Abstract
Formation of a dense microtubule network that impedes cardiac contraction and intracellular transport occurs in severe pressure overload hypertrophy. This process is highly dynamic, since microtubule depolymerization causes striking improvement in contractile function. A molecular etiology for this cytoskeletal alteration has been defined in terms of type 1 and type 2A phosphatase-dependent site-specific dephosphorylation of the predominant myocardial microtubule-associated protein (MAP)4, which then decorates and stabilizes microtubules. This persistent phosphatase activation is dependent upon ongoing upstream activity of p21-activated kinase-1, or Pak1. Because cardiac β-adrenergic activity is markedly and continuously increased in decompensated hypertrophy, and because β-adrenergic activation of cardiac Pak1 and phosphatases has been demonstrated, we asked here whether the highly maladaptive cardiac microtubule phenotype seen in pathological hypertrophy is based on β-adrenergic overdrive and thus could be reversed by β-adrenergic blockade. The data in this study, which were designed to answer this question, show that such is the case; that is, β1- (but not β2-) adrenergic input activates this pathway, which consists of Pak1 activation, increased phosphatase activity, MAP4 dephosphorylation, and thus the stabilization of a dense microtubule network. These data were gathered in a feline model of severe right ventricular (RV) pressure overload hypertrophy in response to tight pulmonary artery banding (PAB) in which a stable, twofold increase in RV mass is reached by 2 wk after pressure overloading. After 2 wk of hypertrophy induction, these PAB cats during the following 2 wk either had no further treatment or had β-adrenergic blockade. The pathological microtubule phenotype and the severe RV cellular contractile dysfunction otherwise seen in this model of RV hypertrophy (PAB No Treatment) was reversed in the treated (PAB β-Blockade) cats. Thus these data provide both a specific etiology and a specific remedy for the abnormal microtubule network found in some forms of pathological cardiac hypertrophy.
Keywords: microtubule, hypertrophy, heart failure
in 1993 a unique cytoskeletal alteration was reported in the hypertrophying heart (51, 52). It was discovered that in myocardium hypertrophying in response to pathological pressure overloading, but not in response to an equivalent degree and duration of physiological volume overloading, there is a persistent increase in microtubule network density that causes contractile dysfunction. This cytoskeletal alteration becomes more pronounced during the deterioration of initially compensatory right ventricular (RV) or left ventricular (LV) pressure overload hypertrophy into the congestive heart failure state, both in animal models of human disease (47, 48) and in human disease itself (58). In addition to contributing to the systolic and diastolic contractile dysfunction that is characteristic of pathological hypertrophy (11), the extensive decoration of cardiomyocyte microtubules with myocardial microtubule-associated protein (MAP)4 that was found to cause this microtubule network densification by stabilizing the microtubules (43) was also found to inhibit the kinesin-based transport of mRNA along microtubules that is required to support the translation of myofibrillar proteins (44). Thus this dense, heavily MAP-decorated microtubule network appears not only to contribute to the progressive contractile dysfunction that is characteristic of pathological hypertrophy but also to undermine the very basis for the compensatory hypertrophic growth response itself.
A search for the etiology of this cytoskeletal alteration led to the discovery, again in pathological pressure overloading but not in physiological volume overloading, of site-specific dephosphorylation of MAP4 that drives its microtubule binding and stabilizing function (10); that is, dephosphorylation of the serine in the KXGS motif of the first of four basic pseudorepeats within the MAP4 microtubule binding domain was both found in pathological hypertrophy and phenocopied each of the major features of the pathological microtubule network when introduced genetically into normal cardiomyocytes. It was then found that this MAP4 dephosphorylation is, in turn, driven by persistent activation of type 1 and type 2 phosphatases, and especially that of protein phosphatase 2A (PP2A) in pathological hypertrophy (10). Furthermore, this phosphatase activation is apparently driven by ongoing activation of the upstream stress-related kinase p21-activated kinase-1, or Pak1. Again, genetic induction of increased activity of upstream Pak1 or downstream PP2A or PP1 in normal cardiomyocytes and/or myocardium reproduced each of the major features of the pathological cardiac microtubule network (7).
This continuous activation of a signaling cascade leading to MAP4 dephosphorylation becomes quite interesting in the context of another progressive change, i.e., in the β-adrenergic system, that is characteristic of the specific pathological setting of decompensated cardiac hypertrophy and failure, especially because increased cardiac microtubules have been seen as a rather general feature of end-stage clinical heart disease (2, 24, 31, 58). Thus, a hallmark of deteriorating intrinsic myocardial function, even well before the onset of overt heart failure, is the increased circulating norepinephrine levels and depleted myocardial norepinephrine content that result from continuous activation of the sympathetic nervous system (9), as well as cardiomyocyte β1-adrenergic receptor downregulation (5), including that caused by MAP4 decoration of microtubules (6). This common pathophysiological setting for both microtubule network densification and increased circulating norepinephrine, coupled with the recent observation of β-agonist activation of cardiac Pak1 (33), led to the hypothesis tested in the present study. This hypothesis is that persistent adrenergic overdrive is the root cause of the microtubule network abnormality and its maladaptive effects and that β-adrenergic blockade would prevent or reverse its occurrence. The data in the present study, generated in our animal model of severe RV pressure overloading wherein persistent microtubule network densification (51, 52) and persistent β-adrenergic overdrive (14) have been well documented, support this hypothesis.
METHODS
RV pressure overload hypertrophy.
This was induced as before (15, 47) by placing a 3.2 mm internal diameter band around the proximal pulmonary artery of adult cats of either sex weighing 3.0–3.8 kg after the induction of anesthesia with ketamine HCl (10 mg/kg im) and meperidine (2.2 mg/kg im). This produced severe RV pressure overloading, with an approximate tripling of RV systolic pressure (Table 1). The LV including the interventricular septum of these pulmonary artery band (PAB) cats served as a same-animal normally loaded control, and the control cats were sham-operated by transiently passing a band around the proximal pulmonary artery. Under the same anesthesia at terminal study 4 wk later, the cats were characterized in terms of the measures given in Table 1. All operative procedures were done under full surgical anesthesia; all procedures and the care of the cats were in accordance with institutional guidelines, which met or exceeded those of the American Physiological Society and the American Association for Accreditation of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committee.
Table 1.
Characteristics of the pulmonary artery band models
| Pulmonary Artery Band |
|||
|---|---|---|---|
| Control | No Treatment | β-Blockade | |
| n | 7 | 11 | 9 |
| RV systolic pressure, mmHg | 24.9 ± 1.7 | 80.0 ± 4.9* | 72.4 ± 7.3* |
| RV weight/body weight, g/kg | 0.58 ± 0.02 | 1.26 ± 0.03* | 1.19 ± 0.04* |
| RV weight/tibial length, g/cm | 0.17 ± 0.01 | 0.33 ± 0.01* | 0.34 ± 0.01* |
| Left ventricle weight/body weight, g/kg | 2.53 ± 0.16 | 2.87 ± 0.07 | 2.60 ± 0.07 |
| Body weight, kg | 3.5 ± 0.2 | 3.4 ± 0.1 | 3.4 ± 0.1 |
| Arteriovenous O2 difference, ml/l | 36.4 ± 4.0 | 41.1 ± 2.3 | 39.6 ± 2.6 |
| RV end-diastolic pressure, mmHg | 4.1 ± 0.6 | 6.0 ± 1.1 | 6.7 ± 1.2 |
| Liver weight/body weight, g/kg | 29.6 ± 1.3 | 31.5 ± 1.3 | 27.7 ± 1.1 |
| Heart rate, beats/min | 191 ± 7 | 195 ± 6 | 155 ± 3*† |
Values are means ± SE. After a one-sample Kolmogorov-Smirnov test showed that the data comprising each of these values were normally distributed, parametric statistical comparisons were made via one-way ANOVA followed by Scheffé's S procedure. RV, right ventricle.
P < 0.01 for difference from control;
P < 0.01 for difference from No Treatment.
Cardiac β-adrenoceptor blockade.
Because increases in both RV mass and RV cardiomyocyte microtubule network density stabilize by 2 wk after PAB placement (49), for the period of 2 wk through 4 wk after surgery there was either no further treatment in half of the PAB cats or treatment by pilling with the nonselective β-adrenergic blocker propranolol HCl extended release (40 mg po b.i.d.) in the other half of the PAB cats. Given recent data suggesting that both the β1- and β2-adrenergic receptor subtypes contribute to nonlocalized cAMP signaling in the failing heart (37), it was necessary to use a nonselective agent. The following protocol allowed us to determine whether our β-adrenergic blockade regimen provided cardiac β- blockade throughout the treatment cycle. As described before in this setting (14), the adequacy of β-adrenergic blockade was assessed by maximally challenging the cats with a β-adrenoceptor agonist (continuous infusion of isoproterenol, 1.5 mg·kg−1·min−1 iv) during ketamine anesthesia (10 mg/kg im) at terminal study 12 h after the last dose of propranolol and immediately before the full anesthesia used for the terminal studies noted above. The percent heart rate increase from baseline in the control group during isoproterenol infusion was 75 ± 13%; it was 72 ± 11% in the untreated PAB cats, and it was 26 ± 7% in the chronically β-blocked PAB cats.
Cardiomyocyte isolation.
Feline cardiomyocytes were isolated separately from the RV and LV of cats as described before (32). After completion of the hemodynamic studies, the cats were heparinized (1,000 units iv) and placed on oxygen. A left thoracotomy was performed, the pericardium was opened, and the heart was rapidly removed, placed in cold buffer solution, and weighed. The aorta was then cannulated, and the coronary arteries were perfused retrograde for 10 min first with a recirculating buffer solution of the composition of (in mmol/l) 130.0 NaCl, 4.8 KCl, 1.2 MgSO4, 1.2 NaH2P04, 4.0 NaHCO3, 0.5 CaCl2, 10.0 HEPES, and 12.5 glucose; second with a nonrecirculating buffer of the same composition but without supplemental calcium; and third with a recirculating calcium-free buffer supplemented with type II collagenase (155 units/ml). Buffer perfusion was terminated when the heart was flaccid. The heart was removed from the cannula, the RV was carefully dissected from the heart, and the RV and remaining myocardium were weighed separately. The LV free wall then was dissected from the heart and weighed; the remaining myocardium was discarded. Cardiocytes from the RV and LV free walls were then isolated and used as described previously (47, 51) or they were maintained for 1 h at 37°C and pH 7.4 in collagenase-free 2.5 mmol/l Ca2+ buffer before defining contractile function.
Cardiomyocyte adenoviral infection.
Freshly isolated and plated adult feline cardiomyocytes were adenovirally infected in Pipers medium (2 ml/35-mm plate) at a multiplicity of infection (MOI) of 1 for 24 h. The cells were then rinsed in serum-free medium and incubated for a further 48 h to permit transgene expression. Parallel control cultures were infected at the same MOI with Ad-β-Gal. Over a MOI range of 1–100 plaque forming units/cell of Ad-β-Gal, there was after 48 h 90% cardiomyocyte infection as determined by immunofluorescence microscopy using a β-Gal antibody; there was no observable cytotoxicity. For the feline cardiomyocyte studies here, cells were infected 48 h earlier at an MOI of 1 either with Ad-β-Gal, an adenovirus encoding bacterial β-galactosidase as a control or with AdKDPak1, an adenovirus encoding kinase-dead Pak1-K299R as a competitive inhibitor of Pak1 activity; AdKDPak1 was a generous gift from Q. Liang (33).
Total, free, and polymerized tubulin.
Myocardial protein fractions were prepared as before (50). For the total tubulin fraction, the myocardium was homogenized in 1% SDS buffer containing (in mM) 10 Tris·HCl (pH 7.4), 0.5 DTT, and 1 Na3VO4, boiled for 5 min, and centrifuged at 16,000 g, 4°C for 10 min; this supernatant was saved as the total protein fraction. For immunoblotting, an equal amount of protein as determined by bicinchoninic acid (BCA) assay (Pierce Biotechnology) was loaded in each lane. For the free tubulin heterodimer and polymerized tubulin (microtubule) fractions, the myocardium was homogenized in a microtubule stabilization buffer containing 50% glycerol, 5% DMSO, 10 mM Na2HPO4, 0.5 mM EGTA, and 0.5 mM MgSO4 and centrifuged at 100,000 g, 25°C for 20 min. The supernatant was saved as the tubulin heterodimer fraction. The pellet was resuspended in 1% SDS buffer 4°C, boiled for 10 min, and centrifuged at 16,000 g for 10 min. This supernatant was saved as the tubulin microtubule fraction. For immunoblotting, to compare the amount of tubulin in the heterodimer versus microtubule fraction of a given sample, an equal proportion of the two fractions from the same sample was applied. For between-sample comparisons, an equal BCA-based amount of protein from each was applied. The blots were incubated for 1 h with the primary antibody. After blots were incubated with peroxidase-labeled secondary antibody, specific protein bands were detected by using horseradish peroxidase in conjunction with enhanced chemiluminescence (Perkin-Elmer). To derive semiquantitative data from these blots, National Institutes of Health ImageJ software was used to provide background-corrected integrated optical density values from scanned images of lightly exposed films.
Total, non-phospho-ser-924 MAP4, and non-phospho-ser-1056 MAP4.
For MAP4, myocardial samples were homogenized in a high-salt buffer of 100 mM Tris·HCl (pH 7.4), 10 mM EGTA, and 0.35 M NaCl containing a protease inhibitor cocktail (No. P8340; Sigma), phosphatase inhibitor cocktails 1 (No. P2850; Sigma) and 2 (No. P5726; Sigma), and 1 mM DTT; immediately put on ice for 20 min; boiled; and centrifuged at 16,000 g, 4°C for 30 min. SDS-PAGE was carried out on the supernatants with equal BCA-based protein loading for each sample using a precast 3–8% gradient Tris-acetate gel (NuPAGE; Invitrogen). Separated proteins were transferred to polyvinylidene difluoride membranes (Immobilon; Millipore); incubated overnight with a 1:1,000 dilution of our antibodies to MAP4 (50) or non-phospho-Ser-924 MAP4 or non-phospho-Ser-1056 MAP4 (validated as described) (7); incubated with peroxidase-labeled secondary antibody for 1 h at room temperature; and visualized via enhanced chemiluminescence (Perkin-Elmer). The sequences of the peptide immunogens used to generate the non-phospho-Ser-924 MAP4 and non-phospho-Ser-1056 MAP4 antibodies (Antagene), as well as antibody specificity in each case (Fig. 4, C and D, in Ref. 10), were given previously (10).
Fig. 4.
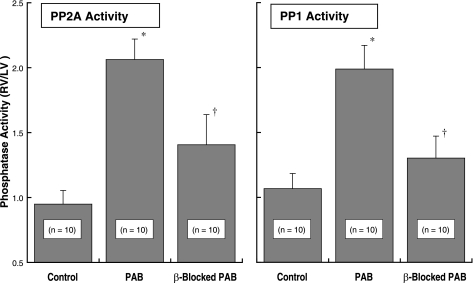
Myocardial activity of protein phosphatase 2A (PP2A) and protein phosphatase 1 (PP1). Myocardial homogenates for these assays were prepared from the same animals as those used to prepare the data in Fig. 1. PP2A activity was measured using an immunoprecipitation assay kit (No. 17-313; Upstate Biotech) as described in the methods. PP1 activity was determined using the PSP Assay System (No. P0780S; New England Biolabs), as is also described in the methods. *P < 0.05 for difference from control; †P < 0.05 for difference from PAB by 1-way ANOVA with Bonferroni post hoc analysis.
Pak1 activity.
To examine this, we took advantage of the fact that autophosphorylation of Pak1 at Threonine-423, a conserved threonine in the activation loop, induces and is essential for Pak1 activity (28, 45). Furthermore, it is known that multiple hypertrophic agents and growth factors activate myocardial Pak1 as determined by phosphorylation of Pak1 at Threonine-423 (27). Therefore, we examined both total and active Pak1 via cellular or myocardial immunoblots prepared with an antibody to total Pak1 (anti-Pak1-sc-881; Santa Cruz Biotechnology), an antibody to total Pak1 (No. 2602S; Cell Signaling), and an antibody to active Pak1 (anti-pThr423 Pak1-No. 2601S; Cell Signalling). Note that the epitope that the total Pak1 antibody recognizes is at a different location than the phospho-threonine antibody; therefore the total Pak1 blot includes all Pak1 regardless of phosphorylation status. For tissue immunoblots, samples from control and PAB cats were extracted with lysis buffer of 50 mM Tris·HCl (pH 7.4), 1% Triton X-100, and 150 mM NaCl containing 1% protease inhibitor cocktail (No. P8340; Sigma), phosphatase inhibitor cocktails 1 (No. 2850; Sigma) and 2 (No. 5726; Sigma), and 1 mM DTT; 20 mg of the protein fraction was used for Pak1 and p-Pak1 immunoblotting. For cellular immunoblots, protein was collected using lysis buffer of 100 mM Tris·HCl, 10 mM EGTA, 2 mM NaCl, 1 mM Na3VO4, and 2% Triton X-100 (pH 7.4) containing a protease inhibitor cocktail (Catalog No. P8340; Sigma) and phosphatase inhibitor cocktails 1 (Catalog No. P2850; Sigma) and 2 (Catalog No. P5726; Sigma), as well as 2 mM of calpain inhibitor E-64. Cells were scraped, homogenized, and centrifuged at 10,000 rpm for 10 min at 4°C. The supernatants were transferred into separate tubes containing SDS sample buffer, after which they were vortexed and boiled for 3 min. Equal amounts of protein were loaded into each lane, and SDS-PAGE was carried out using a precast 7% tris-acetate gel (NuPAGE; Invitrogen). A Fisher recombinant protein ladder was used as a protein size standard (BP3603; Fisher). Separated proteins were transferred onto a polyvinylidene difluoride membrane (Invitrogen), blocked in 2% BSA, and incubated overnight with a 1:200 dilution of a rabbit polyclonal anti-pThr423Pak1 antibody (Catalog No. 2601; Cell Signaling Technology), a 1:1,000 dilution of a rabbit polyclonal anti-Pak1 antibody (Catalog No. 2602; Cell Signaling Technology), or a 1:20,000 dilution of a mouse monoclonal anti-GAPDH antibody (Catalog No. 10R-G109a; Fitzgerald) as a loading control. After this, blots were incubated with a 1:10,000 dilution of either anti-rabbit IgG (Catalog No. WB401B; Promega) or anti-mouse IgG (Catalog No. WB402B; Promega), both conjugated to horseradish peroxidase, and visualized via enhanced chemiluminescence.
PP2A activity.
This was determined as reported elsewhere (22) using a PP2A immunoprecipitation phosphatase assay (No. 17-313; Upstate Biotech) that measures free phosphate with a malachite green dye. Total protein from the RVs and LVs of control and PAB cats was extracted with lysis buffer of 50 mM Tris·HCl, 1% Triton X-100, and 150 mM NaCl (pH 7.4) containing a 1% protease inhibitor cocktail (No. P8340; Sigma). To immunoprecipitate PP2A, lysates containing 200 mg of protein were incubated with 4 mg of anti-PP2A-C subunit antibody (No. 05-421; Upstate Biotech) and 40 ml of protein A-agarose slurry for 2 h at 4°C with constant rocking. The immunoprecipitates were washed three times in Tris-buffered saline and once with Ser/Thr assay buffer containing (in mM) 50 Tris·HCl (pH 7.0) and 100 CaCl2 and resuspended in 20 ml of Ser/Thr assay buffer. The reaction was initiated by the addition of 60 ml of phosphopeptide substrate (750 mM; KRpTIRR). Following incubation for 10 min at 30°C in a shaking incubator, the reaction mixture was centrifuged briefly and the supernatant was transferred to a 96-well microtiter plate. The reaction was terminated by the addition of malachite green phosphate detection solution for 10–15 min at room temperature, and free phosphate was quantified by measuring the absorbance of the mixture at 650 nm using a microplate reader.
PP1 activity.
Protein Ser/Thr phosphatase activity of PP1 was determined as reported elsewhere (41) using the PSP Assay System (No. P0780S; New England Biolabs). Total protein from the RVs and LVs of control and PAB cats was extracted with lysis buffer of 50 mM Tris·HCl, 1% Triton X-100, and 150 mM NaCl (pH 7.4) containing a 1% protease inhibitor cocktail (No. P8340; Sigma); 10 ml of the protein fraction was used for the phosphatase activity assay. The assays were performed in a 50-ml buffer of 50 mM Tris·HCl, 0.1 mM Na2EDTA, 5 mM DTT, 5 mM caffeine, and 0.01% Brij 35 (pH 7.0) at 30°C. The PP1 activity was measured with 32P-labeled myelin basic protein as a substrate in the presence of either 4 nM okadaic acid to block PP2A activity or 1 mM okadaic acid to block both PP1 and PP2A activity (7, 57). Thus PP1 activity was calculated from okadaic acid-sensitive protein phosphatase activity by deducting phosphatase activity with 1 mM okadaic acid from activity with 4 nM okadaic acid. The reaction was initiated by adding 10 ml of 32P-labeled myelin basic protein as a substrate and incubated at 30°C for 10 min. The reaction was terminated by the addition of 200 ml of 20% trichloroacetic acid, cooled on ice for 5 min, and centrifuged; 200 ml of the supernatant was used to count the released 32P in the assay.
Immunofluorescence confocal microscopy.
The cells were fixed and stained as described previously (8). Briefly, cardiomyocytes plated on coverslips were extracted for 1 min in a 1% Triton X-100 buffer containing (in mM) 2 EGTA, 0.1 EDTA, 1 MgSO4, and 100 MES (pH 6.75). They were then rinsed three times in the same buffer with no detergent and fixed in 3.7% formaldehyde in this same buffer for 30 min. After each coverslip was blocked with 10% donkey serum in 0.10 M glycine and 0.05 M PBS for 30 min at room temperature, the cells were incubated at 4°C overnight in a 1:200 dilution of primary antibody in 2% normal donkey serum in PBS. After being washed three times with PBS, the cells were incubated at room temperature for 2 h in a 1:200 dilution of fluorescein-conjugated secondary antibody in 2% normal donkey serum in PBS. Optical sections (0.1 mm) were acquired using a Zeiss LSM510META confocal microscope equipped with a 30 mW Argon laser (458, 477, 488, and 514 nm), a 1 mW Helium-Neon laser (543 nm), a 5 mW Helium-Neon laser (633 nm), and a Plan-Apochromat 63x/1.40 objective to obtain high resolution images. Adobe Photoshop 7.0 software was used for superimposing the laser channels and for cropping and rotating images. To derive semiquantitative data from cardiomyocyte micrographs, the channel of interest was converted to grayscale, the “Lasso” tool in Adobe Photoshop 7.0 software was then used to outline the cell boundary, and the mean pixel intensity within this boundary was found using the “Histogram” tool.
Sarcomere mechanics.
Sarcomere motion in isolated cardiomyocytes was studied using the Ionoptix system as used before (26) (IonOptix, Milton, MA). Freshly isolated cardiomyocytes in 2.5 mM Ca2+ buffer were placed in a custom-made double-jacketed temperature-controlled study chamber that was positioned on an inverted Olympus IX70 microscope (Olympus America, Melville, NY). Temperature was maintained constant at 37 ± 0.1°C by a M3 Lauda water bath (Lauda Wobser, Germany). Two opposing platinum electrodes were mounted in the study chamber to deliver 0.25-Hz, 5-ms DC pulses of alternating polarity at 20% greater than threshold. The cardiomyocyte image that was collected by a 40× objective lens (Olympus) was diverted to a video charge-coupled device MyoCam camera (IonOptix). The charge-coupled device camera was adapted to acquire images at 240 Hz with a sarcomere length measurement time resolution of 4.2 ms. Calibration of the system was performed before each experiment using a standard micrometer slide having a 2 mm line spacing. Sarcomere dynamics were recorded by specialized data acquisition and analysis software (SoftEdge and IonWizard; IonOptix). The signal-to-noise ratio was significantly improved by averaging 10 sequential contraction runs. Only cardiomyocytes with the following characteristics were recorded: single rod-shaped cells that were unattached to any adjacent cell that contracted in response to each electrical stimulus and that were quiescent between stimuli. Standard video edge-detection techniques and Fast Fourier Transform spectral analysis were then used to isolate a well-defined sarcomere pattern in the cell of interest. When after 5 to 10 contractions the extent of shortening was stable, 10 contractions were sampled and averaged to improve signal-to-noise ratios and to yield a final profile of sarcomere length versus time during one cell contraction. Absolute twitch amplitude was measured as the difference between systolic sarcomere length and resting sarcomere length. Shortening velocities of sarcomere length transients were determined by numerical time differentiation.
Data analysis.
Data were expressed as means ± SE; any statistical comparisons are specified in the legends of the table and of the individual figures.
RESULTS
Effects of systemic β-adrenergic receptor blockade during severe pressure overload hypertrophy on components of the cardiac microtubule array.
As seen in Table 1, a band of the diameter used here reliably produces within 4 wk severe RV hypertrophy (47) but not RV failure. Overt myocardial failure with pleural effusion and ascites, as well as abnormally elevated RV end-diastolic pressure and arteriovenous O2 difference, are only seen with tighter banding (47). However, microtubule network densification and cellular contractile dysfunction are characteristic of the level and duration of RVH (47, 51, 52) seen in this study. Indeed, at 2 wk after PAB, when β-blockade was initiated in the PAB + β-blockade group, the amount of RVH (49) and the extent of microtubule changes are already at a new steady state that continues for the subsequent 2 wk (51). Note that throughout this study, including here, propranolol treatment initiated at the midpoint of the 4-wk PAB period, when the hypertrophic growth is complete and the abnormal microtubule phenotype is well established, returns the cytoskeletal changes toward but not to baseline. However, the residual difference is generally not statistically different. In simplest terms, this residual difference may well exist because a competitive β-antagonist is being used and perhaps also because factors other than adrenergic input have a role in this pathophysiology.
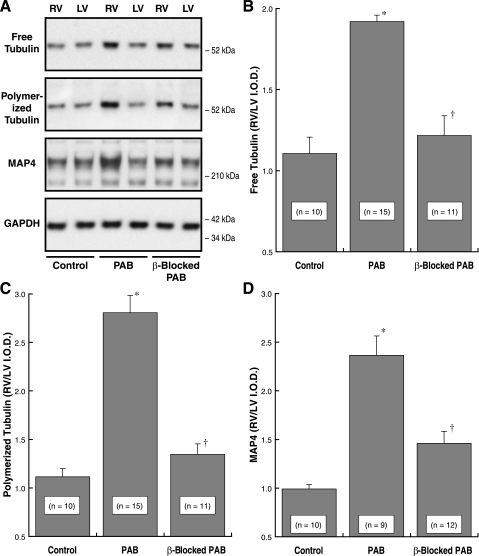
For the two cats whose data are shown as an example in Fig. 1A, the RV systolic pressure was increased from our normal value of 25 ± 2 mmHg to 60 mmHg for the untreated PAB cat and to 65 mmHg for the β-blocked PAB cat treated with propranolol, a nonselective β-adrenergic blocker. In both cats, PAB produced a robust hypertrophic response: our RV/body weight value for normal cats is 0.58 ± 0.02 g/kg; for the untreated PAB cat this value was 1.3 g/kg, and for the β-blocked PAB cat this value was 1.2 g/kg. The most obvious differences in Fig. 1A between the non-β-blocked and β-blocked cats are major reductions in the hypertrophy-induced increases in RV polymerized tubulin and MAP4 in the latter. These conclusions are born out in Fig. 1, B–D, by summary data from these and additional cats whose characteristics are given in Table 1. Despite similar RV pressure overloading and a similar hypertrophic response in the two groups of cats, β-blockade attenuated the hypertrophy-induced increase in free tubulin and especially in polymerized tubulin and MAP4.
Fig. 1.
The effects of β1- and β2-adrenergic receptor blockade on components of the cardiac microtubule network during pressure overload hypertrophy. A: example immunoblots from the right ventricles (RVs) and left ventricles (LVs) of a pair of cats with 4 wk of pulmonary artery band (PAB)-induced RV hypertrophy; 1 animal was β-blocked with propranolol for the final 2 wk, and the other was not β-blocked. A monoclonal anti-β-tubulin antibody (clone DM-1B; Abcam) was used for the tubulin blots, our anti-myocardial microtubule-associated protein (MAP)4 antibody (43) was used for the MAP4 blot, and a monoclonal anti-GAPDH antibody (clone 6C5; Upstate Biotech) was used for the loading control blot. B–D: summary data for the background-corrected integrated optical density (I.O.D.) ratio of RV to LV in immunoblots from the indicated numbers of normal control cats, PAB cats without β-blockade, and PAB cats with β-blockade. *P < 0.05 for difference from control; †P < 0.05 for difference from PAB by 1-way ANOVA with Bonferroni post hoc analysis. For the LVs alone, there was no within-group difference for any of these 3 variables by 1-way ANOVA with Bonferroni post hoc analysis (P = 0.69 for LV free tubulin; P = 0.43 for LV polymerized tubulin; P = 0.29 for LV MAP4; each P value is the minimum value for the 3 comparisons within that group).
Linkage of β-adrenergic stimulation to Pak1 activation in vitro.
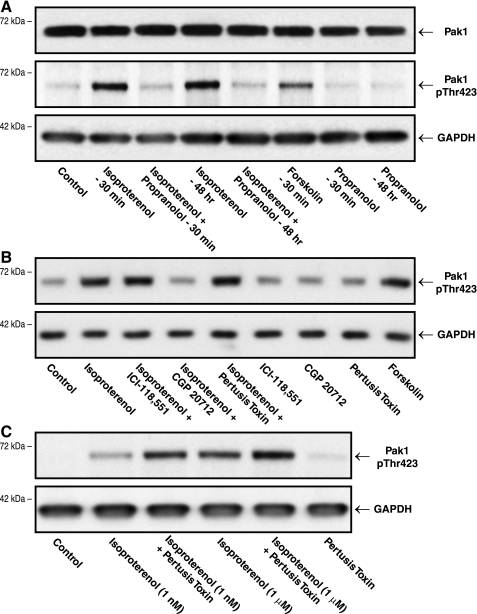
Figure 2A shows that the nonselective β-adrenergic agonist isoproterenol causes increased phosphorylation of Pak1 at Threonine-423, an activation loop threonine whose phosphorylation induces and is required for Pak1 activity (28, 45). Because we are postulating here a linkage between β-adrenergic activation of Pak1 and excess cardiomyocyte microtubules, it is important to note that this effect is present both acutely at 30 min and at the much later time point of 48 h, such that persistent cardiomyocyte β-adrenergic receptor stimulation leads to persistent Pak1 activation. This persistence of Pak1 activation is especially pertinent in the context of our previous in vivo data, which showed ongoing Pak1 activation in hypertrophied myocardium at the latest time point examined, i.e., 10 wk after severe pressure overloading (Fig. 1 in Ref. 7). This isoproterenol effect is blocked by propranolol, which, in turn, has no independent effect on Pak1 activation. Finally, as would be expected (Fig. 1B in Ref. 33), there is no β-agonist or β-antagonist effect on total Pak1.
Fig. 2.
The effect of β-adrenergic stimulation on cardiomyocyte p21-activated kinase-1 (Pak1) activation. As described in methods, the immunoblots were prepared from homogenates of cultured quiescent adult feline cardiomyocytes. A: these cells were untreated (control) or treated with 1 mM isoproterenol for 30 min with or without pretreatment and concurrent treatment with 20 mM propranolol for 30 min, or with 100 nM isoproterenol in the presence or absence of 20 mM propranolol for 48 h. Forskolin was used at a concentration of 1 mM for 30 min, and propranolol alone was used at 20 mM for 30 min or 48 h. B: these cells were untreated (control) or treated with 1 mM isoproterenol for 30 min with or without a 15-min pretreatment and concurrent treatment with 300 nM of the selective β2-antagonist ICI-118,551 hydrochloride (Catalog No. 0821; Tocris), or 300 nM of the selective β1-antagonist CGP 20712 dihydrochloride (Catalog No. 1024; Tocris), or a 3-h pretreatment with 1.5 mg/ml of pertussis toxin (Catalog No. 3097; Tocris). Forskolin was used at a concentration of 1 mM for 30 min. Concentrations of ICI-118,551 and CGP 20712 were chosen based on previous data showing specific antagonism of β2- and β1-receptors, respectively (46), as well as data showing that at 300 nM, for both agents all high affinity binding sites on cardiomyocytes are occupied (29). The concentration of pertussis toxin was chosen based on data showing inhibition of Gi-mediated pathways in adult cardiomyocytes (55). C: these cells were untreated (control) or treated for 30 min with 1 nM or 1 mM isoproterenol with or without a 3-h pretreatment with 1.5 mg/ml of pertussis toxin. Relief from Gi effects enhanced the isoproterenol effects on Pak1 activation. A polyclonal anti-Pak1 antibody (No. 2602S; Cell Signaling) was used for the Pak1 blot; a polyclonal anti-pThr-423Pak1 antibody (No. 2601S; Cell Signaling) was used for the p-Pak1 blots. Note that the epitope that the total Pak1 antibody recognizes is at a different location than that recognized by the phospho-threonine antibody; therefore the total Pak1 blot includes all Pak1 regardless of phosphorylation status. A monoclonal anti-GAPDH antibody (No. 10R-G109a; Fitzgerald) was used for the loading control blots. Data similar to these were gathered in 3 additional experiments.
Note further that Fig. 2A shows that exposure of these cells to forskolin, which increases intracellular cAMP and thus protein kinase A via β-adrenoceptor-independent direct adenylyl cyclase activation, also causes an increase in active, phospho-Thr-423 Pak1. This would suggest that Gs-dependent adrenergic signaling may be at least as important to Pak1 activation as the currently postulated primacy of Gi-dependent adrenergic Pak1 signaling (27). This distinction is important, since the in vivo portion of the present study is predicated on the idea that any beneficial effects of β-blockade on Pak1 activation and downstream cytoskeletal changes are based on blockade of the primarily β1-adrenergic, Gs-mediated effects of the excessive circulating norepinephrine found in heart failure (5, 9). We therefore did the experiment in isolated cardiomyocytes shown in Fig. 2B in an effort to clarify this situation. Figure 2B shows that isoproterenol and forskolin cause a similar increase in activated, phospho-Thr-423 Pak1. Pretreatment with the β2-selective antagonist ICI-118,551 did not affect the isoproterenol-induced increase in phospho-Thr-423 Pak1, but pretreatment with the β1-selective antagonist CGP 20712 reduced the isoproterenol-induced increase in phospho-Thr-423 Pak1. Pretreatment with pertussis toxin, which impairs Gi protein interaction with β-adrenoceptors, did not affect the isoproterenol-induced increase in phospho-Thr-423 Pak1, suggesting that activation is via the β1-adrenoreceptor. Figure 2C shows that when a subsaturating concentration of isoproterenol was used, pertussis toxin pretreatment greatly enhanced Pak1 phosphorylation at Threonine-423, indicating that Pak1 is activated through β1-adrenoreceptors. In summary, these findings in quiescent adult cardiomyocytes from a large mammal show that the nonselective β-adrenergic agonist isoproterenol causes phosphorylation and thus activation of Pak1 primarily through the β1-adrenoceptor and its downstream protein kinase A activity rather than through the β2-adrenoceptor and its downstream phosphoinositide-3-kinase activity (23).
Specificity of β-adrenergic-Pak1 linkage to tubulin polymerization in vitro and effect of systemic β-adrenergic receptor blockade during severe pressure overload hypertrophy on Pak1 signaling of microtubule network densification.
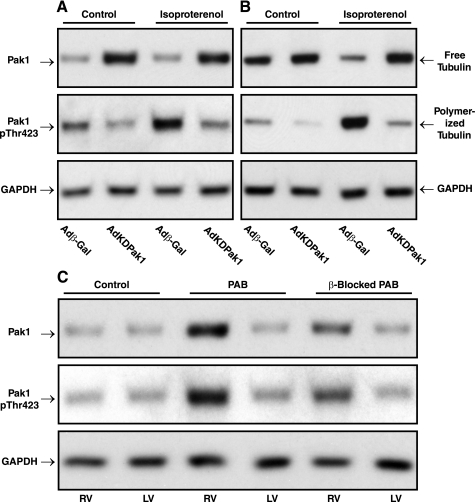
Figure 3A shows that isoproterenol-induced activation of Pak1 in adult feline cardiomyocytes is greatly reduced by prior infection of these cells with AdKDPak1, an adenovirus encoding kinase-dead Pak1. Figure 3B shows that the isoproterenol-induced Pak1 activation is associated with an increase in the ratio of polymerized to free tubulin that, once again, is greatly reduced by prior infection of these cells with AdKDPak1. Note that we had shown previously that cardiomyocyte infection with AdKDPak1 has no independent, nonspecific effect on cardiomyocyte microtubules (Fig. S1 in Ref. 7). Taken together with the data in Fig. 2, these data show that β-adrenergic stimulation, such as that to which the hemodynamically stressed heart is exposed, not only activates Pak1 but also causes tubulin polymerization, apparently through a pathway that depends on active Pak1.
Fig. 3.
Pak1 specificity of β-adrenergic signaling through active Pak1 to increased microtubules in vitro and β-adrenergic blockade of this effect in vivo. A and B: AdKDPak1 infection of normal feline cardiomyocytes, which doubled the amount of total Pak1 in these cells, prevented isoproterenol induction of both Pak1 activation (A) and tubulin polymerization (B). Data similar to these were gathered in additional experiments. C: these immunoblots showing total and active Pak1 were prepared from myocardial homogenates from the RVs and LVs of 4-wk PAB cats with and without β-adrenergic blockade with propranolol for the final 2 wk. For 3 experiments such as that shown in this panel, the densitometric ratio of RV to LV Pak1 was 1.83 ± 0.06 for the untreated PAB cats and 1.33 ± 0.03 for the β-blocked PAB cats. For the ratio of RV to LV p-Pak1, the values were 1.81 ± 0.37 for the untreated PAB cats and 1.26 ± 0.14 for the β-blocked PAB cats. For the 3 panels in this figure, a polyclonal anti-Pak1 antibody (No. 2602S; Cell Signaling) was used for the Pak1 blots, a polyclonal anti-pThr-423Pak1 antibody (No. 2601S; Cell Signaling) was used for the p-Pak1 blots, a monoclonal anti-β-tubulin antibody (clone DM-1B; Abcam) was used for the tubulin blots, and a monoclonal anti-GAPDH antibody (No. 10R-G109a; Fitzgerald) was used for the loading control blots.
Turning from in vitro to in vivo data, and starting upstream at Pak1, the immunoblots in Fig. 3C show that in the pressure-overloaded RV versus the normally loaded same-animal control LV from a PAB cat there is a modest increase over the control cat in the quantity of Pak1 but a greater increase in Pak1 activity as estimated from phosphorylation of Pak1 at Thr423. The right two lanes of these immunoblots show that in a β-blocked PAB cat each of these increases is greatly attenuated by β-adrenergic blockade with propranolol. Thus, these in vivo data are consistent with the in vitro data shown in Fig. 2, A and B.
Effects of systemic β-adrenergic receptor blockade during severe pressure overload hypertrophy on events downstream from Pak1 activation.
Looking next at downstream type 1 and type 2A phosphatase activity, the data in Fig. 4 show that there is a doubling of the activity of both PP2A and PP1 at 4 wk after PAB when comparing the pressure-overloaded RVs with the normally loaded same-animal control LVs. These data are similar to those we reported recently for this model of pressure overload hypertrophy (Fig. 5 in Ref. 7). However, what is uniquely shown here is that each of these increases in phosphatase activity is greatly attenuated by β-adrenergic blockade with propranolol.
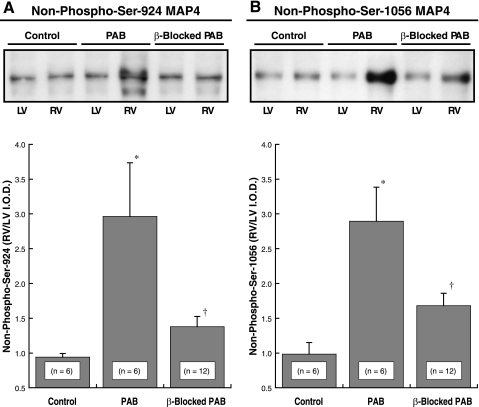
In our study using mass spectrometry to characterize site-specific MAP4 phosphorylation status in hypertrophied myocardium (10), we found MAP4 dephosphorylation at Ser-924 and Ser-1056 in the assembly-promoting region of the COOH-terminal microtubule binding domain. Ser-924 dephosphorylation and to a lesser extent Ser-1056 dephosphorylation increased MAP4 binding to microtubules (Fig. 7 in Ref. 10). We therefore looked here as shown in Fig. 5 yet further downstream from the data shown in Fig. 4 at MAP4 Ser-924 and Ser-1056 dephosphorylation, since this appears to be the major determinant of MAP4-microtubule affinity and thus microtubule network stability and density (10). It is clear from the data in Fig. 5 that pathological hypertrophy-associated MAP4 dephosphorylation at Ser-924 and Ser-1056 is largely prevented by β-adrenergic blockade with propranolol.
Fig. 5.
Non-phospho-Ser-924 and non-phospho-Ser-1056 MAP4. Example immunoblot above and summary immunoblot data below from the RVs and LVs of control cats and of cats with 4 wk of PAB-induced RV hypertrophy are shown; the animals were either β-blocked with propranolol for the final 2 wk or they were not β-blocked. A: example immunoblot and summary data prepared using our anti-non-phospho-Ser-924 MAP4 antibody (10). B: example immunoblot and summary data prepared using our anti-non-phospho-Ser-1056 MAP4 antibody (10). For the 2 immunoblots, Coomassie Blue staining of corresponding gels was used to verify equal protein loading. *P < 0.05 for difference from control; †P < 0.05 for difference from PAB by 1-way ANOVA with Bonferroni post hoc analysis.
Effects of systemic β-adrenergic receptor blockade during severe pressure overload hypertrophy on cardiomyocyte microtubule network density and contractile function.
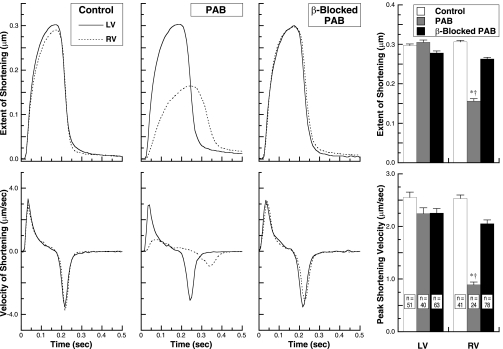
The confocal micrographs in Fig. 6 show that the hypertrophy-associated increase in cardiomyocyte microtubule network density following RV pressure overloading is largely prevented by concurrent β-adrenergic receptor blockade. Likewise, although the PAB cats did not yet exhibit overt right heart myocardial failure, the sarcomere mechanics data in Fig. 7 show that although the contractile function of normally loaded LV cardiomyocytes is unaffected by β-adrenergic receptor blockade, the striking decrease in RV cardiomyocyte contractile function seen after severe RV pressure overloading is prevented by concurrent β-adrenergic receptor blockade. Thus, not only the structural hypertrophy-associated microtubule network densification but also its functional consequence of cellular contractile dysfunction are prevented by β-adrenergic receptor blockade.
Fig. 6.
The effect of β-adrenergic receptor blockade on RV cardiomyocyte microtubule network density during 4 wk of RV pressure overload hypertrophy. The micrographs were prepared from cardiomyocytes isolated from the RVs of control and 4-wk PAB cats with and without β-adrenergic blockade with propranolol for the final 2 wk. A monoclonal anti-β-tubulin antibody (clone DM-1B; Abcam; red) was used to identify the microtubules, and our anti-MAP4 antibody (green) was used for MAP4. The orange to yellow signal shows colocalization of these 2 proteins. For the β-tubulin channel in which the microtubules are labeled, the number inset into each micrograph gives the mean pixel intensity, determined as described in methods, of the microtubule network within the boundary of that cardiomyocyte; each micrograph is a single 0.1 mm confocal section taken at the level of the nuclei. Scale bar = 20 mm. The graph provides summary data for mean pixel intensity for 30 RV cardiomyocytes from each of the 3 groups. Six cells were studied from each of 5 cats in the control, PAB, and β-blocked PAB groups. *P < 0.05 for difference from control; †P < 0.05 for difference from PAB and control by 1-way ANOVA with Bonferroni post hoc analysis.
Fig. 7.
The effect of β-adrenergic receptor blockade on contractile function of normally loaded LV cardiomyocytes and same-animal normally loaded or pressure-overloaded RV cardiomyocytes from the RVs and LVs of control cats and 4-wk PAB cats with and without β-adrenergic blockade with propranolol for the final 2 wk. The number of cats in each group from which these cells were isolated was 4. Example data for the extent and velocity of LV and RV cardiomyocyte sarcomere shortening during contractions sampled at 240 Hz are given in the left 6 panels, and summary data are given for the specified number of cells in each group in the right 2 panels. *P < 0.05 for difference from control RVs; †P < 0.05 for difference from β-blocked PAB RVs by 1-way ANOVA with Bonferroni post hoc analysis.
DISCUSSION
Why was it important to do this study?
The impetus for this study was to discover the root cause of hypertrophy-dependent microtubule network densification and the means for its prevention or reversal. This was not simply because of its intrinsic scientific interest. Instead, and as a matter having major translational potential, apart from microtubule depolymerization we are unaware of anything other than acute pharmacological inotropic stimulation that has anything as dramatic an effect in reversing the contractile dysfunction of pressure-hypertrophied myocardium in vivo or of its constituent cardiomyocytes in vitro as that shown in Figs. 3–5 of our earlier study of microtubule depolymerization in canine aortic stenosis (30), a chronically progressive, clinically highly relevant model of its human analog (58).
Cause(s) of microtubule network densification-STAT3?
An attractively simple candidate etiology for this cytoskeletal abnormality is signal transducer and activator of transcription 3 (STAT3) inhibition of the microtubule destabilizing protein stathmin, where STAT3 stabilizes microtubules by binding to the carboxy-terminal tubulin-interacting domain of stathmin, thereby antagonizing its microtubule destabilizing activity (35). Indeed, there is transient STAT3 tyrosine phosphorylation after acute pressure overloading of the LV in the rat (39), rabbit (34), and mouse (56), as well as more persistent STAT3 phosphorylation in association with a stabilized microtubule network in murine LV hypertrophy and failure (36). However, in the same feline RV pressure overload model used in our earliest work on the cardiac cytoskeleton (51), in much of our subsequent work in this area (11), and here, although there is transient STAT3 recruitment to the cytoskeleton at 2 days after pressure overloading (54), this is no longer significant by 1 wk after pressure overloading when the characteristic indefinitely persistent dense microtubule network is just beginning to form (49). Thus it is not clear that STAT3 has a causal role in producing and more especially in maintaining the hypertrophy-related dense cardiac microtubule network.
Cause(s) of microtubule network densification-MAP4 binding to microtubules?
Apart from this specific problem with a STAT3-dependent mechanism, a more general and crucial problem is that any unifying explanation for the hypertrophy-dependent microtubule phenotype must account not only for increased αβ-tubulin heterodimers and microtubules (51, 52) but also for increased MAP4 and MAP4 decoration of microtubules (43). In thinking about this, especially given the prevalent control of microtubule stability and thus network density in interphase cells by the family of structural MAPs (16), the microtubule-MAP abnormality seen in Alzheimer's disease provided an important insight, since microtubule network density and stability, and its regulation, are just the opposite of what we see in pathological cardiac hypertrophy. Thus, in Alzheimer's disease a neuronal MAP, Tau, dissociates from microtubules and forms characteristic neurofibrillary tangles of paired helical filaments (25). This is caused by hyperphosphorylation of Tau, especially at sites within the microtubule-binding domain, which reduces its affinity for microtubules (53). Specifically, phosphorylation of the single human Tau Ser-262 residue within the KXGS motif of the first microtubule binding domain repeat essentially abolishes microtubule binding of Tau (4), thus destabilizing the microtubule network. In direct contrast, when we used mass spectrometry to examine site-specific phosphorylation of native MAP4 from hypertrophied myocardium, we found striking dephosphorylation of feline MAP4 Ser-924 (10). This serine residue, which is within the KXGS motif of the first microtubule binding domain repeat of MAP4, is homologous to human Tau Ser-262. In an etiologically critical experiment, when we expressed a dephosphormimetic Ser-924 → Ala MAP4 mutant in normal cardiomyocytes, all of the features of the microtubule network seen in pathological pressure overload hypertrophy were reproduced (10).
Does site-specific MAP4 dephosphorylation produce a dense, stable microtubule network?
Because this persistent site-specific MAP dephosphorylation in hypertrophied myocardium increases MAP4-microtubule affinity, this finding could explain both the extensive MAP4 decoration of microtubules and their stabilization (43). Furthermore, as we have discussed elsewhere (10), the increased synthesis of the tubulins and MAP4 that we observe (43, 49) could well be caused by the loss of negative feedback control when the hyperstabilized microtubules and their associated MAP4 are effectively isolated from their ordinarily dynamic intracellular pools. The question then becomes that of what is controlling MAP4 Ser-924 phosphorylation status. Turning again to the analogy with regulation of microtubule stability in Alzheimer's disease, decreased activity of PP2A appears to be primarily responsible for increased phosphorylation of human Tau Ser-262 (17, 21) in that setting. We therefore asked whether a directionally opposite phosphatase dysregulation from that seen in Alzheimer's disease could be responsible for the site-specific MAP4 dephosphorylation that appears to be responsible for microtubule stabilization in pathological cardiac hypertrophy. Our recent data indicate that such is the case, since increased activity of the predominant myocardial protein phosphatases, both that of PP1 and especially that of PP2A, is a striking and persistent feature of severe pressure overload hypertrophy (7). Finally, in asking what is responsible for persistent activation of PP2A in this setting, the observation that activity of an upstream multifunctional enzyme, Pak1, is increased in early cardiac hypertrophy (33) provided another important insight, since in our hands Pak1 activity is persistently increased in severe pressure overload hypertrophy and overexpression of Pak1 in normal cardiomyocytes reproduces the hypertrophic microtubule phenotype (7).
Is activated Pak1 etiologically upstream of increased phosphatase activity?
Of interest in this context, it would appear that Pak1 activation is protective against short-term toxic effects of doxorubicin on isolated neonatal cardiomyocytes (33). Although the following potential etiology for this Pak1 protective effect was not investigated in that study, doxorubicin has striking microtubule disrupting effects in isolated neonatal cardiomyocytes (40), and this microtubule disruption may be acting through effects on MAP4 (3, 20). Thus, in a setting where a toxin is disrupting microtubules, the microtubule stabilizing pathway initiated by Pak1 activation of phosphatases may be protective, whereas in a hypertrophied cardiomyocyte with contractile and transport abnormalities, Pak1 induction of microtubules in excess of a normal baseline is deleterious.
Does increased β-adrenergic activity reproduce the signaling to an endpoint of a dense microtubule network?
The fact that increased β-adrenergic activity is regularly seen in pathological cardiac hypertrophy, including this model of severe RV pressure overloading (14), together with the recent observation that β-adrenergic agonists cause Pak1 activation (33), and that adenosine inhibits the cardiac hypertrophy-dependent microtubule changes (19), likely through its antiadrenergic effects (12), led to the present study; that is, in the setting of this knowledge, we hypothesized and then showed here that chronic β-adrenergic blockade would prevent the abnormal microtubule network from forming during pathological cardiac hypertrophy.
Can β-adrenergic blockade prevent this?
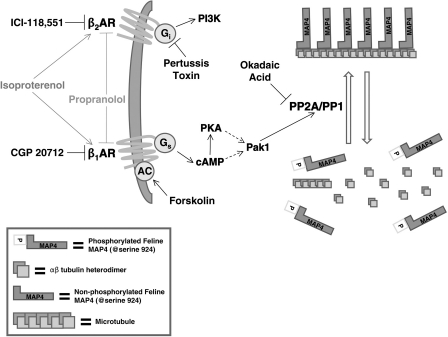
The data in the present study, which was framed in terms of the schema shown in Fig. 8, strongly support this idea. Thus chronic β-adrenergic blockade prevents the increases in free tubulin, microtubules, and MAP4 that otherwise occur quite reliably and predictably in the feline RV pressure overload model that we employed here. Furthermore, our in vitro data in Fig. 2 show that β-adrenergic stimulation activates Pak1 and that active Pak1 causes microtubule polymerization, whereas the balance of the in vivo data in this study shows that β-adrenergic blockade suppresses the ongoing activity of each member of the signaling cascade that we have identified (7) as causing and maintaining via increased phosphatase activity site-specific MAP4 dephosphorylation and consequent microtubule network MAP4 binding, as well as network stabilization and densification (10). Indeed, by establishing in this and the two preceding studies (7, 10) a signaling cascade originating in the β1-adrenergic overdrive characteristic of pathological cardiac hypertrophy that leads to microtubule network densification, we have delineated a basic mechanism that helps to explain the counterintuitive observation that β-adrenergic blockade is beneficial to the failing heart even when characterized by a reduced inotropic state.
Fig. 8.
Signaling pathways mediating β-adrenergic effects on the cardiac microtubule network. The nonselective β-agonist isoproterenol, which is structurally similar to adrenaline, activates both β1- and β2-adrenergic receptors (AR), but as shown by the data in Fig. 2 the predominant effects on Pak1 and then phosphatase activation are mediated through the β1-adrenoceptor-Gs pathway. The nonselective competitive β-antagonist propranolol blocks or reverses the activation of this same pathway. Feline MAP4 Serine-924 is homologous to human MAP4 Serine-914. PI3K, phosphatidylinositol-3-kinase.
Is this an exclusive mechanism?
It should be clear that the data in this study do not constitute a general mechanism explaining the entirety of the well-established beneficial effects of chronic β-adrenergic blockade in pathological myocardial states (38), which are likely based to some extent on preventing the more general cytotoxic cardiomyocyte effects such as we initially observed with sustained β-adrenergic stimulation (32). As an example, β-blockade-mediated reductions in phosphatase activity could increase Ca2+ cycling protein phosphorylation, thus enhancing inotropism via effects on the rate and amplitude of the Ca2+ transient (41). Instead, the data reported here are but one specific, concrete example of the more general improvement in biological function of the cardiac myocyte by β-blockade predicted some time ago (18). Furthermore, and as an explicit example of settings to which these findings do not apply, it is known that there is myocardial microtubule depolymerization in the immediate postischemic period (42), such that prevention of microtubule polymerization by β-blockade cannot explain its beneficial effects in this setting (1). It is nonetheless true that a significant portion of chronic clinical heart disease eventuates as high wall stress pressure overload hypertrophy, and in our hands both in patients (58) and in multiple animal models (12) of this entity the pathological microtubule phenotype is quite characteristic. Furthermore, apart from this rather specific hemodynamic setting that we have studied, other investigators have identified increased microtubules more generally in advanced clinical heart disease (2, 24, 31).
Conclusion
In this context, given the deleterious effects of a dense, MAP4-decorated microtubule network on myocardial contractile and transport function (12), might the prevention of these functional abnormalities favorably impact the natural history of those cardiac diseases in which they would be expected to occur? In terms of practical implications framed within the outlined shown in Fig. 8, where β1- but not β2-adrenergic input initiates Pak1-driven microtubule network densification, this is the most interesting question raised by this entire series of studies of the cardiac cytoskeleton originating in 1993 (51), since it is entirely possible that chronic β-adrenergic blockade instituted early might delay or prevent the appearance of the pathological microtubule phenotype.
GRANTS
This work was supported, in whole or in part, by National Heart, Lung, and Blood Institute Grants HL-094545 and HL-104287 (to G. Cheng) and RHL-092124 (to D. Kuppuswamy) and by a Department of Veterans Affairs Merit Review Grant (to G. Cheng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G. Cheng, H.K., C.F.B., and J.G.W. performed experiments; G. Cheng, H.K., C.F.B., J.G.W., and G. Cooper analyzed data; G. Cheng, H.K., C.F.B., J.G.W., D.K., and G. Cooper approved final version of manuscript; H.K., J.G.W., D.K., and G. Cooper interpreted results of experiments; H.K., C.F.B., J.G.W., D.K., and G. Cooper edited and revised manuscript; J.G.W., D.K., and G. Cooper conception and design of research; G. Cooper prepared figures; G. Cooper drafted manuscript.
REFERENCES
- 1. Abraham WT, Greenberg BH, Yancy CW. Pharmacologic therapies across the continuum of left ventricular dysfunction. Am J Cardiol 102: 21G–28G, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Aquila-Pastir LA, DiPaola NR, Matteo RG, Smedira NG, McCarthy PM, Moravec CS. Quantitation and distribution of β-tubulin in human cardiac myocytes. J Mol Cell Cardiol 34: 1513–1523, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bash-Babula J, Toppmeyer D, Labassi M, Reidy J, Orlick M, Senzon R, Alli E, Kearney T, August D, Shih W, Yang JM, Hait WN. A phase I/pilot study of sequential doxorubicin/vinorelbine: effects on p53 and microtubule-associated protein 4. Clin Cancer Res 8: 1057–1064, 2002 [PubMed] [Google Scholar]
- 4. Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of Tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11: 153–163, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, Stinson EB. β1- And β2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective β1-receptor down-regulation in heart failure. Circ Res 59: 297–309, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Cheng G, Qiao F, Gallien TN, Kuppuswamy D, Cooper G. Inhibition of β-adrenergic receptor trafficking in adult cardiocytes by MAP4 decoration of microtubules. Am J Physiol Heart Circ Physiol 288: H1193–H1202, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Cheng G, Takahashi M, Shunmugavel A, Wallenborn JG, DePaoli-Roach AA, Gergs U, Neumann J, Kuppuswamy D, Menick DR, Cooper G. Basis for MAP4 dephosphorylation-related microtubule network densification in pressure overload cardiac hypertrophy. J Biol Chem 285: 38125–38140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng G, Zile MR, Takahashi M, Baicu CF, Bonnema DD, Cabral F, Menick DR, Cooper G. A direct test of the hypothesis that increased microtubule network density contributes to contractile dysfunction of the hypertrophied heart. Am J Physiol Heart Circ Physiol 294: H2231–H2241, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Chidsey CA, Braunwald E. Sympathetic activity and neurotransmitter depletion in congestive heart failure. Pharmacol Rev 18: 685–700, 1966 [PubMed] [Google Scholar]
- 10. Chinnakkannu P, Samanna V, Cheng G, Ablonczy Z, Baicu CF, Bethard JR, Menick DR, Kuppuswamy D, Cooper G. Site-specific microtubule-associated protein 4 dephosphorylation causes microtubule network densification in pressure overload cardiac hypertrophy. J Biol Chem 285: 21837–21848, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cooper G. Cytoskeletal networks and the regulation of cardiac contractility: microtubules, hypertrophy, and cardiac dysfunction. Am J Physiol Heart Circ Physiol 291: H1003–H1014, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Cooper G. Proliferating cardiac microtubules. Am J Physiol Heart Circ Physiol 297: H510–H511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooper G, Kent RL, McGonigle P, Watanabe AM. β-Adrenergic receptor blockade of feline myocardium. Cardiac mechanics, energetics, and β-adrenoceptor regulation. J Clin Invest 77: 441–455, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper G, Kent RL, Uboh CE, Thompson EW, Marino TA. Hemodynamic versus adrenergic control of cat right ventricular hypertrophy. J Clin Invest 75: 1403–1414, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cooper G, Satava RM, Jr, Harrison CE, Coleman HN. Mechanism for the abnormal energetics of pressure-induced hypertrophy of cat myocardium. Circ Res 33: 213–223, 1973 [DOI] [PubMed] [Google Scholar]
- 16. Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci 23: 307–311, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Drewes G, Mandelkow EM, Baumann K, Goris J, Merlevede W, Mandelkow E. Dephosphorylation of Tau protein and Alzheimer paired helical filaments by calcineurin and phosphatase-2A. FEBS Lett 336: 425–432, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Eichhorn EJ, Bristow MR. Medical therapy can improve the biological properties of the chronically failing heart. A new era in the treatment of heart failure. Circulation 94: 2285–2296, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Fassett JT, Xu X, Hu X, Zhu G, French J, Chen Y, Bache RJ. Adenosine regulation of microtubule dynamics in cardiac hypertrophy. Am J Physiol Heart Circ Physiol 297: H523–H532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fromes Y, Gounon P, Tapiero H, Fellous A. Effects of fluoro-doxorubicin (ME2303) on microtubules: influence of different classes of microtubule-associated proteins. J Protein Chem 15: 561–573, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein Tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer's disease. J Biol Chem 275: 5535–5544, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Guan L, Song K, Pysz MA, Curry KJ, Hizli AA, Danielpour D, Black AR, Black JD. Protein kinase C-mediated down-regulation of cyclin D1 involves activation of the translational repressor 4E-BP1 via a phosphoinositide 3-kinase/Akt-independent, protein phosphatase 2A-dependent mechanism in intestinal epithelial cells. J Biol Chem 282: 14213–14225, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Harney JA, Rodgers RL. Insulin-like stimulation of cardiac fuel metabolism by physiological levels of glucagon: involvement of PI3K but not cAMP. Am J Physiol Endocrinol Metab 295: E155–E161, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klovekorn WP, Schlepper M, Schaper W, Schaper J. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res 86: 846–853, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 1739: 198–210, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Ishibashi Y, Takahashi M, Isomatsu Y, Qiao F, Iijima Y, Shiraishi H, Simsic JM, Baicu CF, Robbins J, Zile MR, Cooper G. Role of microtubules versus myosin heavy chain isoforms in contractile dysfunction of hypertrophied murine cardiocytes. Am J Physiol Heart Circ Physiol 285: H1270–H1285, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Ke Y, Lei M, Solaro RJ. Regulation of cardiac excitation and contraction by p21 activated kinase-1. Prog Biophys Mol Biol 98: 238–250, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. King CC, Gardiner EM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, Bokoch GM. p21-Activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1). J Biol Chem 275: 41201–41209, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Kitagawa Y, Adachi-Akahane S, Nagao T. Determination of β-adrenoceptor subtype on rat isolated ventricular myocytes by use of highly selective β-antagonists. Br J Pharmacol 116: 1635–1643, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koide M, Hamawaki M, Narishige T, Sato H, Nemoto S, DeFreyte G, Zile MR, Cooper G, Carabello BA. Microtubule depolymerization normalizes in vivo myocardial contractile function in dogs with pressure-overload left ventricular hypertrophy. Circulation 102: 1045–1052, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Kostin S, Hein S, Arnon E, Scholz D, Schaper J. The cytoskeleton and related proteins in the human failing heart. Heart Fail Rev 5: 271–280, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Mann DL, Kent RL, Parsons B, Cooper G. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 85: 790–804, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Mao K, Kobayashi S, Jaffer ZM, Huang Y, Volden P, Chernoff J, Liang Q. Regulation of Akt/PKB activity by P21-activated kinase in cardiomyocytes. J Mol Cell Cardiol 44: 429–434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miyamoto T, Takeishi Y, Takahashi H, Shishido T, Arimoto T, Tomoike H, Kubota I. Activation of distinct signal transduction pathways in hypertrophied hearts by pressure and volume overload. Basic Res Cardiol 99: 328–337, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Ng DC, Lin BH, Lim CP, Huang G, Zhang T, Poli V, Cao X. STAT3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J Cell Biol 172: 245–257, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ng DC, Ng IH, Yeap YY, Badrian B, Tsoutsman T, McMullen JR, Semsarian C, Bogoyevitch MA. Opposing actions of extracellular signal-regulated kinase (ERK) and signal transducer and activator of transcription 3 (STAT3) in regulating microtubule stabilization during cardiac hypertrophy. J Biol Chem 286: 1576–1587, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. β2-Adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327: 1653–1657, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Packer M. β-Adrenergic blockade in chronic heart failure: principles, progress, and practice. Prog Cardiovasc Dis 41, Suppl 1: 39–52, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Pan J, Fukuda K, Kodama H, Makino S, Takahashi T, Sano M, Hori S, Ogawa S. Role of angiotensin II in activation of the JAK/STAT pathway induced by acute pressure overload in the rat heart. Circ Res 81: 611–617, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Rabkin SW, Sunga P. The effect of doxorubicin (adriamycin) on cytoplasmic microtubule system in cardiac cells. J Mol Cell Cardiol 19: 1073–1083, 1987 [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez P, Mitton B, Waggoner JR, Kranias EG. Identification of a novel phosphorylation site in protein phosphatase inhibitor-1 as a negative regulator of cardiac function. J Biol Chem 281: 38599–38608, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Sato H, Hori M, Kitakaze M, Iwai K, Takashima S, Kurihara H, Inoue M, Kamada T. Reperfusion after brief ischemia disrupts the microtubule network in canine hearts. Circ Res 72: 361–375, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Sato H, Nagai T, Kuppuswamy D, Narishige T, Koide M, Menick DR, Cooper G. Microtubule stabilization in pressure overload cardiac hypertrophy. J Cell Biol 139: 963–973, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scholz D, Baicu CF, Tuxworth WJ, Xu L, Kasiganesan H, Menick DR, Cooper G. Microtubule-dependent distribution of mRNA in adult cardiocytes. Am J Physiol Heart Circ Physiol 294: H1135–H1144, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Sheehan KA, Ke Y, Wolska BM, Solaro RJ. Expression of active p21-activated kinase-1 induces Ca2+ flux modification with altered regulatory protein phosphorylation in cardiac myocytes. Am J Physiol Cell Physiol 296: C47–C58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sucharov CC, Mariner PD, Nunley KR, Long C, Leinwand L, Bristow MR. A β1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am J Physiol Heart Circ Physiol 291: H1299–H1308, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Tagawa H, Koide M, Sato H, Cooper G. Cytoskeletal role in the contractile dysfunction of cardiocytes from hypertrophied and failing right ventricular myocardium. Proc Assoc Am Physicians 108: 218–229, 1996 [PubMed] [Google Scholar]
- 48. Tagawa H, Koide M, Sato H, Zile MR, Carabello BA, Cooper G. Cytoskeletal role in the transition from compensated to decompensated hypertrophy during adult canine left ventricular pressure overloading. Circ Res 82: 751–761, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Tagawa H, Rozich JD, Tsutsui H, Narishige T, Kuppuswamy D, Sato H, McDermott PJ, Koide M, Cooper G. Basis for increased microtubules in pressure-hypertrophied cardiocytes. Circulation 93: 1230–1243, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Takahashi M, Shiraishi H, Ishibashi Y, Blade KL, McDermott PJ, Menick DR, Kuppuswamy D, Cooper G. Phenotypic consequences of β1-tubulin expression and MAP4 decoration of microtubules in adult cardiocytes. Am J Physiol Heart Circ Physiol 285: H2072–H2083, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Tsutsui H, Ishihara K, Cooper G. Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science 260: 682–687, 1993 [DOI] [PubMed] [Google Scholar]
- 52. Tsutsui H, Tagawa H, Kent RL, McCollam PL, Ishihara K, Nagatsu M, Cooper G. Role of microtubules in contractile dysfunction of hypertrophied cardiocytes. Circulation 90: 533–555, 1994 [DOI] [PubMed] [Google Scholar]
- 53. Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and Tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci 25: 59–68, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Willey CD, Palanisamy AP, Johnston RK, Mani SK, Shiraishi H, Tuxworth WJ, Zile MR, Balasubramanian S, Kuppuswamy D. STAT3 activation in pressure-overloaded feline myocardium: role for integrins and the tyrosine kinase BMX. Int J Biol Sci 4: 184–199, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiao RP, Avdonin P, Zhou YY, Cheng H, Akhter SA, Eschenhagen T, Lefkowitz RJ, Koch WJ, Lakatta EG. Coupling of β1-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ Res 84: 43–52, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Yasukawa H, Hoshijima M, Gu Y, Nakamura T, Pradervand S, Hanada T, Hanakawa Y, Yoshimura A, Ross J, Jr, Chien KR. Suppressor of cytokine signaling-3 is a biomechanical stress-inducible gene that suppresses gp130-mediated cardiac myocyte hypertrophy and survival pathways. J Clin Invest 108: 1459–1467, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yoon SY, Choi JE, Choi JM, Kim DH. Dynein cleavage and microtubule accumulation in okadaic acid-treated neurons. Neurosci Lett 437: 111–115, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Zile MR, Green GR, Schuyler GT, Aurigemma GP, Miller DC, Cooper G. Cardiocyte cytoskeleton in patients with left ventricular pressure overload hypertrophy. J Am Coll Cardiol 37: 1080–1084, 2001 [DOI] [PubMed] [Google Scholar]