Abstract
Amyloidosis represents a group of diseases in which proteins undergo misfolding to form insoluble fibrils with subsequent tissue deposition. While almost all deposited amyloid fibers share a common nonbranched morphology, the affected end organs, clinical presentation, treatment strategies, and prognosis vary greatly among this group of diseases and are largely dependent on the specific amyloid precursor protein. To date, at least 27 precursor proteins have been identified to result in either local tissue or systemic amyloidosis, with nine of them manifesting in cardiac deposition and resulting in a syndrome termed “cardiac amyloidosis” or “amyloid cardiomyopathy.” Although cardiac amyloidosis has been traditionally considered to be a rare disorder, as clinical appreciation and understanding continues to grow, so too has the prevalence, suggesting that this disease may be greatly underdiagnosed. The most common form of cardiac amyloidosis is associated with circulating amyloidogenic monoclonal immunoglobulin light chain proteins. Other major cardiac amyloidoses result from a misfolding of products of mutated or wild-type transthyretin protein. While the various cardiac amyloidoses share a common functional consequence, namely, an infiltrative cardiomyopathy with restrictive pathophysiology leading to progressive heart failure, the underlying pathophysiology and clinical syndrome varies with each precursor protein. Herein, we aim to provide an up-to-date overview of cardiac amyloidosis from nomenclature to molecular mechanisms and treatment options, with a particular focus on amyloidogenic immunoglobulin light chain protein cardiac amyloidosis.
Keywords: heart, cardiomyocytes
this article is part of a collection on Protein Handling. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
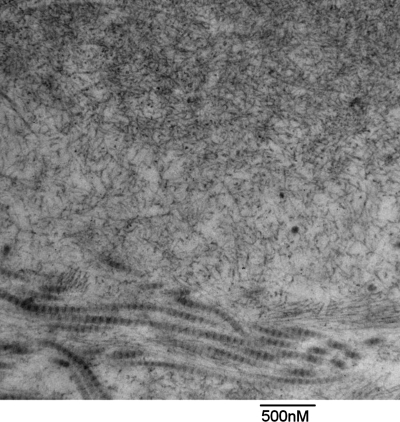
Amyloidosis represents a group of diseases that are characterized by extracellular deposition of amyloid fibrils in organs and tissues (61). These amyloid fibrils, as shown in Fig. 1, are composed of misfolded protein aggregates that arrange themselves in an antiparallel β-sheet form and manifest as insoluble rigid, nonbranching fibrils of 7.5–10 nm in diameter when viewed via electron microscopy (97). The term “amyloid” originated over 150 years ago as a descriptor for the “waxy” material observed in affected tissues, with the misconception that the deposits were from starch-like materials (amyl = “starch,” and -oid = “like”) (54). It was later realized, however, that amyloid fibrils are in fact composed of misfolded proteins that result from either excess production of or specific mutations in precursor proteins. To date, at least 27 distinct amyloid precursor proteins, each with heterogeneous protein sequence, structure, and function, have been identified to be amyloidogenic or capable of misfolding, aggregation, fibril formation, and tissue deposition (96). Abnormal amyloidogenic proteins may be either inherited or acquired through somatic mutations. Despite the diversity of precursor proteins, the final resulting nonbranched fibrils are largely indistinguishable from each other. Diagnosis of amyloidosis has largely centered on the identification of amyloid fibril deposition in pathology specimens using staining with Congo red, thioflavin T, or Alcian blue. With Congo red staining, amyloid fibrils exhibit a classic apple green birefringence when viewed using polarized light (30).
Fig. 1.
Amyloid fibril deposition in heart tissue affected by AL cardiomyopathy. Amyloid fibrils are seen in the upper part of the electron micrograph of heart tissue taken from a patient with AL cardiomyopathy (courtesy of Dr. Federica del Monte). The amyloid fibrils are nonbranched rigid fibrils of a 10 nm diameter. Collagen fibrils, with a much larger diameter are seen in the lower part of the image.
Amyloidosis may manifest as either a localized or systemic disease, depending on whether a single organ or multiple organs are affected. The individual amyloid diseases are classified based on their precursor protein, which translates to specific characteristics of the disease presentation and governs patient treatment plans and prognoses (25, 26, 53). Of the 27 identified precursor proteins which may result in human amyloidoses (Table 1), thus far nine proteins have been shown to potentially result in cardiac involvement. The nomenclature used in this review follows the guidelines set forth by The Nomenclature Committee of the International Society of Amyloidosis (96). Typically, the name starts with “A” for amyloid and is followed by an abbreviation of the precursor protein, e.g., amyloidogenic immunoglobulin light chain protein (AL), the precursor protein for primary amyloidosis, and amyloidogenic transthyretin (ATTR), the precursor protein for senile or familial amyloidosis. The most critical and fatal manifestation of systemic amyloidosis is associated with cardiac involvement, termed “cardiac amyloidosis” or “amyloid cardiomyopathy.” Cardiac amyloidosis, particularly AL cardiomyopathy, results in a rapid decline in cardiac function, with symptomatic heart failure and development of amyloid cardiomyopathy. AL cardiomyopathy is unique in that most standard heart failure therapies, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and digoxin have low efficacy in treating this disease, partly because they are all poorly tolerated. To date, treatment options are limited, resulting in an overall 1.3-yr median survival from diagnosis in AL cardiomyopathy, comparable with the most aggressive forms of cancers (26, 53). In this review, we provide an overview of the current knowledge base and potential molecular mechanisms underpinning these diseases, as well as review recent experimental therapeutic strategies for cardiac amyloidosis with a concentrated focus on AL amyloid cardiomyopathy.
Table 1.
Summary of currently known amyloid precursor proteins and amyloidosis
| Nomenclature | Precursor of Amyloid Fiber | Precursor Protein Production Site | Type | Distribution (Involved Organs) |
|---|---|---|---|---|
| AL (29) | Immunoglobulin light chain | Bone marrow | Acquired | Systemic (kidney, heart, nervous system, liver, spleen, soft tissue) |
| AH (24) | Immunoglobulin heavy chain | Bone marrow | Acquired | Systemic (kidney, heart, liver, spleen, soft tissue) |
| ATTRa (61) | Transthyretin | Liver | Hereditary | Systemic (nervous system, kidney, heart) |
| Acquired | Systemic (heart) | |||
| Aβ2M (42) | β2 microglobulin | Ubiquitous in all nucleated cells | Acquired | Systemic (hemodialysis-associated, mainly joints) |
| AA (83) | Serum amyloid A | Liver | Acquired | Systemic (liver, kidney, spleen, heart, gastrointestinal) |
| AApoAI (61) | Apolipoprotein AI | Liver and small intestine | Hereditary | Systemic (Liver, kidney) |
| AApoAII (61) | Apolipoprotein AII | Liver and small intestine | Hereditary | Systemic (kidney, heart) |
| AApoAIV (8) | Apolipoprotein AIV | Intestine | Hereditary | Systemic (heart) |
| AGel (56) | Gelsolin | Ubiquitous | Hereditary | Systemic (cornea, cranial nerve) |
| ALys (74) | Lysozyme | Ubiquitous | Hereditary | Systemic (kidney, liver, spleen) |
| AFib (99) | Fibrinogenα chain | Liver | Hereditary | Systemic (kidney, sporadic heart) |
| ACys (73) | Cystatin C | Ubiquitous | Hereditary | Systemic (brain arteries and arterioles) |
| ABri/ADan (32) | ABriPP and ADanPP | Brain, kidney, pancreas | Hereditary | Localized (central neuron system) |
| ALect2 (5) | Leukocyte chemotactic factor 2 | Liver | Acquired | Localized (kidney) |
| Aβb (4) | Amyloid β protein precursor | Brain | Acquired Hereditary | Localized (brain) |
| Aprp (31) | Prion | Infection agent | Acquired Hereditary | Localized (brain, nervous system) |
| ACal (80) | Procalcitonin | Thyroid | Acquired | Localized (thyroid) |
| AIAPPc (37, 43) | Islet amyloid polypeptide | Pancreas | Acquired | Localized (Islets of Langerhans) |
| AANF (48) | Atrial natriuretic factor | Atria | Acquired | Localized (atria) |
| Apro (110) | Prolactin | Pituitary | Acquired | Localized (pituitary) |
| AIns (111) | Insulin | Pancreas | Acquired | Localized (pancreas) |
| AMed (39) | Lactadherin | Aortic media | Acquired | Localized (aortic media) |
| Aker (64) | Kerato-epithelin | Eye | Hereditary | Localized (cornea) |
| ALac (1) | Lactoferrin | Eye | Acquired | Localized (cornea) |
| AOaap (65) | Odontogenic ameloblast-associated protein | Teeth | Acquired | Localized (teeth) |
| ASemI (58) | Semenogelin | Seminal vesicles | Acquired | Seminal (vesicles) |
ATTR amyloidosis may represent 2 forms of amyloidosis: familial ATTR amyloidosis caused by mutant transthyretin protein, and acquired or senile ATTR amyloidosis caused by wild-type transthyretin protein.
Aβ amyloidosis may represent 2 forms of amyloidosis: familial Alzheimer's disease and sporadic Alzheimer's disease.
AIAPP amyloid deposition was also found in kidney.
Cardiac Amyloidosis
Cardiac amyloidosis is defined by the presence of extracellular amyloid deposition within the heart, with infiltration occurring in all anatomical distributions, including the atria, ventricles, and perivascular space (most commonly within small vessels) as well as within the valves and conduction system in some cases (28). Amyloid fibril infiltration leads to biventricular wall thickening with impaired relaxation, disruption of ventricular organization, and the loss of ventricular elasticity and often is categorized clinically as a restrictive cardiomyopathy (22).
Cardiac amyloidosis has been traditionally viewed as a rare or orphan disease, particularly compared with acquired cardiovascular diseases such as coronary artery disease and hypertensive heart disease. Ongoing efforts have suggested, though, that the rarity of cardiac amyloidosis may be more a reflection of its underdiagnosis, rather than true incidence. This may be particularly true in the transthyretin (TTR) amyloidoses. In general, diagnosis of amyloid cardiomyopathy has proven to be quite challenging, requiring cardiac biopsy and pathological evaluation for a definitive histological diagnosis in many patients. Cardiac involvement is probably often misdiagnosed during the early stages of the disease, when ventricular wall thickening due to amyloid infiltration may be misdiagnosed as left ventricular hypertrophy due to hypertension. In patients with confirmed amyloid deposition in other organ systems, wall thickening on echocardiography, particularly if associated with low voltage on the electrocardiogram, is highly suggestive of cardiac involvement, and cardiac biopsy is rarely needed in such cases (28). In contrast, senile systemic amyloidosis, and many of the TTR mutations almost exclusively involve the heart, and endomyocardial biopsy are usually needed for a tissue diagnosis. As the treatment strategies for amyloidosis strategies are highly dependent on the type of amyloid precursor protein, precise typing of the amyloid is critical. In many cases, such as gene-positive amyloid with typical findings of ATTR or a young patient with multiorgan involvement and clear evidence of a plasma cell dyscrasia, the diagnosis is clear, but there are many cases in which clinical features are nonspecific or confusing. In such cases the most accurate diagnostic technique appears to be molecular analysis of the amyloid fibrils from a biopsy, using mass spectrometry (105).
Cardiac amyloidosis can be classified as primary (AL), secondary (reactive, AA), hereditary, senile systemic, or isolated atrial amyloidosis (Table 2). This classification is based on the different amyloidogenic precursor proteins that are deposited, and correspondingly, the treatment strategies and prognoses are distinct for each of the different types of cardiac amyloidosis. In developed nations, primary amyloidosis (AL), senile systemic amyloidosis, and familial amyloidosis are most prevalent; the latter two are caused by amyloid fibrils from wild-type and variant TTR (ATTR), respectively. AA is frequently associated with chronic infection and inflammatory conditions and results from amyloid deposition of serum AA proteins. It rarely affects the heart, and when it does, the clinical manifestations tend to be mild. Isolated atrial amyloidosis results from deposition of atrial natriuretic peptide and is generally a late-onset condition, which is of little clinical significance other than being associated with an increased incidence of atrial fibrillation. Rare amyloidogenic precursor proteins may also result in cardiac amyloid deposition, including mutant apoliprotein A1 (40, 41, 70), fibrinogen (100), and gelsolin (12, 60). Additionally, recent studies have demonstrated oligomeric assembly-derived intracellular and extracellular amyloid-like fibril deposition in hearts of patients with idiopathic dilated cardiomyopathy (IDCM). This abnormal protein aggregation was found to affect calcium handling and contributed to cardiac dysfunction (33). Although the fibrils found in these patients with IDCM exhibited positive Congo red staining, the nature of the precursor proteins responsible for these amyloid-like fibrils remains to be determined. Importantly, further investigation is required to establish the causal relationship among amyloid precursor proteins, oligomers, and/or amyloid fiber in the development of cardiac pathology in patients with IDCM. Senile systemic amyloidosis is most associated with aging, and as the general population continues to advance in median age, it is suspected that the incidence of cardiac amyloidosis will only continue to increase.
Table 2.
Summary of common cardiac amyloidosisa
| Nomenclature | Precursor of Amyloid Fiber | Type | Prevalence | Prognosis |
|---|---|---|---|---|
| AL (26) | Immunoglobulin light chain | Primary amyloid cardiomyopathy | 50% in patients with AL amyloidosis | Median survival-11 mo |
| ATTR (28, 46, 79, 101) | Mutant transthyretin | Familial amyloid cardiomyopathy | 30% in patients with familial amyloidosis | Median survival 9–13 yr |
| Wild-type transthyretin | Senile systemic amyloid (SSA) cardiomyopathy | In most of patients with senile amyloidosis | Median survival-75 mo | |
| AA (28) | Serum amyloid A | Secondary amyloid cardiomyopathy | Rare, only 2% in patients with AA amyloidosis | Good |
| AANF (48, 49) | Atrial natriuretic factor | Isolated atrial amyloidosis | Very common | Good |
| More than 40% in people older than 50 |
Other cardiac amyloidosis include: AH (24), AApoAI (40, 41, 61, 69), AApoAIV (7, 8), AFib (99), and AGel (12, 60). However, these are rarely occurring with limited literature.
AL Cardiomyopathy
Primary or AL amyloidosis may be the most common systemic amyloidosis in the US and occurs equally in men and women, mostly in those over 50 years of age. While this disease is thought to be uncommon, the disease incidence is similar to other well-known diseases such as Hodgkin's disease with ∼2,000 to 2,500 new cases each year (25, 26, 95). The underlying etiology of AL amyloidosis is a plasma cell dyscrasia, in which clonal expansion of plasma cells results in overproduction and secretion of monoclonal immunoglobulin light chain protein. This form of plasma cell dyscrasia is similar to that which occurs in multiple myeloma, and as such, 10–15% of myeloma patients also develop AL amyloidosis. The precise mechanisms that regulate light chain protein misfolding and eventual fiber formation are unknown. It has been suggested that these light chain proteins often contain mutations which may facilitate protein misfolding, eventually leading to aggregation and assembly into amyloid fibrils (3, 52).
AL amyloidosis affects multiple organs in addition to the heart, including the kidney, liver, peripheral nervous system, and autonomic nervous system, with heart and kidney involvement most common, occurring in >50% of patients with AL amyloidosis. Cardiac involvement in AL amyloidosis patients is associated with the worst prognosis of any organ involvement (92) because of the development of a rapid AL cardiomyopathy, with overt heart failure and a median survival of <6 mo when untreated (25, 26). Pharmacological treatment strategies for AL cardiomyopathy include supportive care of cardiac function and elimination of the sources of light chain (87). Standard therapies for heart failure, including digoxin, calcium channel blockers, and β-blockers, are generally avoided because of their excess toxicity. Angiotensin-converting enzyme inhibitors and angiotensin-II receptor blockers are also poorly tolerated in patients with AL cardiomyopathy because of the potential for profound hypotension (26, 62). In AL cardiomyopathy, diuretics are often used to manage fluid status, though with great caution to avoid excessively lowering filling pressure and exacerbating insufficient cardiac output (62). Heart transplantation followed by chemotherapy and/or stem cell transplant has been demonstrated to achieve a long-term remission and improve the survival in highly selected patients (17, 34). Heart transplant, however, is oftentimes not a viable option for patients with cardiac involvement due to the high incidence of multiorgan involvement and the rapid progression of disease in patients with AL amyloidosis (85). The mainstay therapeutic options for eliminating amyloidogenic light chain production was for many years the alkylating agents (specifically melphalan). High-dose melphalan with autologous hematopoietic stem cell transplantation was introduced two decades ago and has a good response rate but high morbidity and mortality in patients with severe cardiac amyloidosis, thereby needing careful patient selection (13).
Intriguingly, in patients with AL cardiomyopathy, the reduction of circulating free light chain levels following treatment is generally associated with a better clinical outcome despite a lack of obvious improvement in echocardiographic measurements (21, 88). Peptide biomarkers such as cardiac troponin and NH2-terminal pro-brain natriuretic peptide have been proposed for use as prognostic markers in patients with AL amyloidosis (20, 71). Based on serum levels of cardiac troponin and NH2-terminal pro-brain natriuretic peptide, a simplified staging system was developed to predict the median survival of AL amyloidosis patients (19). This convenient and highly reproducible system facilitates comparison across different studies to obtain information regarding the efficacy of different treatments. While a detailed elaboration of chemotherapy and stem cell transplantation are beyond the scope of this review, bortezomib, a proteasome inhibitor, which was initially developed for treatment of multiple myeloma, has clearly been proving useful. In fact, bortezomib may produce a more rapid response than melphalan. By inhibiting proteasome function, bortezomib triggers endoplasmic reticulum stress, resulting in plasma cell death. Recent studies in patients with AL amyloidosis have shown the efficacy of bortezomib, with or without dexamethasone, in eliciting a hematological response in 71% of patients in only 1.2 mo (median response time) (47, 82, 98). Another promising drug is thalidomide (and the similar agent lenalidomide). The combination of thalidomide with either melphalan or cyclophosphamide-dexamethasone, when administered to AL amyloidosis patients with cardiac involvement, showed increased tolerance and a high hematological response (109). While these reports are very encouraging, these chemotherapy agents are generally associated with neurotoxicity and (occasionally) worsening of heart or kidney function, among other significant side effects (90) and should be used under the supervision of a physician skilled in treating amyloidosis.
Despite the progress made over the past decades, the poor survival associated with AL cardiomyopathy poses a great need for collaborative efforts between clinicians and basic researchers to better understand the molecular mechanisms that promote disease pathogenesis and to develop advanced, targeted therapies.
Mechanisms Underlying Tissue Damage
The pathophysiology of AL cardiomyopathy was hypothesized to arise exclusively from fibril deposition in the extracellular space, with subsequent increased passive stiffness and a parallel loss of cardiac parenchyma. This potential mechanism of disease was further supported by physiological measures of primarily impaired filling and diastolic function in patients with amyloid cardiomyopathy (27, 51). Emerging evidence, however, has now challenged this notion and suggests that fibril deposition represents only one aspect of disease pathology, with light chain precursor proteins or aggregates themselves inducing direct cardiotoxicity, independent of fibril deposition. The first evidence for direct precursor protein toxicity originated with clinical observations that suggested a far worse outcome in patients with AL cardiomyopathy relative to ATTR cardiomyopathy, despite similar degrees of fibril deposition (23). Moreover, reduced circulating serum-free light chain following chemotherapy has correlated with improved cardiac function and prognosis in patients with AL cardiomyopathy, despite unchanged amyloid fibril infiltration in the heart (72). Because of the strong correlation between serum-free light chain levels and prognosis, an assay measuring free light chain levels was developed and is routinely used in managing AL amyloidosis patients, especially those with cardiac involvement. These clinical observations formed the basis for the hypothesis that light chain proteins themselves may be directly cardiotoxic.
The first experimental evidence to support the direct cardiotoxic effects of amyloidogenic proteins originated from work by Liao and colleagues (57), demonstrating that the infusion of amyloidogenic light chain proteins, purified from AL amyloidosis patients with severe cardiac involvement, but not light chain protein from myeloma patients without amyloidosis, resulted in diastolic dysfunction in an isolated mouse heart model. Further work demonstrated the direct cardiac toxicity of amyloidogenic light chain protein in cultured cardiac cells, with amyloidogenic light chain proteins increasing cellular oxidant stress, independent of fibril formation (11). The notion that amyloidogenic light chain proteins themselves may alter cardiac function has been subsequently confirmed by several additional laboratories (63, 64, 94). Migrino and colleagues (63) have found higher levels of serum oxidative stress markers in patients with AL amyloidosis compared with healthy controls. They also found that human arterioles exposed to amyloidogenic light chain proteins results in the generation of reactive oxygen species and impairment of vascular smooth muscle cell relaxation. This was extended in a recent study where Migrino and collaborators (64) reported endothelial dysfunction following amyloidogenic light chain treatment coupled with increased apoptotic cell death of coronary artery endothelial cells. In addition to showing that isolated amyloidogenic light chain protein from patients conferred cardiac toxicity, Sikkink and Ramirez-Alvarado (94) were able to recapitulate cardiac toxicity by using recombinant light chain proteins with sequence specific mutations.
Insight into the mechanism by which amyloidogenic light chain causes toxicity and cellular dysfunction was highlighted in the studies reported by Shi et al. (93). Using both pharmacological inhibition of p38 MAPK and dominant-negative p38α MAPK adenovirus, Shi et al. reported that noncanonical p38 MAPK pathway activation plays a pivotal role in mediating amyloidogenic light chain-induced contractile dysfunction and apoptotic cell death in cultured cardiac cells. These observations were confirmed in vivo in mice. The infusion of amyloidogenic light chains by osmotic minipump increased apoptosis in cardiac tissue, and this was abolished in p38α MAPK dominant-negative mice, suggesting that p38α activation is essential for amyloidogenic light chain protein to execute its cardiac toxicity.
Taken together, the direct cardiac toxicity of amyloidogenic light chain protein has been shown to likely be an important factor in the pathogenesis of AL amyloidosis cardiomyopathy. These experimental data provide a strong notion for the elimination of circulating light chain proteins, either directly or through the removal of source plasma cells. Furthermore, molecular targets such as the p38 MAPK signaling axis offer additional potential targets for future therapeutic interventions to combat this dreadful disease.
Mechanism of Amyloid Fibrillogenesis
What determines the amyloidogenic properties of precursor proteins remains largely unclear. It has been shown that a number of proteins are prone to misfold and form amyloid fibrils with a predominantly antiparallel β-sheet secondary structure. This dynamic equilibrium between a native form and a misfolded form can be influenced by several independent factors, including, but not limited to, specific genetic mutations, abnormally high concentration of protein levels, destabilization of protein structure, dysfunctional protein degradation, or defective quality control machinery because of aging or other pathological conditions (61). In addition, a recent identification of intrinsic disordered protein (IDP) has extended the understanding of protein misfolding involved in amyloidosis (104). In contrast to conventional proteins, IDPs are naturally unfolded proteins that remain functional without having an ordered secondary or tertiary structure and transition to different folding states based on signaling or posttranslational modifications (103). For instance, the monomer of Aβ42, the precursor protein for Alzheimer's disease, remains in unstructured IDP states (without either α-helix or β-sheet structure) and the assembly of Aβ42 fibrils starts with partially refolding to premolten globule-like conformation (50).
While significant efforts have been put forth to understand how precursor proteins undergo a series of conformation changes to misfold and eventually form amyloid fibril, the mechanisms guiding this process, particularly for AL amyloidosis, still remain poorly understood. Researchers have found that amyloidogenic light chain proteins are less stable compared with normal counterparts, which may contribute to protein misfolding (52, 68, 106, 107). That said, amyloidogenic light chain proteins have been found to exhibit a number of mutations without a clear genotype-phenotype association. Recent efforts have focused on those factors that may contribute to decreased thermostability of amyloidogenic light chain protein, including particular amino acid mutations, posttranslational modifications, and extracellular matrix environment (3, 15, 35, 52, 91, 101). Toward this, several reports have demonstrated that a single amino acid mutation present in an amyloidogenic light chain protein may result in significantly less thermostability and enhanced fibril formation (3, 59, 68, 77, 108). While this work on a relatively small number of light chain proteins is certainly encouraging, because of the heterogeneity of this disease, a larger sample size is needed to draw a definitive conclusion. Moreover, by comparing mutated amyloidogenic light chain proteins with normal light chain proteins, ongoing work has sought to determine mutational “hot spots” and protein sites that may alter posttranslational modification, including N-glycosylation (101). When comparing light chain sequences of 50-κ and 91-λ light chain proteins from AL patients, Poshusta et al. (79) identified a significant difference in the number of nonconservative mutation between normal and AL light chain sequences in specific secondary structure elements. Moreover, the distribution of nonconservative mutations was correlated with serum-free light chain levels in AL patients. This may suggest a potential association between nonconservative mutations and amyloidogenesis in AL amyloidosis. The detailed molecular mechanism of how these nonconservative mutations contribute to misfolding and fibrillogenesis still remain to be explored.
In addition to the genetic mutations, several important posttranslational modifications have also been described in amyloidogenic light chain proteins. These posttranslational modifications include disulfide-linked dimerization, S-cysteinylation, glycosylation, fragmentation, S-sulfonation, and 3-chlorotyrosine formation (15). Dimerization has been suggested to be important to maintain the normal structure and stability (2). Davern et al. (16) chemically modified lysine residues of amyloidogenic light chain proteins with sulfo-NHS-biotin, which greatly enhanced binding to cells as well as altered conformational state, suggesting that posttranslational modifications of light chain may contribute to the amyloidogenesis and pathogenesis of the disease. Additionally, glycosaminoglycans (GAGs) have been found to be involved in amyloid fibril deposition, based on mass spectrometry analysis; however, the role of GAGs in the formation of amyloid fibrils remains unclear (67). Recent work from Ren et al. (83) has shown that the presence of GAGs promotes the formation of oligomers and fibrils of amyloidogenic light chain proteins but not non-amyloid myeloma light chain proteins. This highlights the interaction of light chain protein and extracellular matrix environment in the formation of amyloid fibril deposition. Moreover, serum matrix metalloproteinase-9 and tissue inhibitors of matrix metalloproteinase-1 have been suggested to be higher in patients with AL cardiomyopathy compared with patients with ATTR cardiac amyloidosis, suggesting that extracellular matrix proteolysis may play a role in this disease (9). Recent studies have shown that the extracellular chaperone molecule clusterin binds and stabilizes nonnative conformation of proteins to prevent their aggregation (69). Moreover, clusterin has been shown to protect the brain from the development of Alzheimer's disease (69). A recent study has shown that clusterin is preferentially downregulated in the serum from patients with either ATTR or AL amyloidosis, suggesting that the loss of this extracellular chaperone may be associated with fibril deposition and disease pathogenesis (38). While the role of clusterin has not been investigated in the pathogenesis of AL cardiomyopathy, it remains an interesting target for future investigation.
Experimental and Evolving Therapies
The standard and newer therapies for AL cardiomyopathy described above currently include alkylating chemotherapy and high-dose melphalan with autologous hematopoietic stem cell transplantation (73). These treatments, however, are not viable options of all AL patients and are sometimes poorly tolerated (25, 26, 62). Therefore, it is of great importance to translate the basic science-driven understanding of amyloidosis into clinical drug development. The success of target therapy has been witnessed in the management of ATTR cardiomyopathy, in which stabilizing mutant and wild-type TTR with small molecules has proven to be effective in animal models and is currently being evaluated in clinical trials (NCT-00925002, NCT-01171859) (89). Several experimental therapeutic approaches that aim to target amyloidogenic light chain protein or amyloid fibrils resulting from for AL amyloidosis are currently at various stages of development and are described below.
Small RNA interference (RNAi) technology has been used in an attempt to eliminate light chain production and has been found to have high specificity and low toxicity in experimental models (18, 86). Phipps and colleagues (78) used RNAi in an SP2/O mouse myeloma cell line expressing amyloidogenic light chain and found a significant reduction in light chain production without affecting cell viability. Similarly, small interfering RNA (siRNA) targeting amyloidogenic light chain production was able to inhibit the synthesis of light chain protein in vitro, and a single dose of electroporation-mediated siRNA delivery in vivo was demonstrated to effectively reduce circulating light chain levels in an AL light chain secreting plasmacytoma transplant animal model (44). These two studies provide a potential for targeting specific pathological light chain sequences, which may provide opportunities for personalized treatment strategies. Currently, RNAi is being developed for the treatment of TTR amyloidosis in clinical trials (NCT-01148953). Antisense oligonucleotides have been shown to be effective in suppressing TTR expression in an animal model and are currently entering clinical trials for treating TTR amyloidosis in humans (ISIS-TTRRx) (6).
Immunotherapy has also been intensively studied in amyloidosis. Notably, vaccinations targeting Aβ protein have been suggested to reduce amyloid fibril load in mice models of Alzheimer's disease (14, 55, 110). Attempts have also been made to manipulate the immune response to accelerate fibril removal. Amyloid fibrils normally do not elicit a strong immune response, both because of their endogenous properties and the generally poor immunity of the patients (35). Over a decade ago, Hrncic et al. (45) reported that anti-light chain monoclonal antibodies in mice receiving human AL amyloidosis transplantation resulted in the rapid dissolution of amyloid fibrils and neutrophil infiltration. In addition to targeting light chain protein for removal, immunotherapy has also been used to target key proteins known to protect amyloid fibers from degradation. The glycoprotein serum amyloid P component (SAP) has been universally found in all types of amyloid fibril deposition, binding to motifs in the amyloid fibrils in a calcium-dependent manner (67, 99). SAP presence is thought to protect fibrils from proteolysis-mediated degradation (76). As such, by targeting SAP, one might directly disturb amyloid deposition. Using this concept, Bodin et al. (10) recently showed that antibodies targeting SAP in a systemic AA amyloidosis mouse model greatly accelerated the removal of massive visceral amyloid deposits via a complement-dependent and macrophage reaction without significant side effects. In a small study of humans with fibrinogen amyloidosis CPHPC, (R)-1-{6-[(R)-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexa-noyl}pyrrolidine-2 carboxylic acid, a novel bis(d-proline), depleted SAP and resulted in an apparent stabilization of their disease and markedly slowed the progression of renal disease compared with historic controls (36).
These experimental approaches may represent viable therapeutic options, though they remain early in development and require further study before being evaluated in clinical settings.
Closing Remarks
Managing cardiac amyloidosis, particularly AL cardiac amyloidosis, has been a daunting task for clinicians, particularly given the rapid disease progression, reduced therapeutic options, and poor survival. Both basic research and clinical observation have provided compelling evidence for the direct cardiac toxicity of precursor amyloidogenic light chain proteins and have suggested the elimination of precursor proteins via either chemotherapy or stem cell transplantation to be a central goal in the management of AL amyloidosis. Newly introduced chemotherapeutic reagents and regimens coupled with the disease-staging system have also been shown to greatly enhance disease management in patients. As a result, the four-year overall survival in AL amyloidosis patients from date of diagnosis has significantly improved over the past three decades: 21% in 1977–1986, 24% in 1987–1996, and 33% in 1997–2006 (53). While significant advances have been made in the past decade, the overall prognosis associated with AL cardiomyopathy is still very poor. The high one-year mortality in AL amyloidosis patients suggests that early diagnosis of AL cardiomyopathy is paramount for the ultimate survival of patients. The identification and development of effective treatments demands a clear understanding of the molecular mechanisms that promote disease pathogenesis. Recent work has finally begun to make important inroads into understanding amyloid cardiomyopathy (particularly AL cardiomyopathy) and will lead to new potential treatments. Many of these treatments have entered early stages of development and will require close collaboration between basic scientists and clinical investigators to make effective treatment strategies a reality.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute grants HL-086967, HL-093148, and HL-099173 (to R. Liao) and research funds provided by Cardiac Amyloid Program, Brigham and Women's Hospital. S. Mishra is supported by National Institutes of Health T32 Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.G. and S.M. prepared figures; J.G., S.M., and R.L. drafted manuscript; J.G., S.M., R.H.F., and R.L. edited and revised manuscript; R.H.F. and R.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Federica del Monte for providing human AL cardiomyopathy heart samples for electron microscopy.
REFERENCES
- 1. Ando Y, Nakamura M, Kai H, Katsuragi S, Terazaki H, Nozawa T, Okuda T, Misumi S, Matsunaga N, Hata K, Tajiri T, Shoji S, Yamashita T, Haraoka K, Obayashi K, Matsumoto K, Ando M, Uchino M. A novel localized amyloidosis associated with lactoferrin in the cornea. Lab Invest 82: 757–766, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Baden EM, Owen BA, Peterson FC, Volkman BF, Ramirez-Alvarado M, Thompson JR. Altered dimer interface decreases stability in an amyloidogenic protein. J Biol Chem 283: 15853–15860, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baden EM, Randles EG, Aboagye AK, Thompson JR, Ramirez-Alvarado M. Structural insights into the role of mutations in amyloidogenesis. J Biol Chem 283: 30950–30956, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet 377: 1019–1031, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Benson MD, James S, Scott K, Liepnieks JJ, Kluve-Beckerman B. Leukocyte chemotactic factor 2: a novel renal amyloid protein. Kidney Int 74: 218–222, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Benson MD, Kluve-Beckerman B, Zeldenrust SR, Siesky AM, Bodenmiller DM, Showalter AD, Sloop KW. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve 33: 609–618, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Bergstrom J, Murphy C, Eulitz M, Weiss DT, Westermark GT, Solomon A, Westermark P. Codeposition of apolipoprotein A-IV and transthyretin in senile systemic (ATTR) amyloidosis. Biochem Biophys Res Commun 285: 903–908, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Bergstrom J, Murphy CL, Weiss DT, Solomon A, Sletten K, Hellman U, Westermark P. Two different types of amyloid deposits—apolipoprotein A-IV and transthyretin—in a patient systemic amyloidosis with. Lab Invest 84: 981–988, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Biolo A, Ramamurthy S, Connors LH, O'Hara CJ, Meier-Ewert HK, Soo Hoo PT, Sawyer DB, Seldin DC, Sam F. Matrix metalloproteinases and their tissue inhibitors in cardiac amyloidosis: relationship to structural, functional myocardial changes and to light chain amyloid deposition. Circ Heart Fail 1: 249–257, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bodin K, Ellmerich S, Kahan MC, Tennent GA, Loesch A, Gilbertson JA, Hutchinson WL, Mangione PP, Gallimore JR, Millar DJ, Minogue S, Dhillon AP, Taylor GW, Bradwell AR, Petrie A, Gillmore JD, Bellotti V, Botto M, Hawkins PN, Pepys MB. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature 468: 93–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, Apstein CS, Liao R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res 94: 1008–1010, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Chastan N, Baert-Desurmont S, Saugier-Veber P, Derumeaux G, Cabot A, Frebourg T, Hannequin D. Cardiac conduction alterations in a French family with amyloidosis of the Finnish type with the p. Asp187Tyr mutation in the GSN gene. Muscle Nerve 33: 113–119, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Cibeira MT, Sanchorawala V, Seldin DC, Quillen K, Berk JL, Dember LM, Segal A, Ruberg F, Meier-Ewert H, Andrea NT, Sloan JM, Finn KT, Doros G, Blade J, Skinner M. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood 118: 4346–4352, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Citron M. Alzheimer's disease: strategies for disease modification. Nat Rev Drug Discov 9: 387–398 2010 [DOI] [PubMed] [Google Scholar]
- 15. Connors LH, Jiang Y, Budnik M, Theberge R, Prokaeva T, Bodi KL, Seldin DC, Costello CE, Skinner M. Heterogeneity in primary structure, post-translational modifications, and germline gene usage of nine full-length amyloidogenic kappa1 immunoglobulin light chains. Biochemistry 46: 14259–14271, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davern S, Murphy CL, O'Neill H, Wall JS, Weiss DT, Solomon A. Effect of lysine modification on the stability and cellular binding of human amyloidogenic light chains. Biochim Biophys Acta 1812: 32–40, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Dey BR, Chung SS, Spitzer TR, Zheng H, Macgillivray TE, Seldin DC, McAfee S, Ballen K, Attar E, Wang T, Shin J, Newton-Cheh C, Moore S, Sanchorawala V, Skinner M, Madsen JC, Semigran MJ. Cardiac transplantation followed by dose-intensive melphalan and autologous stem-cell transplantation for light chain amyloidosis and heart failure. Transplantation 90: 905–911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dillon CP, Sandy P, Nencioni A, Kissler S, Rubinson DA, Van Parijs L. Rnai as an experimental and therapeutic tool to study and regulate physiological and disease processes. Annu Rev Physiol 67: 147–173, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, Greipp PR, Witzig TE, Lust JA, Rajkumar SV, Fonseca R, Zeldenrust SR, McGregor CG, Jaffe AS. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol 22: 3751–3757, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Dispenzieri A, Kyle RA, Gertz MA, Therneau TM, Miller WL, Chandrasekaran K, McConnell JP, Burritt MF, Jaffe AS. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet 361: 1787–1789, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Dubrey S, Mendes L, Skinner M, Falk RH. Resolution of heart failure in patients with AL amyloidosis. Ann Intern Med 125: 481–484, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, Falk RH. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM 91: 141–157, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Dubrey SW, Cha K, Skinner M, LaValley M, Falk RH. Familial and primary (AL) cardiac amyloidosis: echocardiographically similar diseases with distinctly different clinical outcomes. Heart 78: 74–82, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eulitz M, Weiss DT, Solomon A. Immunoglobulin heavy-chain-associated amyloidosis. Proc Natl Acad Sci USA 87: 6542–6546, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Falk RH. Cardiac amyloidosis: a treatable disease, often overlooked. Circulation 124: 1079–1085, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation 112: 2047–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med 337: 898–909, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis 52: 347–361, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Gertz MA. Immunoglobulin light chain amyloidosis: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol 86: 180–186, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A, Grateau G. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol 79: 319–328, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Ghetti B, Tagliavini F, Takao M, Bugiani O, Piccardo P. Hereditary prion protein amyloidoses. Clin Lab Med 23: 65–85, viii, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Ghiso JA, Holton J, Miravalle L, Calero M, Lashley T, Vidal R, Houlden H, Wood N, Neubert TA, Rostagno A, Plant G, Revesz T, Frangione B. Systemic amyloid deposits in familial British dementia. J Biol Chem 276: 43909–43914, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Gianni D, Li A, Tesco G, McKay KM, Moore J, Raygor K, Rota M, Gwathmey JK, Dec GW, Aretz T, Leri A, Semigran MJ, Anversa P, Macgillivray TE, Tanzi RE, del Monte F. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation 121: 1216–1226, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gibbs SD, Sattianayagam PT, Hawkins PN, Gillmore JD. Cardiac transplantation should be considered in selected patients with either AL or hereditary forms of amyloidosis: the UK National Amyloidosis Centre experience. Intern Med J 39: 786–787; author reply 787–788, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Gillmore JD, Hawkins PN. Drug insight: emerging therapies for amyloidosis. Nat Clin Pract Nephrol 2: 263–270, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Gillmore JD, Tennent GA, Hutchinson WL, Gallimore JR, Lachmann HJ, Goodman HJ, Offer M, Millar DJ, Petrie A, Hawkins PN, Pepys MB. Sustained pharmacological depletion of serum amyloid P component in patients with systemic amyloidosis. Br J Haematol 148: 760–767, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Gong W, Liu ZH, Zeng CH, Peng A, Chen HP, Zhou H, Li LS. Amylin deposition in the kidney of patients with diabetic nephropathy. Kidney Int 72: 213–218, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Greene MJ, Sam F, Soo Hoo PT, Patel RS, Seldin DC, Connors LH. Evidence for a functional role of the molecular chaperone clusterin in amyloidotic cardiomyopathy. Am J Pathol 178: 61–68, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haggqvist B, Naslund J, Sletten K, Westermark GT, Mucchiano G, Tjernberg LO, Nordstedt C, Engstrom U, Westermark P. Medin: an integral fragment of aortic smooth muscle cell-produced lactadherin forms the most common human amyloid. Proc Natl Acad Sci USA 96: 8669–8674, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamidi Asl K, Liepnieks JJ, Nakamura M, Parker F, Benson MD. A novel apolipoprotein A-1 variant, Arg173Pro, associated with cardiac and cutaneous amyloidosis. Biochem Biophys Res Commun 257: 584–588, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Hamidi Asl L, Liepnieks JJ, Hamidi Asl K, Uemichi T, Moulin G, Desjoyaux E, Loire R, Delpech M, Grateau G, Benson MD. Hereditary amyloid cardiomyopathy caused by a variant apolipoprotein A1. Am J Pathol 154: 221–227, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heegaard NH. Beta(2)-microglobulin: from physiology to amyloidosis. Amyloid 16: 151–173, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Hoppener JW, Lips CJ. Role of islet amyloid in type 2 diabetes mellitus. Int J Biochem Cell Biol 38: 726–736, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Hovey BM, Ward JE, Soo Hoo P, O'Hara CJ, Connors LH, Seldin DC. Preclinical development of siRNA therapeutics for AL amyloidosis. Gene Ther 18: 1150–1156, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hrncic R, Wall J, Wolfenbarger DA, Murphy CL, Schell M, Weiss DT, Solomon A. Antibody-mediated resolution of light chain-associated amyloid deposits. Am J Pathol 157: 1239–1246, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jacobson DR, Pastore RD, Yaghoubian R, Kane I, Gallo G, Buck FS, Buxbaum JN. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med 336: 466–473, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Kastritis E, Anagnostopoulos A, Roussou M, Toumanidis S, Pamboukas C, Migkou M, Tassidou A, Xilouri I, Delibasi S, Psimenou E, Mellou S, Terpos E, Nanas J, Dimopoulos MA. Treatment of light chain (AL) amyloidosis with the combination of bortezomib and dexamethasone. Haematologica 92: 1351–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Kaye GC, Butler MG, d'Ardenne AJ, Edmondson SJ, Camm AJ, Slavin G. Isolated atrial amyloid contains atrial natriuretic peptide: a report of six cases. Br Heart J 56: 317–320, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kholova I, Niessen HW. Amyloid in the cardiovascular system: a review. J Clin Pathol 58: 125–133, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kirkitadze MD, Condron MM, Teplow DB. Identification and characterization of key kinetic intermediates in amyloid beta-protein fibrillogenesis. J Mol Biol 312: 1103–1119, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Klein AL, Hatle LK, Burstow DJ, Seward JB, Kyle RA, Bailey KR, Luscher TF, Gertz MA, Tajik AJ. Doppler characterization of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol 13: 1017–1026, 1989 [DOI] [PubMed] [Google Scholar]
- 52. Klimtchuk ES, Gursky O, Patel RS, Laporte KL, Connors LH, Skinner M, Seldin DC. The critical role of the constant region in thermal stability and aggregation of amyloidogenic immunoglobulin light chain. Biochemistry 49: 9848–9857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kumar SK, Gertz MA, Lacy MQ, Dingli D, Hayman SR, Buadi FK, Short-Detweiler K, Zeldenrust SR, Leung N, Greipp PR, Lust JA, Russell SJ, Kyle RA, Rajkumar SV, Dispenzieri A. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin Proc 86: 12–18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kyle RA. Amyloidosis: a convoluted story. Br J Haematol 114: 529–538, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol 6: 108–119 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Levy E, Haltia M, Fernandez-Madrid I, Koivunen O, Ghiso J, Prelli F, Frangione B. Mutation in gelsolin gene in Finnish hereditary amyloidosis. J Exp Med 172: 1865–1867, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liao R, Jain M, Teller P, Connors LH, Ngoy S, Skinner M, Falk RH, Apstein CS. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation 104: 1594–1597, 2001 [PubMed] [Google Scholar]
- 58. Linke RP, Joswig R, Murphy CL, Wang S, Zhou H, Gross U, Rocken C, Westermark P, Weiss DT, Solomon A. Senile seminal vesicle amyloid is derived from semenogelin I. J Lab Clin Med 145: 187–193, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Martin DJ, Ramirez-Alvarado M. Comparison of amyloid fibril formation by two closely related immunoglobulin light chain variable domains. Amyloid 17: 129–136, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maury CP, Baumann M. Isolation and characterization of cardiac amyloid in familial amyloid polyneuropathy type IV (Finnish): relation of the amyloid protein to variant gelsolin. Biochim Biophys Acta 1096: 84–86, 1990 [DOI] [PubMed] [Google Scholar]
- 61. Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med 349: 583–596, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol 29: 1924–1933, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Migrino RQ, Hari P, Gutterman DD, Bright M, Truran S, Schlundt B, Phillips SA. Systemic and microvascular oxidative stress induced by light chain amyloidosis. Int J Cardiol 145: 67–68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Migrino RQ, Truran S, Gutterman DD, Franco DA, Bright M, Schlundt B, Timmons M, Motta A, Phillips SA, Hari P. Human microvascular dysfunction and apoptotic injury induced by AL amyloidosis light chain proteins. Am J Physiol Heart Circ Physiol 301: H2305–H2312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Munier FL, Korvatska E, Djemai A, Le Paslier D, Zografos L, Pescia G, Schorderet DF. Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat Genet 15: 247–251, 1997 [DOI] [PubMed] [Google Scholar]
- 66. Murphy CL, Kestler DP, Foster JS, Wang S, Macy SD, Kennel SJ, Carlson ER, Hudson J, Weiss DT, Solomon A. Odontogenic ameloblast-associated protein nature of the amyloid found in calcifying epithelial odontogenic tumors and unerupted tooth follicles. Amyloid 15: 89–95, 2008 [DOI] [PubMed] [Google Scholar]
- 67. Murphy CL, Wang S, Williams T, Weiss DT, Solomon A. Characterization of systemic amyloid deposits by mass spectrometry. Methods Enzymol 412: 48–62, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Nowak M. Immunoglobulin kappa light chain and its amyloidogenic mutants: a molecular dynamics study. Proteins 55: 11–21, 2004 [DOI] [PubMed] [Google Scholar]
- 69. Nuutinen T, Suuronen T, Kauppinen A, Salminen A. Clusterin: a forgotten player in Alzheimer's disease. Brain Res Rev 61: 89–104, 2009 [DOI] [PubMed] [Google Scholar]
- 70. Obici L, Bellotti V, Mangione P, Stoppini M, Arbustini E, Verga L, Zorzoli I, Anesi E, Zanotti G, Campana C, Vigano M, Merlini G. The new apolipoprotein A-I variant leu(174)→Ser causes hereditary cardiac amyloidosis, and the amyloid fibrils are constituted by the 93-residue N-terminal polypeptide. Am J Pathol 155: 695–702, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Palladini G, Campana C, Klersy C, Balduini A, Vadacca G, Perfetti V, Perlini S, Obici L, Ascari E, d'Eril GM, Moratti R, Merlini G. Serum N-terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation 107: 2440–2445, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Palladini G, Lavatelli F, Russo P, Perlini S, Perfetti V, Bosoni T, Obici L, Bradwell AR, D'Eril GM, Fogari R, Moratti R, Merlini G. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood 107: 3854–3858, 2006 [DOI] [PubMed] [Google Scholar]
- 73. Palladini G, Merlini G. Transplantation vs. conventional-dose therapy for amyloidosis. Curr Opin Oncol 23: 214–220, 2011 [DOI] [PubMed] [Google Scholar]
- 74. Palsdottir A, Snorradottir AO, Thorsteinsson L. Hereditary cystatin C amyloid angiopathy: genetic, clinical, and pathological aspects. Brain Pathol 16: 55–59, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pepys MB, Hawkins PN, Booth DR, Vigushin DM, Tennent GA, Soutar AK, Totty N, Nguyen O, Blake CC, Terry CJ, Feest TG, Zalin AM, Hsuan JJ. Human lysozyme gene mutations cause hereditary systemic amyloidosis. Nature 362: 553–557, 1993 [DOI] [PubMed] [Google Scholar]
- 76. Pepys MB, Herbert J, Hutchinson WL, Tennent GA, Lachmann HJ, Gallimore JR, Lovat LB, Bartfai T, Alanine A, Hertel C, Hoffmann T, Jakob-Roetne R, Norcross RD, Kemp JA, Yamamura K, Suzuki M, Taylor GW, Murray S, Thompson D, Purvis A, Kolstoe S, Wood SP, Hawkins PN. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature 417: 254–259, 2002 [DOI] [PubMed] [Google Scholar]
- 77. Peterson FC, Baden EM, Owen BA, Volkman BF, Ramirez-Alvarado M. A single mutation promotes amyloidogenicity through a highly promiscuous dimer interface. Structure 18: 563–570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Phipps JE, Kestler DP, Foster JS, Kennel SJ, Donnell R, Weiss DT, Solomon A, Wall JS. Inhibition of pathologic immunoglobulin-free light chain production by small interfering RNA molecules. Exp Hematol 38: 1006–1013, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Poshusta TL, Sikkink LA, Leung N, Clark RJ, Dispenzieri A, Ramirez-Alvarado M. Mutations in specific structural regions of immunoglobulin light chains are associated with free light chain levels in patients with AL amyloidosis. PLoS One 4: e5169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, Salvi F, Ciliberti P, Pastorelli F, Biagini E, Coccolo F, Cooke RM, Bacchi-Reggiani L, Sangiorgi D, Ferlini A, Cavo M, Zamagni E, Fonte ML, Palladini G, Salinaro F, Musca F, Obici L, Branzi A, Perlini S. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation 120: 1203–1212, 2009 [DOI] [PubMed] [Google Scholar]
- 81. Reches M, Porat Y, Gazit E. Amyloid fibril formation by pentapeptide and tetrapeptide fragments of human calcitonin. J Biol Chem 277: 35475–35480, 2002 [DOI] [PubMed] [Google Scholar]
- 82. Reece DE, Sanchorawala V, Hegenbart U, Merlini G, Palladini G, Fermand JP, Vescio RA, Liu X, Elsayed YA, Cakana A, Comenzo RL. Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a phase 1 dose-escalation study. Blood 114: 1489–1497, 2009 [DOI] [PubMed] [Google Scholar]
- 83. Ren R, Hong Z, Gong H, Laporte K, Skinner M, Seldin DC, Costello CE, Connors LH, Trinkaus-Randall V. Role of glycosaminoglycan sulfation in the formation of immunoglobulin light chain amyloid oligomers and fibrils. J Biol Chem 285: 37672–37682, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rocken C, Shakespeare A. Pathology, diagnosis and pathogenesis of AA amyloidosis. Virchows Arch 440: 111–122, 2002 [DOI] [PubMed] [Google Scholar]
- 85. Roig E, Almenar L, Gonzalez-Vilchez F, Rabago G, Delgado J, Gomez-Bueno M, Crespo-Leiro MG, Arizon JM, de la Fuente L, Manito N. Outcomes of heart transplantation for cardiac amyloidosis: subanalysis of the Spanish registry for heart transplantation. Am J Transplant 9: 1414–1419, 2009 [DOI] [PubMed] [Google Scholar]
- 86. Sah DW, Aronin N. Oligonucleotide therapeutic approaches for Huntington disease. J Clin Invest 121: 500–507, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sanchorawala V. Light-chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol 1: 1331–1341, 2006 [DOI] [PubMed] [Google Scholar]
- 88. Sanchorawala V, Seldin DC, Magnani B, Skinner M, Wright DG. Serum free light-chain responses after high-dose intravenous melphalan and autologous stem cell transplantation for AL (primary) amyloidosis. Bone Marrow Transplant 36: 597–600, 2005 [DOI] [PubMed] [Google Scholar]
- 89. Sekijima Y, Kelly JW, Ikeda S. Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr Pharm Des 14: 3219–3230, 2008 [DOI] [PubMed] [Google Scholar]
- 90. Seldin DC, Choufani EB, Dember LM, Wiesman JF, Berk JL, Falk RH, O'Hara C, Fennessey S, Finn KT, Wright DG, Skinner M, Sanchorawala V. Tolerability and efficacy of thalidomide for the treatment of patients with light chain-associated (AL) amyloidosis. Clin Lymphoma 3: 241–246, 2003 [DOI] [PubMed] [Google Scholar]
- 91. Selkoe DJ. Folding proteins in fatal ways. Nature 426: 900–904, 2003 [DOI] [PubMed] [Google Scholar]
- 92. Shah KB, Inoue Y, Mehra MR. Amyloidosis and the heart: a comprehensive review. Arch Intern Med 166: 1805–1813, 2006 [DOI] [PubMed] [Google Scholar]
- 93. Shi J, Guan J, Jiang B, Brenner DA, Del Monte F, Ward JE, Connors LH, Sawyer DB, Semigran MJ, Macgillivray TE, Seldin DC, Falk R, Liao R. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci USA 107: 4188–4193, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sikkink LA, Ramirez-Alvarado M. Cytotoxicity of amyloidogenic immunoglobulin light chains in cell culture. Cell Death Dis 1: e98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Simms RW, Prout MN, Cohen AS. The epidemiology of AL and AA amyloidosis. Baillieres Clin Rheumatol 8: 627–634, 1994 [DOI] [PubMed] [Google Scholar]
- 96. Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Westermark P. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid 17: 101–104, 2011 [DOI] [PubMed] [Google Scholar]
- 97. Sipe JD, Cohen AS. Review: history of the amyloid fibril. J Struct Biol 130: 88–98, 2000 [DOI] [PubMed] [Google Scholar]
- 98. Sitia R, Palladini G, Merlini G. Bortezomib in the treatment of AL amyloidosis: targeted therapy? Haematologica 92: 1302–1307, 2007 [DOI] [PubMed] [Google Scholar]
- 99. Skinner M, Vaitukaitis JL, Cohen AS, Benson MD. Serum amyloid P-component levels in amyloidosis, connective tissue diseases, infection, and malignancy as compared to normal serum. J Lab Clin Med 94: 633–638, 1979 [PubMed] [Google Scholar]
- 100. Stangou AJ, Banner NR, Hendry BM, Rela M, Portmann B, Wendon J, Monaghan M, Maccarthy P, Buxton-Thomas M, Mathias CJ, Liepnieks JJ, O'Grady J, Heaton ND, Benson MD. Hereditary fibrinogen A alpha-chain amyloidosis: phenotypic characterization of a systemic disease and the role of liver transplantation. Blood 115: 2998–3007, 2010 [DOI] [PubMed] [Google Scholar]
- 101. Stevens FJ. Four structural risk factors identify most fibril-forming kappa light chains. Amyloid 7: 200–211, 2000 [DOI] [PubMed] [Google Scholar]
- 102. Suhr OB, Herlenius G, Friman S, Ericzon BG. Liver transplantation for hereditary transthyretin amyloidosis. Liver Transpl 6: 263–276, 2000 [DOI] [PubMed] [Google Scholar]
- 103. Uversky VN. Intrinsic disorder in proteins associated with neurodegenerative diseases. Front Biosci 14: 5188–5238, 2009 [DOI] [PubMed] [Google Scholar]
- 104. Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys 37: 215–246, 2008 [DOI] [PubMed] [Google Scholar]
- 105. Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 114: 4957–4959, 2009 [DOI] [PubMed] [Google Scholar]
- 106. Wall J, Schell M, Murphy C, Hrncic R, Stevens FJ, Solomon A. Thermodynamic instability of human lambda 6 light chains: correlation with fibrillogenicity. Biochemistry 38: 14101–14108, 1999 [DOI] [PubMed] [Google Scholar]
- 107. Wall JS, Gupta V, Wilkerson M, Schell M, Loris R, Adams P, Solomon A, Stevens F, Dealwis C. Structural basis of light chain amyloidogenicity: comparison of the thermodynamic properties, fibrillogenic potential and tertiary structural features of four Vlambda6 proteins. J Mol Recognit 17: 323–331, 2004 [DOI] [PubMed] [Google Scholar]
- 108. Wally J, Kica G, Zhang Y, Ericsson T, Connors LH, Benson MD, Liepnieks JJ, Murray J, Skinner M, Comenzo RL. Identification of a novel substitution in the constant region of a gene coding for an amyloidogenic kappa1 light chain. Biochim Biophys Acta 1454: 49–56, 1999 [DOI] [PubMed] [Google Scholar]
- 109. Wechalekar AD, Goodman HJ, Lachmann HJ, Offer M, Hawkins PN, Gillmore JD. Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood 109: 457–464, 2007 [DOI] [PubMed] [Google Scholar]
- 110. Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer's disease. Nat Rev Immunol 6: 404–416, 2006 [DOI] [PubMed] [Google Scholar]
- 111. Westermark P, Eriksson L, Engstrom U, Enestrom S, Sletten K. Prolactin-derived amyloid in the aging pituitary gland. Am J Pathol 150: 67–73, 1997 [PMC free article] [PubMed] [Google Scholar]
- 112. Yumlu S, Barany R, Eriksson M, Rocken C. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol 40: 1655–1660, 2009 [DOI] [PubMed] [Google Scholar]