Abstract
Many studies have implicated the peroxisome proliferator-activated receptor (PPAR) family of nuclear receptor transcription factors in regulating cardiac substrate metabolism and ATP generation. Recently, evidence from a variety of cell culture and organ systems has implicated ubiquitin and small ubiquitin-like modifier (SUMO) conjugation as post-translational modifications that regulate the activity of PPAR transcription factors and their coreceptors/coactivators. Here we introduce the ubiquitin and SUMO conjugation systems and extensively review how they have been shown to regulate all three PPAR isoforms (PPARα, PPARβ/δ, and PPARγ) in addition to the retinoid X receptor and PPARγ coactivator-1α subunits of the larger PPAR transcription factor complex. We then present how the specific ubiquitin (E3) ligases have been implicated and review emerging evidence that post-translational modifications of PPARs with ubiquitin and/or SUMO may play a role in cardiac disease. Because PPAR activity is perturbed in a variety of forms of heart disease and specific proteins regulate this process (E3 ligases), this may be a fruitful area of investigation with respect to finding new therapeutic targets.
Keywords: retinoid X receptor, small ubiquitin-like modifier, ubiquitin, phosphorylation
this article is part of a collection on Post-translational Protein Modification in Metabolic Stress. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
Cardiac energy substrate utilization is transcriptionally controlled, in part, by the peroxisome proliferator-activated receptor (PPAR) family of transcription factors and their coreceptors/coactivators, including PPARα, PPARβ/δ, PPARγ, retinoid X receptor-α (RXRα), and PPARγ coactivator 1α (PGC-1α). Mechanistically, PPARα, PPARβ/δ, and PPARγ form heterodimers with the RXRα and coactivators (e.g., PGC-1α) and repressors [e.g., nuclear receptor corepressor (NCoR)] to regulate the transcription of genes involved in lipid metabolism and energy regulation (21, 48, 62). PPARs interact with their ligand(s), which include long chain fatty acids and their derivatives, whereas RXRα interacts with retinoic acid, both of which enhance the binding of PPAR complex to DNA binding elements in the promoter regions of genes, called PPAR response elements (PPREs) (59). Decreased cardiac PPAR activities have been implicated in the regulation of fatty acid utilization in mouse, rat, and dog models of heart failure (8, 64, 65, 74). The mechanisms by which PPAR is regulated are not entirely clear in the context of heart failure. However, recent studies have reported post-translational modifications of all three PPAR isoforms, the PPAR coactivator RXRα, or the PPAR coactivator PGC-1α, which may explain how PPAR isoform activities are regulated (52, 69, 71). PPARs and their coreceptors/coactivators also play nonmetabolic roles in cardiovascular disease, namely in the regulation of cardiac hypertrophy, myocardial inflammation, extracellular matrix remodeling, and oxidative stress. For example, PPAR activity regulates nuclear factor-κB (NF-κB) (19, 99), c-Jun/ activator protein-1 (42), GATA (20, 33), and nuclear factor of activated T cells (5, 55) in response to hypertrophic and inflammatory stimuli, demonstrating that PPARs can play a protective role in cardiac disease independent of their ability to activate genes involved in fatty acid uptake and β-oxidation. PPARs may also play a role in the development of cardiac fibrosis and protection against cardiac oxidative stress (19, 37, 41, 85). In this review, we highlight recent studies implicating the post-translational regulation of PPAR transcription factors by ubiquitin and small ubiquitin-like modifier (SUMO) and then discuss how these modifications regulate PPAR function. While the studies presented in this review examine largely noncardiomyocyte systems, new studies have demonstrated their applicability to striated muscle in regulating fatty acid and glucose utilization and apoptosis. These findings offer insight into how the post-transcriptional regulation of PPARs may also be applicable to an array of cardiac diseases characterized by altered PPAR activities.
Post-translational Modification by Ubiquitin and SUMO: Parallel Systems
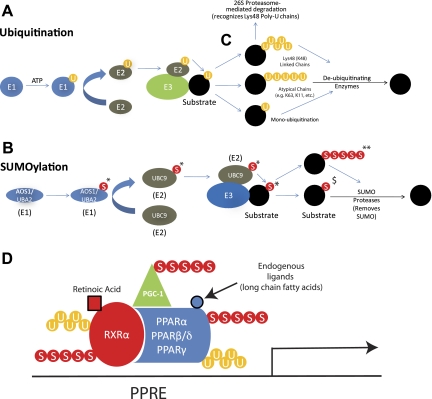
The conjugation of ubiquitin or SUMO is unique among post-translational modifications in that it involves the attachment of another polypeptide (31, 60, 61, 96) instead of the addition of a functional group, such as a phosphate, acetate, lipid, or carbohydrate. The structures of ubiquitin, a 76-amino acid polypeptide, and SUMO, a 101-amino acid polypeptide, are very similar; however, they have only ∼18% sequence homology and differ greatly in their surface charge topology (88). The ubiquitin and SUMO conjugation pathways parallel each other in many aspects, only differing in the specific enzymes involved, as outlined in Fig. 1. In the first step, free ubiquitin or SUMO is covalently linked to the ubiquitin-activating enzyme (E1) in an ATP-dependent reaction (Fig. 1, A and B, far left) (70). Next, ubiquitin or SUMO is transferred from the E1 enzyme to the ubiquitin-conjugating enzyme (E2). Finally, the interaction between the E2 and the ubiquitin or SUMO ligase enzyme (E3) allows the E3 to initiate the transfer of ubiquitin or SUMO from the E2 to a lysine residue on the substrate (18, 32). The E3 is the pivotal component in both the ubiquitin and SUMO conjugation pathways since it confers specificity to the system by directly interacting with the substrate. Therefore, identifying ubiquitin ligase-substrate pairs is integral to understanding the cellular functions affected by ubiquitin or SUMO conjugation. Many E1, E2, and E3 enzymes in the ubiquitin pathway have been identified, whereas only a handful of these enzymes have been identified for the SUMO pathway (31, 43, 66). This distinction parallels our general understanding of the effects of ubiquitination and SUMOylation; i.e., much more is understood about the effects of ubiquitination compared with what is understood about the effects of SUMOylation.
Fig. 1.
Ubiquitination and small uniquitin-like modifier (SUMO)ylation of the peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor (RXR)/PPARγ coactivator 1α (PGC-1α) complex. Ubiquitination (A) and SUMOylation (B) are multistep processes using E1, E2, and E3 enzymes to posttranslationally modify substrates (described in text). C: canonical ubiquitination (top) occurs by ubiquitin chains placed by the E2/E3 complex whereby the interubiquitin links occur through their lysine-48 (K48). Substrates modified with ubiquitin chains linked by their K48 are generally targeted for degradation by the 26S proteasome. Both atypical ubiquitination (linkages between ubiquitin such as K63, middle) and monoubiquitination (bottom) generally target substrates to change localization which can affect activity (discussed in text). D: PPAR/RXR/PGC-1 complex may be ubiquitinated and/or SUMOylated on each of their subunits, allowing for complex and nuanced regulation of PPAR activity. Here we represent a composite theoretical view of the post-translational modifications that may occur on RXRα, PPAR isoforms, and PGC-1 complex during PPAR signaling, indicating the potential complexity by which cardiac PPARs may be regulated. Vertebrates have 4 SUMO isoforms, 3 of which have been found to be involved in post-translational modification of proteins (SUMO-4 is predicted to be a pseudogene). *SUMO-1, SUMO-2, or SUMO-3. SUMO-2 and SUMO-3 (**) have the presence of a motif that allows for the polySUMOylation of substrates, whereas SUMO-1 ($) is mainly involved in monoSUMOylation. U, ubiquitination; S, SUMO; AOS1, activator of SUMO1; UBA2, ubiquitin-like modifier activating enzyme 2; UBC9, ubiquitin-conjugating enzyme 9; PPRE, PPAR response element.
The greatest difference between ubiquitination and SUMOylation lies in the effects that specific conjugation patterns foster on protein activity and cellular localization. The most commonly described ubiquitination pattern is polyubiquitination, where the initial ubiquitin molecule is conjugated to the substrate, followed by the addition of subsequent ubiquitin molecules linked by their lysine located in the 48th amino acid position (see Fig. 1C) (36). When the lysines are serially connected to each other by this canonical lysine number 48, it is referred to as a K48 (K = lysine) polyubiquitin chain. This canonical K48 ubiquitination pattern is recognized by the 26S proteasome, targeting the modified protein for degradation (Fig. 1C) (12, 36, 44). Other recognized ubiquitination patterns include monoubiquitination (the placement of only 1 ubiquitin on a substrate) (12) and polyubiquitination via atypical [non-K48 linked (K63)] ubiquitin chains (27). Monoubiquitination and polyubiquitination (Fig. 1C) via atypical ubiquitin chains have a wider effect on cellular function than polyubiquitination via K48, including translation, cell cycle regulation, signal transduction, protein trafficking, and protein-protein interactions (31, 39, 54, 96, 98). Similar to ubiquitination, SUMOylation patterns define the involvement of SUMO conjugation in cellular processes: polySUMOylation has been linked to the stress response, whereas monoSUMOylation has been primarily associated with transcription regulation, nuclear transport, and regulation of protein-protein interactions (43, 58, 82, 94). Vertebrates have four SUMO isoforms, SUMO1–4, with the largest distinction between isoforms being the presence of a SUMOylation motif in SUMO-2/3 only. This SUMOylation motif allows for the formation of polySUMO chains with SUMO-2/3, but not with SUMO-1 (Fig. 1B) (28). The SUMO-4 isoform shares 87% amino acid homology with SUMO-2, but it lacks introns, suggesting it may be a pseudogene. Whereas endogenous SUMO-4 mRNA has been found in tissues, the protein has not been detected and it is unclear whether it can be conjugated to proteins based on its expected sequence and structure (11, 72, 97).
The post-translational modification of the PPAR complex with ubiquitin and SUMO has been described by a number of investigators. Figure 1D illustrates the many possible SUMO and ubiquitin modifications that may occur on the PPARα, PPARβ/δ, and PPARγ isoforms, the RXRα nuclear receptor, along with the PGC-1α coreceptor. Most of the studies described next investigate these post-translational modifications on individual subunits: the effects of SUMOylation and ubiquitination on PPAR activity likely reflect a composite of the post-translational modification on the individual subunits. Much work remains to be done to determine the molecular details of how specific ubiquitin and SUMO ligases affect the overall PPAR complex function.
Ubiquitination of PPARs
The regulation of PPARα by the ubiquitin proteasome system.
Over the past 10 years, several lines of evidence have implicated the ubiquitin proteasome system (UPS) in the regulation of PPARα activity. In early studies, GFP- or hemagglutinin-tagged PPARα expression vectors were transfected into HeLa cells and analyzed by Western blot analysis for PPARα. These studies revealed that PPARα protein stability is greatly affected by the presence or absence of a selective PPARα ligand (40). Similar observations have been reported in HepG2 cells transfected with PPARα (10). In these cells, cotransfection with PPARα and hemagglutinin-tagged ubiquitin reveals that PPARα can be polyubiquitinated and that this post-translational modification is lost in the presence of multiple PPARα ligands. Likewise, increasing the expression of the RXRα and the CREB binding protein coactivator of PPARα (both PPARα coactivators) in HepG2 cells leads to a decrease in PPARα ubiquitination. Finally, when these cells are treated with MG132, a proteasome inhibitor, PPARα activity is increased, presumably because of the decrease in ubiquitin-mediated proteasomal degradation of PPARα (10).
Recently, additional molecular details surrounding PPARα ubiquitination and its effect on PPARα activity have been reported by Gopinathan et al. (34). In this study, an interesting relationship between PPARα activity and the ratio of PPARα to murine double minute 2 (MDM2, a ubiquitin ligase) was revealed. MDM2 associates with PPARα through the A/B domain of PPARα (Fig. 2A) and the coexpression of MDM2 increases PPARα ubiquitination (34). Increasing MDM2 levels relative to PPARα and PPARβ/δ, but not PPARγ, leads to a decrease in PPAR activity (34), whereas knocking down MDM2 expression levels with small interfering RNA in rat hepatoma cells inhibits mRNA expression of several PPARα targets (34). Increasing MDM2 levels in the presence of Wy-14643 (a PPARα selective agonist) leads to enhanced PPARα activity up to a ratio of MDM2 to PPARα <0.5 to 1; however, ratios of MDM2 to PPARα ≥ 1 inhibits PPARα activity (34). In summary, these studies demonstrate the ubiquitination of PPARα in a ligand-dependent manner and that effect of ubiquitination on PPARα activity depends on the systems studied (Table 1).
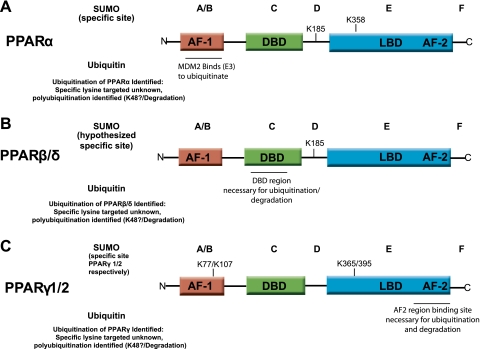
Fig. 2.
Summary of reported SUMOylation sites and regions necessary for ubiquitination on PPAR nuclear receptors. Like all nuclear receptors, PPARα, PPARβ/δ, and PPARγ are modular in structure and contain 5 domains, indicated by A, B, C, D, E, and F. The A/B region contains the activation function-1 region (AF-1) where the activity in the presence of ligand is found (95). AF-1 activation is normally weak but synergizes with the activation function-2 region (AF-2) found in the E domain upon ligand binding, resulting in an increase in transcription activity and gene expression. The DNA binding domain (DBD) is found in the C domain and binds specific DNA sequences in the PPREs found in the promoter regions of genes regulated by PPARs. The D domain contains the hinge region, connecting the DBD to the ligand binding domain (LBD) found in domain E. The LBD contributes to the dimerization of the receptor with RXR and binds coactivator and corepressor proteins here. The E domain also contains the AF-2 domain, whose action is dependent on ligand binding (95). Both domains E and F have been implicated in the dimerization of PPARs with RXR and ligand-dependent transactivation (47, 63). Recent studies have described the specific sites within PPARα, PPARβ/δ, and PPARγ at which SUMOylation occurs (above each PPAR isoform) or, more broadly, that ubiquitination occurs (below each PPAR isoform). The specific amino acids where SUMO modifications have been reported are indicated by the lysine (K) position in the PPAR amino acid sequence. Lysines in the 185, 358, 185, 77/107, and 365/395 positions have been reported as indicated above. For more experimental detail leading to these findings, see Table 1. N, NH2 terminus; C, COOH terminus.
Table 1.
Summary of ubiquitin and SUMO modification of PPARs and the resulting effects on transcription in the presence or absence of ligand
| Nuclear Receptor | Post-translational Modification | Ubiquitin/SUMO Ligase (E3) Identified | Modification Type | Transcriptional Affect | Effect of Ligand | Cell type | References |
|---|---|---|---|---|---|---|---|
| PPARα | Ubiquitination | MDM2 | Polyubiquitination | MDM2-to-PPARα ratio < 0.5: Activation MDM2-to-PPARα ratio ≥ 1.0: Inhibition | Wy14643 is required for MDM2-dependent modulation of PPARα activity | Rat hepatoma | (30) |
| Inhibition of PPARα activity by promoting its degradation | Wy14643 blocks PPARα polyubiquitination | HepG2 | (11, 12, 36) | ||||
| SUMOylation | PIASy | SUMO-1, K185 | Inhibition of PPARα activity by recruiting NCoR corepressor | GW-7647 blocks PPARα monoSUMOylation | COS-7; human hepatoma | (66) | |
| SUMO-2/3, K358 | Not determined | Not determined | NIH3T3; HepG2 | ||||
| SUMO-1, K358 | Inhibition of PPARα activity by recruiting GA-binding protein and histone deacetylases | WY-14643 enhances PPARα monoSUMOylation | NIH3T3; HepG2 | (47) | |||
| PPARβ (also known as PPARδ) | Ubiquitination | Not determined | Polyubiquitination | Inhibition of PPARβ activity by promoting its degradation | PPARβ low and high: GW-501516, L-165041, and cPGI2block PPARβ polyubiquitination | U2OS; human lung cell lines | (28) |
| PPARβ low: GW501516 does not affect PPARβ polyubiquitination | HEK293; mouse fibroblasts | (68) | |||||
| PPARβ high: GW-501516 blocks PPARβ polyubiquitination | |||||||
| PPARγ1/2 | Ubiquitination | Not determined | Polyubiquitination | Inhibition of PPARγ1 activity by promoting its degradation | Thiazolidinediones enhance PPARγ polyubiquitination | Differentiated adipocytes | (34) |
| SUMOylation | PIAS1 & PIASxβ SUMOylates SENP2 deSUMOylates | SUMO-1, K77/K107 SUMO-1, K365/K395 | Inhibition of PPARγ1 activity Inhibition of PPARγ1 activity by recruiting NCoR corepressor | Rosiglitazone enhances PPARγ1 SUMOylation Rosiglitazone has no affect on PPARγ1 deSUMOylation (C2C12) | HEK293; HepG2; NIH3T3; vascular smooth muscle cells; in vivorat cartiod artery; C2C12 | (15, 24, 49, 60, 64, 75, 89) | |
| Rosiglitazone enhances PPARγ1 SUMOylation | Mouse macrophage |
See main text for definition of abbreviations.
The regulation of PPARβ/δ by the UPS.
Like PPARα, the mechanism by which ubiquitination affects PPARβ/δ activity and protein levels has been studied in different cell types. In U2OS human osteosarcoma cells expressing recombinant PPARβ/δ, PPARβ/δ is ubiquitinated and rapidly degraded in the absence of a ligand. However, the addition of PPARβ/δ-specific agonists such as L-165041, GW-501516, and the stable prostaglandin analog carbaprostacyclin PGI2 completely inhibit PPARβ/δ degradation, a process that is reliant on the DNA binding domain of PPARβ/δ (Fig. 2B). When U2OS cells are treated with puromycin (an inhibitor of protein synthesis) and the proteasome inhibitor PS341, PPARβ/δ protein levels are stabilized to levels similar to what is seen with puromycin and L-165041 (a PPARβ/δ agonist) treatment, suggesting that L-165041 blocks ubiquitination and degradation of PPARβ/δ in U2OS cells (30) (Table 1). Independent of basal levels of PPARβ/δ, ligand binding of PPARβ/δ prevents its ubiquitination and subsequent degradation (30).
In contrast to the study described above, studies using mouse fibroblasts demonstrate that the ligand dependency of ubiquitination and degradation of PPARβ/δ is determined by PPARβ/δ protein levels. At low PPARβ/δ protein concentration, PPARβ/δ ubiquitination and degradation is not influenced by the synthetic agonist GW-501516 (77). However, at high PPARβ/δ levels, GW-501516 strongly inhibits the ubiquitination and degradation of PPARβ/δ (77). These findings have implications not only in the biological regulation of PPARβ/δ but also in the experimental design of overexpression systems used to determine the function and regulation of PPARβ/δ.
The regulation of PPARγ by the UPS.
There are two forms of PPARγ that are generated from the same gene by alternative promoter usage, PPARγ1 and PPARγ2. PPARγ1 is found in most cell types, whereas PPARγ2 is found exclusively in adipose tissues (91). Ubiquitination of PPARγ has only been studied in adipocytes (26, 38, 46), and therefore our knowledge of the regulation of PPARγ by the UPS is limited to the PPARγ2 moiety. Like other PPAR family members, the rate at which PPARγ2 is degraded is dependent on its interaction with ligands and appears to be mediated by a ubiquitin-dependent mechanism. However, unlike PPARα and PPARβ/δ, ubiquitin-mediated regulation of PPARγ2 is enhanced rather than inhibited in the presence of PPARγ2-specific ligands. When differentiated adipocyte cells (3T3-F442A) are treated with the PPARγ2 ligand pioglitazone (or other thiazolidinediones), a dose-dependent increase in ubiquitination and a subsequent decrease in the PPARγ2 protein expression are observed (38). When the proteasome is inhibited in this adipocyte cell line, the degradation of PPARγ2 is also inhibited, indicating that the degradation of PPARγ2 in the presence of ligand occurs via proteasomal degradation. Interestingly, PPARγ2 constructs containing mutations in the activation function-1 domain, part of the ligand binding domain, are able to attenuate this ligand-dependent degradation of PPARγ2, supporting the theory that ligand binding to PPARγ2 is necessary for its ubiquitin-mediated proteasomal degradation (38) (Fig. 2C). In the context of the previous two sections, these findings demonstrate that all three members of the PPAR family are degraded by the UPS, albeit by different mechanisms. Table 1 summarizes the studies demonstrating how degradation of PPARα and -β/δ by the UPS is enhanced in the presence of ligand, whereas PPARγ degradation is inhibited, demonstrating the diverse regulation that the UPS has on PPAR isoforms (Table 1).
SUMOylation of PPARs
The regulation of PPARα by SUMOylation.
Evidence from a number of cell studies suggests that SUMOylation of PPARα is a biochemical mechanism by which the cell regulates PPARα activity. In both COS-7 and human hepatoma cells (HuH-7), the SUMO E2 enzyme Ubc9 and the SUMO ligase protein inhibitor of activated STAT (PIASy) SUMOylate PPARα with a SUMO-1 moiety at position K185 (75) (Fig. 2A), resulting in an inhibition of PPARα transcriptional activity. Conversely, substitution of K185 with an arginine (thereby preventing SUMOylation) increases the transcriptional activity of PPARα, indicating the importance of the SUMOylation at K185 in negatively regulating PPARα transcription (75). K185 SUMO-1 is also important for the recruitment of the corepressor protein NCoR (but not NOR2/NCoR2), which results in further repression of PPARα's activity (75). Interestingly, similar to the case of PPARα ubiquitination, SUMOylation of PPARα is dependent on the presence or absence of specific ligands (75), demonstrating a parallel between the process of ubiquitination and SUMOylation of PPARα (Table 1).
SUMOylation of PPARα has also been described to occur in the liver where this post-translational modification results, once again, in the repression of PPARα activity. In studies using a mouse model designed to determine sex-specific PPARα-regulated gene repression, the repression of PPARα target hepatic genes involved in steroid metabolism and immunity were identified in female, but not male, mice (53). Further investigation revealed that SUMO-1 modification of PPARα triggers its interaction with a response element in the target promoter of the steroid oxysterol 7α-hydroxylase cytochrome P4507b1 (CYP7B1), a model of PPAR-regulated gene expression (53). In this reaction, PPARα is SUMOylated at K358 in its ligand binding domain (Fig. 2A), resulting in recruitment of histone deacetylase and DNA and histone methyl transferases to the adjacent Sp1 binding site of the CYP7B1 promoter. This recruitment results in histone methylation and subsequent inhibition of CYP7B1 expression (53), a process that is contingent on the presence of the SUMO ligase PIAS. These observations suggest that PPARα SUMOylation may afford female mice protection against estrogen-induced cholestasis, the most common hepatic disease during pregnancy (53). Interestingly, this pattern of PPARα-mediated gene repression can be recapitulated in male mice treated with the PPARα ligand WY-14643, indicating that, in contrast to K185 SUMOylation of PPARα described above, ligand treatment initiates (rather than inhibits) PPARα SUMOylation on K358. This occurs as a result of a ligand-induced conformational change in the LBD that presents K358 at the surface of the protein (Fig. 2A). Interestingly, the ability of K358-SUMOylated PPARα to repress CYP7B1 expression is independent of PPARα's ability to bind DNA, since the CYP7B1 promoter does not have a PPRE. Instead, K358 SUMOylation of PPARα promotes its interaction with the DNA binding subunit of GA binding protein (GABP), a heterodimeric transcription factor (Table 1). By binding GABP, SUMOylated PPARα promotes DNA methylation and histone acetylation of the CYP7B1 promoter, which leads to the repression of the gene (53). While considerable progress has been made in identifying how SUMOylation regulates PPARα activity, very little is known about the SUMOylation of PPARβ/δ and its effects on activity.
The regulation of PPARβ/δ by SUMOylation.
One study has suggested a potential SUMOylation site of PPARβ/δ in the D region (K185), but evidence for this modification in any system has yet to be reported (Fig. 2B).
The regulation of PPARγ by SUMOylation.
Experimental evidence has suggested that SUMOylation of PPARγ inhibits its activity. While both PPARγ isoforms are reported to be SUMOylated in their activation function-1 (AF-1) domain (Fig. 2C) (84), most SUMOylation studies have focused on PPARγ1 because it is ubiquitously expressed. Initial studies demonstrated that PPARγ1 can be covalently modified by SUMO-1 in HEK293 cells and that the SUMO E3 PIAS could enhance PPARγ1's SUMOylation (68). These studies also found that the lysine residue 107 (K107) within AF-1 region of PPARγ1 is modified by SUMO-1 (Fig. 2C). Other SUMO E3s in the PIAS family, including PIAS1 and PIASxβ, can also SUMOylate PPARγ1 in HEK293 cells (68). Mutating the K107 site enhances PPARγ1 transcriptional activity, suggesting that SUMOylation represses PPARγ1 activity (68). Similarly, PPARγ1-dependent apoptosis is induced by rosiglitazone in HepG2 hepatoblastoma cells when the K107 SUMOylation PPARγ1 mutant (K107R) is expressed, suggesting that PPARγ1 transactivation is modified by SUMO-1 to inhibit downstream PPARγ1-mediated apoptosis (68).
Consistent with the study described above, reporter assays using the K107R mutant PPARγ1 have demonstrated a stronger transactivation of the PPRE promoter regions compared with wild-type in NIH3T3 cells (84, 100). Again, these results suggest that SUMO-1 modification negatively regulates PPARγ1 activity (84, 100). Studies by other groups, aimed at defining the mechanism of SUMOylation-dependent repression of PPARγ1 activity, found that SUMOylation of PPARγ1 does not affect the nuclear localization of PPARγ1; instead, SUMOylation affects PPARγ1's stability and transcriptional activity (25). Interestingly, additional studies using a PPARγ1 phosphorylation mutant at S112 demonstrated that the lack of phosphorylation at this site promotes K107 SUMOylation, increasing the potency of the SUMOylation repressive effects (84, 100). These data demonstrate that SUMOylation of PPARγ1 may depend on the phosphorylation state of PPARγ1, indicating multiple levels of post-translational regulation of PPARγ1 activity.
PPARγ agonists have been shown to inhibit the inflammatory response by blocking the activity of the proinflammatory NF-κB transcription factor in macrophages (73). These studies found that in the presence of ligand, PPARγ1 is SUMOylated at lysine 365 (K365) in the ligand-binding domain (Fig. 2C), targeting PPARγ1 to the NCoR-histone decetylase-3 complex on inflammatory gene promoters (73). This recruits ubiquitination and degradation that mediates the removal of corepressor genes (73). PPARγ1 in addition can bind to PPREs in PPAR regulated genes to enhance their expression. This mechanism may explain how agonist-bound PPARγ1 can effectively inhibit NF-κB target genes. This mechanism contrasts to the PPARγ1 SUMOylation at K107 described above in HepG2 cells, whereby SUMOylation inhibits the transactivation (activation) of PPARγ target genes (14, 56, 68, 84, 100) and PPARγ1 SUMOylation at K365 activates the transrepression (inhibition) of PPARγ induction of target genes (73).
Post-translational Modifications of the PPAR Coreceptor RXRα
The regulation of RXRα by ubiquitination.
Ubiquitination of the PPAR coreceptor RXRα has been reported, although there are a limited number of studies that address this particular post-translational modification (summarized in Table 2). Since PPAR transcription factors work by dimerizing with RXRα, these studies likely have relevance to the post-translational regulation of PPARs. Recent studies have demonstrated that RXR homologs are ubiquitinated by ubiquitin ligases. However, it has not been determined whether this leads to degradation of RXR, which would be expected to inhibit PPAR activity overall. A yeast two-hybrid screen of the Schistosoma mansoni (Sm) cDNA library using SmRXR1 and SmRXR2 as bait identified the RING finger protein Sm seven in absentia (SmSINA) as a potential ubiquitin ligase specific for SmRXRs (22). In vitro ubiquitination assays demonstrated that SmSINA has ubiquitin ligase activity and can polyubiquitinate both SmRXR1 and SmRXR2, targeting them for proteasomal degradation (22). The DNA binding domain of SmRXRs shares 80% homology with mammalian RXRα, but the ligand-binding E domain of SmRXRs shares only 22–25% homology with mammalian RXRα (17). Since SmSINA interacts with the E domains of SmRXR1 and SmRXR2, where S. mansoni and mammals share the least homology in RXR sequence, it is unclear whether SINA-dependent degradation of RXRα would occur in mammals. Another RING finger protein, RNF8, has also been identified as an RXRα-interacting protein using a yeast two-hybrid screen of a human liver cDNA library (90). Interestingly, increasing expression of RNF8 in COS7 cells has no effect on RXRα ubiquitination but increases its transactivation ability, a phenomena that is enhanced by retinoic acid treatment (90). Although this pathway does not lead to RXRα ubiquitination, it is an interesting example of how interaction with a ubiquitin ligase can affect a protein's function in a ubiquitin-independent manner.
Table 2.
Summary of ubiquitin and SUMO modification of PPAR coreceptor RXRα and coactivator PGC-1 α and the resulting effects on transcription
| PPAR Coreceptor/Coactivator | Post-translational Modification | Ubiquitin/SUMO Ligase (E3) Identified | Modification Type | Transcriptional Affect | Effect of Ligand | Cell Type | References |
|---|---|---|---|---|---|---|---|
| RXRα | Ubiquitination | SmSINA and RNF8 (E3 ligase?) Not determined | Polyubiquitination targets Schistosoma mansoni RXR homologues (RXR1, RXR2) for degradation RNF8 enhances RXRα activity independent of ubiquitination polyubiquitination | Not determined Inhibition of RXRα by promoting its degradation | Not determined 9-cis-retinoic acid enhances RXRα polyubiquitination | S. mansoni COS-7 Smooth muscle cells derived from human myometrium and leiomyoma | (21) |
| SUMOylation | SUSP1 (SENP6) SUMO protease (Removes SUMO) | K108 SUMO-1 | SUSP1 deSUMOylation of RXRα enhances its activity. | Not determined | HEK293 | (14) | |
| PGC-1α | Ubiquitination | Not determined | Poly-ubiquitination in nucleus (NH2-terminal pathway?) | Inhibition of PGC-1α by promoting its degradation | — | COS-7; HL-1 (cardiac muscle cell line) | (2, 74) |
| SUMOylation | PIAS1 and PIAS3 SUMOylates SUSP1 (SENP6) SUMO protease (Removes SUMO) | K183 SUMO-1 K183 SUMO-1 | SUMOylation of K183 enhances PGC-1α activity | — | COS-1; HeLa | (71) |
See main text for definition of abbreviations.
Further studies aimed at determining the physiological relevance of RXRα ubiquitination have revealed that RXRα is polyubiquitinated in smooth muscle cells (SMCs) derived from the myometrium and that this ubiquitination is significantly inhibited in cells isolated from leiomyomas, a benign smooth muscle neoplasm of the uterus. Protein lysates from tissue samples from leiomyomas and healthy myometrium contain three RXRα immunoreactive bands: one at 54 kDa, representing the full-length RXRα, and two bands at lower molecular mass at 45 and 42 kDa. Interestingly, the lower molecular mass bands (presumably degradation products) are predominantly in protein lysates from healthy myometrium, whereas the single higher molecular mass band is predominant in protein lysates isolated from leiomyomas, suggesting that RXRα degradation is inhibited in leiomyomas (49). Interestingly, although degradation of RXRα is inhibited in SMCs derived from leiomyomas, it can be rescued by the addition of the RXR ligand 9-cis-retinoic acid, indicating that 9-cis-retinoic acid promotes the degradation of RXRα in leiomyomas (49). These results suggest that RXRα is polyubiquitinated and degraded by the proteasome in SMCs derived from the myometrium and that this process is inhibited during the pathogenesis of leiomyoma, leading to the accumulation RXRα. Furthermore, 9-cis-retinoic acid can attenuate RXRα protein accumulation by restoring RXRα's polyubiquitination and degradation to basal levels, showing the potential of 9-cis-retinoic acid as an efficient treatment of leiomyomas. Since RXRα is the protein PPAR dimerizes with to enhance PPAR activity, it would not be surprising if PPAR activity was increased in leiomyomas, because of increased RXRα protein and activity levels. This has not been determined directly, however.
The regulation of RXRα by SUMOylation.
Similar to RXRα ubiquitination, there are a limited number of studies that address RXRα SUMOylation (Table 2). Choi et al. (13) have shown that SUMOylation of RXRα at K108 within the AF-1 domain in HEK293 cells results in the regulation of its transcriptional activity. RXRα can be conjugated with SUMO-1 at the K108 both in vitro and in vivo (13). Inhibiting the SUMOylation site K108 by mutagenesis of the lysine to an arginine leads to increased RXRα transcriptional activity (13). In addition, preventing K108 SUMOylation also inhibits RXRα transcriptional activity as a coreceptor with PPARγ (13). The SUMO-1-specific protease SUSP1, also known as SENP6, colocalizes with RXRα in the nucleus and removes SUMO-1 from RXRα, but not from the androgen receptor or PPARγ. Increasing SUSP1 expression increases RXRα transcriptional activity, whereas shRNA knockdown of SUSP1 leads to a decrease in RXRα activity (13). These studies suggest that RXRα activity is inhibited by SUMOylation and that SUSP1 plays an important role in enhancing RXRα activity by deSUMOylating it; this mechanism of RXRα regulation can in turn regulate PPARγ transcriptional activity by affecting RXRα's coreceptor activity.
The RXRs are obligate heterodimeric partners necessary for PPAR action, so any effect that ubiquitin or SUMO modification has on RXR activity would likely affect PPAR activity as well. Since both ubiquitination and SUMOylation of RXR are relatively new findings, the broader effects of PPAR isoform activities have not yet been reported. But the effects of RXR modification on overall PPAR activity will reflect a composite of modifications (Fig. 1D). It will therefore be essential to determine how RXR modifications affect PPAR isoform activities, particularly in the context of therapies targeting PPARs which depend on RXR.
Post-translational Modifications of the PPAR Coactivator PGC-1α
In contrast to the PPAR coreceptor RXRα, the post-translational modifications of the coactivator PGC-1α have been thoroughly studied. As with other coactivators, when PGC-1α binds to the PPAR/RXRα coreceptors, it enhances their activity. Hence, post-translational modifications of PGC-1α have a direct bearing on PPAR activity. Known post-translational modifications of PGC-1α include phosphorylation, acetylation, methylation, O-linked-N-acetylglucosylation, ubiquitination, and SUMOylation. These PGC-1α modifications and its modifiers have been recently reviewed by Fernandez-Marcos and Auwerx (23). For the purposes of this review, we will provide an overview in detail of those studies that have focused on the ubiquitination and SUMOylation of PGC-1α.
The regulation of PGC-1α by ubiquitination.
The PPAR coactivator PGC-1α is ubiquitinated and targeted for degradation in the nucleus; however, the details of this mechanism are just starting to be understood. In response to oxidative stress, PGC-1α's subcellular localization changes from a cytoplasmic distribution to a nuclear distribution, promoted by sirtuin-1 deacetylation (2). Moreover, PGC-1's activity is regulated by glycogen synthase kinase-3β, which targets PGC-1α for intranuclear degradation (2). While the details of this process have not been delineated, the phosphorylated form of PGC-1α may make it a unique target for ubiquitination (i.e., the nonphosphorylated form is not ubiquitinated), leading to subsequent degradation and inhibition of its activity (2). Experimental evidence suggests that the COOH-terminal serine-arginine-rich and RNA recognition motif domains of PGC-1α are required for its polyubiquitination and subsequent proteasomal degradation in COS-7 cells (83). Interestingly, when the COOH-terminal region of PGC-1α is expressed alone, it is polyubiquitinated but resistant to proteasomal degradation. Instead, the COOH-terminal fragment forms intranuclear aggregates, tightly complexed with promyelocytic leukemia nuclear bodies, a phenomenon that does not occur with the full-length protein (83). This observation led to the report that proteasomal targeting of polyubiquitinated PGC-1α is dependent on its NH2 terminus, which contains two regions rich in proline (P), glutamic acid (E), serine (S), and threonine (T), termed PEST domains. PEST sequences have been found in proteins with a short intracellular half-life, suggesting they act as a signal for protein degradation, and the above observations would therefore suggest that PEST domains are essential for PGC-1α degradation (76, 83).
Trausch-Azar et al. (92), however, assert that the PEST regions do not play a role in PGC-1α turnover. They instead implicate the NH2-terminal region of PGC-1α as being targeted by the NH2 terminus-dependent ubiquitin proteasome pathway. In the NH2 terminus-dependent pathway, ubiquitin is transferred to the free NH2-terminal residue rather than the ϵ-NH2 group of an internal lysine (16). Using a functional truncated form of PGC-1α, which is predominately nuclear and contains intact activation and the nuclear receptor domains, Trausch-Azar et al. (92) demonstrated that PGC-1α is degraded even when all of the four lysine residues within it are mutated to arginine. Furthermore, this study revealed that whereas the fragment they generated was nuclear and rapidly degraded, the endogenous PGC-1α splice variant, novel truncated (NT) -PGC-1α, is stable and cytoplasmic. This suggests that the cellular localization differences between these two NH2-terminal fragments of PGC-1α is dependent on their ability to interact with chaperones carriers, where NT-PGC-1α may not be able to interact with these chaperones, leading to its exclusion from the nucleus (92). While a post-translational modification of PGC-1α with ubiquitin appears to target it for degradation to inhibit its activity, other post-translational modifications inhibit it through other mechanisms. SUMOylation, for example, inhibits PGC-1α, inhibiting its transcriptional activity by affecting the activity of PGC-1α binding partners.
The regulation of PGC-1α by SUMOylation.
The SUMO ligases PIAS1 and PIAS3 SUMOylate PGC-1α on the conserved lysine residue K183 in the NH2-terminal domain between the PEST motifs, resulting in the inhibition of its transcriptional activity (80). Acetylation, phosphorylation, and ubiquitination do not affect the ability of PGC-1α to be SUMOylated (80). Although SUMOylation does not affect subcellular localization or the stability of PGC-1α, it does attenuate its transcriptional activity, possibly by enhancing its interaction with RIP140, a corepressor (80). Interestingly, mutating the SUMOylation consensus sequence abolished PGC-1α-dependent PPARγ transcriptional activity, demonstrating that SUMOylation of PGC-1α constitutes an additional layer of regulating PPARγ activity in an indirect manner via PGC-1α (80).
The Significance of Post-translational Regulation of the PPAR Complex to Cardiac Disease
The role of PPARs in the heart.
The pathophysiology of common cardiac diseases involves distinct changes in myocardial fuel utilization. The best examples of this include cardiac hypertrophy and heart failure, where the heart switches from the predominant use of fatty acids to glucose as its primary source of energy (93). In both humans and animal models alike, the development of cardiac hypertrophy results in decreases in PPARα expression that parallel a depression of fatty acid utilization (6, 45, 81). Experimental studies using transgenic and knockout animals confirm that PPARα does have a significant role in this metabolic shift. Conversely, the diabetic myocardium essentially does the opposite as the heart undergoing cardiac hypertrophy: it relies almost exclusively on fatty acids and uses glucose to a very small extent (9, 78, 87). While the cardiac changes in diabetic cardiomyopathy are caused by a number of complex mechanisms (89), fatty acid upregulation of PPARα activity has been proposed as one underlying mechanism (35). Overall, these dynamic shifts in myocardial fuel utilization by the regulation PPARα are characteristic in most common cardiac diseases.
A great deal of experimental evidence has placed the regulation of these changes in fatty acid and glucose utilization on the PPAR transcription factors (59). Alterations of all three PPARs in the heart results in derangements in fatty acid and glucose metabolism that result in a significant phenotype or increased susceptibility to insults [for a comprehensive review, see Madrazo and Kelly (59)]. These findings are concrete enough to have evoked a number of therapeutic interventions focusing on partially restoring fatty acid utilization while inhibiting glucose utilization, including PPAR agonists (67). Despite the realization of the importance of PPAR regulation in cardiac disease, the mechanisms by which they are regulated at the level of the cardiomyocyte have not been clearly delineated. Post-translational modifications of PPARs are emerging as one such way PPARs are regulated, including ubiquitination and SUMOylation. Evidence for their regulation in cardiomyocytes is just emerging in studies investigating other types of striated muscle.
Ubiquitination and SUMOylation of PPARs in the heart.
To date, regulation of PPARα, PPARβ/δ, or PPARγ by ubiquitination or SUMOylation in the heart has not been reported. However, there are several examples whereby PPARs are regulated by these post-translational modifications in other closely related types of muscle cells. For example, recent studies have demonstrated the physiological relevance of PPARγ1 SUMOylation in striated muscle cells, suggesting that PPARγ1 SUMOylation may play a role in regulating the extent to which fatty acids are used in the production of ATP. In these studies, PPARγ1 was deSUMOylated by increasing the expression of SENP2 (a SUMO-specific protease/removes SUMO) in C2C12 myotubes (striated muscle cells). By deSUMOylating PPARγ1, an enhanced PPARγ1 activity was detected by the identification of the enhanced expression of the PPARγ1 target genes fatty acid binding protein 3 (FABP3) and fatty acid translocase (CD36), in both the presence and absence of the PPARγ agonist rosiglitazone (15). Similarly, deSUMOylation of PPARγ1 by SENP2 increases the chromatin immunoprecipitation of PPREs of the endogenous PPARγ1 target genes CD36 and FABP3 (14, 15). These observations indicate an important role for both SUMO E3s as well as SUMO-specific proteases (deSUMOylases) in regulating PPARγ activity in skeletal muscle.
Other studies have identified that SUMOylation of PPARγ1 promotes proliferation and migration of vascular SMCs (VSMCs). This has been demonstrated using VSMCs transfected with a PPARγ1 construct in which K107R, the lysine on which SUMOylation occurs, is mutated, thereby inhibited SUMOylation. Inhibiting PPARγ1 SUMOylation in this manner results in a more potent transcriptional inhibition of inducible nitric oxide synthase compared with cells transfected with a wild-type construct (56). In addition, the PPARγ1 K107R mutant is more efficient at inhibiting proliferation and migration compared with wild-type PPARγ1, suggesting that PPARγ1 SUMOylation may play a role in promoting atherosclerosis, since VSMC proliferation and migration play a significant role in determining lesion severity. Indeed, adenoviral expression of the PPARγ1 K107R construct in the carotid arteries of rats after balloon injury results in a significantly decreased intima-to-media ratio compared with that in the controls (56). Consistent with this in vitro data, the PPARγ1 K107R-expressing arteries display a lower proliferation index and higher apoptotic index compared with those in wild-type PPARγ1 (56). These studies demonstrate a role for SUMOylation of PPARγ1 at K107 in VSMCs in the atherosclerotic response.
These examples of PPARγ SUMOylation in regulating the fatty acid oxidation response and apoptosis in striated muscle and vascular smooth muscle, respectively, provide support for the concept that PPARs could be regulated posttranslationally in the heart. This concept of post-translational regulation of PPAR activity in cardiomyocytes in not new. Specifically, PPARα phosphorylation by the MAPK p38 decreases PPARα transcriptional activity (7). Since the p38 pathway is activated in response to cardiac stress, such as that found in cardiac hypertrophy, heart failure, and diabetes, this study implicates PPARα activation as a mechanism by which the heart responds to adverse stimuli. The broader implications of these studies are that the fatty acid and glucose shifts seen in diseases such as cardiac hypertrophy, heart failure, and diabetic cardiomyopathy may be due to these regulatory mechanisms (67) (9, 78, 87). This concept has yet to be tested directly.
The role of PGC-1α in the heart.
In addition to its coactivator function in the PPAR complex, PGC-1α is also a potent regulator of mitochondrial biogenesis. Increased expression of PGC-1α in cardiomyocytes activates mitochondrial biogenesis, oxidative phosphorylation, and respiration (50, 86). The mitochondrial biogenesis enhanced by increased constitutive PGC-1α expression can lead to such sarcomere displacement as to lead to heart failure (50). Even transient increases in PGC-1α expression leads to contractile dysfunction, which is reversible (79).
While excessive PGC-1α expression may have some detrimental effects, it generally is associated with directing beneficial adaptations in the heart and skeletal muscle. For example, long-term exercise leads to increased PGC-1α expression, increased mitochondrial content, and a resistance to fatigue (4). Pressure overload-induced cardiac hypertrophy induces enhanced PGC-1α expression and its target genes of fatty acid oxidation and oxidative phosphorylation (8, 29, 51). Conversely, when PGC-1α is knocked out of the heart and skeletal muscle, the gene expression of oxidative phosphorylation is blunted, leading to reduced mitochondrial activity and decreased ATP (3). Similarly, isolated hearts from PGC-1α−/− mice have decreased cardiac output in response to stimulation (3) and an enhanced mitochondrial susceptibility to apoptotic stimuli (1). These studies indicate a role of PGC-1α in the heart in response to cardiac stresses by supporting both metabolic adaptations (described above) and mitochondrial function (specifically oxidative phosphorylation) as well as the hearts susceptibility to apoptosis. The factors regulating PGC-1α in the heart have not yet been identified.
Ubiquitination and SUMOylation of PGC-1α in the heart.
While regulation of PGC-1α by SUMO has not been identified in cardiomyocytes directly, recent studies have implicated the UPS in the degradation of PGC-1α (92). The steady-state levels of PGC-1α are controlled by dynamic changes in its synthesis and degradation. Its synthesis is regulated by a number of dietary and physiological factors, including exercise (24, 57). When full-length PGC-1α is expressed, it is rapidly degraded (half-life < 30 min). When the NH2-terminal splice variant of PGC-1α (NT-PGC-1α) is expressed, it remains in the cytoplasm and is stable (half-life > 7 h). In the presence of the proteasome inhibitor MG-132, ubiquitin PGC-1α conjugates accumulate, suggesting a role of the UPS in its degradation of PGC-1α (92). These findings suggest that PGC-1α is degraded by the ubiquitin proteasome, targeted by yet to be identified ubiquitin ligases. While much work is necessary to delineate the mechanisms by which the UPS regulates PGC-1α, it appears critical to the regulation of steady-state levels of PGC-1α and the truncated NT-PGC-1α isoforms. The functional consequences of UPS regulation of PGC-1α remain to be determined.
Summary
Results from the studies described above demonstrate several general principles surrounding PPAR post-translational modifications. First, ubiquitination and SUMOylation decrease the expression of PPAR target genes, either by promoting PPAR degradation (via ubiquitination) or recruiting additional proteins to PPAR promoter sequences that inhibit PPAR transactivation function or enhance PPAR transrepression function (SUMOylation) (Table 1). The one exception to this general statement is the case where PPARα-dependent transcription is actually activated by MDM2 at the MDM2-to-PPARα ratio of <0.5 (Table 1). The effect of ligand treatment on post-translational modifications of PPARs does not follow an observable pattern. Instead, ligand treatment can inhibit, enhance, or have no effect on the modification of PPARs depending on PPAR concentration and cell type (Table 1). Adding to the complexity of PPAR regulation by post-translational modifications is the fact that RXRα and PGC-1α, a coreceptor and coactivator, respectively, are also able to be ubiquitinated and SUMOylated (Table 2) in ways that may affect PPAR activity, an end point that needs considerable more study. These studies have real relevance for the use of selective PPAR isotype-specific agonists, which have been shown to regulate cardiac structure and function. However, PPAR-directed therapies often have unexpected effects because of their pleiotropism and bodywide distribution. Understanding the interplay between post-translational modifications, ubiquitin ligase (E3) concentrations, and PPAR activity will help develop more rational means of treating cardiac disease. This may allow for the development of more targeted therapies that may include additional cardiac-specific regulators of PPARs, including ubiquitin and SUMO ligases (E3).
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grant R01-HL-104129 (to M. Willis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.M.W. and M.S.W. conception and design of research; K.M.W. and M.S.W. analyzed data; K.M.W. and M.S.W. interpreted results of experiments; K.M.W. and M.S.W. prepared figures; K.M.W. and M.S.W. drafted manuscript; K.M.W. and M.S.W. edited and revised manuscript; K.M.W. and M.S.W. approved final version of manuscript.
REFERENCES
- 1. Adhihetty PJ, Uguccioni G, Leick L, Hidalgo J, Pilegaard H, Hood DA. The role of PGC-1alpha on mitochondrial function and apoptotic susceptibility in muscle. Am J Physiol Cell Physiol 297: C217–C225, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Anderson RM, Barger JL, Edwards MG, Braun KH, O'Connor CE, Prolla TA, Weindruch R. Dynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell 7: 101–111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab 1: 259–271, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Bao Y, Li R, Jiang J, Cai B, Gao J, Le K, Zhang F, Chen S, Liu P. Activation of peroxisome proliferator-activated receptor gamma inhibits endothelin-1-induced cardiac hypertrophy via the calcineurin/NFAT signaling pathway. Mol Cell Biochem 317: 189–196, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest 105: 1723–1730, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barger PM, Browning AC, Garner AN, Kelly DP. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor alpha: a potential role in the cardiac metabolic stress response. J Biol Chem 276: 44495–44501, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med 10: 238–245, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab 279: E1104–E1113, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Blanquart C, Barbier O, Fruchart JC, Staels B, Glineur C. Peroxisome proliferator-activated receptor alpha (PPARalpha) turnover by the ubiquitin-proteasome system controls the ligand-induced expression level of its target genes. J Biol Chem 277: 37254–37259, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Bohren KM, Nadkarni V, Song JH, Gabbay KH, Owerbach D. A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J Biol Chem 279: 27233–27238, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 243: 1576–1583, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Choi SJ, Chung SS, Rho EJ, Lee HW, Lee MH, Choi HS, Seol JH, Baek SH, Bang OS, Chung CH. Negative modulation of RXRalpha transcriptional activity by small ubiquitin-related modifier (SUMO) modification and its reversal by SUMO-specific protease SUSP1. J Biol Chem 281: 30669–30677, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Chung SS, Ahn BY, Kim M, Choi HH, Park HS, Kang S, Park SG, Kim YB, Cho YM, Lee HK, Chung CH, Park KS. Control of adipogenesis by the SUMO-specific protease SENP2. Mol Cell Biol 30: 2135–2146, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chung SS, Ahn BY, Kim M, Kho JH, Jung HS, Park KS. SUMO modification selectively regulates transcriptional activity of peroxisome proliferator-activated receptor gamma in C2C12 myotubes. Biochem J 433: 155–161, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Ciechanover A, Ben-Saadon R. N-terminal ubiquitination: more protein substrates join in. Trends Cell Biol 14: 103–106, 2004 [DOI] [PubMed] [Google Scholar]
- 17. de Mendonca RL, Escriva H, Bouton D, Zelus D, Vanacker JM, Bonnelye E, Cornette J, Pierce RJ, Laudet V. Structural and functional divergence of a nuclear receptor of the RXR family from the trematode parasite Schistosoma mansoni. Eur J Biochem 267: 3208–3219, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Desterro JM, Thomson J, Hay RT. Ubch9 conjugates SUMO but not ubiquitin. FEBS Lett 417: 297–300, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Diep QN, Benkirane K, Amiri F, Cohn JS, Endemann D, Schiffrin EL. PPAR alpha activator fenofibrate inhibits myocardial inflammation and fibrosis in angiotensin II-infused rats. J Mol Cell Cardiol 36: 295–304, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Ding L, Liang X, Zhu D, Lou Y. Peroxisome proliferator-activated receptor alpha is involved in cardiomyocyte differentiation of murine embryonic stem cells in vitro. Cell Biol Int 31: 1002–1009, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Dowell P, Ishmael JE, Avram D, Peterson VJ, Nevrivy DJ, Leid M. Identification of nuclear receptor corepressor as a peroxisome proliferator-activated receptor alpha interacting protein. J Biol Chem 274: 15901–15907, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Fantappie MR, Osman A, Ericsson C, Niles EG, LoVerde PT. Cloning of Schistosoma mansoni Seven in Absentia (SmSINA)(+) homologue cDNA, a gene involved in ubiquitination of SmRXR1 and SmRXR2. Mol Biochem Parasitol 131: 45–54, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93: 884S–890S, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116: 615–622, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Floyd ZE, Stephens JM. Control of peroxisome proliferator-activated receptor gamma2 stability and activity by SUMOylation. Obes Res 12: 921–928, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem 277: 4062–4068, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Galan JM, Haguenauer-Tsapis R. Ubiquitin lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J 16: 5847–5854, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gareau JR, Lima CD. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol 11: 861–871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol 551: 491–501, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Genini D, Catapano CV. Block of nuclear receptor ubiquitination. A mechanism of ligand-dependent control of peroxisome proliferator-activated receptor delta activity. J Biol Chem 282: 11776–11785, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82: 373–428, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Gong L, Kamitani T, Fujise K, Caskey LS, Yeh ET. Preferential interaction of sentrin with a ubiquitin-conjugating enzyme, Ubc9. J Biol Chem 272: 28198–28201, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Gong ST, Chao IM. Changes in serum thyroglobulin and thyroid autoantibodies in patients with Graves' disease treated with antithyroid drug and their relationship to relapse. J Formos Med Assoc 90: 1155–1162, 1991 [PubMed] [Google Scholar]
- 34. Gopinathan L, Hannon DB, Peters JM, Vanden Heuvel JP. Regulation of peroxisome proliferator-activated receptor-alpha by MDM2. Toxicol Sci 108: 48–58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gottlicher M, Widmark E, Li Q, Gustafsson JA. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci USA 89: 4653–4657, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gregori L, Poosch MS, Cousins G, Chau V. A uniform isopeptide-linked multiubiquitin chain is sufficient to target substrate for degradation in ubiquitin-mediated proteolysis. J Biol Chem 265: 8354–8357, 1990 [PubMed] [Google Scholar]
- 37. Guellich A, Damy T, Lecarpentier Y, Conti M, Claes V, Samuel JL, Quillard J, Hebert JL, Pineau T, Coirault C. Role of oxidative stress in cardiac dysfunction of PPARα−/− mice. Am J Physiol Heart Circ Physiol 293: H93–H102, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J Biol Chem 275: 18527–18533, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol 2: 195–201, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Hirotani M, Tsukamoto T, Bourdeaux J, Sadano H, Osumi T. Stabilization of peroxisome proliferator-activated receptor alpha by the ligand. Biochem Biophys Res Commun 288: 106–110, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Iglarz M, Touyz RM, Viel EC, Paradis P, Amiri F, Diep QN, Schiffrin EL. Peroxisome proliferator-activated receptor-alpha and receptor-gamma activators prevent cardiac fibrosis in mineralocorticoid-dependent hypertension. Hypertension 42: 737–743, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Irukayama-Tomobe Y, Miyauchi T, Sakai S, Kasuya Y, Ogata T, Takanashi M, Iemitsu M, Sudo T, Goto K, Yamaguchi I. Endothelin-1-induced cardiac hypertrophy is inhibited by activation of peroxisome proliferator-activated receptor-alpha partly via blockade of c-Jun NH2-terminal kinase pathway. Circulation 109: 904–910, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Johnson ES. Protein modification by SUMO. Annu Rev Biochem 73: 355–382, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Johnson ES, Ma PC, Ota IM, Varshavsky A. A proteolytic pathway that recognizes ubiquitin as a degradation signal. J Biol Chem 270: 17442–17456, 1995 [DOI] [PubMed] [Google Scholar]
- 45. Karbowska J, Kochan Z, Smolenski RT. Peroxisome proliferator-activated receptor alpha is downregulated in the failing human heart. Cell Mol Biol Lett 8: 49–53, 2003 [PubMed] [Google Scholar]
- 46. Kilroy GE, Zhang X, Floyd ZE. PPAR-gamma AF-2 domain functions as a component of a ubiquitin-dependent degradation signal. Obesity (Silver Spring) 17: 665–673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Komar CM, Braissant O, Wahli W, Curry TE., Jr Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinology 142: 4831–4838, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Kota BP, Huang TH, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacol Res 51: 85–94, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Lattuada D, Vigano P, Mangioni S, Sassone J, Di Francesco S, Vignali M, Di Blasio AM. Accumulation of retinoid X receptor-alpha in uterine leiomyomas is associated with a delayed ligand-dependent proteasome-mediated degradation and an alteration of its transcriptional activity. Mol Endocrinol 21: 602–612, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lehman JJ, Kelly DP. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol 29: 339–345, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Lei B, Lionetti V, Young ME, Chandler MP, d'Agostino C, Kang E, Altarejos M, Matsuo K, Hintze TH, Stanley WC, Recchia FA. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol 36: 567–576, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Leuenberger N, Pradervand S, Wahli W. Sumoylated PPARalpha mediates sex-specific gene repression and protects the liver from estrogen-induced toxicity in mice. J Clin Invest 119: 3138–3148, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li HH, Willis MS, Lockyer P, Miller N, McDonough H, Glass DJ, Patterson C. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest 117: 3211–3223, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li R, Zheng W, Pi R, Gao J, Zhang H, Wang P, Le K, Liu P. Activation of peroxisome proliferator-activated receptor-alpha prevents glycogen synthase 3beta phosphorylation and inhibits cardiac hypertrophy. FEBS J 581: 3311–3316, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Lim S, Ahn BY, Chung SS, Park HS, Cho BJ, Kim M, Choi SH, Lee IK, Lee SW, Choi SJ, Chung CH, Cho YM, Lee HK, Park KS. Effect of a peroxisome proliferator-activated receptor gamma sumoylation mutant on neointimal formation after balloon injury in rats. Atherosclerosis 206: 411–417, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1: 361–370, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Loftus LT, Gala R, Yang T, Jessick VJ, Ashley MD, Ordonez AN, Thompson SJ, Simon RP, Meller R. Sumo-2/3-ylation following in vitro modeled ischemia is reduced in delayed ischemic tolerance. Brain Res 1272: 71–80, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Madrazo JA, Kelly DP. The PPAR trio: regulators of myocardial energy metabolism in health and disease. J Mol Cell Cardiol 44: 968–975, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88: 97–107, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol 135: 1457–1470, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O'Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W. International Union of Pharmacology. LXI Peroxisome proliferator-activated receptors. Pharmacol Rev 58: 726–741, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Michalik L, Desvergne B, Dreyer C, Gavillet M, Laurini RN, Wahli W. PPAR expression and function during vertebrate development. Int J Dev Biol 46: 105–114, 2002 [PubMed] [Google Scholar]
- 64. Morgan EE, Chandler MP, Young ME, McElfresh TA, Kung TA, Rennison JH, Tserng KY, Hoit BD, Stanley WC. Dissociation between gene and protein expression of metabolic enzymes in a rodent model of heart failure. Eur J Heart Fail 8: 687–693, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Morgan EE, Rennison JH, Young ME, McElfresh TA, Kung TA, Tserng KY, Hoit BD, Stanley WC, Chandler MP. Effects of chronic activation of peroxisome proliferator-activated receptor-alpha or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol 290: H1899–H1904, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Muller S, Hoege C, Pyrowolakis G, Jentsch S. SUMO, ubiquitin's mysterious cousin. Nat Rev Mol Cell Biol 2: 202–210, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 356: 1140–1151, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Ohshima T, Koga H, Shimotohno K. Transcriptional activity of peroxisome proliferator-activated receptor gamma is modulated by SUMO-1 modification. J Biol Chem 279: 29551–29557, 2004 [DOI] [PubMed] [Google Scholar]
- 69. Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension 48: 1116–1123, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Okuma T, Honda R, Ichikawa G, Tsumagari N, Yasuda H. In vitro SUMO-1 modification requires two enzymatic steps, E1 and E2. Biochem Biophys Res Commun 254: 693–698, 1999 [DOI] [PubMed] [Google Scholar]
- 71. Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation 106: 606–612, 2002 [DOI] [PubMed] [Google Scholar]
- 72. Owerbach D, McKay EM, Yeh ET, Gabbay KH, Bohren KM. A proline-90 residue unique to SUMO-4 prevents maturation and sumoylation. Biochem Biophys Res Commun 337: 517–520, 2005 [DOI] [PubMed] [Google Scholar]
- 73. Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 437: 759–763, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pellieux C, Aasum E, Larsen TS, Montessuit C, Papageorgiou I, Pedrazzini T, Lerch R. Overexpression of angiotensinogen in the myocardium induces downregulation of the fatty acid oxidation pathway. J Mol Cell Cardiol 41: 459–466, 2006 [DOI] [PubMed] [Google Scholar]
- 75. Pourcet B, Pineda-Torra I, Derudas B, Staels B, Glineur C. SUMOylation of human peroxisome proliferator-activated receptor alpha inhibits its trans-activity through the recruitment of the nuclear corepressor NCoR. J Biol Chem 285: 5983–5992, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rechsteiner M. PEST regions, proteolysis and cell cycle progression. Revis Biol Celular 20: 235–253, 1989 [PubMed] [Google Scholar]
- 77. Rieck M, Wedeken L, Muller-Brusselbach S, Meissner W, Muller R. Expression level and agonist-binding affect the turnover, ubiquitination and complex formation of peroxisome proliferator activated receptor beta. FEBS J 274: 5068–5076, 2007 [DOI] [PubMed] [Google Scholar]
- 78. Rodrigues B, Cam MC, McNeill JH. Myocardial substrate metabolism: implications for diabetic cardiomyopathy. J Mol Cell Cardiol 27: 169–179, 1995 [DOI] [PubMed] [Google Scholar]
- 79. Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res 94: 525–533, 2004 [DOI] [PubMed] [Google Scholar]
- 80. Rytinki MM, Palvimo JJ. SUMOylation attenuates the function of PGC-1alpha. J Biol Chem 284: 26184–26193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 94: 2837–2842, 1996 [DOI] [PubMed] [Google Scholar]
- 82. Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem 275: 6252–6258, 2000 [DOI] [PubMed] [Google Scholar]
- 83. Sano M, Tokudome S, Shimizu N, Yoshikawa N, Ogawa C, Shirakawa K, Endo J, Katayama T, Yuasa S, Ieda M, Makino S, Hattori F, Tanaka H, Fukuda K. Intramolecular control of protein stability, subnuclear compartmentalization, and coactivator function of peroxisome proliferator-activated receptor gamma coactivator 1alpha. J Biol Chem 282: 25970–25980, 2007 [DOI] [PubMed] [Google Scholar]
- 84. Shimizu M, Yamashita D, Yamaguchi T, Hirose F, Osumi T. Aspects of the regulatory mechanisms of PPAR functions: analysis of a bidirectional response element and regulation by sumoylation. Mol Cell Biochem 286: 33–42, 2006 [DOI] [PubMed] [Google Scholar]
- 85. Smeets PJ, Teunissen BE, Willemsen PH, van Nieuwenhoven FA, Brouns AE, Janssen BJ, Cleutjens JP, Staels B, van der Vusse GJ, van Bilsen M. Cardiac hypertrophy is enhanced in PPAR alpha−/− mice in response to chronic pressure overload. Cardiovasc Res 78: 79–89, 2008 [DOI] [PubMed] [Google Scholar]
- 86. St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem 278: 26597–26603, 2003 [DOI] [PubMed] [Google Scholar]
- 87. Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res 34: 25–33, 1997 [DOI] [PubMed] [Google Scholar]
- 88. Su HL, Li SS. Molecular features of human ubiquitin-like SUMO genes and their encoded proteins. Gene 296: 65–73, 2002 [DOI] [PubMed] [Google Scholar]
- 89. Taha M, Lopaschuk GD. Alterations in energy metabolism in cardiomyopathies. Ann Med 39: 594–607, 2007 [DOI] [PubMed] [Google Scholar]
- 90. Takano Y, Adachi S, Okuno M, Muto Y, Yoshioka T, Matsushima-Nishiwaki R, Tsurumi H, Ito K, Friedman SL, Moriwaki H, Kojima S, Okano Y. The RING finger protein, RNF8, interacts with retinoid X receptor alpha and enhances its transcription-stimulating activity. J Biol Chem 279: 18926–18934, 2004 [DOI] [PubMed] [Google Scholar]
- 91. Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 8: 1224–1234, 1994 [DOI] [PubMed] [Google Scholar]
- 92. Trausch-Azar J, Leone TC, Kelly DP, Schwartz AL. Ubiquitin proteasome-dependent degradation of the transcriptional coactivator PGC-1α via the N-terminal pathway. J Biol Chem 285: 40192–40200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. van Bilsen M, Smeets PJ, Gilde AJ, van der Vusse GJ. Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res 61: 218–226, 2004 [DOI] [PubMed] [Google Scholar]
- 94. Vertegaal AC. SUMO chains: polymeric signals. Biochem Soc Trans 38: 46–49, 2010 [DOI] [PubMed] [Google Scholar]
- 95. Warnmark A, Treuter E, Wright AP, Gustafsson JA. Activation functions 1 and 2 of nuclear receptors: molecular strategies for transcriptional activation. Mol Endocrinol 17: 1901–1909, 2003 [DOI] [PubMed] [Google Scholar]
- 96. Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol 2: 169–178, 2001 [DOI] [PubMed] [Google Scholar]
- 97. Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J 428: 133–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wu T, Merbl Y, Huo Y, Gallop JL, Tzur A, Kirschner MW. UBE2S drives elongation of K11-linked ubiquitin chains by the anaphase-promoting complex. Proc Natl Acad Sci USA 107: 1355–1360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yamamoto K, Ohki R, Lee RT, Ikeda U, Shimada K. Peroxisome proliferator-activated receptor gamma activators inhibit cardiac hypertrophy in cardiac myocytes. Circulation 104: 1670–1675, 2001 [DOI] [PubMed] [Google Scholar]
- 100. Yamashita D, Yamaguchi T, Shimizu M, Nakata N, Hirose F, Osumi T. The transactivating function of peroxisome proliferator-activated receptor gamma is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes Cells 9: 1017–1029, 2004 [DOI] [PubMed] [Google Scholar]