Abstract
The current study was undertaken to follow the time course of bone loss in the proximal tibia of rats over several weeks following thoracic contusion spinal cord injury (SCI) of varying severity. It was hypothesized that bone loss would be more pronounced in the more severely injured animals, and that hindlimb weight bearing would help prevent bone loss. Twenty-six female Sprague-Dawley rats (200–225 g, 6–7 weeks old) received standard thoracic (T9) injuries at energies of 6.25, 12.5, 25, or 50 g-cm. The rats were scored weekly for hindlimb function during locomotion. At 0, 2 or 3, and 8 weeks, high-resolution micro-CT images of each right tibia were obtained. Mechanical indentation testing was done to measure the compressive strength of the cancellous bone structure. The 6.25 g-cm group showed near normal locomotion, the 12.5 and 25 g-cm groups showed the ability to frequently or occasionally generate weight-supported plantar steps, respectively, and the 50 g-cm group showed only movement without weight-supported plantar stepping. The 6.25, 12.5 and 25 g-cm groups remained at the same level of bone volume fraction (cancBV/TV=0.24±0.07), while the 50 g-cm group experienced severe bone loss (67%), resulting in significantly lower (p<0.05) bone volume fraction (cancBV/TV=0.11±0.05) at 8 weeks. Proximal tibia cancellous bone strength was reduced by approximately 50% in these severely injured rats. Instead of a linear proportionality between injury severity and bone loss, there appears to be a distinct functional threshold, marked by occasional weight-supported stepping, above which bone loss does not occur.
Key words: bone loss, cancellous, density, locomotion, spinal cord injury
Introduction
Following spinal cord injury (SCI) in humans there is rapid and sustained bone loss below the level of injury (Biering-Sorensen et al., 1988,2009; Dauty et al., 2000; de Bruin et al., 2005; Frey-Rindova et al.; 2000; Garland et al., 1992; Jiang et al., 2006a; Sabo et al., 2001). With complete SCI, the results are worse than when partial motor function is preserved (Garland et al., 2004; Jiang et al., 2006a). In paraplegia, with active sitting, the spine and upper extremities experience frequent loading, and it is primarily in the lower extremities where loads are not present that bone loss occurs. Bone loss has also been shown to be worse in the lower extremities than in the spine or upper extremity following mid-cervical injuries resulting in quadriplegia (de Bruin et al., 2005; Frey-Rindova et al., 2000; Garland et al., 1992; Jiang et al., 2006a; Maïmoun et al., 2005). Even the proximal femur is not as affected as the distal femur and proximal tibia (Jiang et al., 2006a; Needham-Shropshire et al., 1997). Thus, the amount of continued functional loading on a given anatomic region inversely affects the severity of bone loss in humans following SCI.
Not surprisingly, fracture incidence is increased in patients after SCI and with time since SCI due to the decreased mechanical integrity of bone (Eser et al., 2005a; Zehnder et al., 2004). These fractures can complicate recovery, rehabilitation, and activities of daily living (Jiang et al., 2006a). The most common sites of fracture in humans are the distal femur and proximal tibia (Eser et al., 2005b; Garland et al., 2001). These sites are problematic for two reasons. First, high bending moments arise when loads are applied to the distal tibia during falls or transfer maneuvers. Second, these are sites that contain predominantly trabecular bone as opposed to cortical bone. Loss of trabecular bone is typically more severe than loss of cortical bone during the development of osteopenia or osteoporosis (Modlesky et al., 2004; Slade et al., 2005).
The animal literature contains several studies describing the natural course of bone loss in rats following SCI (Jiang et al., 2006b,2007a,2007b,2007c; Liu et al., 2008a,2008b; Minematsu et al., 2003; Morse et al., 2008; Sugawara et al., 1998). These studies, however, did not investigate the relationship between injury severity and the degree or pattern of bone loss. The current study was undertaken to follow the time course of bone loss in the proximal tibia of rats across several weeks following thoracic contusion SCI of varying severity. Bone loss was quantified using in vivo high-resolution three-dimensional micro-CT imaging (Voor et al., 2008). Correlations were examined between mechanical strength of the same bones for which images were obtained and locomotor scores of the animals' walking ability at various time points after injury. It was hypothesized that bone loss would be worse in the more severely injured animals, and that hindlimb weight bearing would help prevent bone loss.
Methods
Twenty-six female Sprague-Dawley rats (200–225 g, 6–7 weeks old) were used in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the University of Louisville. All rats were housed in plastic cages and subjected to a 12-h light/dark cycle. All rats were allowed to move freely in their cages throughout the 8-week experiment. Tap water and a standard rodent diet were available to all rats ad libitum. The animals were anesthetized with pentobarbital (50 mg/kg IP) and the spinal cord was exposed at T9 by laminectomy at the T11 vertebral level. The animals received mild (6.25 g-cm, n=6), moderate (12.5 g-cm, n=7), moderately-severe (25 g-cm, n=7), or severe (50 g-cm, n=6) contusion injuries delivered by the NYU Impactor (W. Young, Rutgers, Newark, NJ) as described previously (Smith et al., 2009). After injury, the muscles and skin were closed in layers with sutures and a topical antibiotic was applied. Each animal also received prophylactic gentamicin sulfate (15 mg/kg IM) on the day of surgery and again 3 and 5 days post-injury. Each animal also received 10 mL of normal saline IP as a fluid replacement strategy. Body temperatures were maintained at 36–37°C using a water-filled heating pad for the duration of the surgery and until all the animals were fully recovered from the anesthetic. Manual bladder expression was performed twice daily for each animal until reflexive voiding had recovered. The injury severities were confirmed at study termination by histological analysis of spinal cord section spared white matter.
Weekly during the study period, the rats were scored for hindlimb function during locomotion using an established open-field locomotor grading protocol (Basso-Beattie-Bresnahan [BBB] rating scale; Basso et al., 1995,1996). The animals were observed for 4 min by two evaluators who were blinded to the animal's injury status, and given a composite score based on various movements.
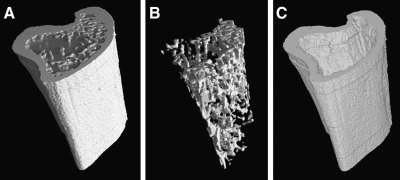
At 0, 2 or 3, and 8 weeks, high-resolution images were collected using a customized micro-CT system (ACTIS 150/225 system; BIR Inc., Lincolnshire, IL). Half of the animals were scanned at 0 weeks to establish the baseline bone parameters. Each animal was anesthetized with isoflurane (2–5% in oxygen) and placed in a custom animal holder that allows the left hindlimb to be flexed and the right to be extended, along with the tail, into the lower column of the imaging tube. While in the holder the animal was supplied with isoflurane using a nose cone. The metaphysis of each right tibia was scanned over a 3-mm distance below the knee joint (Fig. 1). The three-dimensional image data were processed to reveal the volume fractions within the metaphysis occupied by cancellous and cortical bone tissue (Fig. 2). Both the cancellous and cortical bone of the metaphysis contribute to the fracture strength of the proximal tibia (Xu et al., 2009).
FIG. 1.
The custom in vivo micro-CT scanner was used to scan the proximal tibial metaphysis at a nominal resolution of 28 μm.
FIG. 2.
The 3-mm section of the proximal metaphysis was reconstructed in three dimensions (A), and then segmented to isolate cancellous bone (B) and cortical bone (C).
After sacrifice, the left tibias were trimmed to expose the cancellous bone of the proximal tibial metaphysis corresponding to the same region scanned on the right tibias, and an indentation test was performed by advancing a flat-tipped cylindrical post (1.5 mm diameter) axially into the cut surface at a rate of 1 mm/min to measure the compressive strength of the cancellous bone.
After sacrifice, the spinal cords were fixed in paraformaldehyde, cryopreserved in sucrose, and sectioned for histological evaluation of the lesions as described previously (Magnuson et al., 2005; Smith et al., 2009). The percentage area of spared cross-sectional white matter at the injury epicenter was calculated for each animal (Magnuson et al., 2005).
Statistical analysis
Statistical analyses were performed as repeated-measures analysis of variance (ANOVA) followed by Tukey's HSD post-hoc t-tests and Pearson correlations. Additionally, binomial proportion tests were done at 3 and 8 weeks to test the difference between the number of animals that could or could not establish weight support in the 25 and 50 g-cm injury groups. As our hypothesis was that hindlimb weight bearing would help prevent bone loss, this additional analysis was done to distinguish any differences between the two most severe injury groups with respect to weight support. All values are stated as mean±standard deviation.
Results
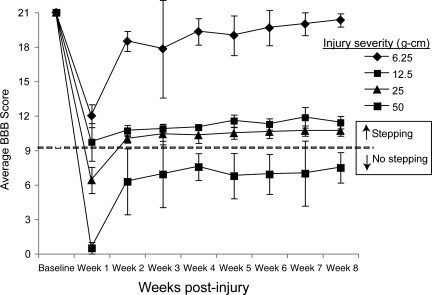
As expected, all injured rats exhibited significant improvements in hindlimb function during locomotion over the first 2–3 weeks post-injury. Locomotor scores (BBB) reached a plateau by week 3, and were steady for the remainder of the 8-week study (Fig. 3). The mildly-injured (6.25 g-cm) group showed nearly normal locomotion, while both moderately-injured groups (12.5 and 25 g-cm) showed the ability to frequently or occasionally generate weight supported plantar steps, respectively. The severely injured (50 g-cm) group showed extensive hindlimb movement (sweeping involving all three joints) without any weight-supported plantar stepping.
FIG. 3.
Graph of the Basso-Beattie-Bresnahan (BBB) locomotor score for each rat group shows the recovery of each group over time, with continuing lack of plantar weight support in the severely-injured (50 g-cm) group, as indicated by a BBB score below 10 (dashed line at 9.5).
All 26 animals survived for the duration of the 8-week study. Upon histological inspection of the spinal cord sections, three animals were removed from the 6.25 g-cm injury group because they maintained 100% spared white matter, and one animal was moved from the 25 g-cm group to the 50 g-cm group because it only had 1.5% spared white matter. The locomotor (BBB) scores of the rats were well correlated (r2=0.898, p<0.05) with the percentage of spared white matter at the injury epicenters at 8 weeks. At both 3 and 8 weeks the 25 g-cm group had significantly more animals that could establish weight support (z=5.07; p<0.001) than the 50 g-cm group.
The rats in all four injury groups lost more than 25% of their cancellous bone volume fraction within 3 weeks, compared to pre-injury baseline (Fig. 4A), mostly due to growth and consolidation of the metaphyseal cancellous bone, and possibly due to transient disuse as noted in the early locomotor scores (Fig. 3). The mildly-injured (6.25 g-cm) and moderately-injured (12.5 and 25 g-cm) groups lost approximately 25% of their cancellous bone relative to baseline (p<0.01) in the first 2 weeks, while they were recovering plantar hindlimb stepping, and then remained at the same level of bone volume fraction through 8 weeks (cancellous bone volume/total volume [cancBV/TV]=0.24±0.07). Conversely, the severely-injured (50 g-cm) group continued to lose bone, and experienced severe bone loss (67%) after 8 weeks, resulting in significantly lower (p<0.01) bone volume fraction (cancBV/TV=0.11±0.05) relative to both baseline and the milder injury groups (Fig. 4A).
FIG. 4.
(A) The cancellous bone volume fraction in the proximal tibia metaphysis decreased approximately 25% in each injury severity group over the first few weeks (p<0.01) relative to baseline. The severely-injured group lost a significant amount of bone over 3 weeks (*p<0.01), and lost 67% of its cancellous bone over 8 weeks (**p<0.01) relative to baseline. The 8-week bone volume fraction in the severely-injured group was also significantly different than the less-severely-injured groups (p<0.01). (B) The cortical bone volume fraction in the proximal tibia metaphysis decreased approximately 11% in each injury severity group over the first few weeks (p<0.01) relative to baseline. The severely-injured group lost a significant amount of bone over 3 weeks (*p<0.01), and lost 32% of its cortical bone over 8 weeks (**p<0.01) relative to baseline. The 8-week cortical bone volume in the severely-injured group was also significantly different than the less-severely-injured groups (p<0.01). (C) The mechanical indentation strength of the proximal tibia metaphyseal cancellous bone decreased significantly (p<0.05), by approximately 50% in the severely-injured group (50 g-cm) compared to the other groups.
The results for cortical bone were similar. The mildly-injured (6.25 g-cm) and moderately-injured (12.5 and 25 g-cm) groups lost approximately 11% of their cortical bone in the first 2 weeks, while they were recovering plantar hindlimb stepping, and then remained at the same level of bone volume fraction for the duration of the study (cortical bone volume/total volume [cortBV/TV]=0.58±0.05). The severely-injured (50 g-cm) group experienced greater bone loss (32%), resulting in significantly lower (p<0.01) bone volume fractions (cortBV/TV=0.43±0.03) at the 8-week time point (Fig. 4B).
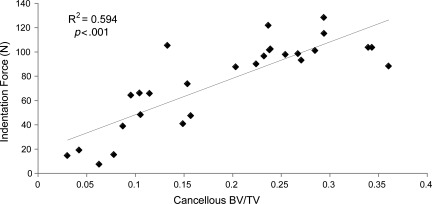
The indentation testing for cancellous bone strength after 8 weeks (Fig. 4C) correlated well with the cancellous bone volume fraction (Fig. 5). The strength of the cancellous bone of the proximal tibial metaphysis was reduced by approximately 50% in the severely-injured rats compared to the groups with mild and moderate injuries.
FIG. 5.
The indentation strength and the cancellous bone volume fraction are correlated (r2=0.594) at 8 weeks post-spinal cord injury, and the correlation is significant (p<0.001; BV/TV, bone volume/total volume).
Discussion
The goals of this study were to quantify bone changes in the hindlimbs of adult female rats after contusive mid-thoracic spinal cord injuries of various severities, and to examine relationships between bone loss and functional status of the partially paralyzed hindlimbs. We found that animals with mild and moderate injuries experienced a decrease in cancellous bone volume fraction of approximately 25%, and recovered the functional capacity to generate hindlimb plantar weight support during stance by 3 weeks post-injury. The mild and moderate injury groups had no further bone loss through 8 weeks. The group with severe injuries remained below the locomotor grading threshold of at least occasional weight-supported plantar hindlimb stepping (BBB score 10), and continued to lose bone until they had lost 67% of their cancellous bone by 8 weeks. These results closely match the results of Morse and associates (2008), for whom the distal femur was found to lose 48% of its cancellous bone and 35% of its cortical bone after only 10 days following a similar 50 g-cm impact injury to the thoracic spinal cord.
This study reveals that over a wide range of injury severity, there appears to be a distinct threshold for bone loss which is related to the degree of compromise of hindlimb weight bearing. Though all or some of the severe bone loss may be related to disuse, the result is not surprising, due to the well-known observation of disuse-related bone loss in both animal and human models (Sievänen, 2010; Weinreb et al., 1991; Zeng et al., 1996). The finding that only minimal walking ability was able to maintain almost normal bone, however, was not expected. Some of the early bone loss could have been caused by transient disuse during the time it took to recover walking ability in the mild and moderate injury groups. It is also possible that some of the observed decrease in volume fraction is normal for aging and growing animals such as those used in this study (Jiang et al., 2007b; Waarsing et al., 2006). Another possibility is that neurological injury results in changes in the bone tissue environment that can have a negative impact on bone mass regardless of the injury severity (Morse et al., 2008). Nevertheless, we found that groups of animals that achieved a mean BBB score of 10 (indicating occasional [5–50%] plantar hindlimb stepping with weight support) by post-injury week 3 were able to prevent a severe net loss of bone in the proximal tibia.
It is clearly a limitation of the present study that there were no uninjured animals evaluated at each time point for direct comparison. Findings from similar studies (Jiang et al., 2007b; Morse et al., 2008), when used for reference, indicate that the bone loss observed in the mild and moderate injury groups was slight compared to what would be expected from normal growth and aging.
The data on bone response to its mechanical environment are very interesting. It has long been believed that bone forms to meet its function. In particular, bone structure will be guided by “Wolff's Law” to align itself with the trajectories of principal stress (Lanyon and Rubin, 1984; Wolff, 1892). Several studies have shown that the magnitude of the loading is important to the preservation or building of bone mass, but other studies have shown that low-amplitude, high-frequency strain can be anabolic in bone (Rubin et al., 2001). These data are especially relevant because the magnitude of the loading need not be greater than normal to elicit a beneficial response in humans (Rubin et al., 2002a,2002b,2004). The evidence from animal studies is that vibrations induced through a platform can cause an increase in cancellous bone mass in the leg (Rubin et al., 2002a). Because relatively low-amplitude strain applied through ground reaction forces improves bone mass, it is apparent that normal walking (weight bearing) is an excellent stimulus for bone mass preservation in the lower extremities.
Normal bone remodeling (turnover) involves a relative balance between osteoclastic resorption of bone and osteoblastic formation of bone on active bone surfaces. It is believed that osteoclasts are actively inhibited by osteocytes and osteoblast-like lining cells that experience normal strain signals from functional load-bearing activity (Mullender and Huiskes, 1995,1997; You et al., 2008). There are several potential mechanisms by which osteocytes inhibit osteoclasts, such as through the release of transforming growth factor-β (Heino et al., 2002), or through matrix extracellular phosphoglycoprotein (Kulkarni et al., 2010). Conversely, when this inhibitory signaling is interrupted by a decrease in strain, the osteoclastic resorption is upregulated, resulting in net bone loss. The osteocytes that see reduced strain release more receptor activator of nuclear factor-κB ligand (RANKL), resulting in greater bone loss (Xiong et al., 2011). Furthermore, when bone is unloaded such as in disuse or SCI, the osteocytes release sclerostin, which inhibits new bone formation by competing with the Wnt signaling pathway involving osteoblasts. Bone that is loaded in vivo will release less sclerostin, thus allowing new bone formation (Robling et al., 2008). There are even some potential therapeutic measures that may be helpful to bone following SCI. For example, human recombinant osteoprotegerin (hrOPG) inhibits the RANKL pathway to ostoclastogenesis, thus preventing bone loss with paralysis (Aliprantis et al., 2011). Future work in this area of SCI and bone quality should investigate the interplay of the various cellular and signaling pathways that control bone remodeling.
Regardless of the mechanism by which bone is lost after SCI, load bearing plays a role in the final result. As the bone architecture is weakened by bone loss and remodeling, the strain on the bone tissue increases again, not because of increased loading, but because any level of loading is stressing and straining a weaker skeleton. In other words, the new strain signal controls bone mass at a new balance “set-point.” In the total absence of load-bearing activity, this eventual increase in strain signal does not occur, resulting in continued net bone loss.
Conclusion
This is the first study to follow and compare the time course of bone loss following spinal cord injuries of different severities. Instead of a linear proportionality between injury severity and bone loss, there appears to be a distinct threshold, marked by occasional weight-supported stepping, above which bone loss is limited. In the most-severely-injured animals (i.e., those unable to achieve weight-supported stepping), rapid bone loss was observed as early as 2–3 weeks, and the bone loss was significant by 8 weeks. Future work should focus on differentiating between the mechanisms of bone loss or preservation as they relate specifically to weight-supported stepping and injury severity.
Acknowledgments
This work was supported by the Kentucky Spinal Cord and Head Injury Research Trust and by National Institutes of Health grants R01 NS052292 and P20 RR15576.
Author Disclosure Statement
No competing financial interests exist.
References
- Aliprantis A.O. Stolina M. Kostenuik P.J. Poliachik S.L. Warner S.E. Bain S.D. Gross T.S. Transient muscle paralysis degrades bone via rapid osteoclastogenisis. FASEB J. 2011 doi: 10.1096/fj.11-196642. Epub before print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neutrotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Biering-Sorensen F. Bohr H. Schaadt O. Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia. 1988;26:293–301. doi: 10.1038/sc.1988.44. [DOI] [PubMed] [Google Scholar]
- Biering-Sørensen F. Hansen B. Lee B.S. Non-pharmacological treatment and prevention of bone loss after spinal cord injury: a systematic review. Spinal Cord. 2009;47:508–518. doi: 10.1038/sc.2008.177. [DOI] [PubMed] [Google Scholar]
- Dauty M. Perrouin Verbe B. Maugars Y. Dubois C. Mathe J.F. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27:305–309. doi: 10.1016/s8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- de Bruin E.D. Vanwanseele B. Dambacher M.A. Dietz V. Stussi E. Long-term changes in the tibia and radius bone mineral density following spinal cord injury. Spinal Cord. 2005;43:96–101. doi: 10.1038/sj.sc.3101685. [DOI] [PubMed] [Google Scholar]
- Eser P. Frotzler A. Zehnder Y. Denoth J. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch. Phys. Med. Rehabil. 2005a;86:498–504. doi: 10.1016/j.apmr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Eser P. Frotzler A. Zehnder Y. Schiessl H. Denoth J. Assessment of anthropometric, systemic, and lifestyle factors influencing bone status in the legs of spinal cord injured individuals. Osteoporos. Int. 2005b;16:26–34. doi: 10.1007/s00198-004-1638-x. [DOI] [PubMed] [Google Scholar]
- Frey-Rindova P. de Bruin E.D. Stussi E. Dambacher M.A. Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38:26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]
- Garland D.E. Adkins R.H. Kushwaha V. Stewart C. Risk factors for osteoporosis at the knee in the spinal cord injury population. J. Spinal Cord Med. 2004;27:202–206. doi: 10.1080/10790268.2004.11753748. [DOI] [PubMed] [Google Scholar]
- Garland D.E. Adkins R.H. Stewart C.A. Ashford R. Vigil D. Regional osteoporosis in women who have a complete spinal cord injury. J. Bone Joint Surg. [Am.] 2001;83:1195–1200. doi: 10.2106/00004623-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Garland D.E. Stewart C.A. Adkins R.H. Hu S.S. Rosen C. Liotta F.J. Weinstein D.A. Osteoporosis after spinal cord injury. J. Orthop. Res. 1992;10:371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- Heino T.J. Hentunen T.A. Väänänen H.K. Osteocytes inhibit osteoclastic bone resorption through transforming growth factor-beta: enhancement by estrogen. J. Cell Biochem. 2002;85:185–197. doi: 10.1002/jcb.10109. [DOI] [PubMed] [Google Scholar]
- Jiang S.D. Dai L.Y. Jiang L.S. Osteoporosis after spinal cord injury. Osteoporosis Int. 2006a;17:180–192. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- Jiang S.D. Jiang L.S. Dai L.Y. Changes in bone mass, bone structure, bone biomechanical properties, and bone metabolism after spinal cord injury: a 6-month longitudinal study in growing rats. Calcif. Tissue Int. 2007a;80:167–175. doi: 10.1007/s00223-006-0085-4. [DOI] [PubMed] [Google Scholar]
- Jiang S.D. Jiang L.S. Dai L.Y. Effects of spinal cord injury on osteoblastogenesis, osteoclastogenesis and gene expression profiling in osteoblasts in young rats. Osteoporos. Int. 2007c;18:339–349. doi: 10.1007/s00198-006-0229-4. [DOI] [PubMed] [Google Scholar]
- Jiang S.D. Jiang L.S. Dai L.Y. Spinal cord injury causes more damage to bone mass, bone structure, biomechanical properties and bone metabolism than sciatic neurectomy in young rats. Osteoporos. Int. 2006b;17:1552–1561. doi: 10.1007/s00198-006-0165-3. [DOI] [PubMed] [Google Scholar]
- Jiang S.D. Shen C. Jiang L.S. Dai L.Y. Differences of bone mass and bone structure in osteopenic rat models caused by spinal cord injury and ovariectomy. Osteoporos. Int. 2007b;18:743–750. doi: 10.1007/s00198-006-0299-3. [DOI] [PubMed] [Google Scholar]
- Kulkarni R.N. Bakker A.D. Everts V. Klein-Nulend J. Inhibition of osteoclastogenesis by mechanically loaded osteocytes: involvement of MEPE. Calcif. Tissue Int. 2010;87:461–468. doi: 10.1007/s00223-010-9407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyon L.E. Rubin C.T. Static vs dynamic loads as an influence on bone remodelling. J. Biomech. 1984;17:897–905. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- Liu D. Li H. Zhao C.Q. Jiang L.S. Dai L.Y. Changes of substance P-immunoreactive nerve fiber innervation density in the sublesional bones in young growing rats at an early stage after spinal cord injury. Osteoporos. Int. 2008b;19:559–569. doi: 10.1007/s00198-007-0481-2. [DOI] [PubMed] [Google Scholar]
- Liu D. Zhao C.Q. Li H. Jiang S.D. Jiang L.S. Dai L.Y. Effects of spinal cord injury and hindlimb immobilization on sublesional and supralesional bones in young growing rats. Bone. 2008a;43:119–125. doi: 10.1016/j.bone.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Magnuson D.S. Lovett R. Coffee C. Gray R. Han Y. Zhang Y.P. Burke D.A. Functional consequences of lumbar spinal cord contusion injuries in the adult rat. J. Neurotrauma. 2005;22:529–543. doi: 10.1089/neu.2005.22.529. [DOI] [PubMed] [Google Scholar]
- Maïmoun L. Couret I. Mariano-Goulart D. Dupuy A.M. Micallef J.P. Peruchon E. Ohanna F. Cristol J.P. Rossi M. Leroux J.L. Changes in osteoprotegerin/RANKL system, bone mineral density, and bone biochemicals markers in patients with recent spinal cord injury. Calcif. Tissue Int. 2005;76:404–411. doi: 10.1007/s00223-004-0048-6. [DOI] [PubMed] [Google Scholar]
- Minematsu A. Yoshimura O. Maejima H. Miyamoto T. Change of bone mechanical strength in rats after spinal cord injury over a short term. Hiroshima J. Med. Sci. 2003;52:21–25. [PubMed] [Google Scholar]
- Modlesky C.M. Majumdar S. Narasimhan A. Dudley G.A. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J. Bone Miner. Res. 2004;19:48–55. doi: 10.1359/JBMR.0301208. [DOI] [PubMed] [Google Scholar]
- Morse L. Teng Y.D. Pham L. Newton K. Yu D. Liao W.L. Kohler T. Müller R. Graves D. Stashenko P. Battaglino R. Spinal cord injury causes rapid osteoclastic resorption and growth plate abnormalities in growing rats (SCI-induced bone loss in growing rats) Osteoporos. Int. 2008;19:645–652. doi: 10.1007/s00198-007-0494-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullender M.G. Huiskes R. Osteocytes and bone lining cells: which are the best candidates for mechano-sensors in cancellous bone? Bone. 1997;20:527–532. doi: 10.1016/s8756-3282(97)00036-7. [DOI] [PubMed] [Google Scholar]
- Mullender M.G. Huiskes R. Proposal for the regulatory mechanism of Wolff's law. J. Orthop. Res. 1995;13:503–512. doi: 10.1002/jor.1100130405. [DOI] [PubMed] [Google Scholar]
- Needham-Shropshire B.M. Broton J.G. Klose K.J. Lebwohl N. Guest R.S. Jacobs P.L. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 3. Lack of effect on bone mineral density. Arch. Phys. Med. Rehabil. 1997;78:799–803. doi: 10.1016/s0003-9993(97)90190-8. [DOI] [PubMed] [Google Scholar]
- Robling A.G. Niziolek P.J. Baldridge L.A. Condon K.W. Allen M.R. Alam I. Mantila S.M. Gluhak-Heinrich J. Bellido T.M. Harris S.E. Turner C.H. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- Rubin C. Recker R. Cullen D. Ryaby J. McCabe J. McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J. Bone Miner. Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- Rubin C. Turner A.S. Bain S. Mallinckrodt C. McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- Rubin C. Turner A.S. Mallinckrodt C. Jerome C. McLeod K. Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002a;30:445–452. doi: 10.1016/s8756-3282(01)00689-5. [DOI] [PubMed] [Google Scholar]
- Rubin C. Turner A.S. Müller R. Mittra E. McLeod K. Lin W. Qin Y.X. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J. Bone Miner. Res. 2002b;17:349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- Sabo D. Blaich S. Wenz W. Hohmann M. Loew M. Gerner H.J. Osteoporosis in patients with paralysis after spinal cord injury: a cross sectional study in 46 male patients with dual-energy x-ray absorptiometry. Arch. Orthop. Trauma Surg. 2001;121:75–78. doi: 10.1007/s004020000162. [DOI] [PubMed] [Google Scholar]
- Sievänen H. Immobilization and bone structure in humans. Arch. Biochem. Biophys. 2010;503:146–152. doi: 10.1016/j.abb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Slade J.M. Bickel C.S. Modlesky C.M. Majumdar S. Dudley G.A. Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free postmenopausal women. Osteoporos. Int. 2005;16:263–272. doi: 10.1007/s00198-004-1665-7. [DOI] [PubMed] [Google Scholar]
- Smith R.R. Brown E.H. Shum-Siu A. Whelan A. Burke D.A. Benton R.L. Magnuson D.S. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J. Neurotrauma. 2009;26:1017–1027. doi: 10.1089/neu.2008-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara H. Linsenmeyer T.A. Beam H. Parsons J.R. Mechanical properties of bone in a paraplegic rat model. J. Spinal Cord Med. 1998;21:302–308. doi: 10.1080/10790268.1998.11719539. [DOI] [PubMed] [Google Scholar]
- Voor M.J. Yang S. Burden R.L. Waddell S.W. In vivo micro-CT scanning of a rabbit distal femur: repeatability and reproducibility. J. Biomech. 2008;41:186–193. doi: 10.1016/j.jbiomech.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waarsing J.H. Day J.S. Verhaar J.A. Ederveen A.G. Weinans H. Bone loss dynamics result in trabecular alignment in aging and ovariectomized rats. J. Orthop. Res. 2006;24:926–935. doi: 10.1002/jor.20063. [DOI] [PubMed] [Google Scholar]
- Weinreb M. Rodan G.A. Thompson D.D. Immobilization-related bone loss in the rat is increased by calcium deficiency. Calcif. Tissue Int. 1991;48:93–100. doi: 10.1007/BF02555873. [DOI] [PubMed] [Google Scholar]
- Wolff J. The Law of Bone Remodeling. New York: Springer; 1892. 1986 (translation of the German 1892 edition). [Google Scholar]
- Xiong J. Onal M. Jilka R.L. Weinstein R.S. Manolagas S.C. O'Brien C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q. Voor M.J. Merten K. Hamilton K. Duncan S. Stone K. Falcone J. Pierce W. Metaphyseal fracture strength as a better measure of bone quality in osteoporosis. Proceedings of the Mid-America Orthopaedic Association, 27th Annual Meeting; Apr 22–26;2009 ; Amelia Island, FL. 2009. [Google Scholar]
- You L. Temiyasathit S. Lee P. Kim C.H. Tummala P. Yao W. Kingery W. Malone A.M. Kwon R.Y. Jacobs C.R. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder Y. Lüthi M. Michel D. Knecht H. Perrelet R. Neto I. Kraenzlin M. Zäch G. Lippuner K. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos. Int. 2004;15:180–189. doi: 10.1007/s00198-003-1529-6. [DOI] [PubMed] [Google Scholar]
- Zeng Q.Q. Jee W.S. Bigornia A.E. King J.G., Jr. D'Souza S.M. Li X.J. Ma Y.F. Wechter W.J. Time responses of cancellous and cortical bones to sciatic neurectomy in growing female rats. Bone. 1996;19:13–21. doi: 10.1016/8756-3282(96)00112-3. [DOI] [PubMed] [Google Scholar]