Abstract
Nanog is a homeodomain transcription factor associated with the acquisition of pluripotency. Genome analyses of lower and higher vertebrates revealed that the existence of Nanog is restricted to gnathostomata but absent from agnatha and invertebrates. To elucidate the function of Nanog in nonmammalia, we identified the Danio rerio ortholog of Nanog and characterized its role in gain and loss of function experiments. We found Nanog to be crucial for survival of early zebrafish embryos, because depletion of Nanog led to gastrulation defects with subsequent lethality. Mouse Nanog overexpression could rescue these defects. Vice versa, zebrafish Nanog was found to promote proliferation and to inhibit differentiation of mouse embryonic stem cells in the absence of leukemia inhibitory factor. These findings indicate functional conservation of Nanog from teleost fishes to mammals. However, Nanog was lost in the genome of the anurans Xenopus laevis and Xenopus tropicalis. Phylogenetic analysis revealed that deletion probably occurred in a common anuran ancestor along with chromosomal translocations. The closest homologs of Nanog in Xenopus are the Vent proteins. We, therefore, investigated whether the Xvent genes might substitute for Nanog function in Xenopus. Although we found some similarities in phenotypes after overexpression and in the regulation of several marker genes, Xvent1/2 and Nanog cannot substitute each other. Depletion of Nanog in zebrafish cannot be rescued by ectopic expression of Xvent, and Xvent depletion in Xenopus cannot be overcome by ectopic expression of zebrafish Nanog.
Introduction
The undifferentiated cell state in vertebrate embryogenesis and the maintenance of pluripotency in embryonic stem (ES) cells are characterized by the expression of factors, such as Oct4, Nanog, Dax1, Tert, or Dppa1-5. Expression of Oct4 appears to be a prerequisite for the maintenance of the undifferentiated state and self-renewal in mammalian ES cells [1–3], but despite an increasing knowledge about target gene regulations and molecular interactions in different species, the current data do not allow us to get a general view of signaling in maintaining pluripotency. Comparing the genes involved in pluripotency from an evolutionary point of view, the situation becomes even more complicated. In placental mammals (eutheria), only one Oct4 encoding gene (known as Pou5f1) exists, whereas monotremes (protheria) and marsupials (metatheria) additionally posses a Pou2 gene. In the amphibian Xenopus, the Pou2 gene has even been triplicated (Oct60, Oct25, Oct91) [4,5], whereas the Pou5f1 gene is lacking [6]. In some other vertebrates, such as chicken, zebrafish, or axolotl, either a Pou2 or the Pou5f1 gene exists [7]. In addition, several Oct4 regulation cascades are modified between mammals and nonmammals [6,8]. The pluripotency-associated factor, Dppa5, is completely absent in lower vertebrates [9]. Dppa2 and Dppa4 exist in lower vertebrates, such as Anolis (reptile) or Xenopus (amphibian), in form of only one homolog [10]. For a handful of other factors, which are associated with the maintenance of stem cell character, that is, Zfp206, Rex1, Dax1, Klf4, Dppa1, Dppa3, and even for Nanog, the data obtained from lower species are insufficient. Since there is also a stemness-like or a pluripotent state described for nonmammalian organisms [11,12], it is still not clear how this status is maintained in these species. Do these factors exist in lower vertebrates, and are they embedded in the same signaling cascades as in higher vertebrates?
In the current study, we focus on the homeobox transcription factor Nanog, another master regulator of pluripotency [13–15]. Nanog was identified by screening mouse cDNA libraries for ES cell specific transcripts [16,17] and by expression cloning from an ES cell cDNA library as a factor maintaining cytokine-independent self-renewal of murine ES cells [even in the absence of leukemia inhibitory factor (LIF)] [13]. Although Nanog is unable to re-induce stem cell character without Oct4 [18], Nanog is re-expressed in fully reprogrammed induced pluripotent stem cells and promotes transfer of pluripotency after cell fusions [19,20]. This indicates that Nanog is a central player in the core transcriptional regulatory circuitry of pluripotency [14,21,22] and is a gateway to the pluripotent ground state [15]. Nanog−/− mice embryos are lethal, and ES cells form extraembryonic endoderm at the expense of epiblast. Since Nanog is still expressed in Oct4−/− mouse embryos, it was suggested that these 2 transcription factors act in parallel pathways [13]. Although Oct4 cannot substitute Nanog in self-renewal of murine ES cells in the absence of LIF [23], these 2 factors share a high overlap of target genes [14,21]. In addition, a mutual protein binding between Oct4 and Nanog was found, recruiting HDAC1/2 and NuRD, Sin3A, Pml, and Mta1/2 to form repression complexes called NODE (Nanog and Oct4 associated deacetylase) [24].
A vertebrate homolog of Nanog in nonmammalian organisms was first reported for chicken [25,26]. It was postulated that Nanog is an amniote-specific gene and is absent from anamniotes and invertebrates [25]. However, Nanog homologs have recently been reported for the teleost fish (medaka) [27,28] and for the urodele amphibian axolotl [29]. It was suggested that the mechanisms governing pluripotency might be conserved from urodele amphibians to mammals [29]. However, this does not consider the situation in fish, where Nanog is present, or in the anuran amphibian Xenopus tropicalis, where a Nanog homolog is missing [30]. Therefore, it would be interesting to know which parts of Nanog function are still conserved between different species and which genes may account for Nanog function in Xenopus.
Here, we characterize the Nanog gene of Danio rerio and demonstrate by gain and loss of function (LOF) experiments that Nanog function is essential for zebrafish embryogenesis. We show that Nanog knockdown in zebrafish can be rescued by murine Nanog, whereas the closest sequence homologs to Nanog proteins in Xenopus, the homeobox transcription factors Xvent1 and Xvent2 [31,32], fail to substitute for Nanog function in D. rerio. Additionally, we demonstrate that zebrafish Nanog in contrast to Xvent2 promotes proliferation and inhibits differentiation of mouse ES cells even in the absence of LIF. These findings indicate that Nanog is functionally conserved from bony fishes (teleosts) to mammals.
Materials and Methods
Database analyses
Sequence analyses, EST, and genomic database search and sequence alignments were done by using bioinformatic programs: www.ncbi.nlm.nih.gov/blast; www.ensembl.org/index.html; www.ebi.ac.uk/clustalw/; http://genome.wustl.edu/tools/blast.
Antisense morpholino oligonucleotides
D.r. NanogMO (5′-ATCTTCCAGTCCGCCATTTCGCCGT-3′) (GeneTools) is directed against the translation start site (reverse complementary is underlined). Xvent1 and Xvent2 morpholino oligonucleotides (MOs) were previously reported [33]. For control injections in Xenopus, a control MO (ctrlMO) derived from the human β-globin gene (5′-CCTCTTACCTCAGTTACAATTTATA-3′) was used. For controls in zebrafish, a ctrlMO (5′-ATGCAAACAATACATACACCTCGTG-3′) was used. In vitro test of the morpholino specificity of NanogMO was done as previously described [10].
Cloning of genes and in vitro transcription
A Nanog clone of D. rerio was obtained from imaGenes (accession no. BC162318). The open reading frame was cloned into pCS2 expression vector. For rescue experiments, silent mutations were introduced in the MO-binding site of D.r. Nanog (mut-Nanog: 5′-cgGaattcATGTCaCGgCAcGAgGG-3′) via polymerase chain reaction (PCR) using mutated primers. The translation start is underlined; silent mutations are shown in lowercase. For GST fusion proteins, Nanog open reading frames (murine and D. rerio) were cloned into pGEX-4T-1 vector. Nanog mutants (HD+: 164–179 aa; ΔN: 194–384 aa; ΔC: 1–261 aa) and Nanog ΔHD, lacking aa 195–261, were cloned into pCS2 vector. For RNA injection, plasmids were transcribed with SP6. Digoxigenin labeled antisense RNA for in situ hybridization was obtained with T7 polymerase.
Protein expression and GST-pulldown assay
GST-fusion proteins were expressed in E. coli BL21 (DE3) Plus (Stratagene) and purified in batch with Glutathione-Sepharose™ 4B (Amersham Biosciences) under native conditions according to the manufacturer's protocol. Purified proteins were dialyzed overnight at 4°C (50 mM Tris-HCl, pH 8.0, 1 mM EDTA, 1 mM DTT, and 20% glycerol). 35S-methionine labeled proteins were translated with TNT®T7/SP6 Coupled Reticulocyte Lysate System (Promega). Pulldown analyses were done as previously described [34].
Electrophoretic mobility shift assay
Electrophoretic mobility shift assays (EMSAs) were performed with double-stranded oligonucleotides of the murine Tcf3 promoter (5′-AAACCTGTTAATGGGAGCGCAT-3′ [35]). Oligonucleotides were 5′-end-labeled with γ-ATP using T4 polynucleotide kinase and subsequently annealed. Gel-purified probes were incubated with Xvent2 or Nanog proteins (murine or Danio) for 25 min on ice in 30 μL binding buffer [36] containing the unspecific competitor poly(dIC). For specific competition, unlabeled probes were used. Samples were separated on 8% nondenaturing polyacrylamide gels and analyzed by autoradiography (BAS 1500–Fuji).
ES cell culture
In general, mouse ES cells (E14.1; 129/Ola-derived) were cultured with (DMEM+GlutaMax, 10% FCS, 1.5 g/L Na2CO3, 10 μg/L LIF (SIGMA), and 0.1% penicillin/streptomycin) on irradiated neomycin resistant mouse embryonic feeder cells (MEFs). M.m. Nanog, D.r. Nanog, and Xvent2 sequences were integrated into the eukaryotic expression vector pFIEN, and ES cells were electroporated (Biorad Gene Pulser, 240V) with recombinant constructs or pFIEN vector as control (40 μg). To obtain stably transfected clones, the ES cells were selected with G418 (250 μg/mL; Gibco) for 8 days and then sorted for GFP expression (BD, FACS Aria).
For differentiation assays, ES cells were trypsinized, separated from MEFs and 2×103 cells/40 μL differentiation media [IMDM (Gibco), 1.5 g/L Na2CO3, 10% FCS, 0.1% penicillin/streptomycin, 20 μL/L monothioglycerol, 1.1× nonessential amino acids] were incubated as hanging drops to form embryoid bodies. For controls, 10 μg/L LIF was added to the differentiation media to inhibit differentiation. At day 2 of incubation, embryoid bodies were transferred on gelatinized 24-well plates with differentiation media. Differentiation of embryoid bodies was monitored at day 3 by morphology and at day 4 by AP staining (Alkaline Phosphatase Substrate Kit III; Vector). Images were taken with a Keyence microscope.
BrdU and cell tracking assays
Stably transfected ES cells were dissociated with trypsin and re-seeded at a density of 5×105 cells. Cells were incubated in 10 μM BrdU solution (APC BrdU Flow Kit; BD Biosciences) in phosphate-buffered saline (PBS), harvested at different time points, re-trypsinized, and fixed in Cytofix/Cytoperm (BD Sciences) buffer. Washing, re-fixation, and BrdU staining were performed according to the manufacturer's manual. For counterstaining, cells were incubated in 7-amino-actinomycin (BD Biosciences) and analyzed on FACSCalibur.
For cell tracking, the same amounts of cells were incubated for 45 min in 15 μM Cell Tracker Red CMPTX (Promega). Dye working solution was replaced, and cells were washed with PBS. Cells were trypsinized. Washing, incubation, and fixation were performed according to the manufacturer's protocol (Promega). Cells were analyzed on FACSCalibur.
Real-time reverse transcriptase-PCR
Total RNA extraction, purification, first-strand cDNA synthesis, and real-time reverse transcriptase (RT)-PCR were done as previously described [10]. Data are presented in relative units. Primers for RT-PCR are listed in Supplementary Table S1A, B; Supplementary Data are available online at www.liebertonline.com/scd.
Embryonic staging and in situ hybridization
Embryonic stages were determined for Xenopus [35] and for zebrafish [37]. Whole mount in situ hybridizations were performed according to standard procedures [38,39].
Results
Nanog is present in gnathostomata except for Xenopus
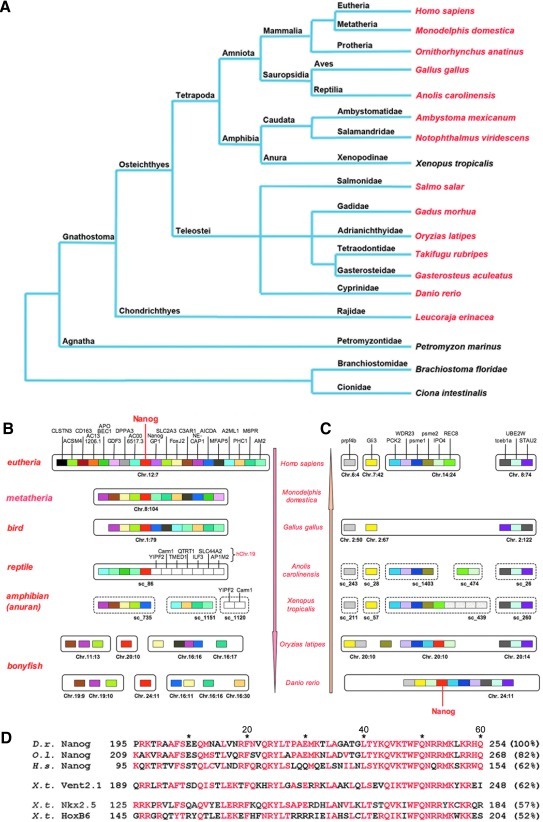
To identify Nanog orthologs in lower vertebrates, we performed BLAST analyses with genomic, EST, and protein databases from different species. We detected Nanog orthologs not only in pro- and metatheria (platypus and opossum), but also in several bony fishes (Cyprinidae: D. rerio; Gasterosteidae: stickleback; Gadidae: Atlantic cod; Salmonidae: salmon; tetradontidae: fugu and tetraodon) (Fig. 1A). The EST CO049347 of the little skate (Leucoraja erinace) gives rise to the assumption that Nanog already exists in cartilaginous fishes. However, we were unable to detect Nanog in the genome of the lamprey (Petromyzon marinus), a representative of jaw-less vertebrates (agnatha), in acranial vertebrates (Branchiostoma), and tunicates [urochordata (ie, Ciona)]. In addition, Nanog-like genes do not exist in non-vertebrates, for example, insects (D. melanogaster) or nematodes (Caenorhabditis elegans). Thus, Nanog appeared in chordates with the evolution of jaw (gnathostomata).
FIG. 1.
Nanog exists in gnathostomata but not in Xenopodinae. (A) Phylogenetic tree and verification of Nanog. Species in red letters: Nanog is present. Species in black letters: Nanog is missing. (B) Synteny analysis of human Nanog and its neighboring genes in lower vertebrates created by the Ensembl database (Anolis carolinensis, version 1.0; Xenopus tropicalis, version 4.1). (C) Synteny analysis of fish Nanog and neighboring genes in higher vertebrates. (D) Alignment of the homeodomain (HD) of Nanog from zebrafish, medaka, human, and the HD of Xvent2.1, Nkx2.5, and HoxB6 from X. tropicalis. Amino acids (PAM homology) are shown in red (3 of 6). Percentages of homologies to zebrafish Nanog are given in brackets. D.r., Danio rerio; O.l., Oryzias latipes; H.s., Homo sapiens; X.t., Xenopus tropicalis.
Interestingly, Nanog is lacking in the genome of X. tropicalis [30]. Since we also failed to detect corresponding ESTs for Nanog in Xenopus laevis, we suppose that Nanog is absent from the entire genus Xenopus and perhaps from the complete pipidian subfamily Xenopodinae. To understand how Nanog was deleted from the Xenopus genome, we performed an extensive synteny analysis of the Nanog loci (Fig. 1B). The human Nanog is located at chromosome 12:7. The genomic surroundings of Nanog are almost identical from human to chicken. Nevertheless, we found in pro- and eutheria some chromosomal inversions and in chicken, the absence of distinct genes, such as GDF3 and Dppa3. Even in the genome of the reptile Anolis carolinensis, Nanog can be located with partially existing synteny in scaffold (sc) 86. Upstream of the Anolis Nanog gene, there are orthologous neighboring genes, such as AM2 or APOBEC1. Downstream of Nanog are 7 genes, whose human orthologs can be found on human chromosome 19. The ortholog genes in X. tropicalis are located within sc_1120. Another block of genes neighboring Nanog in A. carolinensis can be found in X. tropicalis on sc_735 and sc_1151, but no Nanog gene was found within these scaffolds. In teleost fishes, the synteny is completely lost. We checked the surroundings of the Nanog gene in D. rerio and found them to be identical to fugu, tetraodon, and medaka Nanog genes, but there is no conservation of the Nanog locus in comparison to non-fishes. The Nanog gene of D. rerio is located at chromosome 24:11. By tracking the opposite way, we then checked the genomic surroundings of the Nanog in teleost fishes (medaka and zebrafish) and analyzed the location of orthologous genes in other vertebrates (Fig. 1C). The genes surrounding the fish Nanog genes are found in higher vertebrates as orthologous on different chromosomes indicating various chromosomal translocations and rearrangements within the Nanog locus. Such chromosomal translocations are even found in X. tropicalis, but again there is no evidence for a Nanog gene coupled to any of these neighboring genes in this species.
Divergence of Nanog proteins in different species and relationship to Xvents
Murine and human Nanog were described as 3-domain proteins containing a homeodomain (HD), a serine enriched N-terminal, and a C-terminal transactivator domain containing several tryptophan repeats [40,41]. The tryptophan repeats were shown to be crucial for dimerization, proliferation, the maintenance of Nanog-dependent cell self-renewal, and for binding of Nac1 [42–44]. However, when comparing the mammalian Nanog proteins to those of medaka or chicken, they are highly divergent. Therefore, we asked for common characteristics of all Nanog proteins and aligned the orthologs from different species (Supplementary Fig. S1). In addition to rather high rates of identity inside the HD, we found conserved aspartate/glutamate stretches and serine/threonine residues in both the C- and the N-terminus. The C-terminally located tryptophan repeats are mainly found in eutherians, but 2 conserved residues are still found in pro- and metatheria (pos. 198 and 203 of the mouse protein).
Inspection of the Nanog HD reveals a striking aspect being characteristic for the BarH subfamily of HD proteins [45], that is a specific amino acid exchange from an aliphatic nonpolar residue, such as valine or isoleucine, to the polar residue threonine (pos. 241 of the zebrafish protein) (Fig. 1D). This exchange can only be detected in a few Xenopus HD proteins, such as the Vent proteins and Hox11, but not in Nkx or distal-less (Dll) proteins, which also share substantial identities with the HD of Nanog. The highest rates of identity for Nanog proteins exist with Xenopus Vent proteins. Xvent2.1 from X. tropicalis (we used this name for gene1 of Xvent2, because all Xvent1/2/3 genes were found to be duplicated in X. tropicalis) is not a Nanog protein, but belongs to the BarH class [45] and is closely related to Nanog (Fig. 1D; Supplementary Fig. S1). However, a crucial attribute of the Nanog HD is a highly conserved aliphatic, nonpolar residue in helix α2 (M at pos. 225 in zebrafish Nanog), which contributes to DNA binding through minor groove contacts [36]. All other HD proteins, including the Vent proteins, have a basic amino acid (mostly lysine or arginine) at this position. Further, we found 3 invariant positions in the Nanog HD (zebrafish Q218, Y237, and K238) that were exchanged in Xvent2.1. Further, a triplet of amino acids (tρφ), which is invariant in all Nanog proteins, is partially conserved in Xvent2.1 (S at pos. 71 in murine Nanog) (Supplementary Fig. S1), but not in other BarH proteins or in the related Dll proteins (data not shown). We also found several S/T residues in the N-terminus of Xvent2.1 as described for Nanog. However, the 2 invariant amino acids flanking a polar residue adjacent to the HD domain of Nanog at the C-terminus, a basic and a tryptophan residue (b_ρ_W), are not detected in Xvent2.1.
Expression pattern and functional characterization of D.r. Nanog
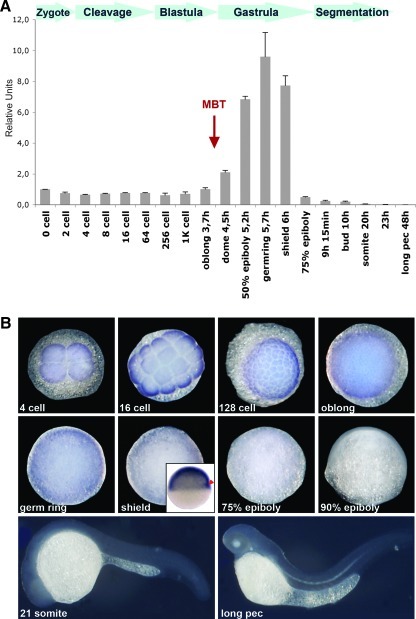
To investigate the role of Nanog in lower vertebrates, we analyzed its function in zebrafish. The full coding sequence of D.r. Nanog was obtained from the image clone BC162318. The corresponding protein contains 384 amino acids. We determine the temporal expression pattern of Nanog by real-time RT-PCR. Nanog RNA can already be detected in unfertilized eggs (Fig. 2A). The amount of transcript is strongly increased at the beginning of gastrulation, peaks at germ ring stage, and is then decreasing. When segmentation starts at 9 h postfertilization, Nanog transcripts are greatly diminished. From somite stage onward, Nanog cannot be detected anymore. We next examined the spatial expression pattern by whole mount in situ hybridization (Fig. 2B). Nanog can already be detected in 4-, 16-, and 128-cell stages. High levels of Nanog are observed during gastrulation, but at the end of gastrulation (75% epiboly), transcripts are already strongly diminished. From segmentation onward (90% epiboly), Nanog cannot be detected anymore.
FIG. 2.
Nanog expression during zebrafish embryogenesis. (A) Temporal expression of D.r. Nanog analyzed by real-time RT-PCR. Histone H4 is used as internal control, and expression is given in relative units. (B) Expression analysis of D.r. Nanog by whole mount in situ hybridization. Four-cell to 75% epiboly are viewed from top, the insert shows a lateral view of the shield (red arrowhead). About 90% epiboly to long pec stage are shown laterally. RT-PCR, reverse transcriptase–polymerase chain reaction.
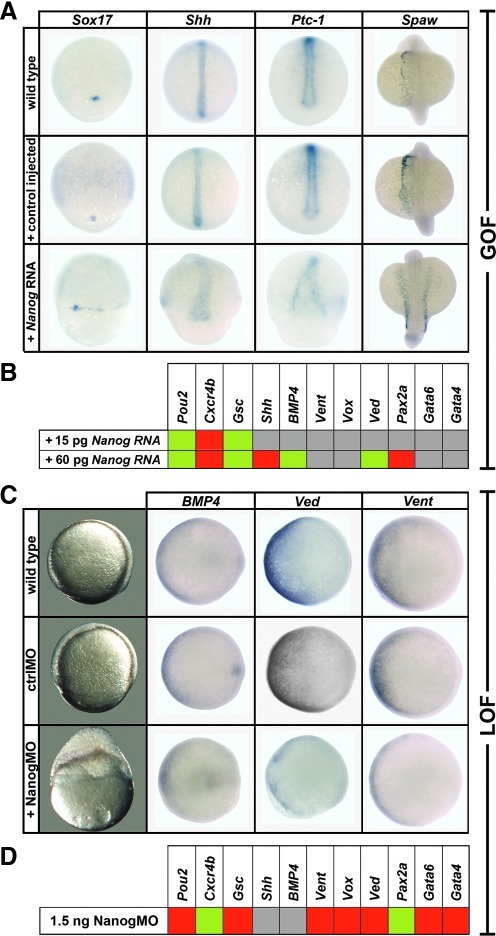
Next, we performed gain of function studies by Nanog overexpression in zebrafish embryos. Injection of D.r. Nanog RNA did not prevent gastrulation, and embryos did not display obvious abnormalities during gastrulation (data not shown). To obtain more precise information on genetic dysregulation, several marker genes were analyzed by in situ hybridization (Fig. 3A; Supplementary Table S2A, S2B). We tested Sox17 (endodermal marker), sonic hedgehog (Shh), a marker for neural ectoderm, and its target Ptc-1. We found in all cases disturbed expression patterns with a loss of the boundaries of their expression domains. Southpaw (Spaw), a nodal-related gene, which is normally expressed at the left side at somite stages [46], is bilaterally expressed in D.r. Nanog RNA injected embryos. Marker gene analysis via real-time RT-PCR (Fig. 3B, for details see Supplementary Table S3A) revealed that Shh was repressed in embryos injected with higher amounts of Nanog RNA. Pou2 was upregulated, even at a low dose of Nanog RNA. This result is consistent with the situation in mammals, where Nanog stimulates Oct4 expression and vice versa [14,44]. We observed no change of ventral markers (BMP4, Vent, Vox, or Ved) at low amounts of Nanog RNA, but an upregulation of Ved (ventrally expressed dharma/bozozok antagonist) and BMP4 at higher amounts of Nanog RNA. Interestingly, goosecoid (Gsc), a marker of dorsal mesoderm, is also upregulated. This finding indicates that Nanog signaling does not only shift the cells toward a ventral fate, but also acts in a more complex manner. Moreover, we observed a strong decrease of the neuroectodermal marker and key regulator of midbrain-hindbrain boundary, Pax2a [47]. Surprisingly, the chemokine receptor Cxcr4b, known as a guidance molecule of primordial germ cells in zebrafish and medaka [48,49], was repressed. This was unexpected, as Cxcr4b was shown to be a direct target of Nanog in medaka and was downregulated after Nanog depletion [50]. Gata4 and Gata6, which were shown to be regulators of endodermal cell fate in Xenopus [51], are not significantly changed, although they are repressed by Nanog in human ES cells [52,53].
FIG. 3.
Gain of function (GOF) and loss of function (LOF) analyses of D.r. Nanog. (A) Whole mount in situ hybridization of wild-type, control (unrelated nucleotide sequence), and Nanog RNA (400 pg) injected embryos hybridized with Sox17 (80% epiboly), Shh (90% epiboly), Ptc-1 (90% epiboly), or Spaw (12 somite stage). (B) Real-time RT-PCR of D.r. Nanog RNA injected embryos at 6 somite stage for indicated marker genes relative to wild-type embryos. (C) Uninjected control, ctrlMO, and NanogMO (1.5 ng) injected embryos at 90% epiboly (lateral view) and in situ hybridization (animal view, the ventral side is left orientated) with BMP4, Ved, and Vent (D) Real-time RT-PCR of NanogMO injected embryos in relation to ctrlMO injected embryos for indicated marker genes at 80% epiboly. Histone H4 is used as internal control. Green: upregulation (≥1.5), red: decrease (≤0.75), gray: no change in transcript level (0.75<×<1.5). MO, morpholino oligonucleotide; ctrl MO, control MO.
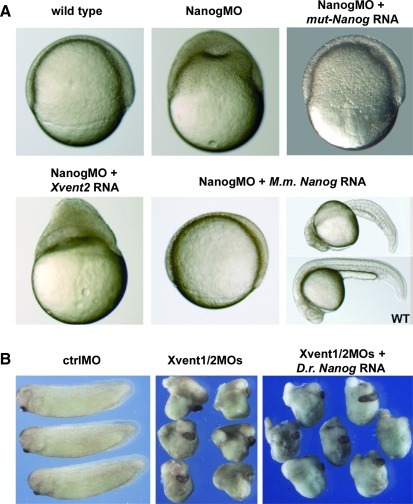
LOF studies were performed by using the morpholino (MO) based knockdown strategy. Specificity of NanogMO was demonstrated in vitro by coupled transcription/translation assays with D.r. Nanog RNA and mutated Nanog (mut-Nanog) RNA, containing 11 silent mutations in the putative MO binding site, in the presence of NanogMO or an unrelated ctrlMO (Supplementary Fig. S2). Translation of D.r. Nanog RNA is blocked by NanogMO but not by ctrlMO. In addition, NanogMO cannot suppress translation of mut-Nanog RNA. NanogMO or ctrlMO were then injected in zebrafish embryos at one cell stage. Although NanogMO injection does not result in disturbed embryogenesis before gastrulation (data not shown), we found the epiboly progression to be strongly impaired in 99% of injected embryos (Fig. 3C, Supplementary Table S2C). Although the blastoderm continues to become thin and has almost covered the yolk cells at 90% epiboly in uninjected and ctrlMO injected embryos, the epiblast in the NanogMO injected embryos is strongly thickened and covered only approximately half of the yolk. Later on, NanogMO injected embryos die, whereas control siblings develop normally. To ensure that the phenotypes yielded by NanogMO injections are specific for Nanog depletion and not a toxic side effect of the NanogMO, we performed rescue experiments. In contrast to NanogMO injection alone, we found that NanogMO and mut-Nanog RNA co-injected embryos overcome critical stages and develop further (Supplementary Table S2C; see also Fig. 6A).
FIG. 6.
Mouse Nanog rescues zebrafish embryos after Nanog knockdown. (A) Co-injection of 400 pg mouse Nanog RNA and 1.5 ng NanogMO in zebrafish embryos prevents the knockdown dependent gastrulation defect and subsequent death, whereas co-injection of 400 pg Xvent2 RNA cannot do so. Upper: uninjected control (90% epiboly), NanogMO injected and knockdown embryos co-injected with mut-Nanog RNA. Lower: knockdown embryos co-injected with Xvent2 RNA or co-injected with mouse Nanog RNA at 90% epiboly and at somitogenesis. (B) Co-injection of D.r. Nanog RNA cannot rescue the Xvent1/2MOs (45 ng) caused phenotype in X. laevis embryos. Left: uninjected X. laevis embryos, stage 29, middle: Xvent1MO and Xvent2MO injected embryos. Right: Xvent1MO and Xvent2MO injected embryos co-injected with 100 pg D.r. Nanog RNA.
We also analyzed Nanog depleted embryos by in situ hybridization and RT-PCR for the expression of ventral marker genes, such as BMP4, Ved, and Vent (Fig. 3C, D; Supplementary Tables S2D and S3B). Although BMP4 expression, responsible for the specification of ventro-posterior cell fate in the late phase of BMP signaling [54], is almost unaffected, the maternally expressed Ved [55] is strongly repressed. In addition, Vent and Vox, which are transcribed at midblastula transition (MBT), are decreased in Nanog depleted embryos. Gata4 and Gata6 expression is also decreased (Fig. 3D). We further observe in Nanog depleted embryos a repression of Gsc and Pou2, confirming the results that Nanog overexpression can activate Gsc and Pou2 expression (Fig. 3B, D). This is in contrast to medaka, where Nanog depletion has no influence on Oct4 expression level [27]. In addition, Pax2a and Cxcr4b, which were repressed by overexpression, are strongly upregulated in Nanog depleted embryos (Fig. 3B, D).
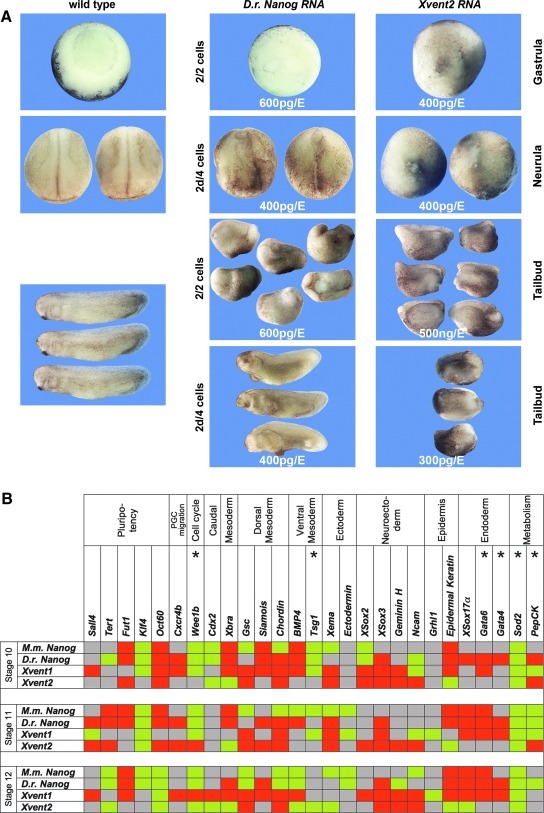
Since the Vent genes display a close sequence homology to zebrafish Nanog and a similar temporal expression profile with transcriptional activation around MBT [31,32], we asked whether overexpression of zebrafish Nanog and Xvent in Xenopus results in the same or a similar phenotype. Bilateral injections of D.r. Nanog or Xvent2 RNA at 2-cell stage delay blastopore formation (Fig. 4A, Supplementary Table S2E). Dorsal injections of D.r. Nanog or Xvent2 RNA in 4-cell stage embryos lead to lacking head structures (Fig. 4A). We also performed real-time RT-PCR for selected marker genes at different developmental stages after injection of mouse and D.r. Nanog as well as Xvent1/2 RNA (Fig. 4B, Supplementary Table S3C). We focused on markers for the undifferentiated state (Oct60, Tert, and Klf4), germ layer formation (such as Xbra, Gsc, and Siamois), cell cycle (Wee1b), and metabolism (PepCK, Sod2), including genes that by microarray analyses were reported to be changed by Nanog in ES cells (marked by asterisks) [56]. Importantly, mouse and D.r. Nanog RNA injections mainly lead to identical regulations. Although many genes are similarly affected by Xvent RNA injections, that is, Sod2, Wee1b, XSox3, or Oct60, we found an inverse regulation of Gsc and Sox2 (Fig. 4B).
FIG. 4.
Comparison of Xvent and D.r. Nanog overexpression in Xenopus laevis. (A) Overexpression of Nanog or Xvent2 in X. laevis embryos results in similar phenotypes. Embryos are shown at stage 11 (vegetal view), at stage 18 (dorsal view), and at stage 29 (lateral view). RNAs were bilaterally injected at indicated amounts into 2-cell stage embryos or into the 2 dorsal cells at 4-cell stage, respectively. (B) Schematic presentation of real-time RT-PCR data derived from overexpression of Nanog and Xvent1/2 in X. laevis at stages 10, 11, and 12. M.m. Nanog (400 pg), D.r. Nanog (400 pg), Xvent1 (350 pg), or Xvent2 RNA (350 pg) were bilaterally injected into 2-cell stage embryos. Histone H4 was used as internal control. Green: upregulation (≥1.5), red: decrease (≤0.75), gray: no change in transcript level (0.75<×<1.5). Marker genes that were shown to be regulated by Nanog in ES cells [56] are indicated by asterisks. ES, embryonic stem.
Murine and D.r. Nanog show similar protein interactions and substitute for each other in vivo
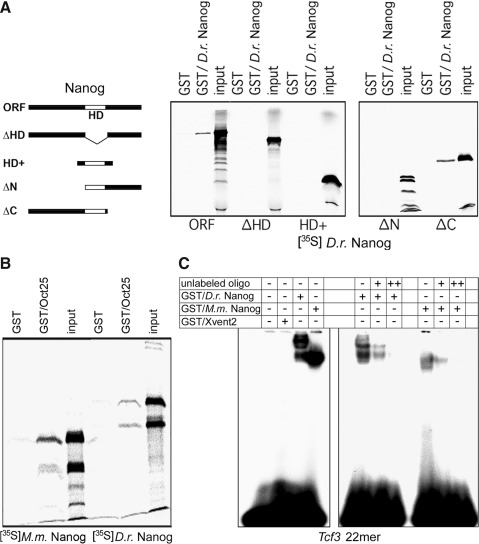
Mammalian Nanog acts as a dimer in the maintenance of self-renewal in the absence of LIF, and dimerization of Nanog was shown to be dependent on C-terminally located tryptophan repeats [42,43]. Axolotl Nanog was reported to be unable for dimerization, because it lacks the tryptophan repeats [29]. Since these repeats are obviously specific for mammals (Supplementary Fig. S1), they are also absent from zebrafish. However, by GST-pulldown analyses, we found that D.r. Nanog can self-associate (Fig. 5A). Size exclusion chromatography of radiolabeled D.r. Nanog on Sephadex G-100 revealed that 70% of labeled protein eluted as dimers and 30% as monomers (data not shown). We prepared deletion constructs of Nanog to delineate the putative dimerization domain and found that the N-terminal region as well as the HD are required for the dimerization process (Fig. 5A). Noteworthy, in contrast to mammalian and axolotl Nanog, the N-terminal region of zebrafish Nanog displays an enrichment of tryptophan residues (Supplementary Fig. S1). We next investigated whether D.r. Nanog also physically interacts with the Pou2 homologous Xenopus protein Oct25. Mammalian and axolotl Nanog bind to Oct4 [22,24,29]. GST-pulldown analyses with Oct25 and murine or zebrafish Nanog demonstrated that both HD transcription factors interact with Oct25 (Fig. 5B).
FIG. 5.
Protein and DNA binding properties of Nanog. (A) Scheme of Nanog deletion mutants used for pulldown analysis of GST-Nanog. White box: HD; ΔHD: deletion of the HD; HD+: HD of Nanog and surrounding 5 amino acids. Note that dimerization of D.r. Nanog does not require the C-terminus. (B) Oct25 interacts with mouse as well as with D.r. Nanog. 35S methionine-labeled mouse and zebrafish Nanog proteins were incubated with the GST-fusion protein of Oct25. (C) Electrophoretic mobility shift assay of a 22mer of the murine Tcf-3 promoter. D.r. and murine Nanog but not Xvent2 interact with the Nanog binding site.
Next, we tested by EMSA a 22mer of the murine Tcf3 promoter, which was shown to be sufficient for murine Nanog binding [36], for binding of D.r. Nanog and Xvent2. The reported consensus binding sequences of murine Nanog (TAATGG, [36]) and Xvent2 (CTAATT, [57,58]) show a certain overlap. However, we found that the murine as well as the zebrafish Nanog can bind the 22mer, whereas Xvent2 does not interact with this binding site (Fig. 5C). This difference in DNA binding between Xvent and Nanog might be due to the already mentioned amino acid exchange within the α2 helix of the HD (basic residue→aliphatic, nonpolar residue).
We then asked whether D.r. Nanog, mouse Nanog, and Xvent can substitute each other after LOF in zebrafish or Xenopus embryos, respectively. First, we tried to overcome NanogMO dependent gastrulation defects and subsequent death by co-injection with mutated D.r. Nanog, mouse Nanog, or Xvent2 RNA (Fig. 6A). Although the blastoderm in Nanog depleted embryos remains thick and does not cover the yolk, co-injection of mouse Nanog RNA successfully substituted for zebrafish Nanog, as the gastrulation defects were reverted, and the embryos survived (Fig. 6A; Supplementary Table S2C). However, co-injection of Xvent2 RNA at different amounts does neither inhibit nor attenuate the NanogMO based gastrulation defects. Vice versa, we depleted Xvent1 and Xvent2 in Xenopus embryos by MO injections and tried to rescue the wild-type phenotype by co-injection of Danio Nanog RNA (Fig. 6B). Co-injections of Xvent1MO/Xvent2MO result in hyper-dorsalized embryos with strongly reduced body axis [33]. Co-injections of murine (data not shown) or zebrafish Nanog RNA at various concentrations did never restore dorso-ventral body axis or attenuate the Xvent1/2 depletion phenotype. Therefore, we conclude that Nanog and Xvent cannot substitute each other in gastrulation processes of zebrafish and in dorso-ventral patterning of X. laevis.
D.r. Nanog but not Xvent promotes proliferation and LIF-independent self-renewal of mouse ES cells
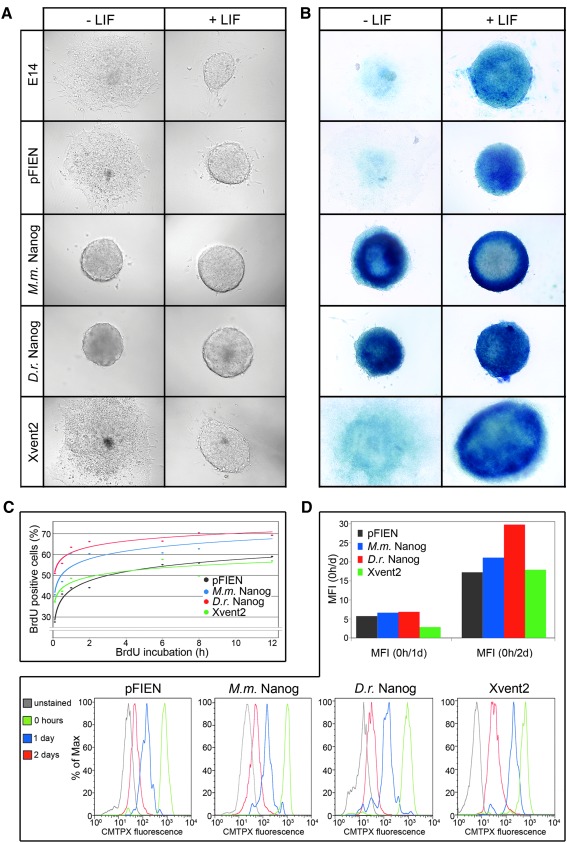
To test whether M.m. Nanog, D.r. Nanog, or Xvent2 have the same effects regarding cell differentiation and cell proliferation in ES cells, we generated stably transfected mouse ES cell lines of these constructs (Supplementary Fig. S3). The effects on cell differentiation were monitored by embryoid body assays, where the morphology and the loss of pluripotency were analyzed. With LIF, that is inhibiting differentiation of ES cells, embryoid bodies of all tested cell lines kept a round, undifferentiated shape, and mainly consisted of pluripotent cells. Without LIF, control cell lines (E14 and pFIEN vector control) and Xvent2 overexpressing cells started differentiation and lost their pluripotent state, which was indicated by the outgrowing and expanding morphology and the decreased blue AP staining of the embryoid bodies after 4 days of culturing (Fig. 7A, B). In contrast, M.m. Nanog as well as D.r. Nanog overexpression were able to inhibit differentiation, visible by the still round shape and blue AP staining of the cultured embryoid bodies. This could be further substantiated by an expression analysis of some selected marker genes in the absence of LIF. Although the pluripotency genes Pou5f1 and Sox2 are highly expressed in D.r. Nanog and M.m. Nanog transfected cells, the differentiation markers T (brachyury) and Sox17 exhibited a strong increase in Xvent2 and pFIEN vector transfected cells (Supplementary Fig. S4).
FIG. 7.
Mouse and zebrafish Nanog promote self-renewal and cell proliferation. (A) Morphology of embryoid bodies after 3 days with (+) and without (−) LIF (leukemia inhibitory factor). (B) Alkaline phosphatase (AP) staining of embryoid bodies with and without LIF after 4 days of culturing. (C) Nanog promotes cell proliferation. Percentages of BrdU positive ES cells detected with FACSCalibur in correlation to the incubation time in hours. (D) Histograms of fluorescence intensity of transfected ES cells treated with Cell Tracker Red CMPTX, in correlation to the percentages of cell numbers analyzed on FACSCalibur, and diagram presenting the ratio of mean fluorescence intensity (MFI) of 0 h to 1 or 2 days after removal of the cell tracker.
Previous studies implicate an important role of Nanog in cell proliferation not only for the mammalian [42] but also for the axolotl and medaka protein [27,29]. To test whether D.r. Nanog and Xvent2 also enhance cell proliferation, we performed BrdU and cell tracking assays with the transfected ES cell lines. We observed a much higher level of BrdU incorporation in mouse Nanog and D.r. Nanog transfected cells in contrast to Xvent2 or vector transfected ES cells (Fig. 7C). To verify these results, we also performed a cell tracking assay. ES cells were incubated with Red CMPTX as cell tracker. A high proliferation rate results in a higher basic value of fluorescence intensity coupled with an enhanced decrease of fluorescence intensity, when the cell tracker is removed. We here observed that fluorescence intensities after 45 min incubation were higher and decreased much faster in D.r. and mouse Nanog transfected ES cells than in pFIEN or Xvent2 transfected cells (Fig. 7D). Accordingly, the ratio of mean fluorescence intensity of 0 h/1 day and, especially 0 h/2 day after removal of cell tracker, was higher in the mouse and D.r. Nanog than in the control and Xvent2 transfected cells (Fig. 7D). Both experimental approaches, BrdU assay and cell tracking, suggest that murine as well as D.r. Nanog enhance proliferation.
Discussion
The Nanog gene is present in amphibia but lacking from the anuran Xenopus
EST data and genomic linkage analyses suggest a model according to which Nanog arose when gnathostomata evolved. In amphibians, Nanog was already identified in the newt Notophthalmus viridescens [59] and in the axolotl Ambystoma mexicanum [29], but Nanog was reported to be absent from the X. tropicalis genome [30]. Since we were also unable to identify ESTs for Nanog in X. laevis, we assume that Nanog is missing from the whole genus Xenopus. The genomic linkage analysis indicates that the Nanog gene has been deleted during some chromosomal translocations in Xenopodinae. During evolution, Nanog passed at least 2 chromosomal rearrangements, first from Osteichthyes to Tetrapodae, and second within amphibians, where Nanog was depleted from anurans or, more specifically, from the Xenopus genome.
Characterization of Nanog in D. rerio
The Nanog gene in zebrafish is maternally expressed and strongly upregulated after the blastula stage of development. Transcripts are detected in the shield, are most abundant during gastrula stage, but are rapidly degraded by 75% epiboly. The murine Nanog expression was detected during embryogenesis from the morula stage onward, is later restricted to the epiblast, and excluded from primitive endoderm [16,17]. Mouse Nanog is detectable in the primitive streak, but is downregulated in cells ingressing through the streak to form mesoderm and definitive endoderm [60]. It is enriched in pluripotent cell lines, such as ES cells, embryonic germ cells, and embryonic carcinoma cells, but absent from somatic tissues [13,17]. Overexpression of zebrafish Nanog revealed an upregulation of the pluripotency factor Pou2 and of ventral (BMP4, Ved) and dorsal-mesodermal (Gsc) markers, whereas the Cxcr4b gene was downregulated. Vice versa, knockdown of Nanog led to an increase of Cxcr4b and Pax2a, whereas Pou2, Gsc, Vent, Vox, Ved, Gata4, and Gata6 were downregulated. Nanog overexpression does not affect the phenotype during gastrulation, but in situ hybridizations revealed a delocalized expression of Sox17, Shh, Ptc-1, and Spaw. Similar to that previously reported for medaka [27], Nanog knockdown in zebrafish prevents epibolic movements during gastrulation and is lethal.
The proliferative effect of Nanog is evolutionarily conserved
D.r. Nanog forms homodimers and maintains self-renewal of mouse ES cells in the absence of LIF. This finding correlates to the effects of murine Nanog, whereas axolotl Nanog was shown to maintain self-renewal, when tryptophan residues in the C-terminus were introduced [29]. Moreover, we found that murine Nanog is able to rescue depletion of zebrafish Nanog during gastrulation. These findings indicate that both D.r. Nanog and mouse Nanog behave as functional homologs.
An important aspect of Nanog function is the proliferative effect that was already described for mammalian, medaka, and axolotl Nanog [27,29,42]. Here, we demonstrate by BrdU incorporation and cell tracking that D.r. Nanog transfected mouse ES cells show a significant increase of proliferation. We, therefore, postulate that the regulation of proliferation is an important common role for all Nanog proteins which has been evolutionarily conserved.
Is Nanog in frogs replaced by Xvents?
How can an organism, such as Xenopus, survive without an important gene for vitality? Either some basic developmental processes in anurans/Xenopodinae have evolutionarily been changed, which made the Nanog gene dispensable, or the function(s) of Nanog gene might have been replaced by functionally related genes. Therefore, we have asked whether Xenopus Vent genes could have compensated for the missing Nanog. Sequence homologies, especially regarding the BarH specific threonine residue and the conserved serine residue outside the HD, which cannot be found in any other BarH proteins, suggest that Vent and Nanog arose from a common ancestor. In addition, the temporal expression profiles of zebrafish Nanog and Xenopus Vents are very similar. Overexpression of zebrafish Nanog and Xenopus Vents in frogs revealed similar phenotypes, and many developmentally important genes were regulated in the same manner. Moreover, zebrafish Nanog as well as Xvent2 can dimerize and bind to Oct25. However, there are other important genes, such as the dorsalizing genes Gsc and Chordin, which are regulated by Nanog and Xvent in an opposite way. Moreover, Nanog stimulates cell proliferation, whereas Xvent has no stimulating effect. Finally, we could show that neither Nanog LOF in zebrafish can be overcome by Xvent overexpression nor LOF of Xvents in frogs can be overcome by zebrafish Nanog overexpression. In contrast, murine Nanog is still able to rescue the depletion of Nanog in zebrafish embryos during gastrulation. These experiments clearly demonstrate that Nanog function in Xenopus cannot simply be substituted by Xvent genes. Either there are other genes involved or Nanog function is per se dispensable in Xenopus. The identification of Nanog from additional amphibians, in particular of other anurans, would help in understanding the role of Nanog in embryogenesis and why it is dispensable in Xenopus.
Supplementary Material
Acknowledgments
The authors thank Alpaslan Tasdogan for help in the proliferation assays, Christian Klein (Max Delbrueck Center for Molecular Medicine) for providing zebrafish embryos for the Nanog in situ hybridization, and Janine Ziermann (Institute of Biology, Leiden University) for providing EST sequences of Triturus carnifex and helpful discussion. This work was supported by grants to W.K. (SFB 497/A1), M.P. (Marie Curie IRG No. 268333), and K.B. (ESF/M.v.W. Scholarship).
Author Disclosure Statement
There are no conflicts of interest relating to this work for any of the authors.
References
- 1.Chambers I. Silva J. Colby D. Nichols J. Nijmeijer B. Robertson M. Vrana J. Jones K. Grotewold L. Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- 2.Masui S. Nakatake Y. Toyooka Y. Shimosato D. Yagi R. Takahashi K. Okochi H. Okuda A. Matoba R, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 3.Pan GJ. Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42–49. doi: 10.1038/sj.cr.7310125. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y. Siegel D. Knöchel W. Xenopus POU factors of subclass V inhibit activin/nodal signaling during gastrulation. Mech Dev. 2006;123:614–625. doi: 10.1016/j.mod.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Morrison GM. Brickman JM. Conserved roles for Oct4 homologues in maintaining multipotency during early vertebrate development. Development. 2006;133:2011–2022. doi: 10.1242/dev.02362. [DOI] [PubMed] [Google Scholar]
- 6.Niwa H. Sekita Y. Tsend-Ayush E. Grützner F. Platypus Pou5f1 reveals the first steps in the evolution of trophectoderm differentiation and pluripotency in mammals. Evol Dev. 2008;10:671–682. doi: 10.1111/j.1525-142X.2008.00280.x. [DOI] [PubMed] [Google Scholar]
- 7.Frankenberg S. Pask A. Renfree MB. The evolution of class V POU domain transcription factors in vertebrates and their characterisation in a marsupial. Dev Biol. 2010;337:162–170. doi: 10.1016/j.ydbio.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Onichtchouk D. Geier F. Polok B. Messerschmidt DM. Mössner R. Wendik B. Song S. Taylor V. Timmer J. Driever W. Zebrafish Pou5f1-dependent transcriptional networks in temporal control of early development. Mol Syst Biol. 2010;6:354. doi: 10.1038/msb.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierre A. Gautier M. Callebaut I. Bontoux M. Jeanpierre E. Pontarotti P. Monget P. Atypical structure and phylogenomic evolution of the new eutherian oocyte- and embryo-expressed KHDC1/DPPA5/ECAT1/OOEP gene family. Genomics. 2007;90:583–594. doi: 10.1016/j.ygeno.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Siegel D. Schuff M. Oswald F. Cao Y. Knöchel W. Functional dissection of XDppa2/4 structural domains in Xenopus development. Mech Dev. 2009;126:974–989. doi: 10.1016/j.mod.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Petitte JN. Liu G. Yang Z. Avian pluripotent stem cells. Mech Dev. 2004;121:1159–1168. doi: 10.1016/j.mod.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Fan L. Crodian J. Liu X. Aleström A. Aleström P. Collodi P. Zebrafish embryo cells remain pluripotent and germ-line competent for multiple passages in culture. Zebrafish. 2004;14:43–51. doi: 10.1089/154585404774101644. [DOI] [PubMed] [Google Scholar]
- 13.Chambers I. Colby D. Robertson M. Nichols J. Lee S. Tweedie S. Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 14.Loh YH. Wu Q. Chew JL. Vega VB. Zhang W. Chen X. Bourque G. George J. Leong B, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 15.Silva J. Nichols J. Theunissen T. Guo G. van Oosten AL. Barrandon O. Wray J. Yamanaka S. Chambers I. Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang SH. Tsai MS. Chiang MF. Li H. A novel NK-type homeobox gene, ENK (early embryo specific NK), preferentially expressed in embryonic stem cells. Gene Expr Patterns. 2003;3:99–103. doi: 10.1016/s1567-133x(03)00005-x. [DOI] [PubMed] [Google Scholar]
- 17.Mitsui K. Tokuzawa Y. Itoh H. Segawa K. Murakami M. Takahashi K. Maruyama M. Maeda M. Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 18.Yates A. Chambers I. The homeodomain protein Nanog and pluripotency in mouse embryonic stem cells. Biochem Soc Trans. 2005;33:1518–1521. doi: 10.1042/BST0331518. [DOI] [PubMed] [Google Scholar]
- 19.Okita K. Ichisaka T. Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 20.Silva J. Chambers I. Pollard S. Smith A. Nanog promotes transfer of pluripotency after cell fusion. Nature. 2006;441:997–1001. doi: 10.1038/nature04914. [DOI] [PubMed] [Google Scholar]
- 21.Boyer LA. Lee TI. Cole MF. Johnstone SE. Levine SS. Zucker JP. Guenther MG. Kumar RM. Murray HL, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J. Rao S. Chu J. Shen X. Levasseur DN. Theunissen TW. Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 23.Chambers I. Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23:7150–7160. doi: 10.1038/sj.onc.1207930. [DOI] [PubMed] [Google Scholar]
- 24.Liang J. Wan M. Zhang Y. Gu P. Xin H. Jung SY. Qin J. Wong J. Cooney AJ. Liu D. Songyang Z. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat Cell Biol. 2008;10:731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
- 25.Cañón S. Herranz C. Manzanares M. Germ cell restricted expression of chick Nanog. Dev Dyn. 2006;235:2889–2894. doi: 10.1002/dvdy.20927. [DOI] [PubMed] [Google Scholar]
- 26.Lavial F. Acloque H. Bertocchini F. Macleod DJ. Boast S. Bachelard E. Montillet G. Thenot S. Sang HM, et al. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development. 2007;134:3549–3563. doi: 10.1242/dev.006569. [DOI] [PubMed] [Google Scholar]
- 27.Camp E. Sánchez-Sánchez AV. García-España A. Desalle R. Odqvist L. Enrique O'Connor J. Mullor JL. Nanog regulates proliferation during early fish development. Stem Cells. 2009;27:2081–2091. doi: 10.1002/stem.133. [DOI] [PubMed] [Google Scholar]
- 28.Wang D. Manali D. Wang T. Bhat N. Hong N. Li Z. Wang L. Yan Y. Liu R. Hong Y. Identification of pluripotency genes in the fish Medaka. Int J Biol Sci. 2011;7:440–451. doi: 10.7150/ijbs.7.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon JE. Allegrucci C. Redwood C. Kump K. Bian Y. Chatfield J. Chen YH. Sottile V. Voss SR. Alberio R. Johnson AD. Axolotl Nanog activity in mouse embryonic stem cells demonstrates that ground state pluripotency is conserved from urodele amphibians to mammals. Development. 2010;137:2973–2980. doi: 10.1242/dev.049262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellsten U. Harland RM. Gilchrist MJ. Hendrix D. Jurka J. Kapitonov V. Ovcharenko I. Putnam NH. Shu S, et al. The genome of the Western clawed frog Xenopus tropicalis. Science. 2010;328:633–636. doi: 10.1126/science.1183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gawantka V. Delius H. Hirschfeld K. Blumenstock C. Niehrs C. Antagonizing the Spemann organizer: role of the homeobox gene Xvent-1. EMBO J. 1995;14:6268–6279. doi: 10.1002/j.1460-2075.1995.tb00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onichtchouk D. Gawantka V. Dosch R. Delius H. Hirschfeld K. Blumenstock C. Niehrs C. The Xvent-2 homeobox gene is part of the BMP-4 signalling pathway controlling dorsoventral patterning of Xenopus mesoderm. Development. 1996;122:3045–3053. doi: 10.1242/dev.122.10.3045. [DOI] [PubMed] [Google Scholar]
- 33.Sander V. Reversade B. De Robertis EM. The opposing homeobox genes Goosecoid and Vent1/2 self-regulate Xenopus patterning. EMBO J. 2007;26:2955–2965. doi: 10.1038/sj.emboj.7601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Y. Siegel D. Oswald F. Knöchel W. Oct25 represses transcription of nodal/activin target genes by interaction with signal transducers during Xenopus gastrulation. J Biol Chem. 2008;283:34168–34177. doi: 10.1074/jbc.M803532200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieuwkoop PD. Faber J. Normal Table of Xenopus laevis (Daudin) 2nd. North Holland: Amsterdam, Elsevier; 1967. [Google Scholar]
- 36.Jauch R. Ng CK. Saikatendu KS. Stevens RC. Kolatkar PR. Crystal structure and DNA binding of the homeodomain of the stem cell transcription factor Nanog. J Mol Biol. 2008;376:758–770. doi: 10.1016/j.jmb.2007.11.091. [DOI] [PubMed] [Google Scholar]
- 37.Kimmel CB. Ballard WW. Kimmel SR. Ullmann B. Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 38.Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- 39.Concordet JP. Lewis KE. Moore JW. Goodrich LV. Johnson RL. Scott MP. Ingham PW. Spatial regulation of a zebrafish patched homologue reflects the roles of sonic hedgehog and protein kinase A in neural tube and somite patterning. Development. 1996;122:2835–2846. doi: 10.1242/dev.122.9.2835. [DOI] [PubMed] [Google Scholar]
- 40.Pan GJ. Pei DQ. Identification of two distinct transactivation domains in the pluripotency sustaining factor nanog. Cell Res. 2003;13:499–502. doi: 10.1038/sj.cr.7290193. [DOI] [PubMed] [Google Scholar]
- 41.Oh JH. Do HJ. Yang HM. Moon SY. Cha KY. Chung HM. Kim JH. Identification of a putative transactivation domain in human Nanog. Exp Mol Med. 2005;37:250–254. doi: 10.1038/emm.2005.33. [DOI] [PubMed] [Google Scholar]
- 42.Ma T. Wang Z. Guo Y. Pei D. The C-terminal pentapeptide of Nanog tryptophan repeat domain interacts with Nac1 and regulates stem cell proliferation but not pluripotency. J Biol Chem. 2009;284:16071–16081. doi: 10.1074/jbc.M109.005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullin NP. Yates A. Rowe AJ. Nijmeijer B. Colby D. Barlow PN. Walkinshaw MD. Chambers I. The pluripotency rheostat Nanog functions as a dimer. Biochem J. 2008;411:227–231. doi: 10.1042/BJ20080134. [DOI] [PubMed] [Google Scholar]
- 44.Wang J. Trowbridge JJ. Rao S. Orkin S. Proteomic Studies of Stem Cells. StemBook [Internet]. Harvard Stem Cell Institute; Cambridge, MA: 2008. [PubMed] [Google Scholar]
- 45.Reig G. Cabrejos ME. Concha ML. Functions of BarH transcription factors during embryonic development. Dev Biol. 2007;302:367–375. doi: 10.1016/j.ydbio.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Long S. Ahmad N. Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development. 2003;130:2303–2316. doi: 10.1242/dev.00436. [DOI] [PubMed] [Google Scholar]
- 47.Lun K. Brand M. A series of no isthmus (noi) alleles of the zebrafish pax2.1 gene reveals multiple signaling events in development of the midbrain-hindbrain boundary. Development. 1998;125:3049–3062. doi: 10.1242/dev.125.16.3049. [DOI] [PubMed] [Google Scholar]
- 48.Sasado T. Yasuoka A. Abe K. Mitani H. Furutani-Seiki M. Tanaka M. Kondoh H. Distinct contributions of CXCR4b and CXCR7/RDC1 receptor systems in regulation of PGC migration revealed by medaka mutants kazura and yanagi. Dev Biol. 2008;320:328–339. doi: 10.1016/j.ydbio.2008.05.544. [DOI] [PubMed] [Google Scholar]
- 49.Knaut H. Werz C. Geisler R. Nüsslein-Volhard C. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 2003;421:279–282. doi: 10.1038/nature01338. [DOI] [PubMed] [Google Scholar]
- 50.Sánchez-Sánchez AV. Camp E. Leal-Tassias A. Atkinson SP. Armstrong L. Díaz-Llopis M. Mullor JL. Nanog regulates primordial germ cell migration through Cxcr4b. Stem Cells. 2010;28:1457–1464. doi: 10.1002/stem.469. [DOI] [PubMed] [Google Scholar]
- 51.Afouda BA. Ciau-Uitz A. Patient R. GATA4, 5 and 6 mediate TGFbeta maintenance of endodermal gene expression in Xenopus embryos. Development. 2005;132:763–774. doi: 10.1242/dev.01647. [DOI] [PubMed] [Google Scholar]
- 52.Hyslop L. Stojkovic M. Armstrong L. Walter T. Stojkovic P. Przyborski S. Herbert M. Murdoch A. Strachan T. Lako M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–1043. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- 53.Babaie Y. Herwig R. Greber B. Brink TC. Wruck W. Groth D. Lehrach H. Burdon T. Adjaye J. Analysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells. 2007;25:500–510. doi: 10.1634/stemcells.2006-0426. [DOI] [PubMed] [Google Scholar]
- 54.Stickney HL. Imai Y. Draper B. Moens C. Talbot WS. Zebrafish bmp4 functions during late gastrulation to specify ventroposterior cell fates. Dev Biol. 2007;310:71–84. doi: 10.1016/j.ydbio.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilardelli CN. Pozzoli O. Sordino P. Matassi G. Cotelli F. Functional and hierarchical interactions among zebrafish vox/vent homeobox genes. Dev Dyn. 2004;230:494–508. doi: 10.1002/dvdy.20073. [DOI] [PubMed] [Google Scholar]
- 56.Singh AM. Hamazaki T. Hankowski KE. Terada N. A heterogeneous expression pattern for Nanog in embryonic stem cells. Stem Cells. 2007;25:2534–2542. doi: 10.1634/stemcells.2007-0126. [DOI] [PubMed] [Google Scholar]
- 57.Schuler-Metz A. Knöchel S. Kaufmann E. Knöchel W. The homeodomain transcription factor Xvent-2 mediates autocatalytic regulation of BMP-4 expression in Xenopus embryos. J Biol Chem. 2000;275:34365–34374. doi: 10.1074/jbc.M003915200. [DOI] [PubMed] [Google Scholar]
- 58.Trindade M. Tada M. Smith JC. DNA-binding specificity and embryological function of Xom (Xvent-2) Dev Biol. 1999;216:442–456. doi: 10.1006/dbio.1999.9507. [DOI] [PubMed] [Google Scholar]
- 59.Maki N. Suetsugu-Maki R. Tarui H. Agata K. Del Rio-Tsonis K. Tsonis PA. Expression of stem cell pluripotency factors during regeneration in newts. Dev Dyn. 2009;238:1613–1616. doi: 10.1002/dvdy.21959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hart AH. Hartley L. Ibrahim M. Robb L. Identification, cloning and expression analysis of the pluripotency promoting nanog genes in mouse and human. Dev Dyn. 2004;230:187–198. doi: 10.1002/dvdy.20034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.