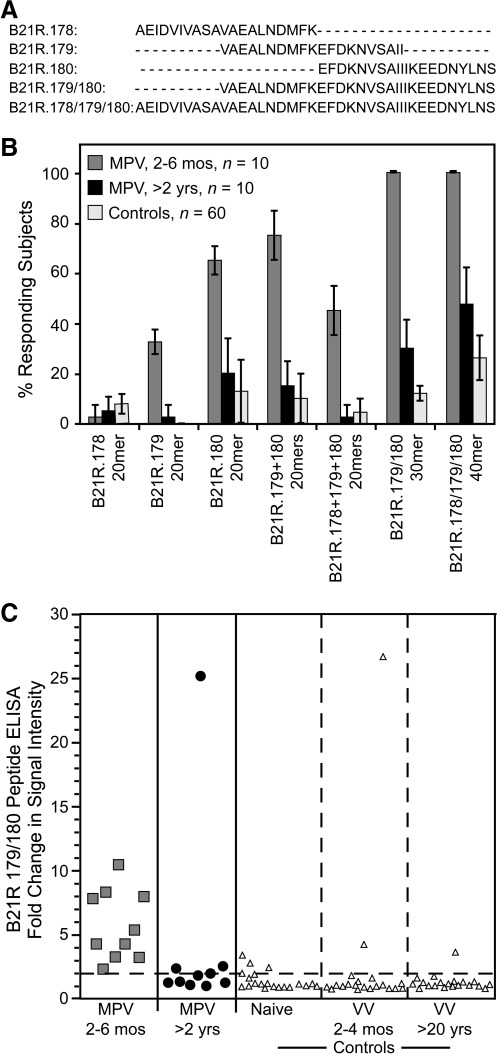
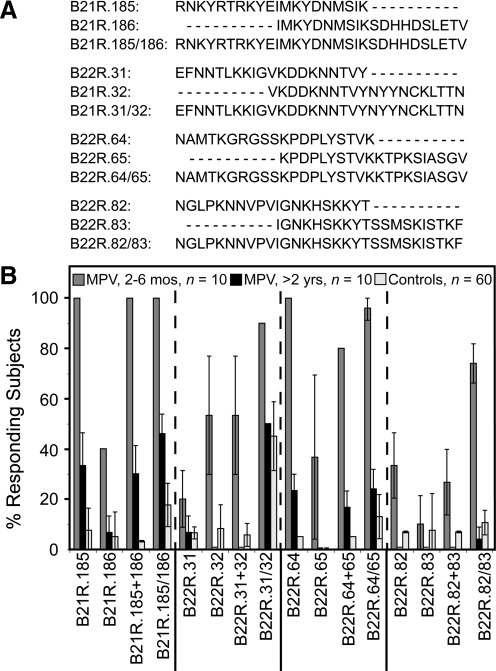
The monkeypox outbreak in the Midwestern United States provided the opportunity to test different approaches to orthopoxvirus-specific diagnostics (Hammarlund et al. 2005). Since there are no other orthopoxviruses in the U.S. that are known to routinely infect humans, we could measure the specificity of our diagnostic tests using orthopoxvirus-seronegative serum samples, or serum samples from subjects who had been immunized either recently or in the distant past with vaccinia virus (VV), which represents an antigenically-related orthopoxvirus and a stringent negative control for preparing MPV-specific diagnostic tests. In these studies, we followed a stepwise approach to developing peptide reagents for serological detection of MPV infection (Fig. 1). Although orthopoxviruses are closely related and have highly-conserved genomes (Lefkowitz et al. 2005), there are several genes encoded by MPV that are absent, truncated, or mutated in vaccinia, and this provides an opportunity for developing differential diagnostics that can be used to distinguish antiviral immunity stemming from prior infection by VV and MPV (Gispen and Brand-Saathof 1976; Esposito et al. 1977; Turner and Baxby 1979; Harper et al. 1979; Arita and Tagaya 1980; Hammarlund et al. 2005; Dubois and Slifka 2008). We previously identified 20mer peptides from the MPV B21R protein (an 1879-AA protein that is absent from the VV genome) that were used to diagnose recent MPV infection (2–12 months post-infection) (Hammarlund et al. 2005). While screening this library of overlapping 20mers, we identified several overlapping peptides that formed clusters of strong serological reactivity. This suggested that adjacent peptides might represent components of a larger epitope, and that combining adjacent peptides might improve assay sensitivity. We investigated this in the current study by mixing combinations of 2 or 3 overlapping individual 20mers together in the same ELISA well, or by synthesizing 30mer or 40mer peptides that spanned the same region as the individual overlapping 20mer peptides (Fig. 2a). Peptides of 30 AA or 40 AA were synthesized representing MPV-Zaire B21R.179/180 (AAs 1780–1810), and B21R.178/179/180 (AAs 1770–1810), respectively, and tested by ELISA for sensitivity and specificity (Fig. 2b). Similarly to previously published results (Hammarlund et al. 2005), the 20mer peptide, B21R.180, was detected with 65% sensitivity by MPV samples obtained 2–6 months post-infection, and with 87% specificity. However, antiviral antibody titers often decline sharply during the first 1–3 years after infection or vaccination before reaching more durable plateau levels (Amanna et al. 2007), and we found that total orthopoxvirus-reactive antibody titers (measured using a VV lysate for ELISA) decreased nearly 80%, from 5090±2283 ELISA units 2–6 months post-MPV infection, to 1132±508 ELISA units >2 years post-infection (24–30 months). The decline in total orthopoxvirus-specific antibody levels may explain why only 20% of MPV samples obtained >2 years post-infection could be detected with any of the 20mer B21R.178, B21R.179, or B21R.180 peptides, a frequency similar to that observed with negative controls (Fig. 2). Mixing equal concentrations of overlapping 20mer peptides in the same ELISA well resulted in modestly improved sensitivity in one case (e.g., B21R.179 + B21R.180 peptides), but decreased sensitivity in another case (e.g., B21R.178 + B21R.179 + B21R.180 peptides), and this appeared to depend largely on the antigenicity of the epitopes that were combined. Increasing peptide length from 20 AA to 30 AA greatly improved sensitivity, from 65% to 100% for recent MPV samples (2–6 months post-infection), and sensitivity improved from 20% to 30% among MPV samples at >2 years post-infection, while maintaining 88% specificity among orthopoxvirus-naïve or VV-immune controls. Peptide B21R.178/179/180 (40mer) identified subjects 2–6 months post-infection with 100% sensitivity, and demonstrated marked improvement of sensitivity for samples examined >2 years post-infection (48%), but this result coincided with a substantial increase in reactivity with control samples (i.e., decreased specificity to 74%). The strength of the ELISA signal for samples tested on B21R.179/180 indicated that MPV-specific serum antibodies measured at 2–6 months post-infection could be readily distinguished from control samples using an assay cut-off defined as twofold over background, although positive samples analyzed >2 years post-infection tended to cluster near this threshold (Fig. 2c). Use of other cut-off values resulted in decreased sensitivity or specificity. For example, a diagnostic cut-off of 3.0 reduced the sensitivity of the assay from 100% to 90% for recent MPV samples, and from 30% to 10% for MPV samples >2 years post-infection, with 93% specificity. Lowering the diagnostic cut-off to 1.5 maintained 100% sensitivity for recent MPV samples, and increased sensitivity from 30% to 50% for MPV samples >2 years post-infection, but resulted in poor specificity (80%). Based on these results, we found that a diagnostic cut-off of 2.0 provided the best combination of sensitivity and specificity. Long peptides are often more difficult to synthesize at high purity compared to shorter peptides, and some 40mer peptides could not be commercially synthesized (data not shown). Nonetheless, the overall balance of sensitivity and specificity appeared to be greatest when 30mer peptides were used. Based on these results, we synthesized an additional 30mer peptide based on an MPV B21R epitope, B21R.185/186 (Fig. 3a), and tested it by ELISA (Fig. 3b). Similarly to previous work (Hammarlund et al. 2005), the B21R.185 20mer identified primary MPV-specific reactivity at 2–6 months post-infection with 100% sensitivity, and 92% specificity. However, MPV-specific antibodies in serum samples obtained >2 years post-infection could only be detected with 33% sensitivity using this peptide. Elongating this peptide to 30 AA (B21R.185/186) maintained 100% sensitivity for recent MPV samples, and improved identification of samples obtained >2 years post-MPV infection to 46% sensitivity, but specificity fell to 82% (Fig. 3b).
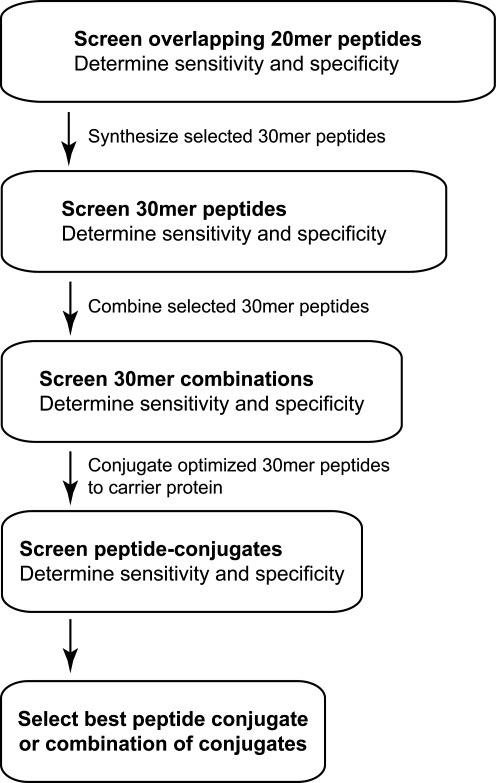
FIG. 1.
Stepwise approach to optimization of peptide-based diagnostic ELISA tests. Initial screening of potential peptide targets is performed using 20-AA peptides with 10 AA of overlap. Peptides 20 AA in length provide the best combination of cost for performing high-throughput screening and feasibility in terms of successful peptide synthesis. Peptides that show high sensitivity and specificity alone or in combination are further optimized by elongating selected peptides to 30 AA in length, and rescreening the peptides alone or in combination to determine sensitivity and specificity. If further refinement is needed, conjugation of the 30-mer peptides to a carrier protein such as BSA is performed, and the reagents are screened individually as well as in combination for sensitivity and specificity. This stepwise approach to developing peptide-based detection reagents may be readily applied to other diagnostic platforms.
FIG. 2.
Effect of peptide length on sensitivity and specificity. Selected 20mer peptides from peptide screens of MPV B21R protein were identified in a previous report as being highly immunogenic (Hammarlund et al. 2005). (A) Wells of ELISA plates were coated with 20mer peptides with the indicated overlap in primary amino acid sequences, alone or in the indicated combinations. Alternatively, 30mer or 40mer peptides that spanned the same region as the individual overlapping peptides were used to assess the sensitivity and specificity of the assay. (B) Serum or plasma samples were diluted 1:50 from primary monkeypox virus (MPV) subjects at 2–6 months post-infection (MPV, 2–6 mos, n=10), or at 24–30 months post-infection (MPV, >2 yrs, n=10), and MPV-negative control subjects (Controls, n=60 total), and tested in duplicate by peptide ELISA. Samples were considered positive for MPV B21R peptide reactivity if they scored ≥2-fold over background in duplicate wells. Error bars represent 95% confidence intervals that were established based on 3–4 independent experiments. (C) Signal intensity was determined for the optimal 30mer peptide, B21R.179/180, and the graph shows a representative experiment. Signal intensity is defined as the fold increase in ELISA OD480 over background (i.e., the OD490 for test peptide/OD490 for irrelevant peptide) for each sample. The dashed line indicates a ratio of 2.0, and this represents the diagnostic cut-off value for determining seropositive/seronegative status. The 60 control subjects included: orthopoxvirus-naïve (Naïve, n=20), vaccinia-immune subjects at 2–4 months post-vaccination (vaccinia virus [VV], 2–4 mos, n=20), and vaccinia-immune subjects at >20 years post-vaccination (VV, >20 yrs, n=20). The percentage of responding MPV-specific samples provided the assay sensitivity, and 100% minus the percentage of responding control samples provided the assay specificity, as described in the material and methods section.
FIG. 3.
Analysis of diagnostic 20mer and 30mer peptides for monkeypox virus (MPV) diagnostics. (A) Peptides consisting of 20 AA in length that exhibited high sensitivity and specificity in preliminary screens of the MPV B21R and VAR B22R overlapping peptide libraries were prepared as 30mer peptides comprised of neighboring amino acid sequences as shown here. (B) Representative experiment showing the peptides (20 AA) tested alone or in combination by ELISA, and compared with 30mer peptide composites to determine if increasing the peptide length would improve assay sensitivity and specificity, as described in Figure 2. Confidence intervals (95%) were established based on 3–5 independent experiments (MPV, 2–6 mos, n=10, MPV subjects at 2–6 months post-infection; MPV, >2 yrs, n=10, MPV subjects at 24–30 months post-infection; Controls, n=60 total, MPV-negative control subjects).
Identification of additional peptide reagents
Supplementary 20mer peptides were identified by first screening a peptide library based on the variola major (VAR) B22R protein, a homolog related to MPV B21R. VAR Bangladesh B22R shares 84% identity with MPV Zaire B21R (Poxvirus Bioinformatics Resource Center, www.poxvirus.org), but is absent from the vaccinia genome. Thus these proteins contain cross-reactive epitopes that might be useful for dual detection of virulent orthopoxviruses (e.g., both MPV and VAR). Using the 1st International Anti-Smallpox Serum Standard as a source of acute VAR serum (2–5 weeks post-rash onset), we identified 40 reactive peptides that scored positive by ELISA and showed >85% specificity (data not shown). Three regions of B22R reacted strongly with the acute smallpox-immune serum, and the three most promising peptide epitopes were synthesized as 30mers for further analysis (Fig. 3a). Of the B22R-derived 30mers tested, B22R.64/65 and B22R.82/83 each cross-reacted with MPV serum samples at 2–6 months post-infection with ≥70% sensitivity, and demonstrated >85% specificity (Fig. 3b). The third peptide, B22R.31/32, reacted with 45% of controls and was removed from further consideration due to low specificity.
Sensitivity and specificity of peptides used in combination
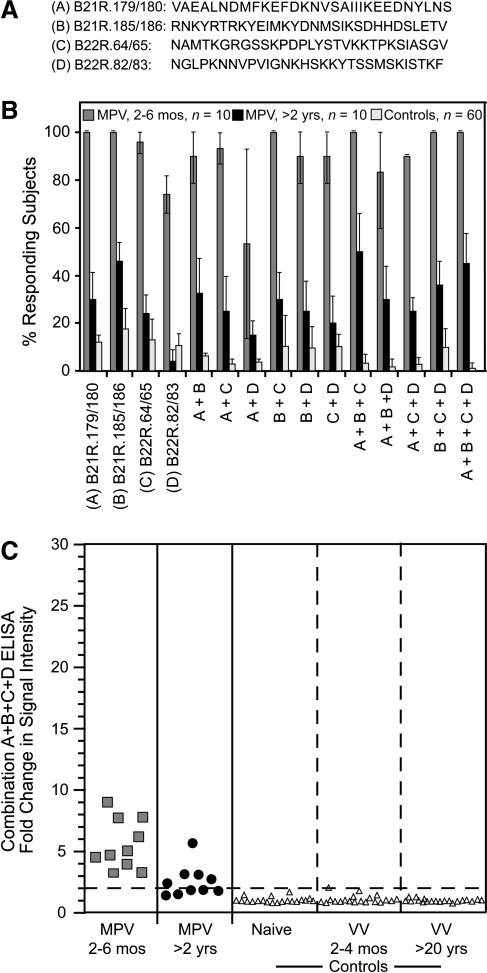
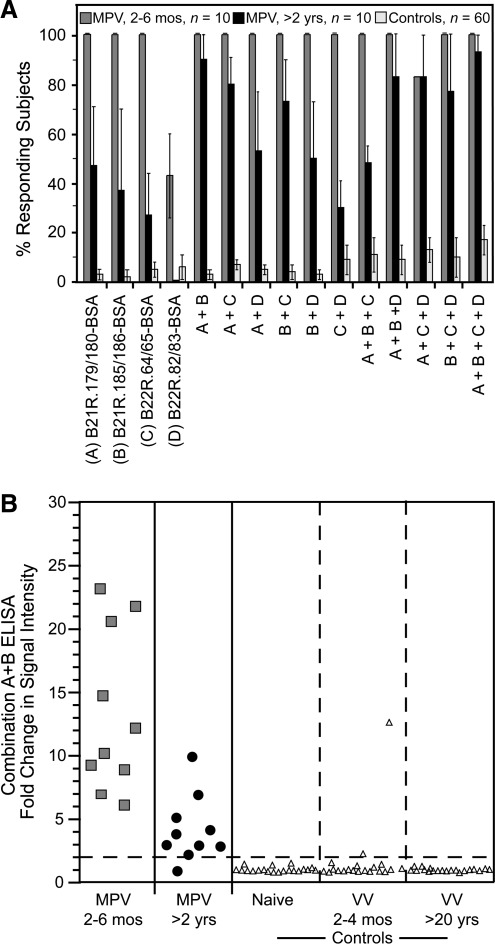
Peptide-based ELISA using selected 30mer peptides consistently resulted in increased sensitivity relative to 20mer peptides (Figs. 2b and 3b). We next addressed whether mixing combinations of 30mer peptides would further improve sensitivity and specificity. Selected peptides (Fig. 4a) were coated on ELISA plates alone or in combination (Fig. 4b). All recent MPV samples examined at 2–6 months post-infection responded to B21R.179/180 and B21R.185/186 (100% sensitivity), compared to 13% and 18%, of control samples, respectively (i.e., 87% and 82% specificity). B22R.64/65 and B22R.82/83 identified MPV-specific antibodies in 96% and 74% of recent MPV samples, respectively, with 87–89% specificity. B22R.64/65 shares 23/30 AA with the corresponding sequence in B21R. This region was not identified as diagnostic in the analysis of the B21R library, possibly because the corresponding peptides in the B21R library were offset by two AA relative to the B22R peptides, and it is possible that an antibody-binding epitope may have been split between the overlapping peptides used in the initial screen. B22R.82/83 shares 28/30 AA with the corresponding sequence in B21R and similarly was not identified as having diagnostic potential in the initial analysis of B21R peptides. Of the selected 30mer peptides tested individually, B21R.185/186 detected MPV samples obtained >2 years post-infection with the greatest sensitivity (46%). Combining peptides in the same ELISA well did not greatly improve sensitivity, but provided improved specificity in several cases. Six of 11 peptide combinations reacted with <5% of control samples (i.e., >95% specificity), including the combination of B21R.179/180 + B21R.185/186 + B22R.64/65 + B22R.82/83, which identified recent MPV infection with 100% sensitivity, MPV samples examined at >2 years post-infection demonstrated 45% sensitivity, and analysis of control samples showed 99% specificity. Analysis of the signal intensity of this combination of peptides showed that recent MPV subjects were readily distinguishable from control subjects (Fig. 4c). Additionally, although the overall percentage of positive subjects did not change substantially, the signal strength of MPV-specific reactivity in serum samples analyzed >2 years post-MPV infection improved (Fig. 4c) relative to that observed with the B21R.179/180 peptide alone (Fig. 2b).
FIG. 4.
Comparison of optimal 30mer peptides alone or in combination for monkeypox virus (MPV) diagnostics. (A) Amino acid sequences of individual 30mer peptides identified as having high sensitivity and specificity. (B) Samples from controls (Controls, n=60 total), and MPV-immune subjects at 2–6 months post-infection (MPV, 2-6 mos, n=10), and 24–30 months post-infection (MPV, >2 yrs, n=10), were tested for reactivity to individual peptides or combinations of peptides by ELISA as indicated. Confidence intervals (95%) were established based on 3–5 independent experiments. (C) Strength of signal against the most optimal four-peptide combination, B21R.179/180 + B21R.185/186 + B22R.64/65 + B22R.82/83, for the indicated groups are shown in a representative experiment, as described in Figure 2. In this experiment, specificity was 100% since 0/60 controls scored positive as indicated by the dashed line [Naïve, (n=20), orthopoxvirus-naïve subjects; VV 2–4 mos, (n=20), vaccinia-immune subjects at 2–4 months post-vaccination; VV, >20 yrs, (n=20), vaccinia-immune subjects at >20 years post-vaccination].
Conjugation of peptides to BSA improves assay sensitivity
A common challenge in peptide-based diagnostics is the inability of small peptides to simultaneously bind solid surface substrates and display accessible epitopes to the antibodies being tested. We postulated that improved presentation of antigenic epitopes might result in improved assay performance. Consequently, a carrier protein, bovine serum albumin (BSA), was conjugated to optimized B21R and B22R peptides. Peptides were also conjugated to keyhole limpet hemocyanin, but preliminary studies demonstrated high non-specific binding to control samples and poor reproducibility (data not shown). For this reason, subsequent studies were performed with peptide-BSA conjugates. Individual peptide-BSA conjugates were coated on ELISA plates alone or in combination, and tested for reactivity with MPV serum samples as well as negative control samples. B21R.179/180-BSA, B21R.185/186-BSA, and B22R.64/65-BSA each detected recent MPV samples (2–6 months post-infection) with 100% sensitivity (Fig. 5a). Conjugation of B22R.82/83 to BSA resulted in decreased sensitivity for detecting recent MPV (43% sensitivity, compared to 74% sensitivity using unconjugated peptide; Fig. 4a). However, conjugation of the MPV B21R peptides to BSA resulted in markedly increased sensitivity for detecting MPV-specific serum antibodies at >2 years post-infection. The B21R.179/180-BSA conjugate identified MPV subjects at >2 years post-infection with the highest sensitivity by itself (47%), but several different combinations of peptide-BSA conjugates further increased assay sensitivity, reaching up to 93% detection of MPV samples tested at >2 years post-infection. However, improved sensitivity coincided with decreased specificity for some peptide-BSA conjugate combinations (Fig. 5a) compared to unconjugated peptide combinations (Fig. 4b). The best balance of sensitivity and specificity was provided by the combination of B21R.179/180-BSA + B21R.185/186-BSA, which identified MPV-specific serum antibodies at 2–6 months post-infection with 100% sensitivity, and MPV-specific antibodies at >2 years post-infection with 90% sensitivity. This combination of peptide conjugates also provided 97% overall specificity. Peptide conjugation to BSA not only improved assay sensitivity, but also greatly increased the relative signal strength (Fig. 5b) relative to unconjugated peptides (Fig. 4c).
FIG. 5.
Analysis of 30mer peptide reagents after conjugation to a carrier protein, bovine serum albumin (BSA). Optimized 30mer peptides were conjugated to BSA and tested by ELISA to determine the effect on diagnostic sensitivity and specificity. (A) Wells of ELISA plates were coated with the indicated peptide-BSA conjugates either alone or in combination, and the ELISA was performed as described in Figure 2. Confidence intervals (95%) were established based on 3 independent experiments. (B) Strength of signal against the optimal B21R.179/180-BSA + B21R.185/186-BSA combined in a single well for the above groups in a representative experiment [MPV, 2–6 mos, n=10, MPV subjects at 2–6 months post-infection; MPV, >2 yrs, n=10, MPV subjects at 24–30 months post-infection; Controls, n=60 total, MPV-negative control subjects; Naïve, (n=20), orthopoxvirus-naïve subjects; VV 2–4 mos, (n=20), vaccinia-immune subjects at 2–4 months post-vaccination; VV, >20 yrs, (n=20), vaccinia-immune subjects at >20 years post-vaccination].
Discussion
In the present study, we describe a stepwise process for identifying and optimizing diagnostic peptide reagents for ELISA-based detection assays. Using this strategy, we showed that a combination of unconjugated 30 AA peptides can reliably identify MPV infection at 2–6 months post-infection with high sensitivity (100%), and MPV infection at >2 years post-infection with up to 45% sensitivity and 99% specificity. Furthermore, we found that conjugating peptides to a carrier protein greatly improved assay sensitivity with samples containing lower MPV-specific antibody titers, and an optimized combination of two peptide-BSA conjugates provided 100% sensitivity for retrospective detection of MPV infection at 2–6 months post-infection, and 90% sensitivity for detection of MPV >2 years post-infection, while still maintaining 97% specificity. The peptide-based IgG diagnostic reagents described in the current study may be useful for detecting prior MPV infection years after recovery, and provides important insight into the seroprevalence of MPV in endemic regions.
We previously identified 20mer peptides from the MPV B21R protein by ELISA that were used to identify MPV infection at 2–4 months post-infection with high sensitivity and specificity (Hammarlund et al. 2005). Additional cross-reactive peptides were similarly identified by screening a library of VAR B22R peptides using a combination of MPV-immune serum and the International Anti-Smallpox Serum Standard. Since smallpox was eradicated over 30 years ago, no individual high-titer acute-phase serum samples were available for screening peptide libraries, and therefore the International Anti-Smallpox Serum Standard was used for these purposes. The Anti-Smallpox Serum Standard consists of pooled sera from 63 acutely-infected smallpox subjects obtained at 2–5 weeks after onset of rash. One drawback to using a pooled serum sample is that it represents the average intensity of the antibody binding with the target antigen, but cannot be used to determine what percentage of subjects within the group had peptide-specific serum antibodies. However, identification of reactive peptides following acute VAR infection may be useful for diagnostics aimed at evaluating a potential orthopoxvirus outbreak. As noted following retrospective analysis of the 2003 U.S. MPV outbreak, orthopoxvirus-specific IgG antibodies may be detected as early as 2 days post-rash onset (Karem et al. 2005). If available, further studies using banked serum samples obtained during acute VAR infection will be necessary to determine the true sensitivity of this approach to early VAR detection, but the results provided here indicate that these reagents have high specificity (97–99% depending on the assay conditions), and may be useful for future development of advanced serological tests.
A broadly applicable diagnostic test must be able to detect infection regardless of the etiological strain. The subjects in the current study were infected with MPV-USA, the sequence of which had not been reported prior to our previous study in which the MPV-Zaire B21R library was utilized (Hammarlund et al. 2005). Fortunately, the diagnostic peptides described here are well conserved among all 7 sequenced strains of MPV (Poxvirus Bioinformatics Resource Center, www.poxvirus.org). The MPV-USA B21R protein (accession no. AAY97788) differs from MPV-Zaire B21R by one amino acid (B21R 1862D-E) within the B21R.185/186 epitope, although this substitution does not appear to affect antibody binding, since our subjects were infected with MPV-USA and the peptide reagent was based on the MPV-Zaire sequence. Thus, the B21R peptides described in the current study are likely to have utility for identifying infection resulting from many or all known MPV strains.
Immunodiagnostic tests utilizing peptides offer several advantages over diagnostic tests that rely on more complex biological materials. For instance, synthetic peptides represent chemically defined antigens, and because they are not derived from biological material, assay standardization and validation are often greatly simplified. Peptide reagents also offer flexibility in terms of antigen specificity, including species-specific diagnostics as well as broad pan-species diagnostics. For example, peptide-based applications ranging from HIV subtype-specific assays to those that simultaneously detect HIV and common co-infections such as hepatitis B virus have been described (Alcaro et al. 2003). Full-length proteins are often effective reagents for immunodiagnostics because they contain multiple epitopes. However, high sensitivity may come at the expense of assay specificity, since these molecules may also contain cross-reactive epitopes. By screening overlapping peptides within an immunogenic protein, we were able to maintain the highly specific epitopes while purging peptide epitopes that are cross-reactive or that demonstrate poor specificity. Although diagnostics based on a single peptide may lack sensitivity due to the dependence on a single antibody epitope, we have demonstrated that elongating peptides and combining multiple peptides can reduce or eliminate this potential problem. Combining 30mer peptides resulted in improved assay performance, with 100% sensitivity for identifying MPV infection at 2–6 months post-infection, as well as high specificity, ranging from 90% to 99% depending on the peptide combination used in the analysis.
Human monkeypox was once considered a sporadic disease of west and central Sub-Saharan Africa, centered primarily in remote locations in the Democratic Republic of Congo (Nalca et al. 2005). However, cases have been reported in other African countries as well, including the eastern African nation of Sudan (Damon et al. 2006). Increased travel and a progressively more connected global community could lead to an increased incidence of MPV outside its previous geographical range. Indeed, the 2003 MPV outbreak in the United States marked the first report of MPV in the Western Hemisphere (Hutson et al. 2007). Furthermore, although variola is extinct in nature, there is concern that variola or monkeypox virus could be engineered as biological weapons. It is possible that, if validated, the diagnostic reagents developed in this study could be used in conjunction with clinical algorithms (Seward et al. 2004) and direct virological tests (Sejvar et al. 2004) in the event of an accidental or deliberate release of these viruses into a susceptible population. Moreover, use of these reagents alone or in combination with other confirmatory tests (e.g., virus neutralization assays, post-adsorption ELISA, or Western blot; Dubois and Slifka 2008; Goldberg et al. 2008) could prove effective for determining seroprevalence of MPV immunity in at-risk populations. The peptides developed through these techniques detected MPV-specific IgG between 2 months and 30 months post-infection with high sensitivity and specificity, and represents a marked improvement in species-specific retrospective diagnosis of orthopoxvirus infection. Furthermore, this work represents progress toward the development of next-generation diagnostic formats, such as lateral flow assays that may be more amenable to epidemiological surveys in remote locations, as well as rapid, point-of-care diagnosis of MPV infection.
Acknowledgments
We thank the volunteers for their time and participation in this research study, M. Lewis for blood collection and processing, and J. Fitchen for insightful discussions.
This study was supported in part by Public Health Service grant R43 AI063675 (to M.K.S.), and Oregon National Primate Research Center grant RR000163 (to M.K.S.).
Author Disclosure Statement
Oregon Health and Science University (OHSU) E.H. and M.K.S. have a financial interest in Najít Technologies, Inc., a company that may have a commercial interest in the results of this research and technology. OHSU, E.H., and M.K.S. have submitted a patent application for this diagnostic approach. These potential conflicts of interest have been reviewed and managed by OHSU and the Integrity Program Oversight Council.
References
- Alcaro MC. Peroni E. Rovero P, et al. Synthetic peptides in the diagnosis of HIV infection. Curr Protein Pept Sci. 2003;4:285–290. doi: 10.2174/1389203033487117. [DOI] [PubMed] [Google Scholar]
- Amanna IJ. Carlson NE. Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- Anderson SG. Skegg J. The international standard for anti-smallpox serum. Bull World Health Organ. 1970;42:515–523. [PMC free article] [PubMed] [Google Scholar]
- Arita M. Tagaya I. Virion polypeptides of poxviruses. Arch Virol. 1980;63:209–225. doi: 10.1007/BF01315028. [DOI] [PubMed] [Google Scholar]
- Baxby D. The surface antigens of orthopoxviruses detected by cross-neutralization tests on cross-absorbed antisera. J Gen Virol. 1982;58:251–262. doi: 10.1099/0022-1317-58-2-251. [DOI] [PubMed] [Google Scholar]
- Crotty S. Felgner P. Davies H, et al. Cutting edge: Long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Damon IK. Roth CE. Chowdhary V. Discovery of monkeypox in Sudan. N Engl J Med. 2006;355:962–963. doi: 10.1056/NEJMc060792. [DOI] [PubMed] [Google Scholar]
- Demkowicz WE. Maa JS. Esteban M. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J Virol. 1992;66:386–398. doi: 10.1128/jvi.66.1.386-398.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois ME. Slifka MK. Retrospective analysis of monkeypox infection. Emerg Infect Dis. 2008;14:592–599. doi: 10.3201/eid1404.071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito JJ. Obijeski JF. Nakano JH. Serological relatedness of monkeypox, variola, and vaccinia viruses. J Med Virol. 1977;1:35–47. doi: 10.1002/jmv.1890010107. [DOI] [PubMed] [Google Scholar]
- Esposito JJ. Obijeski JF. Nakano JH. The virion and soluble antigen proteins of variola, monkeypox, and vaccinia viruses. J Med Virol. 1977;1:95–110. doi: 10.1002/jmv.1890010203. [DOI] [PubMed] [Google Scholar]
- Fenner F. Henderson DA. Arita I. Jezek Z. Ladnyi ID. The Pathogenesis, Immunology, and Pathology of Smallpox and Vaccinia. Geneva: World Health Organization; 1988. Smallpox and its eradication; p. 1469. [Google Scholar]
- Gispen R. Analysis of pox-virus antigens by means of double diffusion: a method for direct serological differentiation of cowpox. J Immunol. 1955;74:134–141. [PubMed] [Google Scholar]
- Gispen R. Brand-Saathof BB. Hekker AC. Monkeypox-specific antibodies in human and simian sera from the Ivory Coast and Nigeria. Bull World Health Organ. 1976;53:355–360. [PMC free article] [PubMed] [Google Scholar]
- Gispen R. Brand-Saathof B. Three specific antigens produced in vaccinia, variola, and monkeypox infections. J Infect Dis. 1974;129:289–295. doi: 10.1093/infdis/129.3.289. [DOI] [PubMed] [Google Scholar]
- Gispen R. Huisman J. Brand-Saathof B, et al. Immunofluorescence test for persistent poxvirus antibodies. Arch Gesamte Virusforsch. 1974;44:391–395. doi: 10.1007/BF01251021. [DOI] [PubMed] [Google Scholar]
- Gnann JW., Jr. McCormick JB. Mitchell S, et al. Synthetic peptide immunoassay distinguishes HIV type 1 and HIV type 2 infections. Science. 1987;237:1346–1349. doi: 10.1126/science.2888192. [DOI] [PubMed] [Google Scholar]
- Goldberg TL. Chapman CA. Cameron K, et al. Serologic evidence for novel poxvirus in endangered red colobus monkeys, western Uganda. Emerg Infect Dis. 2008;14:801–803. doi: 10.3201/eid1405.071686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E. Lewis MW. Carter SV, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11:1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- Hammarlund E. Lewis MW. Hansen SG, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Harper L. Bedson HS. Buchan A. Identification of orthopoxviruses by polyacrylamide gel electrophoresis of intracellular polypeptides. I. Four major groupings. Virology. 1979;93:435–444. doi: 10.1016/0042-6822(79)90247-2. [DOI] [PubMed] [Google Scholar]
- Hutchinson HD. Ziegler DW. Wells DE, et al. Differentiation of variola, monkeypox, and vaccinia antisera by radioimmunoassay. Bull World Health Organ. 1977;55:613–623. [PMC free article] [PubMed] [Google Scholar]
- Hutson CL. Lee KN. Abel J, et al. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am J Trop Med Hyg. 2007;76:757–768. [PubMed] [Google Scholar]
- Jezek Z. Marennikova SS. Mutumbo M, et al. Human monkeypox: a study of 2,510 contacts of 214 patients. J Infect Dis. 1986;154:551–555. doi: 10.1093/infdis/154.4.551. [DOI] [PubMed] [Google Scholar]
- Jezek Z. Nakano JH. Arita I, et al. Serological survey for human monkeypox infections in a selected population in Zaire. J Trop Med Hyg. 1987a;90:31–38. [PubMed] [Google Scholar]
- Jezek Z. Szczeniowski M. Paluku KM, et al. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987b;156:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- Jones-Trower A. Garcia A. Meseda CA, et al. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology. 2005;343:128–140. doi: 10.1016/j.virol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Karem KL. Reynolds M. Braden Z, et al. Characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12:867–872. doi: 10.1128/CDLI.12.7.867-872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karem KL. Reynolds M. Hughes C, et al. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin Vaccine Immunol. 2007;14:1318–1327. doi: 10.1128/CVI.00148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein KR. Atas JG. Collins J. Testing emergency medical personnel response to patients with suspected infectious disease. Prehospital Disaster Med. 2004;19:256–265. doi: 10.1017/s1049023x00001850. [DOI] [PubMed] [Google Scholar]
- Kulesh DA. Baker RO. Loveless BM, et al. Smallpox and pan-orthopox virus detection by real-time 3′-minor groove binder TaqMan assays on the roche LightCycler and the Cepheid smart Cycler platforms. J Clin Microbiol. 2004;42:601–609. doi: 10.1128/JCM.42.2.601-609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz EJ. Upton C. Changayil SS, et al. Poxvirus Bioinformatics Resource Center: a comprehensive Poxviridae informational and analytical resource. Nucleic Acids Res. 2005;33(Database Issue):D311–D316. doi: 10.1093/nar/gki110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MW. Graham MB. Hammarlund E, et al. Monkeypox without exanthem. N Engl J Med. 2007;356:2112–2114. doi: 10.1056/NEJMc062788. [DOI] [PubMed] [Google Scholar]
- Liang FT. Steere AC. Marques AR, et al. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J Clin Microbiol. 1999;37:3990–3996. doi: 10.1128/jcm.37.12.3990-3996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marennikova SS. Malceva NN. Habahpaseva NA. ELISA—a simple test for detecting and differentiating antibodies to closely related orthopoxviruses. Bull World Health Organ. 1981;59:365–369. [PMC free article] [PubMed] [Google Scholar]
- Meyer H. Perrichot M. Stemmler M, et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J Clin Microbiol. 2002;40:2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalca A. Rimoin AW. Bavari S, et al. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41:1765–1771. doi: 10.1086/498155. [DOI] [PubMed] [Google Scholar]
- Oladepo DK. Klapper PE. Marsden HS. Peptide based enzyme-linked immunoassays for detection of anti-HSV-2 IgG in human sera. J Virol Methods. 2000;87:63–70. doi: 10.1016/s0166-0934(00)00152-x. [DOI] [PubMed] [Google Scholar]
- Olson VA. Laue T. Laker MT, et al. Real-time PCR system for detection of orthopoxviruses and simultaneous identification of smallpox virus. J Clin Microbiol. 2004;42:1940–1946. doi: 10.1128/JCM.42.5.1940-1946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porstmann T. Nugel E. Henklein P, et al. Two-colour combination enzyme-linked immunosorbent assay for the simultaneous detection of HBV and HIV infection. J Immunol Methods. 1993;158:95–106. doi: 10.1016/0022-1759(93)90262-6. [DOI] [PubMed] [Google Scholar]
- Putz MM. Alberini I. Midgley CM, et al. Prevalence of antibodies to Vaccinia virus after smallpox vaccination in Italy. J Gen Virol. 2005;86:2955–2960. doi: 10.1099/vir.0.81265-0. [DOI] [PubMed] [Google Scholar]
- Reed KD. Melski JW. Graham MB, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Rimoin AW. Kisalu N. Kebela-Ilunga B, et al. Endemic human monkeypox, Democratic Republic of Congo, 2001–2004. Emerg Infect Dis. 2007;13:934–937. doi: 10.3201/eid1306.061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoin AW. Mulembakani PM. Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale TA. Melski JW. Stratman EJ. Monkeypox: an epidemiologic and clinical comparison of African and US disease. J Am Acad Dermatol. 2006;55:478–481. doi: 10.1016/j.jaad.2006.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ. Chowdary Y. Schomogyi M, et al. Human monkeypox infection: a family cluster in the midwestern United States. J Infect Dis. 2004;190:1833–1840. doi: 10.1086/425039. [DOI] [PubMed] [Google Scholar]
- Seward JF. Galil K. Damon I, et al. Development and experience with an algorithm to evaluate suspected smallpox cases in the United States, 2002–2004. Clin Infect Dis. 2004;39:1477–1483. doi: 10.1086/425500. [DOI] [PubMed] [Google Scholar]
- Shin SY. Lee MK. Kim SY, et al. The use of multiple antigenic peptide (MAP) in the immunodiagnosis of human immunodeficiency virus infection. Biochem Mol Biol Int. 1997;43:713–721. doi: 10.1080/15216549700204521. [DOI] [PubMed] [Google Scholar]
- Sofi Ibrahim M. Kulesh DA. Saleh SS, et al. Real-time PCR assay to detect smallpox virus. J Clin Microbiol. 2003;41:3835–3839. doi: 10.1128/JCM.41.8.3835-3839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD. Ershler WB. Janowski M, et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med. 2008;121:1058–1064. doi: 10.1016/j.amjmed.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. Baxby D. Structural polypeptides of Orthopoxvirus: their distribution in various members and location within the virion. J Gen Virol. 1979;45:537–545. doi: 10.1099/0022-1317-45-3-537. [DOI] [PubMed] [Google Scholar]