Abstract
The effects of differential expression of titin isoforms on sarcomere length (SL)-dependent changes in passive force, maximum Ca2+-activated force, apparent cooperativity in activation of force (nH), Ca2+ sensitivity of force (pCa50), and rate of force redevelopment (ktr) were investigated in rat cardiac muscle. Skinned right ventricular trabeculae were isolated from wild-type (WT) and mutant homozygote (Ho) hearts expressing predominantly a smaller N2B isoform (2,970 kDa) and a giant N2BA-G isoform (3,830 kDa), respectively. Stretching WT and Ho trabeculae from SL 2.0 to 2.35 μm increased passive force, maximum Ca2+-activated force, and pCa50, and it decreased nH and ktr. Compared with WT trabeculae, the magnitude of SL-dependent changes in passive force, maximum Ca2+-activated force, pCa50, and nH was significantly smaller in Ho trabeculae. These results suggests that, at least in rat ventricle, the magnitude of SL-dependent changes in passive force, maximum Ca2+-activated force, pCa50, nH, and ktr is defined by the titin isoform.
Keywords: titin filaments, cardiac muscle, myocardial contractility, length-dependent activation
it is well established that for a given free Ca2+ concentration ([Ca2+]free), intact and chemically skinned ventricular myocardial preparations generate more force when sarcomere length (SL) is increased within a normal physiological range (from ∼1.8 to 2.4 μm; Refs. 2, 29, 35). This SL-dependent change in Ca2+ sensitivity of force, also referred to as length-dependent activation, facilitates the regulation of ventricular output of the heart in response to changes in ventricular filling on a beat-to-beat basis (Frank-Starling's law of the heart; Refs. 2, 34). Despite intense research, the precise mechanism for SL-dependent changes in Ca2+ sensitivity remains unclear. However, several lines of evidence suggest that the SL-dependent changes in Ca2+ sensitivity of force may be primarily due to SL-induced changes in interfilament lattice spacing (distance between thin and thick filament; Refs. 19, 32, 33, 42, 58) and SL-induced orientational ordering of the myosin heads perpendicular to the thick filament backbone as assessed by the intensity of the M3 meridonal reflection from X-ray diffraction patterns of electrically stimulated myocardium (15). That is, when SL is increased from short to long length, the interfilament lattice spacing decreases, whereas orientational ordering of the myosin heads perpendicular to the thick filament backbone increases, which in turn increases the probability of myosin cross-bridge binding to actin and generation of more force directly or indirectly via cooperative activation of the thin filament (16, 19, 21). It has been proposed that titin, the third filamentous protein of the cardiac sarcomere, plays a prominent role in defining the SL-induced changes in contractile properties of myocardium (8, 9, 20, 24).
Titin, also referred to as connectin, is a high molecular mass (3,000–4,000 kDa) extensible sarcomeric protein in striated muscle (57). Its N and C termini are embedded in the Z disc and M line, respectively, in each half sarcomere (26). The extensible region of the titin molecule, which gives rise to passive force when sarcomeres are stretched (7, 27, 63), resides in the I band and consists of three segments: 1) the tandem immunoglobin (Ig) segment (serially linked Ig-like domains); 2) the PEVK segment (rich in proline [P], glutamate [E], valine [V], and lysine [K]); and 3) the N2A and N2B unique amino acid sequence (N2A-U and N2B-U; Ref. 41). In cardiac muscle, the magnitude of SL-dependent changes in passive force, Ca2+ sensitivity of force and lateral separation of thick and thin filaments (interfilament lattice spacing) varies with the length and phosphorylation status of these segments (24, 25, 30, 38, 39, 64). For example, in bovine myocardium, SL-dependent changes in passive force (an increase), Ca2+ sensitivity of force (an increase), and interfilament lattice spacing (a decrease) are less pronounced in atria expressing primarily the N2BA isoform, with a longer Ig segment, a PEVK segment of variable length, and both N2B-U and N2A-U segments, than in ventricles expressing 50% of N2BA isoform and 50% of N2B isoform, with a shorter Ig segment, a shorter PEVK segment, and only the N2B-U segment (24). Phosphorylation of N2B -U segment by protein kinase A in rat skinned ventricular preparations (64) and bovine skinned left ventricular and atrial preparations (25) and by protein kinase G in human skinned ventricular preparations (39) has been reported to diminish SL-dependent changes in passive force. In contrast, protein kinase Cα-catalyzed phosphorylation of the PEVK segment in porcine and mouse skinned left ventricular preparations has been shown to potentiate SL-dependent changes in passive force (30).
In the present study, we investigated the SL dependencies of force production and cross-bridge cycling kinetics in rat ventricular myocardium expressing different titin isoforms (28). Triton X-100 permeabilized right ventricular trabeculae isolated from rat hearts expressing primarily either a shorter N2B isoform [wild type (WT)] or a giant N2BA-G isoform [homozygote (Ho)] were exposed to a range of Ca2+ concentration under isometric conditions at two different SLs (2.0 or 2.35 μm) to determine passive force, maximum Ca2+-activated force, Ca2+ sensitivity of force production (pCa50), apparent cooperativity in activation of force production (Hill coefficient, nH), and apparent rate of force redevelopment (ktr) following unloaded shortening and rapid restretch.
MATERIALS AND METHODS
Animals.
The WT (n = 9) and Ho (n = 8) rats used in the present study were generated as described previously (28). Briefly, both the Sprague-Dawley and Fisher 344 strain were obtained from Harlan Sprague-Dawley (Indianapolis, IN). The original Sprague-Dawley rats with mutation were crossed with Fisher 344 inbreeds and subsequently crossed with Brown-Norway rats. Thus, the current rats were ∼50% Brown-Norway rats, 25% Fisher 344 rats, and 25% Sprague-Dawley rats. All procedures involving animal care and handling were approved by the University of Wisconsin-Madison Animal Care and Use Committee.
Preparation of skinned trabeculae.
Right ventricular trabeculae were isolated as described previously (45). Briefly, the hearts were removed from rats (either sex; 100–300 g) anesthetized with inhaled isoflurane and pinned down to the base of a dissecting dish filled with modified Ringer solution (in mmol/l): 120 NaCl, 19 NaHCO3, 1.2 Na2HPO4, 1.2 MgSO4, 5 KCl, 1 CaCl2, and 10 glucose pH 7.4 at 22°C preequilibrated with 95% O2-5% CO2. After the right ventricles were cut open, the hearts were exposed to fresh Ringer solution containing 30 mM 2,3-butanedione monoxime for 30 min (2× solution change). 2,3-Butanedione monoxime is thought to act as a “chemical phosphatase” (62) or as an agent with “phosphatase-like activity” (3) and has been shown to reduce the basal phosphorylation of myosin regulatory light chain in rat and mouse myocardium (11, 45, 50, 54, 56). The right ventricular trabeculae were then dissected free, tied to sticks to hold muscle length fixed, and transferred to relaxing solution (in mmol/l: 100 KCl, 20 imidazole, 7 MgCl2, 2 EGTA, 4 ATP, 0.5 PMSF, 0.04 leupeptin, and 0.01 E64 pH 7.0 at 4°C) containing 1% Triton X-100. After skinning overnight, the trabeculae were washed in fresh relaxing solution (∼1 h) and then stored at −20°C in relaxing solution containing glycerol (50:50 vol/vol). The left ventricles were cut into smaller pieces, snap frozen in liquid nitrogen, and used for protein analysis.
Experimental solutions, apparatus, and protocols.
Solution compositions used for mechanical measurements were calculated using the computer program of Fabiato (14). Both pCa 9.0 and preactivating solution contained the following (in mmol/l): 100 N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES), 15 creatine phosphate, 4.66 ATP, 0.04 leupeptin, and 0.01 E64. In addition, pCa 9.0 solution contained the following (in mmol/l): 7 EGTA, 0.02 CaCl2, 5.49 MgCl2 (1 free Mg2+), and 65.54 potassium propionate, where as preactivating solution contained the following (in mmol/l): 0.07 EGTA, 5.29 MgCl2 (1 free Mg2+), and 86.01 potassium propionate. pCa 4.5 solution contained the following (in mmol/l): 100 BES, 15 creatine phosphate, 4.72 ATP, 7 EGTA, 7.01 CaCl2, 5.29 MgCl2 (1 free Mg2+), and 51.61 potassium propionate. pH of all solutions was adjusted to 7.0 with KOH (22°C). A range of Ca2+ activating solutions (pCa 6.5 to 5.0) was prepared by mixing solutions of pCa 9.0 and pCa 4.5.
On the day of an experiment, skinned trabeculae were washed in relaxing solution for 30 min before they were cut free from the sticks and their ends were trimmed. The trimmed trabeculae were then transferred to a stainless steel experimental chamber containing pCa 9.0 solution (43). The ends of each trabecula were attached to the arms of a motor (model 312B; Aurora Scientific) and force transducer (model 403; Aurora Scientific), as described earlier (43). The chamber assembly was then placed on the stage of an inverted microscope (Olympus) fitted with a ×40 objective and a CCTV camera (model WV-BL600; Panasonic). The light from a halogen lamp was used to illuminate the skinned preparations. Bitmap images of the preparations were acquired using an AGP 4X/2X graphics card and associated software (ATI Technologies) and were used to assess mean SL during the course of each experiment. Changes in force and motor position were sampled (16-bit resolution, DAP5216a; Microstar Laboratories) at 2.0 kHz using SLControl software developed in this laboratory (http://www.slcontrol.com). Data were saved to computer files for later analysis.
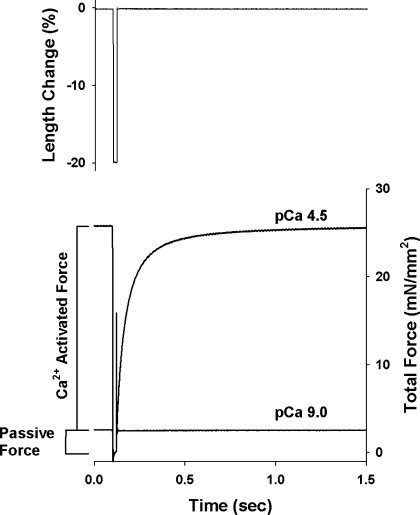
Active force-pCa and ktr-pCa/force relationships were first established at SL of ∼2.00 μm and then at 2.35 μm as described previously (45). Briefly, the skinned trabeculae were stretched to a mean SL of ∼2.00 μm in pCa 9.0 solution. After the length and width were measured, the preparations were transferred first to preactivating solution, then to Ca2+ activating solution, and finally back to pCa 9.0 solution. Once in the Ca2+-activated solution, steady-state force and the apparent rate constant of force redevelopment (ktr) were measured simultaneously using the modified multistep protocol developed by Brenner and Eisenberg (5) described in detail previously (47) and illustrated in Fig. 1. Briefly, after force reached a steady level in activating solution (pCa 6.0–4.5), the length of the preparation was rapidly reduced by ∼20%, held for ∼20 ms, and then restretched back to its original length. As a result of restretch, there was an initial transient increase, followed by a decrease in force (seen as a spike in the force trace) and subsequent slow recovery of force nearing the initial steady-state level. The ktr reported in the present study is the rate constant of force redevelopment after the spike. The drop in force recorded in solution of pCa 9.0 was taken as passive force and was therefore subtracted from the drop in total force at each pCa to yield Ca2+-activated force (P). The protocol was repeated to establish active force-pCa and ktr-pCa/relative active force at SL of 2.35 μm only if the drop in maximum Ca2+-activated force in pCa 4.5 solution was <20%. Upon the completion of mechanical measurements, the trabeculae were cut free at the points of attachment, placed in SDS sample buffer, and stored at −80°C until subsequent protein analysis.
Fig. 1.
Experimental protocol for determination of passive force, Ca2+-activated force and rate constant of force redevelopment (ktr) in rat skinned right ventricular trabeculae. Changes in force recorded before, during, and after (bottom) a step change in length (top) of rat skinned right ventricular trabecula. Once active force reached a steady state in solution of pCa 4.5, muscle length was rapidly reduced by 20%. Ca2+-activated force was determined by subtracting the passive force measured at pCa 9.0 from the total force generated at pCa 4.5. After 20 ms of unloaded shortening, the preparation was restretched to its original length. The ktr is the apparent rate constant of force redevelopment after the force spike.
To determine titin isoform expression in WT and Ho myocardium, the left ventricular myocardial preparations were suspended in SDS sample buffer and electrophoresed using large format SDS agarose gels as described previously (61). Titin isoform patterns were essentially identical in right and left ventricles from respective groups (data not shown). To determine the myosin heavy chain (MHC) isoform expression in WT and Ho myocardium, the left ventricular preparations stored in SDS samples were electrophoresed using large format gels with 3.5% acrylamide stacking and 5% resolving gel (60). To determine myofibrillar protein composition in WT and Ho myocardium, the trabeculae from mechanical experiments stored in SDS sample buffer were electrophoresed using 10–12.5% Tris·HCl Precast Criterion gels (Bio-Rad, Hercules, CA). The gels were silver stained using methods described previously (49) with minor modifications as follows: the gels were 1) incubated for 20 min in fixing solution containing 50% methanol and 10% acetic acid; 2) washed for 20 min (4 times ddH2O changes) with ddH2O; 3) incubated for 1.5 min in 0.01% sodium thiosulfate solution and then rinsed four times with ddH2O; 4) incubated for 20 min in 0.09% silver nitrate solution and then rinsed four times with ddH2O; 5) incubated in developing solution containing 0.0004% sodium thiosulfate, 2% potassium carbonate, and 0.0068% formaldehyde until proteins were visible and then rinsed four times with ddH2O; and 6) incubated for 20 min in destaining solution containing 10% methanol and 10% acetic acid, rinsed four times in ddH2O, and finally rinsed (with slow rotation) overnight in ddH2O. The gels were then imaged using GDS-8000 (UVP BioImaging Systems).
To ensure that the differences in mechanical properties between WT and Ho trabeculae were not confounded by differential phosphorylation state of myofibrillar proteins, we separated the myofibrillar proteins from four of each WT and Ho left ventricles using 10% Tris·HCl Precast Criterion gels (Bio-Rad) and six of each WT and Ho left ventricles using large format SDS agarose gels as described above. Both types of gels were first stained with Pro-Q Diamond to detect phosphoproteins and finally with SYPRO Ruby to detect myofibrillar proteins as described in details previously (10, 51). To avoid minor differences in protein loading between WT and Ho samples on the level of phosphorylation of myofibrillar proteins, we loaded on the same gel a range of concentrations per sample and then determined the slope of integrated optical density (IOD) vs. concentration (μg) for both the phosphoproteins and proteins as described previously (10, 51).
Data analysis.
Cross-sectional areas of skinned trabeculae were calculated by assuming that the trabeculae were cylindrical and by calculations using the widths measured from video images of the mounted preparations. Each Ca2+-activated force (P) at pCa between 6.3 and 5.4 was expressed as a fraction of the maximum Ca2+-activated force (Po) developed by the same preparations at pCa 4.5, i.e., P/Po. To determine the Ca2+ sensitivity of isometric force (pCa50), force-pCa data were fitted with the Hill equation: P/Po = {[Ca2+]n/(kn + [Ca2+]n)}, where n is the slope (Hill coefficient), and k is the Ca2+ concentration for half-maximal activation (pCa50). The ktr was determined by linear transformation of the half-time of force recovery [ktr = −ln 0.5 × (t1/2)−1], as described previously (47).
All data are presented as means ± SE. Statistical analysis of the data was done using either paired or unpaired t-tests. P values <0.05 were taken as indicating significant differences.
RESULTS
Myofibrillar protein profile of WT and Ho rat myocardium.
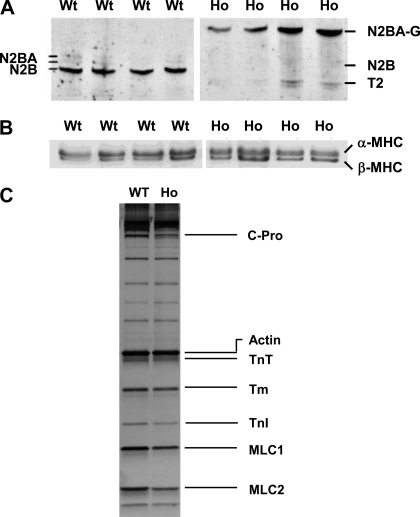
Figure 2 shows a typical SDS-PAGE analysis of rat ventricular tissue for expression profile of titin isoforms (A), MHC isoforms (B), and other myofibrillar proteins (C). It is apparent from Fig. 2A that the WT ventricles express a predominantly fast migrating N2B isoform whereas Ho ventricles express a predominantly slow N2BA-G isoform, an observation consistent with a previous study (28). The estimated molecular mass for N2B and N2BA-G isoform was 2,970 and 3,830 kDa, respectively (28). The estimated molecular mass for N2B was similar to that reported for the N2B isoform expressed in rat (44) and mouse (40) ventricles, whereas that for the N2BA-G isoform was significantly higher than that reported for N2BA isoform expressed in bovine, porcine, and human heart (7, 24). The expression of MHC isoforms (Fig. 2B) was similar in WT (68 ± 4% α-MHC and 32 ± 4% β-MHC) and in Ho (59 ± 4% α-MHC and 41 ± 4% β-MHC) ventricles. SDS-PAGE analysis of WT and Ho trabeculae did not reveal any detectable differences in the expression of either ventricular myosin light chains (i.e., MLC-1 and MLC-2) or thin filament regulatory proteins (i.e., TnT, Tm, and TnI; Fig. 2C).
Fig. 2.
SDS-PAGE analysis of wild-type (WT) and homozygote (Ho) rat ventricles. Typical expression profile of titin isoforms (A), myosin heavy chain isoforms (B), and other myofilament proteins (C) in WT and Ho ventricles examined by SDS electrophoresis as described in methods and materials. A: left ventricles (4 each) from WT and Ho rats were analyzed for titin isoforms. WT ventricles expressed predominantly a shorter N2B isoform whereas the Ho ventricles expressed predominantly a giant N2BA-G isoform. T2 is the degradation product of titin. B: WT ventricles expressed 68 ± 4% α- and 32 ± 4% β-myosin heavy chain (MHC) and the Ho ventricles expressed 59 ± 4% α- and 41 ± 4% β-MHC. C: no major differences were visible in expression profile of other myofibrillar proteins between WT and Ho ventricles. C-Pro, myosin binding protein-C; TnT, cardiac troponin T; α-Tm, α-tropomyosin; TnI, cardiac troponin I; MLC-1, ventricular myosin light chain 1; MLC-2, ventricular myosin light chain 2. Protein loading was not homogenous between lanes.
Phosphorylation state of myofibrillar proteins.
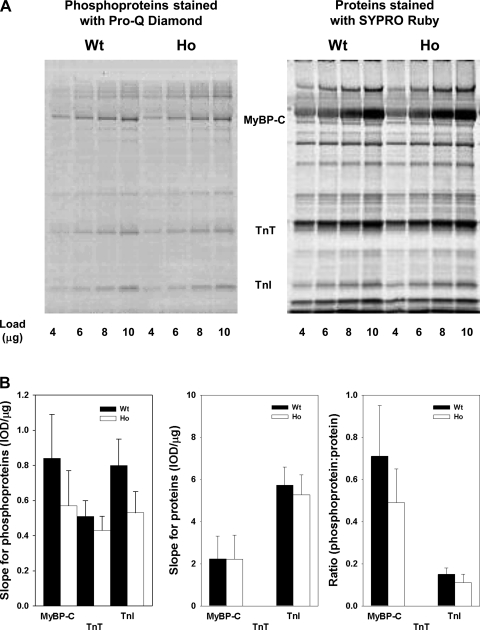
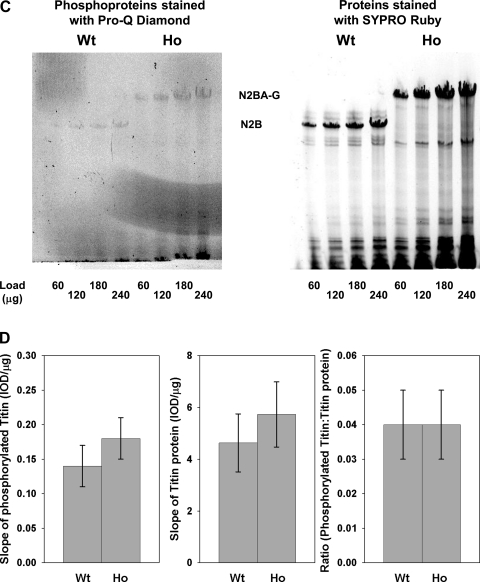
Figure 3A shows a typical example of phosphoproteins (left) and proteins (right) in WT and Ho samples stained with Pro-Q Diamond and SYPRO ruby, respectively. In both WT and Ho samples, several myofibrillar proteins were found to exist in basally phosphorylated state. Specifically, the prominent phosphoproteins detected by Pro-Q Diamond staining included: myosin binding protein C (MyBP-C), troponin T (TnT), and troponin I (TnI). Figure 3B shows the slope of phosphoproteins (left) and proteins (middle), determined by plotting the IOD vs. concentration of WT and Ho samples, and the ratio of the slope of phosphoprotein:protein (right). The values of the slope of phosphorylated MyBP-C, TnT, and TnI and the ratio of phosphorylated protein slope:total protein slope were slightly higher in WT than Ho samples, but the difference was not statistically significant. Figure 3C shows a typical example of titin isoforms expressed in WT and Ho ventricles and stained with Pro-Q Diamond (left) and SYPRO Ruby (right). Figure 3D shows the slope of phosphorylated titin isoforms (left) and total protein (middle), determined by plotting the IOD vs. concentration of WT and Ho samples, and the ratio of phosphorylated titin isoform:total protein (right). To account for the difference in molecular mass between N2BA-G (3.83 MDa) and N2B (2.97 MDa), a factor of 1.29 was incorporated in determining the slopes of both phosphorylated and total protein for Ho samples. We found that the values of both the slope of phosphorylated (left) and total protein (middle) for N2BA-G titin isoform expressed by Ho myocardium were higher than N2B titin isoform expressed by WT myocardium, whereas the ratio of phosphorylated:total protein (bottom) was similar for N2BA-G and N2B. Thus we believe that under our experimental conditions, such a small difference in the degree of phosphorylation of myofibrillar proteins between WT and Ho myocardium is unlikely to have profound effect on the mechanical parameters measured in the present study.
Fig. 3.
Phosphorylation state of myofibrillar proteins in WT and Ho ventricles. Representative 10% Tris·HCl Precast Criterion gel (A) and large format SDS agarose gel (C) stained with Pro-Q Diamond to detect phosphoproteins (left) and SYPRO Ruby to detect total protein (right). B and D, left: slope of phosphoproteins. B and D, middle: shows the slope of proteins determined from regression analysis of plots of integrated optical density (IOD) vs. protein concentration (μg). B and D, right: ratio of the slope of phosphoprotein:total protein. Due to lack of clear delineation of TnT band following SYPRO Ruby staining, the slope of TnT protein (B, middle) and the ratio of the slope of phosphorylated TnT:TnT protein (B, right) were not determined. Each bar represents the mean and the error bar the SE.
SL-dependent changes in passive and maximum Ca2+-activated force.
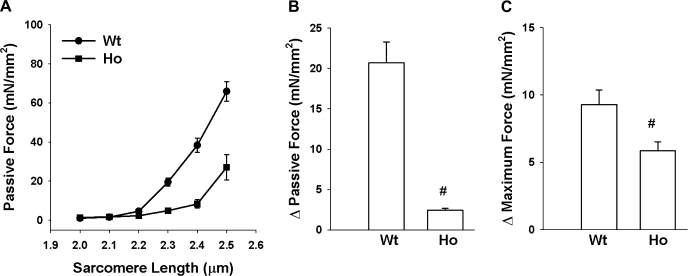
Passive forces in skinned right ventricular trabeculae isolated from WT and Ho rat hearts were measured at SL 2.0 and 2.35 μm in a solution of pCa 9.0. At SL 2.0 μm, passive force was significantly lower in Ho than WT trabeculae (Table 1). Upon stretching the trabeculae from SL 2.0 to 2.35 μm, passive force increased from 1.95 ± 0.25 to 22.64 ± 2.74 mN/mm2 in WT trabeculae and from 1.23 ± 0.11 to 3.68 ± 0.28 mN/mm2 in Ho trabeculae (Table 1). Statistical analysis indicated that the passive force at SL 2.35 μm was also significantly lower in Ho than in WT trabeculae. The magnitude (i.e., difference in passive force at 2.0 and 2.35 μm) of SL-dependent changes in passive force was WT > Ho trabeculae (Fig. 4B).
Table 1.
SL-dependent changes in passive force, maximum Ca2+-activated force, pCa50, nH, and maximum rate of force redevelopment in Wt and Ho rat skinned myocardium
| SL, μm | Resting Force, mN/mm2 | Maximum Ca2+-Activated Force, mN/mm2 | Hill Coefficient (nH) | Ca2+ Sensitivity of Force (pCa50) | Maximum Rate of Force Redevelopment (ktr, s−1) |
|---|---|---|---|---|---|
| WT (n = 13) | |||||
| 2.00 | 1.95 ± 0.25 | 25.99 ± 2.76 | 3.12 ± 0.08 | 5.69 ± 0.02 | 17.09 ± 0.97 |
| 2.35 | 22.64 ± 2.74* | 35.23 ± 3.20* | 2.71 ± 0.04* | 5.81 ± 0.02* | 14.96 ± 1.17* |
| Ho (n = 11) | |||||
| 2.00 | 1.23 ± 0.11† | 16.51 ± 1.25† | 2.89 ± 0.10 | 5.67 ± 0.01 | 9.28 ± 0.79† |
| 2.35 | 3.68 ± 0.28*† | 22.36 ± 1.70*† | 2.75 ± 0.07* | 5.72 ± 0.01*† | 8.05 ± 0.59*† |
All valuses are expressed as means ± SE, with the number of skinned trabeculae given in parentheses. Resting force was measured at pCa 9.0. Maximum force and the rate constant of force redevelopment (ktr) were measured at pCa 4.5. pCa50 and nH values were derived by fitting the force-pCa relationships to a Hill equation. WT, wild type; Ho, homozygote.
Significantly different from values recorded at sarcomere length (SL) = 2.0 μm.
Significantly different from values recorded in WT at similar SL.
Fig. 4.
Magnitude of sarcomere length (SL)-dependent changes in passive and maximum Ca2+-activated force in WT and Ho trabeculae. A: passive force was measured by sequentially stretching WT (n = 4; ●) and Ho (n = 4; ■) trabeculae to various SL in pCa 9.0 solution. B and C: passive and maximum Ca2+-activated forces were measured in pCa 9.0 and 4.5 solution respectively, first at SL 2.0 μm and then at 2.35 μm in WT (n = 13) and Ho (n = 11) trabeculae. Magnitude of SL-dependent changes in passive force (B) and SL-dependent changes in maximum Ca2+-activated force (C) was determined by subtracting values recorded at SL 2.0 μm from those recorded at 2.35 μm. Open bar represents the mean and the error bars the SE. #Significantly different from values determined in WT trabeculae.
Maximum Ca2+-activated force generated by WT and Ho skinned right ventricular trabeculae in solution of pCa 4.5 was measured at SL 2.0 and 2.35 μm. At SL 2.0 μm, the maximum Ca2+-activated force generated by Ho was determined to be significantly lower than WT trabeculae (Table 1). When trabeculae were stretched from SL 2.0 to 2.35 μm, there was a significant increase in maximum Ca2+-activated force in WT (from 25.99 ± 2.76 to 35.23 ± 3.20 mN/mm2) and Ho (16.51 ± 1.25 to 22.36 ± 1.70 mN/mm2) trabeculae (Table 1). Statistical analysis indicated that the maximum Ca2+-activated force generated by Ho was also significantly lower than WT trabeculae at SL 2.35 μm. The magnitude (i.e., difference in maximum Ca2+-activated force at 2.0 and 2.35 μm) of SL-dependent changes in maximum Ca2+-activated force was WT (9.30 ± 1.07 mN/mm2) > Ho (5.85 ± 0.68 mN/mm2) trabeculae (Fig. 4C).
SL-dependent changes in apparent cooperativity in activation of force and Ca2+ sensitivity of force.
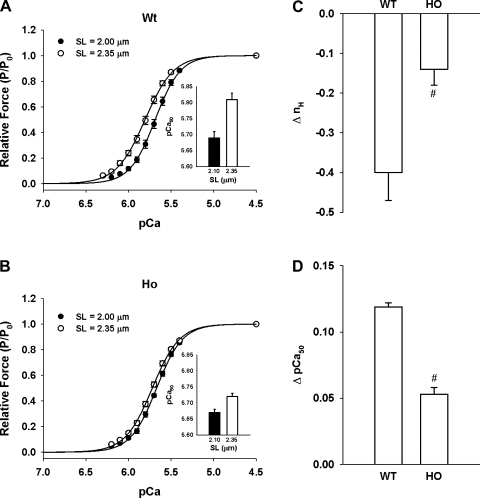
At SL 2.0 and 2.35 μm, the force-pCa relationships in WT (Fig. 5A) and Ho (Fig. 5B) trabeculae were sigmoidal and were fit to the Hill equation described in methods and materials to yield the Hill coefficient (nH), an index of apparent cooperativity in activation of force, and pCa50, an index of Ca2+ sensitivity of force. At SL 2.0 μm, the values of nH and pCa50 determined in Ho were not significantly different from those determined in WT trabeculae (Table 1). When trabeculae were stretched from SL 2.0 to 2.35 μm, the nH values significantly decreased from 3.12 ± 0.08 to 2.71 ± 0.04 in WT and from 2.89 ± 0.10 to 2.75 ± 0.07 in Ho, whereas pCa50 values significantly increased from pCa 5.69 ± 0.02 to 5.81 ± 0.02 in WT and from pCa 5.67 ± 0.01 to 5.72 ± 0.01 in Ho trabeculae (Table 1 and Fig. 5, A and B, inset). Statistical analysis of the data indicated that the values of pCa50, but not nH, determined in Ho were significantly lower than those determined in WT trabeculae at SL 2.35 μm. The magnitude (i.e., differences in nH and pCa50 at SL 2.0 and 2.35 μm) of SL-dependent changes in apparent cooperativity in activation of force was WT (0.40 ± 0.07 U) > Ho (0.14 ± 0.06 U; Fig. 5C) and in Ca2+ sensitivity of force was WT (0.12 ± 0.01 pCa U) > Ho (0.05 ± 0.01 pCa U) trabeculae (Fig. 5D).
Fig. 5.
SL-dependent changes in apparent cooperativity in force generation (nH) and Ca2+ sensitivity of force (pCa50) in WT and Ho rat skinned right ventricular trabeculae. Force-pCa relationships of WT (A; n = 13) and Ho (B; n = 11) rat skinned right ventricular trabeculae were determined first at short SL (2.00 μm; ●) and then at long SL (2.35 μm; ○). Solid lines were generated using the Hill equation described in methods and materials. Fitted values for nH and pCa50 for WT trabeculae were 2.94 and pCa 5.69, respectively, at short SL and 2.59 and pCa 5.81, respectively, at long SL and for Ho trabeculae were 2.86 and pCa 5.67, respectively, at short SL and 2.73 and pCa 5.72, respectively, at long SL. Inset: pCa50 values determined at short (black bars) and long (white bars) SL. Magnitude of SL-dependent changes in nH (C) and pCa50 (D) was determined by subtracting values recorded at short from those recorded at long SL. Data points are the means ± SE. #Significantly different from values determined in WT trabeculae.
SL-dependent changes in rate of force redevelopment (ktr).
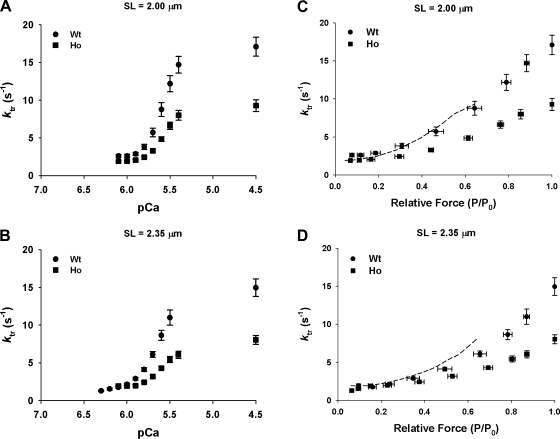
Figure 6 shows Ca2+-dependent (A and B) and force-dependent (C and D) changes in ktr observed in WT and Ho at short (2.0 μm) and long (2.35 μm) SLs. At submaximal (pCa 5.8–5.4) and maximum (pCa 4.5) [Ca2+]free, Ho trabeculae redeveloped forces at a slower rate than WT trabeculae. At pCa 4.5, Ho trabeculae redeveloped maximum force at a rate of 9.28 ± 0.79 s−1 at short SL and 8.05 ± 0.59 s−1 at long SL whereas WT trabeculae redeveloped maximum force at 17.09 ± 0.97 s−1 at short SL and 14.96 ± 1.17 s−1 at long SL (Table 1). At submaximal [Ca2+]free, Ho trabeculae redeveloped forces also at slower rates than WT trabeculae, and thus depressed the ktr-pCa relationship at short (Fig. 6A) and long (Fig. 6B) SL. A similar trend was observed when ktr values were replotted against steady-state isometric force (as a function of maximum force; Fig. 6, C and D). However, when ktr values recorded in Ho trabeculae were replotted against steady-state force expressed as a fraction of maximum force recorded in WT trabeculae (dashed line), the ktr-force relationship in Ho trabeculae was found to be similar to WT trabeculae, suggesting that the right shift in the relationship was because the maximum force generated by Ho trabeculae was ∼35% less than WT trabeculae and that Ho trabeculae most likely redevelop forces at similar rates as WT trabeculae.
Fig. 6.
Ca2+- and force-dependent changes in apparent rate constant of force redevelopment (ktr) at 2.00 and 2.35 μm in WT and Ho rat skinned right ventricular trabeculae. Rate of force redevelopment (ktr) was measured as described in methods and materials at submaximal (pCa 6.3–5.4) and maximum (pCa 4.5) free Ca2+ concentration ([Ca2+]free) first at short (2.00 μm; A and C) and then at long (2.35 μm; B and D) SL in WT (●) and Ho (■) skinned trabeculae. A and B: ktr-[Ca2+]free (pCa) relationship established in WT and Ho trabeculae. C and D: ktr-relative force (forces expressed as a fraction of maximum Ca2+-activated force recorded at each SL) relationship established in WT and Ho trabeculae. Dashed line in C and D shows the ktr-relative force (forces expressed as a fraction of mean maximum Ca2+-activated force recorded at each SL in WT trabeculae) relationship in Ho trabeculae factoring in the depression of maximum force.
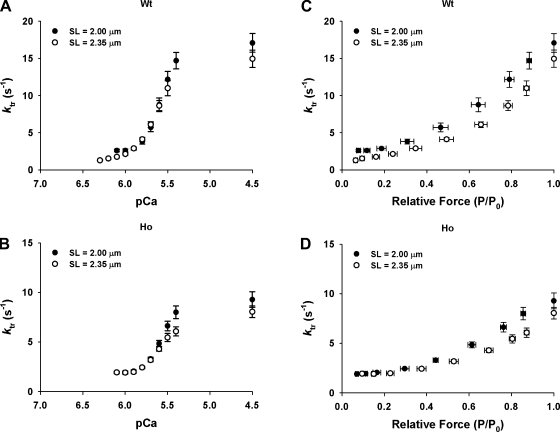
Figure 7 shows the ktr-pCa (A and B) and ktr-relative force (C and D) relationships established first at SL 2.0 and then at 2.35 μm in WT and Ho trabeculae. While the ktr-pCa relationships established at SL 2.0 μm were indistinguishable from those established at 2.35 μm in both types of trabeculae, the ktr-relative force relationships established at SL 2.35 μm lie to the right of those established at SL 2.0 μm in both types of trabeculae. That is, the WT and Ho trabeculae redeveloped submaximal and maximal forces at a slower rate at SL 2.35 than 2.0 μm. Thus WT and Ho trabeculae exhibited a SL-dependent decrease in apparent rate of force redevelopment.
Fig. 7.
SL-dependent changes in ktr-pCa and ktr-relative force relationships in WT and Ho rat skinned right ventricular trabeculae. ktr-pCa and ktr-relative force relationships were established first at short (2.00 μm; ●) and then at long (2.35 μm; ○) SL in WT (A and C) and Ho (B and D) rat skinned right ventricular trabeculae.
DISCUSSION
In the present study, we used rat skinned ventricular trabeculae expressing either a smaller N2B isoform (2,970 kDa; WT) or a giant N2BA-G isoform (3,830 kDa; Ho) to examine the effects of titin isoforms on SL-dependent changes in both the steady-state and dynamic contractile properties. The main findings of this study are as follows: 1) at both short (2.0 μm) and long (2.35 μm) SL, the values of passive force, maximum Ca2+-activated force, and rates of force redevelopment were significantly lower in Ho than WT trabeculae and 2) stretching the skinned trabeculae from short (2.0 μm) to long (2.35 μm) SL increased i) the passive force, with the magnitude of SL-dependent changes in passive force being WT > Ho; ii) the maximum Ca2+-activated force, with the magnitude of SL-dependent changes in maximum Ca2+-activated force being WT > Ho; and iii) Ca2+ sensitivity of force, with the magnitude of SL-dependent changes in Ca2+ sensitivity of force being WT > Ho; and decreased i) the apparent cooperativity in activation of force, with the magnitude of SL-dependent changes in apparent cooperativity in activation of force being WT > Ho; and ii) the rate of force redevelopment. While an earlier study (24) used skinned bovine atrial preparations expressing a larger N2BA and ventricular preparations expressing both a smaller N2B and a larger N2BA isoforms to examine the effects of titin isoforms on SL-dependent changes in steady-state contractile properties (24), the work reported here allowed a within species (rat) and tissue (ventricle) comparison of the role of titin isoforms where the expression of other contractile protein isoforms appear to be constant.
Effects of titin isoforms on passive and maximum Ca2+-activated force.
Compared to WT trabeculae expressing predominantly a short N2B isoform, passive force was reduced by ∼35% at short SL and 85% at long SL in Ho trabeculae expressing predominantly a giant N2BA-G isoform. These results are consistent with those of Wu et al. (63), who found passive force to be higher in mouse ventricular preparations, expressing predominantly the N2B isoform; lower in bovine atrial preparation, expressing predominantly the N2BA isoform; and intermediate in bovine ventricular preparations, expressing both N2B and N2BA isoforms.
In cardiac muscle, passive force is generated as a result of sequential extension of Ig, PEVK, and N2B unique segments (spring element) in the I band. At any given physiological SL (1.8–2.4 μm), the amount of passive force generated will depend on the degree of extension of the spring element (53). Because of the larger size of the spring element in N2BA than N2B (18), at any given SL, one would anticipate the degree of extension of the spring element to be smaller in myocardial preparations expressing more N2BA than N2B isoform. As a consequence, the passive force generated by myocardial preparations expressing either predominantly N2BA isoform (bovine atrial preparations and Ho trabeculae) or both N2B and N2BA isoforms (bovine ventricular preparations) will be lower than preparations expressing predominantly N2B isoform (mouse ventricular preparations and WT trabeculae). Although the greater reduction in passive force at long SL in Ho than bovine atrial preparations predicts the presence of a larger spring element in N2BA-G than in the N2BA isoform (or additional length due to insertion of more Ig domains by alternative splicing), confirmatory experiments dealing with sequence analysis have yet to be performed.
Near total replacement of a short N2B isoform with a giant N2BA-G isoform reduced the maximum Ca2+-activated force by ∼35% at both SLs (Table 1). A similar reduction in maximum Ca2+-activated force was apparent in bovine atrial preparations expressing a long N2BA isoform in an earlier study (24), although the authors did not comment on the difference in maximum Ca2+-activated force between bovine atrial and ventricular preparations expressing both the N2B and N2BA isoforms. It is tempting to speculate that the size and incomplete straightening of both N2BA and N2BA-G restrict access of myosin binding sites on actin, hence reducing the maximum Ca2+-activated force.
Effects of titin isoforms on SL-dependent changes in passive and active force.
Both the WT and Ho trabeculae exhibited SL-dependent increases in passive force, maximum Ca2+-activated force, and Ca2+ sensitivity of force, confirming earlier observations in rat ventricular trabeculae and myocytes (16, 36, 42), mouse ventricular myocytes (6, 9, 13, 37), bovine atrial strips (24), bovine and porcine ventricular strips (13, 24, 52), and human ventricular myocytes (13, 55). However, the effects of stretching WT and Ho trabeculae from a short to long length on passive force, maximum Ca2+-activated force, and Ca2+ sensitivity of force differed quantitatively. That is, the magnitudes of the SL-dependent increase in passive force (∼21 and ∼3 mN/mm2), maximum Ca2+-activated force (∼9 and ∼6 mN/mm2) and Ca2+ sensitivity of force (∼0.12 and ∼0.05 pCa U) were larger in WT and smaller in Ho trabeculae. Based on previous studies showing a larger increase in the magnitude of SL-dependent increase in passive force in myocardium expressing a short N2B titin isoform (22, 23, 35) than myocardium expressing a long N2BA titin isoform (24), it is reasonable to conclude that the differences in the magnitude of SL-dependent changes in passive force between WT and Ho trabeculae reported here occurred because WT trabeculae were expressing predominantly the short N2B titin isoform whereas Ho trabeculae were expressing predominantly the giant N2BA-G titin isoform (Fig. 2A). The similarity between the magnitude of SL-dependent changes in passive force (WT > Ho) and both the maximum Ca2+-activated force and Ca2+ sensitivity of force (WT > Ho) reported here and the linear correlation between the magnitude of SL-dependent increase in passive force and both the maximum Ca2+-activated force and Ca2+ sensitivity of force reported previously (9, 23, 24, 48) suggests that the differences in the magnitude of SL-dependent changes in maximum Ca2+-activated force and Ca2+ sensitivity of force between WT and Ho trabeculae may also be due to differential expression of titin isoforms. A striking difference between earlier studies in rat (12, 16, 35, 36), mouse (13, 40), porcine (13), bovine (24), and human (13, 55) ventricular muscles and the present study is the effect of SL on nH, an index of apparent cooperativity in the activation of force. While earlier studies reported no significant SL-dependent decreases in nH, we observed a significant SL-dependent decrease in nH in WT (∼0.41 U) and Ho (0.14 U) trabeculae. The similarity between the order of SL-dependent increase in passive force (WT > Ho) and SL-dependent decrease in nH (WT > Ho) suggests that the decrease in nH may also be modified by titin isoforms.
Effects of titin isoforms on SL-dependent changes in cross-bridge cycling kinetics.
Irrespective of SL, the rate of force redevelopment (ktr) varied with the level of activating [Ca2+]free (or force) in WT and Ho trabeculae, increasing as [Ca2+]free (or force) was elevated from submaximal to maximal levels (Fig. 6). These Ca2+- and force-dependent changes in ktr reported are consistent with previous results from rat (45, 46), mouse (13, 47), bovine, porcine (13), and human (13) myocardium. Compared with WT trabeculae, submaximal and maximal forces redeveloped at slower rates in Ho trabeculae at both SL (Fig. 6). Thus, when ktr values were plotted against steady-state force, expressed as a fraction of maximum force, the curvilinear ktr-force relationship established in Ho trabeculae was found to be to the right of that in WT trabeculae (Fig. 6, C and D) at both SL. However, when ktr values recorded in Ho trabeculae were replotted against steady-state forces expressed as a fraction of maximum force recorded in WT trabeculae (dashed line), the ktr-force relationship in Ho trabeculae was found to be similar to WT trabeculae, suggesting that the right shift in ktr-force relationship was because of reduced number of cross bridges involved in force generation and that cross-bridge cycling kinetics is likely to be similar in both Ho and WT trabeculae. Previous studies (17) have shown that in rat hearts, a reduction in the ratio of fast α-MHC vs. slow β-MHC expression as a consequence of aging slows the rate of force redevelopment. Since the ratio of fast α-MHC vs. slow β-MHC expression was similar in Ho and WT trabeculae (Fig. 2B), the slower rate of force redevelopment in Ho than WT trabeculae is very unlikely to be due to a reduction in fast α-MHC vs. slow β-MHC expression.
In WT and Ho trabeculae, the ktr-pCa relationships established at long SL were similar to those established at short SL (Fig. 7, A and B). A similar observation was reported by Adhikari et al. (1) in rat myocardium and by Edes et al. (13) in mouse myocardium, both of which are known to express primarily the N2B titin isoform (40), and human and pig myocardium, both of which are known to express a mixture of N2B and N2BA titin isoforms (24, 44). Collectively, the results suggest that titin isoforms most likely have no significant effect on Ca2+ dependence of ktr in myocardium. On the other hand, the ktr -force relationships established at long SL were to the right of those established at short SL in WT and Ho trabeculae (Fig. 7, C and D), i.e., at equivalent forces, ktr values tended to be lower at long than short SL in WT and Ho trabeculae. Such an effect could be a manifestation of greater spread of cross-bridge-induced recruitment of cross bridge at long SLs due to enhanced cross-bridge binding and the increased time required to complete this component of activation. This effect of SL on the ktr-force relationship is also similar to that reported earlier in rat trabeculae by Adhikari et al. (1) and in mice, human, and pig myocardium by Edes et al. (13). The comparable SL-dependent decrease in ktr in WT and Ho trabeculae suggests that titin isoforms are unlikely to have major impact on SL-dependent decrease in ktr.
Possible mechanisms by which SL potentiates force production in rat myocardium.
For a given [Ca2+]free, the ability of WT and Ho rat myocardium to generate more force at long SL can be explained by considering the two-state kinetic model of cross-bridge interaction proposed by Huxley (31) and modified by Brenner (4). In this model, multiple states of the cross-bridge kinetic scheme are reduced to just two states, i.e., the rate of transition of cross bridges from nonforce-generating state to force-generating state is described by fapp, whereas gapp describes the rate of transition of cross bridges from the force generating state back to the nonforce-generating state. Steady isometric force (P) is then equal to N × F × [fapp/(fapp + gapp)], where N is the number of cycling cross bridges, F is the average force per cross bridge, and ktr = fapp + gapp. Thus, for a given [Ca2+]free, the ability of rat myocardium to generate more force at long SL could be due to an increase in N or F or fapp or a some combination of the three parameters. At long SL, a reduction in lattice spacing (9, 23, 24) and an increase in myosin head orientation perpendicular to the thick filament backbone (15) will bring the myosin cross bridges closer to binding sites on actin and may thus increase N and accelerate cross-bridge cycling kinetics. The SL-induced increase in N may arise as a result of an increase in either the probability of myosin cross-bridge binding to actin or the binding affinity of TnC for Ca2+, an effect that is seen when the distance between myosin and actin is reduced on stretching myocardium from short to long lengths (19), or both. While there is no direct evidence in favor for titin isoform-dependent changes in the orientation of myosin heads perpendicular to the thick filament backbone, Fukuda et al. (24) reported that the magnitude of SL-dependent decrease in lattice spacing is significantly greater in bovine ventricle, expressing a mixture of N2B and N2BA titin isoforms, than bovine atria, expressing predominantly N2BA titin isoform. Assuming this to be true for rat myocardium and that N varies linearly with both lattice spacing and the orientation of myosin heads perpendicular to the thick filament backbone, then the order of SL-dependent increase in N of WT > Ho would predict a similar order of SL-dependent increase in Ca2+ sensitivity of force as the one reported here. The greater proximity of myosin cross bridge to actin would also facilitate cooperative binding of myosin cross bridges to actin, which would be manifested as a decrease in the steepness of the force-pCa relationship. At present we have no explanation for how bringing myosin cross bridges closer to actin increases F. SL-induced predisposition of myosin cross bridges to attach to actin would predict an increase in fapp. Wannenburg et al. (59) reported no significant effect of SL on rate of cross-bridge detachment in rat myocardium. Assuming this to be true, under our experimental conditions then an increase in fapp would be possible only if the values of ktr were larger at long than at short SL. However, the SL-dependent decrease in ktr reported here and by Adhikari et al. (1) fails to support this idea and suggests that a decrease in rate of cross-bridge detachment or a longer time for the spread of cooperative activation (see previous paragraph) has to occur to account for a role of cross-bridge cycling kinetics in potentiating force production at long SL. Thus the SL-dependent increase in Ca2+ sensitivity of force in rat myocardium is most likely to be due to some combination of an increase in N and F and a simultaneous increase in fapp and a decrease in gapp.
In summary, our results provides evidence in support of the idea that titin isoforms play a prominent role in modulating SL-dependent changes in Ca2+-activated force, a cellular mechanism by which the heart regulates ventricular output in response to changes in ventricular filling on a beat-to beat basis (Frank-Starling's law of the heart). The magnitude of the SL-dependent increase in Ca2+-activated force and decrease in the apparent cooperativity of activation of force was largest in ventricles expressing predominantly a short N2B isoform and smallest in ventricles expressing predominantly a giant N2BA-G isoform whereas the SL-dependent decrease in ktr was similar in both types of ventricles, suggesting that cross-bridge cycling kinetics may not be the primary mechanism responsible for increasing Ca2+-activated force at long SL.
GRANTS
This work was supported by the College of Agricultural and Life Sciences, University of Wisconsin-Madison and National Heart, Lung, and Blood Institute Grants HL-77196 (to M. L. Greaser) and R37-HL-82900 (to R. L. Moss).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.P., J.M.P., R.L.M., and M.L.G. conception and design of research; J.R.P. and J.M.P. performed experiments; J.R.P. and J.M.P. analyzed data; J.R.P., J.M.P., R.L.M., and M.L.G. interpreted results of experiments; J.R.P. and J.M.P. prepared figures; J.R.P. drafted manuscript; J.R.P., J.M.P., R.L.M., and M.L.G. edited and revised manuscript; J.R.P., J.M.P., R.L.M., and M.L.G. approved final version of manuscript.
REFERENCES
- 1. Adhikari BB, Regnier M, Rivera AJ, Kreutziger KL, Martyn DA. Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophys J 87: 1784–1794, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol 17: 821–840, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Barth Z, Strauss JD, Dohet C, Rüegg JC. The Ca2+ sensitizer EMD 53998 antagonizes the effect of 2, 3-butanedione monoxime on skinned cardiac muscle fibres. Eur J Pharmacol 296: 285–289, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85: 3265–3269, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenner B, Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci USA 83: 3542–3546, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cazoral O, Szilagyi S, Vignier N, Salazar G, Kramer E, Vassort G, Carrier L, Lacampagne A. Length and protein kinase A modulations of myocytes in cardiac myosin binding protein C-deficient mice. Cardiovasc Res 69: 370–380, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitas K, Labeit S, Granzier H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res 86: 59–67, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Cazorla O, Vassort G, Garnier D, Guennec JL. Length modulation of active force in rat cardiac myocytes: is titin the sensor? J Mol Cell Cardiol 31: 1215–1227, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Cazorla O, Wu Y, Irving TC, Granzier H. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ Res 88: 1028–1035, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Chen PP, Patel JR, Rybakova IN, Walker JW, Moss RL. Protein kinase A-induced myofilament desensitization to Ca2+ as a result of phosphorylation of cardiac myosin-binding protein C. J Gen Physiol 136: 615–627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colson BA, Locher MR, Bekyarova T, Patel JR, Fitzsimons DP, Irving TC, Moss RL. Differential roles of myosin regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol 588: 981–993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dobesh DP, Konhilas JP, de Tombe PP. Cooperative activation in cardiac muscle: impact of sarcomere length. Am J Physiol Heart Circ Physiol 282: H1055–H1062, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Edes IF, Czuriga D, Csanyi G, Chlopicki S, Recchia FA, Borbely A, Galajda Z, Edes I, van der Velden J, Stienen GJM, Papp Z. Rate of tension redevelopment is not modulated by sarcomere length in permeabilized human, murine, and porcine cardiomyocytes. Am J Physiol Regul Integr Comp Physiol 293: R20–R29, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol 157: 378–417, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Farman GP, Gore D, Allen EJ, Schoenfelt K, Irving TC, de Tombe PP. Myosin head orientation: a structural determinant for the Frank-Starling relationship. Am J Physiol Heart Circ Physiol 300: H2155–H2160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fitzsimons DP, Moss RL. Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circ Res 83: 602–607, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Fitzsimons DP, Patel JR, Moss RL. Aging-dependent depression in the kinetics of force development in rat skinned myocardium. Am J Physiol Heart Circ Physiol 276: H1511–H1519, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Freiburg A, Trombitas K, Hell W, Cazorla O, Fougerousse F, Centner T, Kolmerer B, Witt C, Beckmann JS, Gregorio CC, Granzier H, Labeit S. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res 86: 1114–1121, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Fuchs F, Wang YP. Sarcomere length versus interfilament spacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding. J Mol Cell Cardiol 28: 1375–1383, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Fukuda N, Granzier H. Role of the giant elastic protein titin in the Frank-Starling mechanism of the heart. Curr Vasc Pharmacol 2: 135–139, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Fukuda N, Kajiwara H, Ishiwata S, Kurihara S. Effects of MgADP on length dependence of tension generation in skinned rat cardiac muscle. Circ Res 86: 1–6, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Fukuda N, Sasaki D, Ishiwata S, Kurihara S. Length dependence of tension generation in rat skinned cardiac muscle: role of titin in the frank-starling mechanism of the heart. Circulation 104: 1639–1645, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle. Pflügers Arch 449: 449–457, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle. J Physiol 553: 147–154, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol 125: 257–271, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Furst DO, Osborn M, Nave R, Weber K. The organization of the titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z-line extends close to the M-line. J Cell Biol 106: 1563–1572, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Granzier HK, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J 68: 1027–1044, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greaser ML, Warren CM, Esbona K, Guo W, Duan Y, Parrish AM, Krzesinski PR, Norman HS, Dunning S, Fitzsimons DP, Moss RL. Mutation that dramatically alters rat titin isoform expression and cardiomyocyte passive tension. J Mol Cell Cardiol 44: 983–991, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hibberd MG, Jewell BR. Calcium-and length-dependent force production in rat ventricular muscle. J Physiol 329: 527–540, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, Labeit S, Granzier H. PKC phosphorylation of titin's PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res 105: 631–638, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huxley AF. Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7: 257–318, 1957 [PubMed] [Google Scholar]
- 32. Irving T, Wu Y, Berkyarova T, Farman GP, Fukuda N, Granzier H. Thick-filament strain and interfilament spacing in passive muscle: effect of titin-based passive tension. Biophys J 100: 1499–1508, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Irving TC, Konhilas J, Perry D, Fischetti R, de Tombe PP. Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. Am J Physiol Heart Circ Physiol 279: H2568–H2573, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Katz AM. Ernest Henry Starling, his predecessors, and the “Law of the Heart”. Circulation 106: 2986–2992, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Kentish JC, Ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relationships of intact and skinned trabeculae from rat right ventricle: influence of calcium concentrations on these relations. Circ Res 58: 755–768, 1986 [DOI] [PubMed] [Google Scholar]
- 36. Konhilas JP, Irving TC, de Tombe PP. Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ Res 90: 59–65, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, de Tombe PP. Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol 547: 951–961, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kruger M, Linke WA. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil 27: 435–444, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Kruger M, Sebastian K, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dosRemedios CG, Linke W. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin spring. Circ Res 104: 87–94, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Lee EJ, Peng J, Radke M, Gotthardt M, Granzier HL. Calcium sensitivity and the Frank-Starling mechanism of the heart are increased in titin N2B region-deficient mice. J Mol Cell Cardiol 49: 449–458, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Linke WA, Rudy DE, Centner T, Gautel M, Witt C, Labeit S, Gregorio CC. I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure. J Cell Biol 146: 631–644, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McDonald K, Moss RL. Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length. Circ Res 77: 199–205, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Moss RL, Swinford AE, Greaser ML. Alterations of the Ca2+ sensitivity of tension development by single skeletal muscle fibers at stretched lengths. Biophys J 43: 115–119, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Neagoe C, Kulke M, del Monte F, Gwathmey JK, de Tombe PP, Jajjar RJ, Linke WA. Titin isoform switch in ischemic human heart disease. Circulation 106: 1333–1341, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Olsson MC, Patel JR, Fitzsimons DP, Walker JW, Moss RL. Basal myosin light chain phosphorylation is a determinant of Ca2+ sensitivity of force and activation dependence of the kinetics of myocardial force development. Am J Physiol Heart Circ Physiol 287: H2712–H2718, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Palmer S, Kentish JC. Roles of Ca2+ and crossbridge kinetics in determining the maximum rates of Ca2+ activation and relaxation in rat and guinea pig skinned trabeculae. Circ Res 83: 179–186, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Patel JR, Fitzsimons DP, Buck SH, Muthuchamy M, Wieczorek DF, Moss RL. PKA accelerates rate of force development in murine skinned myocardium expressing α- or β-tropomyosin. Am J Physiol Heart Circ Physiol 280: H2732–H2739, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Patrick SM, Hoskins AC, Kentish JC, White E, Shiels HA, Cazorla O. Enhanced length-dependent Ca2+ activation in fish cardiomyocytes permits a large operating range of sarcomere lengths. J Mol Cell Cardiol 48: 917–924, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem 68: 850–858, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Stelzer JE, Patel JR, Moss RL. Acceleration of stretch activation in murine myocardium due to phosphorylation of myosin regulatory light chain. J Gen Physiol 128: 261–272, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stelzer JE, Patel JR, Moss RL. Protein kinase A-mediated acceleraion of stretch activation response in murine myocardium is eliminated by ablation of cMyBP-C. Circ Res 99: 884–890, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Terui T, Sodnomtseren M, Matsuba D, Udaka J, Ishiwata S, Ohtsuki I, Kurihara S, Fukuda N. Troponin and titin coordinately regulate length-dependent activation in skinned porcine ventricular muscle. J Gen Physiol 131: 275–283, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trombitas K, Redkar A, Centner T, Wu Y, Labeit S, Granzier H. Extensibility of isoforms of cardiac titin: variation in contour length of molecular subsegments provides a basis for cellular passive stiffness diversity. Biophys J 79: 3226–3234, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Turnbull L, Hoh JFY, Ludowyke RI, Rossmanith GH. Troponin I phosphorylation enhances crossbridge kinetics during β-adrenergic stimulation in rat cardiac tissue. J Physiol 542: 911–920, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van der Velden J, de Jong JW, Owen VJ, Burton PBJ, Stienen GJM. Effect of protein kinase A on calcium sensitivity of force and its sarcomere length dependence in human cardiomyocytes. Cardiovasc Res 46: 487–495, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Venema RC, Raynor RL, Noland TAJ, Kuo JF. Role of protein kinase C in the phosphorylation of cardiac myosin light chain 2. Biochem J 294: 401–406, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang K, McClure J, Tu A. Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci USA 76: 3698–3702, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang YP, Fuchs F. Osmotic compression of skinned cardiac and skeletal muscle bundles: effects on force generation, Ca2+ sensitivity and Ca2+ binding. J Mol Cell Cardiol 27: 1235–1244, 1995 [DOI] [PubMed] [Google Scholar]
- 59. Wannenburg T, Janssen PML, Fan D, de Tombe PP. The frank-starling mechanism is not mediated by changes in rate of cross-bridge detachment. Am J Physiol Heart Circ Physiol 273: H2428–H2435, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Warren CM, Greaser ML. Method for cardiac myosin heavy chain separation by sodium dodecyl sulfate gel electrophoresis. Anal Biochem 320: 149–151, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Warren CM, Krzesinski PR, Greaser ML. Vertical agrose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis 24: 1695–1702, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Wiggens JR, Reiser J, Fitzpatrick DF, Bergey JL. Inotropic actions of diacetyl monoxime in cat ventricular muscle. J Pharmacol Exp Ther 212: 217–224, 1980 [PubMed] [Google Scholar]
- 63. Wu Y, Cazolra O, Labeit D, Labeit S, Granzier H. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J Mol Cell Cardiol 32: 2151–2162, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res 90: 1181–1188, 2002 [DOI] [PubMed] [Google Scholar]