Abstract
Background
Mutations in key tumor suppressor genes such as tumor protein 53 (TP53) and phosphatase and tensin homolog deleted on chromosome ten (PTEN) are the main genetic alterations in cancers. TP53 mutations have been found in most patients with non-small cell lung cancer (NSCLC), whereas PTEN mutations are rarely found in lung cancer, though most NSCLCs lack PTEN protein synthesis. However, the signaling involved in radio- and chemotherapy of NSCLC with wild-type PTEN and nonfunctional p53 is not clearly understood.
Methods
In this study, we established a xenograft tumor model with H358 NSCLC cells expressing wild-type PTEN, but nonfunctional p53. Protein expression and phosphorylation of PTEN and its downstream signal molecules in NSCLC tissues were detected by Western blot.
Results
We demonstrated that radiation and paclitaxel alone inhibited tumor growth, but a combined therapy of radiation and paclitaxel was more effective in inhibiting NSCLC tumor growth. Interestingly, both radiation and paclitaxel significantly increased PTEN protein expression and phosphorylation. Further identification of the affected PTEN downstream molecules showed that Akt phosphorylation at Ser473 and Thr308 residues was significantly decreased, whereas Bax and cleaved caspase-3 levels were significantly increased in tumor tissues treated with both radiation and paclitaxel. The combined treatment was more effective than either treatment alone in regulating the studied molecules. We also found that paclitaxel, but not radiation, inhibited phosphoinositide 3-kinase (PI3K) activity.
Conclusions
Our study suggested that a PTEN-PI3K-Akt-Bax signaling cascade is involved in the therapeutic effect of combined radiation/paclitaxel treatment in NSCLC without p53 expression. Our study also suggested that PTEN is an ideal target in tumors with wild-type PTEN and a lack of functional p53.
Key words: non-small cell lung cancer, radiation, paclitaxel, PTEN, p53
Introduction
Lung cancer is the leading cause of cancer-related mortality in both men and women worldwide.1 Several histological types have been identified and are classified into two groups: small cell lung cancer and non-small cell lung cancer (NSCLC). More than 80% of lung cancers are NSCLC.2 Only 30% of patients with NSCLC are diagnosed with early-stage disease that is amenable for surgical and adjuvant therapy.3 Despite advances in surgical technology, the 5-year survival rate for early-stage NSCLC is only 55%–80%.4 For unresectable diseases, the 5-year survival rate is only 2%–5%.4 Chemotherapy and radiotherapy are currently the main treatments for late-stage NSCLC patients. Particularly, the application of stereotactic body radiotherapy significantly improves the 5-year survival rate.5 Paclitaxel is a natural compound originally isolated from pacific yew tree bark and now used to treat a variety of cancers, including lung cancer.6 Although a combination of radiation and paclitaxel therapy has been evaluated in lung cancer treatment in several clinical trials,7–9 the molecular foundation responsible for radiation/paclitaxel therapy has not yet been elucidated. Recently, significant progress has been made in understanding the molecular mechanism of lung cancer. Recent efforts that target therapy against the molecules involved in tumor progression and metastasis benefit overall survival.10 Therefore, identification of the signal molecules involved in the treatment of radiation/paclitaxel will benefit the targeting therapy of NSCLC.
The tumor suppressor gene tumor protein 53 (TP53) is a key element in cell apoptosis, cell-cycle arrest, and in maintaining genomic stability. p53 protein exerts its role through activating the transcription of hundreds of genes by binding to specific sequences at their promoters.11 TP53 mutations are the most frequent gene abnormalities leading to inactivation of p53, which were identified in up to 70% of NSCLC.1,2 Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is also a key tumor suppressor gene, and mutations of the PTEN gene have been widely observed in a variety of malignancies.12 However, PTEN mutations have been reported as occurring rarely in NSCLC (∼10%), but PTEN protein synthesis is found to be reduced in most cases of lung cancer.13 PTEN and p53 are thought to be involved in sustaining cellular homeostasis through complex interactions between them. For example, p53 directly binds to the PTEN promoter and regulates its transcription, whereas PTEN, in turn, stabilizes p53 by directly binding to p53 and enhances p53 activity through either phosphatase-dependent or phosphatase-independent mechanisms.14 Inactivation of p53 function often correlates with increased malignancy, poor survival, and resistance to treatment.15 Therefore, it is important to analyze PTEN signaling in NSCLC tumor cells with lacking functional p53, which may enhance our understanding on NSCLC carcinogenesis. By considering that about 60% of NSCLC patients have TP53 mutations, but have no PTEN mutations, the establishment of a therapeutic strategy that induces PTEN expression will benefit the survival of NSCLC patients.
Ionizing radiation (IR) is thought to kill cells by causing direct DNA damage, specifically double-strand breaks. The generation of reactive oxygen species (ROS) in the cell by IR may also indirectly damage DNA. ROS can lead to several types of DNA damage.16 It has been demonstrated that DNA damage can activate PTEN,17 while ROS controls the expression of various tumor suppressor genes.18 For instance, radiation has been revealed to significantly upregulate PTEN gene expression.19,20 Various chemotherapeutic agents used to treat cancer also mediate their effects through the production of ROS, which induces DNA damage.18 For example, paclitaxel has been reported as inducing DNA damage in tumor cells.21,22 In cancer cells that lack PTEN function, the phosphoinositide 3-kinase (PI3K)-Akt signaling pathway is usually activated to enhance tumor cell survival.23 In normal cells, functional PTEN dephosphorylates PI-(3,4,5)-triphosphate (IP3) generated by the activation of the PI3K, thereby negatively regulating the PI3K-Akt-mTOR pathway and leading to G1 cell-cycle arrest and apoptosis.13 IP3 is the activator of phosphorylation of Akt at Ser473 and Thr308 residues.24 Akt negatively regulates apoptosis by negatively regulating Bax levels.25
In this study, we established the xenograft tumor mouse model by subcutaneously inoculating H358 cells into the right hind limbs of Balb/C nude mice. H358 is a tumor cell line of NSCLC with wild-type PTEN and null p53.26 The efficacy of combined radiation and paclitaxel therapy was evaluated by comparing the results of the combined treatment with radiation and paclitaxel alone. The activation of PTEN and its downstream signaling was investigated in tumor tissues.
Materials and Methods
Cell culture
H358, a human NSCLC cell line with wild-type PTEN and null p53 expression (null p53), was obtained from American Type Culture Collection and grown in RPMI-1640 medium with 10% fetal bovine serum at 37°C, 5% CO2.
Animals
Balb/C nude mice (BALB/c, nu/nu) at the weight of 20–22 g were provided by the Animal Center of Shanghai Biological Science Institution and housed in rooms under standard lighting conditions and temperature. Water and food were provided ad libitum. All animal experiments were conducted under an approved protocol from the Central South University and performed in accordance with the animal care guidelines of the Chinese Council.
Tumor growth delay study
Approximately 5×106 H358 tumor cells were subcutaneously injected into the right hind limbs of mice. After the tumors had grown to 7–8 mm in diameter, the mice were randomly divided into four groups: control, paclitaxel alone, radiation alone, and radiation plus paclitaxel treatment group. Each treatment group consisted of 10 animals. The mice in the control group were given a saline injection and sham irradiation. The mice in the radiation alone and radiation plus paclitaxel groups were irradiated with a 4 MeV linear accelerator (Varian) at a dose rate of 2 Gy/min centered on a tumor mass with other body parts covered. Three doses were given at 6 Gy each for 3 continuous days. After radiation, the mice in the paclitaxel alone and radiation plus paclitaxel groups were injected with paclitaxel (i.p.) at a dose of 8 mg/kg once a week for 5 weeks. The tumors were measured in two dimensions every 2 days, and tumor volume (V) was calculated using the following formula: V=(1/2) S2×L (S, the shortest dimension; L, the longest dimension).27 The animals were euthanized when they exhibited signs of morbidity or when the subcutaneous tumor reached a size that required sacrifice (35 days after radiation). The tumor specimens were snap frozen and maintained at −80°C.
Tumor tissue homogenate and Western blot
The tumor tissues were homogenized, and Western blot was performed as previously described.28 The antibodies for site-specific phosphorylation of the PI3K p85/p55 subunit, phospho-Akt Ser473, phospho-Akt Thr308, total Akt, PTEN, phospho-PTEN (Ser380/Thr382/383), Bax, cleaved caspase-3 (CC3), and the peroxidase-labeled secondary antibody were purchased from Cell Signaling Technology. To control for loading efficiency, the blots were stripped and reprobed with α-tubulin antibody (Sigma-Aldrich). The images were scanned with Adobe photoshop (Adobe) and quantified with NIH Image J (see http://rsb.info.nih.gov). Expressions of all proteins were evaluated relative to α-tubulin expression (i.e., relative density=subunit/α-tubulin levels). Background correction values were subtracted from each lane to minimize the variability across membranes.
Silencing of PTEN expression and Hochest33342 staining
The target sequence for PTEN RNAi was previously reported.29 The PTEN and control siRNA duplex sequences were purchased from Qiagen. H358 cells were passaged 24 hours before transfection. Cells in six-well plates were transfected with control siRNA or PTEN siRNA using Lipfectamine 2000 following the user manual (Invitrogen). Forty-eight (48) hours later, the cells were irradiated for 4 Gy and continually cultured for 24 hours or incubated with 1 mM of H2O2 for 24 hours. The cells were then subjected to Hochest33342 staining as previously described.27
Statistical analysis
Data were analyzed using Statistical Package for the Social Science, version 14.0. The tumor growth data were analyzed by one-way analysis of variance followed by the Bonferroni paired t-test to assess the statistical significance of difference between different treatment groups. Statistical analysis for Western blot data was done with the two-tailed Student's t-test. All data are presented as mean±standard error of the mean. A p<0.05 was considered statistically significant.
Results
Combination of radiation and paclitaxel treatment significantly inhibited H358 tumor growth
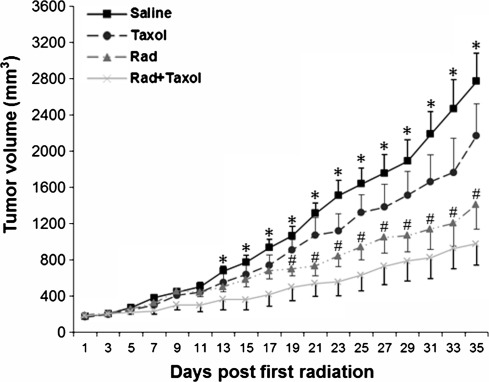
After the H358 tumor reached 7–8 mm in diameter, the mice were given direct irradiation on the tumor mass followed by a paclitaxel injection. Tumor growth was subsequently measured. As shown in Figure 1, although tumor growth was inhibited in paclitaxel-injected mice, it is less efficient than radiation alone and the combined treatment, suggesting that H358 cells were resistant to paclitaxel. Relatively, radiation was much better at inhibiting tumor growth at later time points compared with a paclitaxel injection (p<0.05 from day 19). However, the combined treatment of radiation and paclitaxel was much more effective than either radiation alone or paclitaxel alone groups while significantly inhibiting tumor growth at 13 days post first radiation. The inhibition was increasingly more significant with time (p<0.05 from day 13 to 19, p<0.01 from day 21 to 29, and p<0.001 from day 31 to 35 vs. control group).
FIG. 1.
Tumor growth delay by radiation/paclitaxel treatments. H358 cells (5×106) were injected (s.c.) into the flanks of nude mice. The measurement of tumor sizes was conducted every 2 days. Bars represent±standard error of the mean. *p<0.01 between combination treatment and control groups. #p<0.05 between radiation alone and control group. Taxol, paclitaxel; Rad, radiation. n=10.
Radiation/paclitaxel significantly upregulated PTEN expression and phosphorylation and reduced PI3K activity in H358 tumor tissues
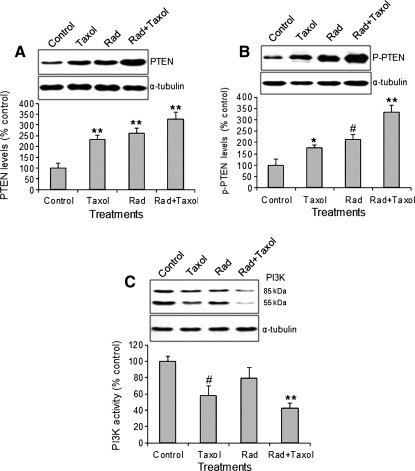
Previous studies have demonstrated that radiation significantly upregulates PTEN expression in cultured cells.19,20 To explore whether the antitumor effect of combined treatment was mediated by PTEN signaling-induced apoptosis, we measured the levels of total PTEN (Fig. 2A) and phosphorylated PTEN protein (Fig. 2B) after treatments. Although paclitaxel and radiation alone had significantly increased total PTEN and phospho-PTEN levels, the combined treatment was more effective and resulted in a 2.5- and 3.2-fold increase in protein levels compared with either of the two single treatments, respectively. PI3K is a key downstream molecule of PTEN.13 We further tested PI3K activation determined by site-specific phosphorylation of the PI3K p85/p55 subunit.24 Radiation alone exhibited no significant effect on PI3K activity, whereas paclitaxel significantly reduced PI3K activity. The combined treatment resulted in 62% reduction in PI3K activity (Fig. 2C).
FIG. 2.
Western blot of phosphatase and tensin homolog deleted on chromosome ten (PTEN) and phosphoinositide 3-kinase (PI3K) activity. (A) Total PTEN levels. (B) Phospho-PTEN levels. (C) Phospho-PI3K p85/p55 subunit. *p<0.05, #p<0.01, **p<0.001 versus control. Taxol, paclitaxel; Rad, radiation. n=10.
Radiation/paclitaxel significantly reduced Akt phosphorylation in H358 tumor tissues
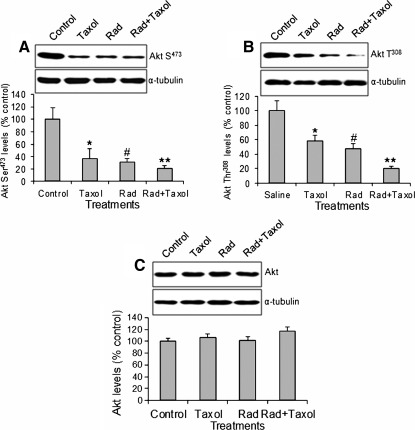
PI3K is the main activator of Akt and PI3K-produced IP3 activates Akt through phosphorylation of Akt at Ser473 and Thr308 residues.24 As shown in Figure 3, all treatments significantly decreased the levels of Akt phosphorylation at Ser473 and Thr308 residues (Fig. 3A, B). The combined treatment was more effective in downregulating Akt phosphorylation than either treatment alone. In contrast, all treatments had no effect on total Akt levels (Fig. 3C), suggesting that Akt activity, but not Akt expression, was downregulated.
FIG. 3.
Western blot of Akt expression and phosphorylation. (A) Akt Ser473 levels. (B) Akt Thr308 levels. (C) Total Akt levels. *p<0.05, #p<0.01, **p<0.001 versus control. Taxol, paclitaxel; Rad, radiation. n=10.
Radiation/paclitaxel significantly upregulated Bax and CC3 levels in H358 tumor tissues
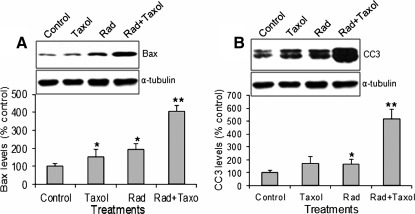
Akt is an important survival factor. Thus, inhibition of Akt activity should induce apoptosis. We measured Bax protein level and caspase-3 activation (cleaved 17 and 19 kDa fragment). Although paclitaxel and radiation alone increased Bax (Fig. 4A) and CC3 levels (Fig. 4B), the combined treatment was more effective.
FIG. 4.
Bax and cleaved caspase-3 (CC3) levels. (A) Bax levels. (B) CC3 levels (17 and 19 kDa). *p<0.05, **p<0.001 versus control. Taxol, paclitaxel; Rad, radiation. n=10.
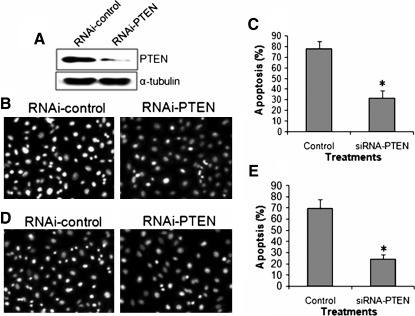
Silencing of PTEN expression in cultured H358 cells inhibited apoptosis induced by radiation and 1 mM H2O2 treatments
To further investigate the role of PTEN in tumor cell apoptosis induced by radiation and reactive oxygen stress, we knocked down PTEN expression and observed apoptosis in H358 cells under the treatment of radiation (4 Gy) and H2O2 (1 mM). Results demonstrated that PTEN siRNA transfection significantly inhibited PTEN expression (Fig. 5A). The knockdown of PTEN expression significantly inhibited radiation-induced apoptosis (Fig. 5B, C). Similarly, the knockdown of PTEN expression significantly inhibited H2O2-induced apoptosis in H358 tumor cells (Fig. 5D, E). This result suggested that radiation- and oxygen-stress-induced apoptosis in H358 cells was mediated by PTEN gene expression.
FIG. 5.
Knockout of PTEN expression inhibited radiation and H2O2-induced apoptosis in H358 cells. (A) Western blot of PTEN protein expression in cultured H358 cells transfected with control siRNA (RNAi-control) or PTEN siRNA (RNAi-PTEN). (B) Representative of Hochest33342 staining of H358 cells treated with radiation and transfection of control siRNA and PTEN siRNA. A significant decrease in apoptosis was observed in irradiated H358 cells transfected with PTEN siRNA compared with control siRNA (C). (D) Representative of Hochest33342 staining of H358 cells treated with 1 mM H2O2 and transfection of control siRNA and PTEN siRNA. A significant decrease in apoptosis was observed in H2O2-treated H358 cells transfected with PTEN siRNA compared with control siRNA (E). *p<0.001 versus control. n=5.
Discussion
Cancer patients with wild-type p53 usually have a better survival rate compared with patients with mutated p53 when treated with chemotherapy.30 However, about 70% of NSCLCs have been reported to have TP53 gene mutations. In contrast, PTEN mutations are rarely found in lung cancer, but most NSCLCs lack PTEN protein synthesis. Importantly, p53 and PTEN regularly interact with each other. This raises a question of whether anticancer agents could effectively establish their therapeutic effect on NSCLC via PTEN, but independent of p53. The current study answered this question. The therapeutic effect could be effectively established by a combination of radiation and paclitaxel through the upregulation of PTEN expression, PTEN activity, and activity of downstream molecules in NSCLC without p53. Although the antitumor effect of radiation and paclitaxel has been evaluated in lung cancer patients in clinical trials,7–9 the molecular mechanism involved in the treatment, particularly its correlation with p53 or PTEN, remains unknown. In this study, we established a tumor xenograft animal model by using NSCLC cells expressing wild-type PTEN, but not p53. With this animal model, we confirmed the therapeutic effect of combined radiation and paclitaxel treatment on NSCLCs lacking functional p53. Although radiation alone significantly inhibited tumor growth, combined therapy was more effective. Importantly, we elucidated the molecular mechanism involved in the treatment of NSCLC with radiation and paclitaxel. Our study, therefore, suggests that antitumor therapy can be established by targeting PTEN expression and/or activity in NSCLC patients with the p53 mutation.
It is widely accepted that radiation causes initial DNA damage by directly breaking double-stranded DNA and damages DNA further by inducing ROS production. DNA damage activates a lot of genes, which subsequently leads to DNA repair in normal tissue, while also activating tumor suppressor genes to induce cell apoptosis underlying radiotherapy and chemotherapy. For example, radiation-induced DNA damage can activate PTEN.17,18 PTEN possesses a carboxy terminal, noncatalytic regulatory domain with three phosphorylation sites (Ser380, Thr382, and Thr383). Phosphorylation at these sites increases PTEN stability and positively affects PTEN's biological activity.31,32 Therefore, it is not surprising that in this study, radiation increased PTEN protein level and PTEN activity. Interestingly, paclitaxel was previously revealed as exerting its therapeutic role by blocking mitosis through stabilizing spindle microtubule dynamics33 and by inhibiting integrins' and HSP90's functions.34 A recent study demonstrated that paclitaxel can induce caspase-independent mitotic death relating to p73, but not p53 activation.35 Our study is the first which demonstrates that paclitaxel exerts an antitumor effect on NSCLC without p53 expression via initiating PTEN signaling. Paclitaxel has been demonstrated as inducing DNA damage in tumor cells.21,22 We, therefore, proposed that paclitaxel-induced DNA damage also activates PTEN. Generally, PTEN dephosphorylates PI3K-produced IP3 and subsequently regulates the PI3K-Akt-mTOR pathway.13 In this study, we also observed that paclitaxel, but not radiation, activated PI3K, identified by the site-specific phosphorylation of the PI3K p85/p55 subunit.24 However, how paclitaxel activates PI3K is currently unclear.
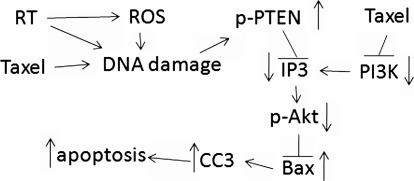
Although different kinases are responsible for Akt phosphorylation at serine 473 and threonine 308 residues, PI3K-mediated full activation of Akt requires phosphorylation on both residues.24 In this study, Akt phosphorylation on both Thr308 and Ser473 was significantly decreased in radiation alone, paclitaxel alone, and in their combination. The Akt phosphorylation levels were obviously associated with the PTEN activity, suggesting that a decrease in Akt phosphorylation is PTEN activity dependent. The PI3K-Akt pathway is involved in the regulation of cell survival, apoptosis, and growth.8 Therefore, decreased Akt activity may be responsible for the antitumor effect of radiation and paclitaxel on NSCLC through inducing tumor cell apoptosis. Bax is an apoptotic protein negatively regulated by Akt, but positively regulated by p53.25 Caspase-3 is one of the most widely studied effector caspases. Activated caspase-3 plays pivotal roles in both the death receptor pathways induced by caspase-8 and the mitochondrial pathway involving caspase-9.36 We, therefore, measured the Bax protein and CC3 levels in NSCLC tissues. As expected, the levels of Bax and activated caspase-3 were significantly upregulated in radiation alone, paclitaxel alone, and the combined treatment. Moreover, Bax and activated caspase-3 levels positively correlated with PTEN activity, but negatively correlated with the tumor growth and Akt phosphorylation. Our study suggested a PTEN-PI3K-Akt-Bax signal pathway, which mediated the therapeutic effect of radiation and paclitaxel in p53-dysfunctional NSCLC (Fig. 6).
FIG. 6.
Schematic representation of the signaling pathways involved in radiation/paclitaxel treatment in non-small cell lung cancer without p53. Radiation (RT) produces reactive oxygen species (ROS), which leads to DNA damage. DNA damage stimulates PTEN activation, subsequently inhibits PI3K-Akt activity, which results in high Bax and activated caspase-3 levels and tumor cell apoptosis.
Although p53 is the key target of irradiation and most chemotherapy agents,37 we found in this study that PTEN is a direct target of radiation and paclitaxel in NSCLC tumor cells lacking functional p53. The therapeutic effect of radiation and paclitaxel was mediated by the upregulation of PTEN activity and its downstream signaling. We proposed that radiation leads to direct DNA damage or indirect DNA damage by ROS, and paclitaxel also induces DNA damage. DNA damage then either stimulates PTEN expression or decreases PTEN degradation by upregulating its phosphorylation. The activated PTEN inhibits PI3K activity, and subsequent Akt activity. The loss of Akt activity results in high Bax levels, which induces tumor cell apoptosis (Fig. 5C). Interestingly, paclitaxel also inhibited PI3K activity in an unknown mechanism. Our in vitro study demonstrated that the silencing of PTEN expression in H358 cells significantly inhibited radiation and H2O2-induced cell apoptosis (Fig. 5). Therefore, our study not only confirmed the therapeutic role of radiation and paclitaxel in p53-abnormal NSCLC, but also elucidated its molecular mechanism. Importantly, our study suggested that PTEN can be a key therapeutic target independent of p53.
In conclusion, combined radiation and paclitaxel treatment is more effective in inhibiting tumor growth in NSCLC with p53 abnormality than radiation and paclitaxel treatment alone. Both radiation and paclitaxel achieve a therapeutic effect by inducing tumor cell apoptosis via the activation of PTEN-PI3K-Akt-Bax signaling. PTEN can be an ideal target in treating NSCLC with lack of p53.
Acknowledgments
This work has been supported by the Chinese National Natural Science Foundation (Grant No. 30800518 to Yuxiang Chen) and the New Teachers of Doctor Fund Project of Ministry of Education (Grant No. 200805331090 to Yuxiang Chen).
Disclosure Statement
All authors declare no conflicts of interest.
References
- 1.Shao C. Lu C. Chen L, et al. p53-Dependent anticancer effects of leptomycin B on lung adenocarcinoma. Cancer Chemother Pharmacol. 2011;67:1369. doi: 10.1007/s00280-010-1434-6. [DOI] [PubMed] [Google Scholar]
- 2.Regina S. Valentin JB. Lachot S, et al. Increased tissue factor expression is associated with reduced survival in non-small cell lung cancer and with mutations of TP53 and PTEN. Clin Chem. 2009;55:1834. doi: 10.1373/clinchem.2009.123695. [DOI] [PubMed] [Google Scholar]
- 3.Reungwetwattana T. Eadens MJ. Molina JR. Chemotherapy for non-small-cell lung carcinoma: From a blanket approach to individual therapy. Semin Respir Crit Care Med. 2011;32:78. doi: 10.1055/s-0031-1272872. [DOI] [PubMed] [Google Scholar]
- 4.Edward HL. The Cancer-Matrix Manual. 5th. Madison, WI: Advanced Medical Publishing; 2010. p. 208. [Google Scholar]
- 5.Chang JY. Roth JA. Stereotactic body radiation therapy for stage I non-small cell lung cancer. Thorac Surg Clin. 2007;17:251. doi: 10.1016/j.thorsurg.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Schmittel A. Second-line therapy for small-cell lung cancer. Expert Rev Anticancer Ther. 2011;11:631. doi: 10.1586/era.11.7. [DOI] [PubMed] [Google Scholar]
- 7.Carter DL. Garfield D. Hathorn J, et al. A randomized phase III trial of combined paclitaxel, carboplatin, and radiation therapy followed by weekly paclitaxel or observation for patients with locally advanced inoperable non-small-cell lung cancer. Clin Lung Cancer. 2011. http://dx.doi.org/10/1016/jclc.2001.10.08. http://dx.doi.org/10/1016/jclc.2001.10.08 [Epub ahead of print] [DOI] [PubMed]
- 8.Chen WC. Kim J. Kim E, et al. A phase II study of radiotherapy and concurrent paclitaxel chemotherapy in breast-conserving treatment for node-positive breast cancer. Int J Radiat Oncol Biol Phys. 2012;82:14. doi: 10.1016/j.ijrobp.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 9.Horn L. Bernardo P. Sandler A, et al. A phase II study of paclitaxel+etoposide+cisplatin+concurrent radiation therapy for previously untreated limited stage small cell lung cancer (E2596): A trial of the Eastern Cooperative Oncology Group. J Thorac Oncol. 2009;4:527. doi: 10.1097/JTO.0b013e31819c7daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bearz A. Berretta M. Lleshi A, et al. Target therapies in lung cancer. J Biomed Biotechnol. 2011;2011:921231. doi: 10.1155/2011/921231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee EY. Muller WJ. Oncogenes and tumor suppressor genes. Cold Spring Harb Perspect Biol. 2010;2:a003236. doi: 10.1101/cshperspect.a003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steelman LS. Bertrand FE. McCubrey JA. The complexity of PTEN: Mutation, marker and potential target for therapeutic intervention. Expert Opin Ther Targets. 2004;8:537. doi: 10.1517/14728222.8.6.537. [DOI] [PubMed] [Google Scholar]
- 13.Jin G. Kim MJ. Jeon HS, et al. PTEN mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small cell lung cancers. Lung Cancer. 2010;69:279. doi: 10.1016/j.lungcan.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Andjelkovic T. Bankovic J. Stojsic J, et al. Coalterations of p53 and PTEN tumor suppressor genes in non-small cell lung carcinoma patients. Transl Res. 2011;157:19. doi: 10.1016/j.trsl.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Mogi A. Kuwano H. TP53 mutations in non-small cell lung cancer. J Biomed Biotechnol. 2011;2011:583929. doi: 10.1155/2011/583929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acharya A. Das I. Chandhok D, et al. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid Med Cell Longev. 2010;3:23. doi: 10.4161/oxim.3.1.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pu T. Zhang XP. Liu F, et al. Coordination of the nuclear and cytoplasmic activities of p53 in response to DNA damage. Biophys J. 2010;99:1696. doi: 10.1016/j.bpj.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta SC. Hevia D. Patchva S, et al. Upsides and downsides of ROS for cancer: The roles of ROS in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4414. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escrivà M. Peiró S. Herranz N, et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol Cell Biol. 2008;28:1528. doi: 10.1128/MCB.02061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang HQ. Wang J. Yuan ZY, et al. The drug-resistance to gefitinib in PTEN low expression cancer cells is reversed by irradiation in vitro. J Exp Clin Cancer Res. 2009;28:123. doi: 10.1186/1756-9966-28-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branham MT. Nadin SB. Vargas-Roig LM, et al. DNA damage induced by paclitaxel and DNA repair capability of peripheral blood lymphocytes as evaluated by the alkaline comet assay. Mutat Res. 2004;560:11. doi: 10.1016/j.mrgentox.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Weigel TL. Lotze MT. Kim PK, et al. Paclitaxel-induced apoptosis in non-small cell lung cancer cell lines is associated with increased caspase-3 activity. J Thorac Cardiovasc Surg. 2000;119:795. doi: 10.1016/S0022-5223(00)70016-X. [DOI] [PubMed] [Google Scholar]
- 23.LoPiccolo J. Blumenthal GM. Bernstein WB, et al. Targeting the PI3K/Akt/mTOR pathway: Effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X. Mi J. Wetsel WC, et al. PI3 kinase is involved in cocaine behavioral sensitization and its reversal with brain area specificity. Biochem Biophys Res Commun. 2006;340:1144. doi: 10.1016/j.bbrc.2005.12.114. [DOI] [PubMed] [Google Scholar]
- 25.Sasabe E. Tatemoto Y. Li D, et al. Mechanism of HIF-1alpha-dependent suppression of hypoxia-induced apoptosis in squamous cell carcinoma cells. Cancer Sci. 2005;96:394. doi: 10.1111/j.1349-7006.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon YK. Kim HP. Han SW, et al. KRAS mutant lung cancer cells are differentially responsive to MEK inhibitor due to AKT or STAT3 activation: Implication for combinatorial approach. Mol Carcinog. 2010;49:353. doi: 10.1002/mc.20607. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X. Kon T. Wang H, et al. Enhancement of hypoxia-induced tumor cell death in vitro and radiation therapy in vivo by use of small interfering RNA targeted to hypoxia-inducible factor-1alpha. Cancer Res. 2004;64:8139. doi: 10.1158/0008-5472.CAN-03-2301. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X. Lee TH. Davidson C, et al. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacology. 2007;32:377. doi: 10.1038/sj.npp.1301101. [DOI] [PubMed] [Google Scholar]
- 29.Nho RS. Xia H. Diebold D, et al. PTEN regulates fibroblast elimination during collagen matrix contraction. J Biol Chem. 2006;281:33291. doi: 10.1074/jbc.M606450200. [DOI] [PubMed] [Google Scholar]
- 30.Russo A. Bazan V. Iacopetta B, et al. TP53-CRC Collaborative Study Group. The TP53 colorectal cancer international collaborative study on the prognostic and predictive significance of p53 mutation: Influence of tumor site, type of mutation, and adjuvant treatment. J Clin Oncol. 2005;23:7518. doi: 10.1200/JCO.2005.00.471. [DOI] [PubMed] [Google Scholar]
- 31.Vazquez F. Ramaswamy S. Nakamura N, et al. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres J. Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 33.Orr GA. Verdier-Pinard P. McDaid H, et al. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzpatrick FA. Wheeler R. The immunopharmacology of paclitaxel (Taxol), docetaxel (Taxotere), and related agents. Int Immunopharmacol. 2003;3:1699. doi: 10.1016/j.intimp.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Kitagawa K. Niikura Y. Caspase-independent mitotic death (CIMD) Cell Cycle. 2008;7:1001. doi: 10.4161/cc.7.8.5720. [DOI] [PubMed] [Google Scholar]
- 36.Budihardjo I. Oliver H. Lutter M, et al. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y. p53 and its downstream proteins as molecular targets of cancer. Mol Carcinog. 2006;45:409. doi: 10.1002/mc.20231. [DOI] [PubMed] [Google Scholar]