Abstract
It is generally accepted that the endothelium regulates vascular tone independent of the activity of the sympathetic nervous system. Here, we tested the hypothesis that the activation of sympathetic nerves engages the endothelium to oppose vasoconstriction. Local inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ signals (“pulsars”) in or near endothelial projections to vascular smooth muscle (VSM) were measured in an en face mouse mesenteric artery preparation. Electrical field stimulation of sympathetic nerves induced an increase in endothelial cell (EC) Ca2+ pulsars, recruiting new pulsar sites without affecting activity at existing sites. This increase in Ca2+ pulsars was blocked by bath application of the α-adrenergic receptor antagonist prazosin or by TTX but was unaffected by directly picospritzing the α-adrenergic receptor agonist phenylephrine onto the vascular endothelium, indicating that nerve-derived norepinephrine acted through α-adrenergic receptors on smooth muscle cells. Moreover, EC Ca2+ signaling was not blocked by inhibitors of purinergic receptors, ryanodine receptors, or voltage-dependent Ca2+ channels, suggesting a role for IP3, rather than Ca2+, in VSM-to-endothelium communication. Block of intermediate-conductance Ca2+-sensitive K+ channels, which have been shown to colocalize with IP3 receptors in endothelial projections to VSM, enhanced nerve-evoked constriction. Collectively, our results support the concept of a transcellular negative feedback module whereby sympathetic nerve stimulation elevates EC Ca2+ signals to oppose vasoconstriction.
Keywords: calcium signaling; myoendothelial junction; inositol 1,4,5-trisphosphate receptors; endothelium; vascular smooth muscle; endothelial cells
sympathetic nerves regulate vascular tone through the corelease of ATP and norepinephrine (NE) at neurovascular junctions (41). NE acts on vascular smooth muscle (VSM) cell (VSMC) Gq-coupled α1-adrenergic receptors [the predominant α-adrenergic receptor isoform in mesenteric VSM (21, 43)] to activate phospholipase C (PLC) and elevate diacylglycerol and inositol 1,4,5-trisphosphate (IP3), thereby activating PKC and IP3 receptors (IP3Rs) in the sarcoplasmic reticulum. ATP released at sympathetic nerve-muscle junctions activates VSMC ionotropic purinergic P2X receptors, specifically P2X1 receptors (P2X1Rs), and causes an influx of extracellular Ca2+ and Na+ into the cell, creating an excitatory junction potential that, if sufficiently large, activates voltage-dependent Ca2+ channels (VDCCs) and promotes contraction (23, 33, 46).
Activation of purinergic and adrenergic receptors induces two temporally distinct Ca2+ events in VSMCs: fast, rapid-onset junctional Ca2+ transients and slower-onset Ca2+ waves (23, 24). Junctional Ca2+ transients reflect P2X1R-mediated Ca2+ influx (11, 24, 25), whereas Ca2+ waves are propagating elevations in Ca2+ caused primarily by IP3-mediated Ca2+ release from the sarcoplasmic reticulum (10, 12, 15, 18, 45) that may also contribute to VSM contraction (31, 46).
In addition to perivascular sympathetic nerves, luminal endothelial cells (ECs) also modulate VSMC function through the production of vasodilators such as nitric oxide (NO) and through EDHF (6, 32). However, more recent evidence has suggested that the converse pathway, signaling from VSMCs to ECs, may be important for the regulation of arterial diameter in response to stimuli, such as KCl and phenylephrine (PE), that cause VSMC contraction (4). This observation is consistent with previous reports (4, 14, 37, 42) showing that adrenergic receptor activation by exogenous agonists not only elevates intracellular Ca2+ in VSMCs but also increases Ca2+ in the ECs lining these vessels. A previous report (16) has described spontaneous local EC Ca2+ events in rat mesenteric arteries after the stimulation of VSM with PE, without further defining the location of these Ca2+ events.
The current thinking is that this VSM-to-endothelium signal is communicated via the passage of a factor (e.g., Ca2+ or IP3) through myoendothelial gap junctions, which connect VSMCs to endothelial projections through the internal elastic lamina, to elevate EC Ca2+ (4, 16, 22). Dora et al. (4) first postulated that α1-adrenergic agonist-induced increases in endothelial Ca2+ resulted from the movement of Ca2+ from VSMCs to ECs through myoendothelial gap junctions. However, other studies (19, 22) have shown that elevating VSM Ca2+ by membrane depolarization does not affect endothelial Ca2+. Instead, recent reports (16, 22) have shown that inhibition of IP3 generation within VSMCs or blockade of EC IP3Rs prevents vasoconstrictor-evoked increases in bulk Ca2+ in ECs in intact rat mesenteric arteries, suggesting a role for IP3 in VSM-to-endothelium communication. Although these studies indicated that bath-applied PE can affect EC Ca2+ signaling through the engagement of smooth muscle α-adrenergic receptors, it is not known whether sympathetic nerve stimulation can also influence endothelial function.
Endothelial projections to smooth muscle have a distinctive structural architecture containing endoplasmic reticulum elements, gap junctions, IP3Rs, and intermediate-conductance (IK) Ca2+-sensitive K+ channels (KCa3.1 channels) (27, 30, 36). Using confocal microscopy and connexin (Cx)40Bac-GCaMP2 mice (27, 39), which express the Ca2+ biosensor GCaMP2 specifically in ECs, our laboratory has recently identified and characterized IP3-mediated Ca2+-release events, termed Ca2+ pulsars, within endothelial projections of mesenteric arteries to smooth muscle, establishing this approach as a powerful tool for monitoring EC Ca2+ signaling at or near the connection points to smooth muscle.
In this study, we tested the hypothesis that brief sympathetic nerve stimulation can engage the vascular endothelium to oppose vasoconstriction. To accomplish this, we determined the effects of sympathetic nerve stimulation on EC Ca2+ signaling in intact mesenteric arteries using the GCaMP2 mouse model. Our results are consistent with a model in which NE released from sympathetic nerves activates VSMC α1-adrenergic receptors to engage EC IP3Rs in myoendothelial projections and subsequently activate EC IK channels to oppose vasoconstriction.
EXPERIMENTAL PROCEDURES
Mouse models and tissue preparations.
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Vermont. Wild-type C57BL/6 and transgenic Cx40Bac-GCaMP2 mice were used. The Cx40Bac-GCaMP2 mouse expresses the Ca2+ biosensor GCaMP2 under the control of the Cx40 promoter and thus expresses GCaMP2 only in ECs in the vasculature (27, 39). Mesenteric artery tissue was dissected, and third-order mouse mesenteric arteries were cleaned of connective tissue and removed into HEPES (10 mM)-buffered saline (pH 7.4) at 4°C. For imaging experiments, arteries were slit open longitudinally and mounted on a Sylgard block with the endothelial surface exposed to the bath solution; this en face preparation facilitated the detection and analysis of Ca2+ signals.
Solutions.
Experiments using isolated third-order mesenteric arteries were performed in physiological saline solution [containing (in mM) 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2 CaCl2, 1.2 MgCl2, 7 glucose, and 0.023 EDTA] equilibrated with 95% O2-5% CO2 before and during the experiments. Except where indicated, experiments were performed in the presence of capsaicin (1 μM), atropine (10 μM), and MRS-2179 (3 μM) to block sensory nerves, muscarinic receptors, and purinergic P2Y1 receptors, respectively.
Electrical field stimulation.
Two platinum electrodes (0.25-mm diameter, World Precision Instruments) were placed in parallel on either side of a mesenteric artery (or en face preparation) and connected to a Grass S48 stimulator. After equilibration at 35°C, perivascular nerves of pressurized (80 mmHg) arteries or en face arterial preparations were stimulated by trains of electrical pulses (50–150 V, 0.25-ms pulse, 15 Hz, 5-s train duration). A submaximal voltage was used and was varied initially to some extent until a stable response was achieved. The voltage remained constant throughout both imaging experiments and diameter measurements. The train duration was 5 s to minimize tissue movement for Ca2+ measurements in the en face preparation (9, 25).
Diameter measurements of isolated mesenteric arteries.
Arteries were cannulated to size-matched micropipettes on an arteriograph system, pressurized to 80 mmHg using an electronic servo-pressure transducer (Living Systems Instrumentation, Burlington, VT), and continuously perfused with oxygenated, prewarmed (37°C) physiological saline solution. All ion channel blockers were added to the bath solution. Luminal vessel diameter was monitored using a charge-coupled device camera and edge-detection software (IonOptix). Electrical field stimulation (EFS)-evoked constrictions were analyzed using MiniAnalysis (Synaptosoft, Fort Lee, NJ) software. EFS-evoked constrictions were measured as the area under the curve (AUC) and calculated for the region between the baseline and maximum constriction from the point of EFS application to 95% recovery of the diameter to pre-EFS values. Both fast and slow decay phases of constriction were included in the calculations of AUC and thereby provide an index of overall constrictor responses. At least three constrictions per artery were averaged.
Ca2+ imaging.
Ca2+ imaging was performed on third-order mesenteric artery en face preparations using an Andor Technology Nipkow spinning-disk confocal system coupled to a Nikon Eclipse E600 FN upright microscope with a ×60 water-dipping objective (numerical aperture: 1.0); images were acquired at 15–30 images/s. Cx40Bac-GCaMP2 mice were used to detect pulsars, which are local, stationary, IP3R-mediated events (27). Pulsar area, measured at 50% peak amplitude, was found to be ∼14 μm2, as previously observed (27). GCaMP2 was excited at 488 nm with a solid-state laser, and fluorescence emission was collected using a 527.5/49-nm band-pass filter. Ca2+ pulsars were measured offline by detecting an increase in the fractional fluorescence (F/Fo) that was significantly above background noise (F/Fo > 1.2) using custom-designed software. F/F0 was evaluated in 9 × 9-pixel regions of interest in the collected image positioned at points corresponding to peak pulsar amplitude; F0 was obtained from the same region of interest in 10 images without activity. The kinetic properties of pulsars were analyzed using our custom software, which was written by A. D. Bonev (27), and pulsar frequency was determined as number of pulsar events over time per field of view. All events that occurred during EFS, including those that occurred during tissue movement, are presented in histograms. Because some images exhibited movement during EFS, for the sake of consistency, all events that occurred during the 5-s stimulation were excluded in the statistical analysis shown in Fig. 2, which compares the 20-s time bracket after EFS (starting at the end of the EFS pulse) to the 20-s time bracket before EFS for each pharmacological intervention.
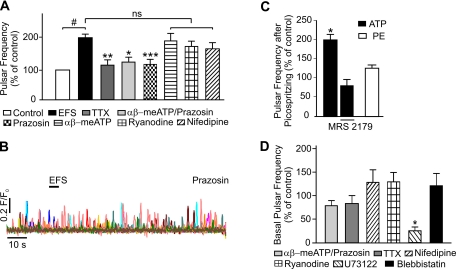
Fig. 2.
Inhibition of α-adrenergic receptors prevents EFS-induced increases in Ca2+ pulsar frequency. A: summary of the effects of various agents on EFS-induced Ca2+ pulsar frequency, comparing the 20-s interval starting immediately after the end of the EFS pulse with the 20-s interval before EFS (control). Events that occurred during EFS were excluded from this analysis because some images showed excessive movement during EFS. EFS significantly increased pulsar activity compared with unstimulated controls. This increase in EC Ca2+ pulsars was prevented by pretreatment with 1 μM TTX (n = 8), 500 nM prazosin/10 μM αβ-meATP (n = 6), or 500 nM prazosin alone (n = 18), which reduced pulsar frequency to levels that were not significantly different from unstimulated controls. EFS-induced pulsar activity was not significantly changed by treatment with 10 μM αβ-meATP (n = 10), 10 μM ryanodine (n = 6), or 5 μM nifedipine (n = 5). ns, Not significant. #P < 0.01 vs. unstimulated controls; *P < 0.05, **P < 0.001, and ***P < 0.0001 vs. EFS alone. B: representative fluorescence (F/F0) traces illustrating the lack of effect of EFS on Ca2+ pulsar activity in the presence of prazosin (500 nM). C: summary graph depicting the twofold elevation in pulsar frequency induced by picospritzing 10 μM ATP directly on endothelial cells (ECs) in an en face arterial preparation (n = 3, *P < 0.05). ATP had no effect in the presence of 3 μM MRS-2179 (n = 3), which was routinely included in bath solutions. Picospritzing 100 μM phenylephrine (PE; n = 3) on ECs in an en face preparation did not increase Ca2+ pulsar frequency, indicating that neurally released norepinephrine (NE) does not act directly on the endothelium. D: effects of drug treatments on basal pulsar activity. αβ-meATP (10 μM) + prazosin (500 nM), TTX (1 μM), nifedipine (5 μM), ryanodine (10 μM), and blebbistatin (50 μM) had no significant effects on basal pulsar activity (n = 3–6). Application of 10 μM U-73122 significantly reduced basal pulsar activity (n = 3–4; *P < 0.05).
Picospritzing.
ATP or PE was directly applied to the endothelial surface of an en face mesenteric artery preparation by picospritzing for 5 s at a pressure of 0.3 bar. Stimulation/involvement of VSM signaling mechanisms was avoided by adjusting the picospritzer to a distance of ∼10 μm above the endothelium.
Data analysis and statistics.
Prism (GraphPad Software, La Jolla, CA) and OriginPro7.5 software were used for statistical tests and preparation of graphs. Data are expressed as means ± SE unless otherwise noted. For Fig. 1, F and G, means ± SE were calculated using the descriptive “statistics on rows” tool in OriginPro 7.5. For Figs. 2–4, two-tailed paired t-tests were used for the comparison of paired experiments, and two-way repeated-measures ANOVAs followed by Bonferroni post tests were used for the comparison of two or more groups, as appropriate. One-sample t-tests were used to compare a single sample to a hypothetical value. P values of <0.05 were considered statistically significant. Each experimental treatment condition was performed at least three times on at least three separate animals. Two-sample independent t-tests were applied to compare biophysical characteristics (amplitude, duration, and decay and rise times) of pulsars at existing sites before nerve stimulation to those at newly recruited sites after nerve stimulation.
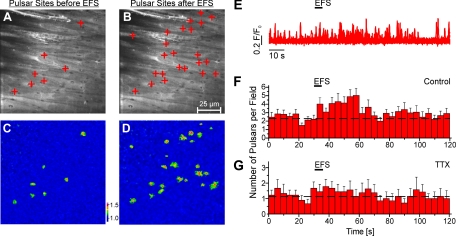
Fig. 1.
Stimulation of nerves increases Ca2+ pulsar frequency in the endothelium of third-order mesenteric arteries from connexin40Bac-GCaMP2 mice. A and B: en face arterial preparations. The plus signs indicate pulsar sites in a field over 20 s before (A) and after (B) electrical field stimulation (EFS). C and D: pseudocolor images showing the initiation sites of Ca2+ pulsars (corresponding to the composite images in A and B) 20 s before (C) and after (D) EFS. The color-coded scale represents the fractional fluorescence (F/F0). E: representative fluorescence (F/F0) traces illustrating the effect of EFS on Ca2+ pulsars originating from pulsar sites. F and G: histograms of before, during, and after EFS under control conditions (n = 16 fields, 8 arteries; F) and in the presence of 1 μM TTX (n = 9 fields, 5 arteries; G). Each bar includes SEs and corresponds to pulsars per field during 4 s. The dashed lines indicate the mean frequency before stimulation.
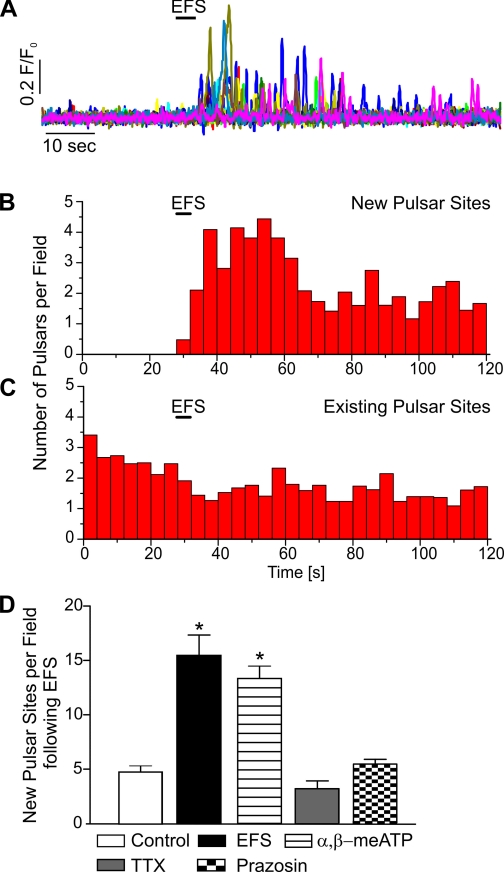
Fig. 3.
Stimulation of sympathetic nerves induces the recruitment of new EC Ca2+ pulsar sites. A: representative traces of the time course of Ca2+ pulsar activity at newly recruited sites after EFS. B and C: histograms of Ca2+ pulsars at newly recruited pulsar sites (n = 11 fields, 5 arteries; B) and preexisting sites (n = 34 fields, 15 arteries; C). D: summary graph showing a significantly increased number of new Ca2+ pulsar sites per field over the course of 20 s after EFS (n = 13, P < 0.0001) compared with the time control in the absence of EFS (n = 3). The number of new Ca2+ pulsar sites after EFS was also significantly increased in the presence of 10 μM αβ-meATP (n = 27, *P < 0.0001) but not in the presence of 1 μM TTX (n = 12) or 500 nM prazosin (n = 21).
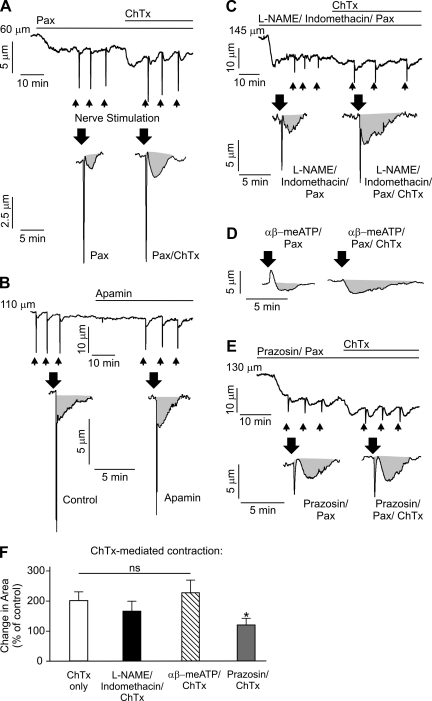
Fig. 4.
Intermediate-conductance (IK) channels oppose nerve-induced constriction via a mechanism that is dependent on vascular smooth muscle cell α-adrenergic receptor activation and independent of nitric oxide synthase/cyclooxygenase. A–E: representative diameter changes in pressurized (80 mmHg, 166 ± 25-μm maximal diameter and 129 ± 23-μm resting diameter, n = 34 arteries) third-order mesenteric arteries in response to EFS (indicated by arrows). Gray areas indicate area under the curve, which was calculated as an integral of the constriction. A: application of 100 nM charybdotoxin (ChTx) in the presence of 1 μM paxilline (Pax) increased constriction to identical nerve stimulation by twofold (n = 6 arteries). B: application of 300 nM apamin did not cause vasoconstriction (1.4 ± 1.0%, n = 5 arteries) and did not significantly alter nerve-induced vasoconstriction (P > 0.05, n = 5 arteries). This increase in EFS-induced constriction produced by inhibition of IK channels with ChTx was not significantly effected by pretreatment with 100 μM N-nitro-l-arginine methyl ester (l-NAME)-10 μM indomethacin-1 μM Pax (n = 5 arteries; P > 0.05; C) or 10 μM αβ-meATP-1 μM Pax (n = 5 arteries, P > 0.05; D) but was abolished by pretreatment with 500 nM prazosin-1 μM Pax (n = 5 arteries, *P < 0.05; E). Although 10 μM αβ-meATP completely abolished peak constriction in response to EFS, its effects on the area under the curve were minimal. F: summary graph illustrating the changes in luminal diameter upon EFS. For all experiments with ChTx, arteries were first incubated with 1 μM Pax to block smooth muscle large-conductance channels. Control corresponds to pretreatment of vessels with the indicated pathway inhibitors (in the presence of Pax).
RESULTS
Stimulation of sympathetic nerves acts through VSM α-adrenergic receptors to promote Ca2+ signaling in ECs.
Intracellular Ca2+ was measured in ECs in third-order mesenteric arteries from Cx40Bac-GCaMP2 mice, which express the genetically encoded Ca2+ biosensor GCaMP2 specifically in the vascular endothelium (27, 39). En face preparations were used in these experiments; the field of view was ∼120 × 136 μm and contained ∼14 ECs. Inhibitors of muscarinic receptors, purinergic P2Y receptors, and sensory nerves were included in the bath solution (see experimental procedures).
In the absence of nerve stimulation, mesenteric artery ECs exhibited a basal level of IP3-mediated Ca2+ signals that manifested as transient, stationary IP3R-mediated Ca2+ events (Ca2+ pulsars) primarily at holes in the internal elastic lamina (Fig. 1; see also Ref. 27). Nerve stimulation by EFS (15 Hz, 5-s train) increased the frequency of Ca2+ pulsars about twofold for about 20 s after EFS (Figs. 1, A–F, and 2A). EC pulsar frequency increased within 2–5 s after the start of the stimulation pulse and returned to basal levels ∼40 s after the cessation of nerve stimulation (Fig. 1F), consistent with the time course of smooth muscle Ca2+ signaling observed in third-order mesenteric arteries after EFS (23). Approximately 81% percent of pulsars were located in the vicinity (<2.5 μm) of holes in the internal elastic lamina.
The transient increase in EC Ca2+-signaling responses to EFS was prevented by TTX (1 μM, 5-min incubation), a blocker of neuronal Na+ channels (Figs. 1G and 2A). Stimulation of sympathetic nerves leads to the release of NE and ATP, which activate α1-adrenergic and P2X1Rs, respectively, in VSMCs (41). Prazosin, an inhibitor of α1-adrenergic receptors, prevented the EFS-induced increase in EC Ca2+ signaling (Fig. 2, A and B). In contrast, the P2X1R agonist/antagonist αβ-meATP (10 μM), which transiently activates and then chronically desensitizes P2X1Rs, had no effect on EFS-induced increases in EC Ca2+ signaling (Fig. 2A). These results are consistent with the idea that the activation of VSMC α1-adrenergic receptors by nerve-released NE leads to an increase in EC Ca2+ signaling.
It is nonetheless conceivable that NE and ATP released by sympathetic nerves might directly affect EC Ca2+ signaling. To test this, we directly picospritzed ATP (10 μM) or PE (100 μM) onto the endothelium of an en face arterial preparation and measured changes in EC Ca2+ signals (Fig. 2C). PE did not affect EC Ca2+ signaling when picospritzed onto the endothelium, suggesting that the third-order mesenteric artery ECs used in this study do not express the molecular machinery necessary to respond directly to NE, a result consistent with a previous report (14). However, picospritzed ATP did cause a significant elevation in EC Ca2+ signaling, an effect that was blocked by preincubation with the P2Y1 receptor antagonist MRS-2179 (3 μM, 10-min incubation; Fig. 2C). Thus, because MRS-2179 is routinely included in all bath solutions (see experimental procedures) (44) and ECs are unresponsive to PE, it is unlikely that neurally derived NE or ATP acts directly on the vascular endothelium under the experimental conditions used.
Recent studies (17, 22) have indicated that VSM can communicate with the endothelium through myoendothelial gap junctions in an IP3-dependent manner. To explore this possibility, we tested the effects of U-73122 (10 μM), an inhibitor of PLC. Inhibition of PLC greatly reduced basal Ca2+ pulsar activity, as previously reported (27) (see also Fig. 2D), and EFS had no effect.
The lack of effect of P2X1R inhibition on EFS-induced EC Ca2+ signaling suggests that P2X1R-mediated Ca2+ influx and activation of VDCCs by excitatory junction potentials are not required for EFS-induced EC Ca2+ signaling, further suggesting that VSMC intracellular Ca2+ is not the primary mediator of VSM-to-endothelium signaling. To further explore this issue, we tested the effects of antagonists of VDCCs and ryanodine receptors (RyRs) on nerve-induced increases in EC Ca2+ signaling. Pretreatment with the VDCC blocker nifedipine (5 μM) minimized movement in response to EFS but did not affect the increase in EC Ca2+ pulsar activity induced by sympathetic nerve stimulation (Fig. 2A). Blebbistatin (50 μM), a selective inhibitor of myosin-actin interactions, also minimized contraction without affecting EC Ca2+-signaling responses to EFS (2.3-fold increase in pulsar frequency after EFS, P < 0.05, n = 4 arteries). Likewise, the RyR blocker ryanodine (10 μM) failed to block the increase in EC Ca2+ pulsar activity induced by the stimulation of sympathetic nerves (Fig. 2A). Basal EC Ca2+ signaling was also unaffected by nifedipine, blebbistatin, ryanodine, prazosin, and αβ-meATP (Fig. 2D). Collectively, these results suggest that neither increases in smooth muscle global Ca2+ nor elevated RyR-mediated Ca2+ spark activity makes a major contribution to communicating sympathetic nerve input to the endothelium, implying a potential role for IP3 in transducing this signal.
Sympathetic nerve stimulation recruits new EC Ca2+-signaling sites.
We (27) have previously shown that the endothelial-dependent vasodilator ACh enhances Ca2+ pulsar activity by inducing new sites and by increasing pulsar frequency at existing sites. Thus, EFS could increase EC Ca2+ signaling by affecting activity at existing sites, by recruiting new sites, or both. Here, we found that EFS considerably increased the number of new pulsar sites, with the transient increase in pulsar activity peaking at 4 sites/field per 4 s after EFS, as measured over 20 s (Fig. 3, A and B). The amplitude, duration, and rise and decay times of Ca2+ pulsars were not significantly changed upon nerve stimulation (Table 1). Interestingly, in contrast to stimulation with ACh, EFS did not increase the frequency of pulsars at existing sites (Fig. 3C and Table 1). Therefore, as shown in Fig. 3D, the increase in frequency is due to the appearance of new sites. The residual number of new sites in the presence of TTX (Fig. 3D) provides a measure of the frequency of spontaneously occurring new sites over the duration of the experiment. The addition of prazosin reduced the number of new sites to basal levels (i.e., that observed in the presence of TTX), indicating that inhibition of α1-adrenergic receptors prevented the recruitment of new pulsar sites. αβ-meATP did not affect EFS induction of new sites (Fig. 3D). Collectively, these observations indicate that the recruitment of new pulsar sites after sympathetic nerve stimulation is mediated by VSMC adrenergic signaling pathways.
Table 1.
Comparison of Ca2+ pulsars from the mesenteric endothelium before and after nerve stimulation
| Ca2+ Pulsars |
||
|---|---|---|
| Parameters | Before EFS | New sites after EFS |
| Amplitude, F/F0 | 1.37 ± 0.02 | 1.37 ± 0.02 |
| Rise time, ms | 194 ± 10 | 189 ± 8 |
| Duration, ms | 278 ± 12 | 251 ± 10 |
| Half-time for decay, ms | 175 ± 11 | 153 ± 8 |
| Frequency, Hz | 0.08 ± 0.006 | 0.07 ± 0.003 |
The Ca2+ pulsar parameters shown are peak amplitude [in fractional fluorescence (F/F0)], rise time (measured as the 10%-to-90% signal interval), duration (measured as 50% peak signal width), and half-time for decay of the signal. Kinetic characteristics of Ca2+ pulsars were calculated from 43 and 96 pulsars recorded in 5 fields over 20-s time courses before and after nerve stimulation, respectively. EFS, electrical field stimulation. “After EFS” refers to Ca2+ pulsars originating from new, EFS-induced pulsar sites.
EC IK channels regulate nerve-evoked constriction.
IK channels and IP3Rs are colocalized to endothelial projections (27, 35), suggesting the potential for local communication between Ca2+ pulsars and Ca2+-sensitive IK channels. To test the hypothesis that EFS-induced IP3R-mediated Ca2+ signals in ECs activate IK channels to oppose nerve-evoked constriction, we exploited a combination of pharmacological agents with selectivity against the K+ channels expressed in ECs and smooth muscle cells. The bee venom toxin apamin was used to selectively block small-conductance (SK) Ca2+-sensitive K+ channels (KCa2.3 channels) (2, 28, 40), which are also expressed in ECs. IK channels were blocked using the synthetic inhibitor Tram-34 and the scorpion toxin charybdotoxin (ChTx), which also blocks large-conductance (BK) Ca2+-sensitive K+ channels, but not SK channels, with high affinity (3, 28). In experiments using ChTx, the potential effects of BK channels, which are expressed in VSMCs but not in normal ECs (8, 20, 26, 34), were eliminated using the selective blocker paxilline (13, 28, 29). Elevation of intravascular pressure to 80 mmHg induced myogenic tone, constricting these mesenteric arteries by 22.7 ± 0.8% (n = 34). Block of IK channels with Tram-34 (10 μM) or ChTx (100 nM, in the presence of paxilline) caused a small, but significant, constriction when added alone (6.3 ± 0.9% and 4.8 ± 0.6% for Tram-34 and ChTx, respectively, n = 5 arteries; Fig. 4). Block of IK channels also increased EFS-induced vasoconstriction, measured as an integral of the constriction (AUC; Fig. 4, A and F), by approximately twofold, but did not significantly change peak amplitude (n = 5 arteries, P > 0.05). In contrast, application of the SK channel blocker apamin (300 nM) did not cause vasoconstriction (1.4 ± 1.0%, n = 5 arteries) and did not significantly alter nerve-induced vasoconstriction (P > 0.05, n = 5 arteries; Fig. 4B). These results suggest that EC IK channels tonically oppose pressure-induced constriction as well as EFS-induced constriction.
In addition to SK/IK channel-dependent EDHF, the endothelium releases the vasoactive factors NO and prostanoids through the action of NO synthase (NOS) and cyclooxygenase (COX), respectively (5, 32). Block of NOS with N-nitro-l-arginine methyl ester [l-NAME (100 μM)] and COX with indomethacin (10 μM) did not significantly affect nerve-induced vasoconstriction, suggesting that NOS/COX does not play a significant role in opposing nerve-induced vasoconstriction in these mesenteric arteries (P > 0.05, n = 5 arteries). Consistent with this interpretation, block of IK channels in the presence of l-NAME (100 μM)/indomethacin (10 μM) resulted in an increase in nerve-induced vasoconstriction comparable to that observed under control conditions (Fig. 4, C and F). This suggests that IK channels regulate nerve-evoked constriction in small mesenteric arteries independent of NOS/COX activity.
To confirm that the VSMC α1-adrenergic receptor pathway, as described above (see Fig. 2), is functionally linked with IK-dependent attenuation of nerve-evoked constriction, we preincubated pressurized mesenteric arteries with prazosin (500 nM) or the purinergic receptor blocker αβ-meATP (10 μM) and measured changes in EFS-evoked constrictions in the presence and absence of the IK channel blocker ChTx (100 nM). Prazosin reduced the sustained component of EFS-evoked constrictions, measured as the AUC, by 37.9 ± 2.4% (n = 5 arteries, P < 0.05). The initial rapid constriction, which did not make a major contribution to the total AUC, was eliminated by αβ-meATP (n = 4 arteries; Fig. 4D), confirming that this transient contraction represents the response to an excitatory junction potential caused by the rapid influx of Na+/Ca2+ through P2X1Rs. In arteries pretreated with αβ-meATP (in the presence of 1 μM paxilline), ChTx induced an increase in vasoconstriction comparable to that observed in the absence of αβ-meATP (Fig. 4, D and F). In contrast, the increase in vasoconstriction due to IK channel block was abolished in the presence of prazosin (500 nM; Fig. 4, E and F), indicating that the VSMC α1-adrenergic receptor signaling pathway is the primary contributor to the local increase in Ca2+ pulsar events at myoendothelial projections, subsequent activation of proximate IK channels, and regulation of vascular tone.
DISCUSSION
Here, we propose the novel concept that brief stimulation of sympathetic nerves induces an increase in IP3-mediated Ca2+ pulsars in the endothelial projections to smooth muscle. Our data support a model in which sympathetic nerve stimulation does not activate EC Ca2+ directly but instead induces heterocellular communication from VSMCs to ECs, leading to increased EC Ca2+ pulsar activity at myoendothelial projections (Fig. 5). Sympathetic nerve-induced Ca2+ pulsars signal locally to activate EC IK channels, engaging a NOS/COX-independent EDHF mechanism that opposes nerve-induced vasoconstriction. Each of the sympathetic nerve-evoked responses (increased EC Ca2+ pulsar activity, IK channel activation, and regulation of vasoconstriction) is mediated by VSMC α1-adrenergic signaling.
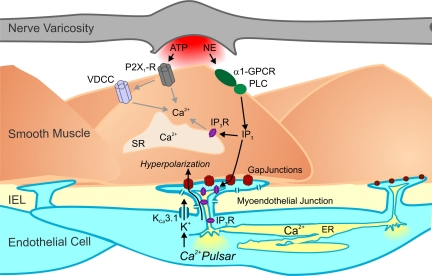
Fig. 5.
Model of negative feedback regulation by the endothelium in response to sympathetic nerve stimulation. Activation of sympathetic nerves leads to the corelease of ATP and NE, which activate vascular smooth muscle cell purinergic receptors (P2X1Rs) and α1-adrenergic receptors [α1-G protein-coupled receptors (α1-GPCR)], respectively. The subsequent elevation of smooth muscle inositol 1,4,5-trisphosphate (IP3) by the activation of α1-adrenergic receptors is transmitted through myoendothelial gap junctions to IP3 receptors (IP3Rs) in the endothelial projections to increase intracellular Ca2+ (pulsars). This, in turn, activates colocalized EC IK channels, providing a hyperpolarizing influence on the smooth muscle to oppose vasoconstriction. VDCC, voltage-dependent Ca2+ channels; PLC, phospholipase C; SR, sarcoplasmic reticulum; IEL, inner elastic lamina; ER, endoplasmic reticulum.
Our finding that VSMC α-adrenergic receptor activation increases EC Ca2+ signaling is consistent with previous reports (4, 14, 37, 42) showing that VSMC adrenergic receptor activation by bath-applied PE increases Ca2+ in the ECs lining these vessels. Activation of α1-adrenergic receptors elevates the levels of both smooth muscle IP3 and intracellular Ca2+, which could permeate myoendothelial gap junctions to activate IP3Rs in the endothelial projections and thereby increase Ca2+ pulsar activity. Our data do not support the idea that the movement of Ca2+ underlies this transcellular signal. Sympathetic stimulation leads to the release of ATP and NE. ATP signaling through VSMC P2X1Rs does not appear to be involved in this transcellular communication, indicating that the elevation of VSMC Ca2+ via extracellular Ca2+ influx is not sufficient to drive this process. The failure of inhibitors of RyRs and VDCCs to prevent the signaling of sympathetic nerves to the endothelium, as reported here, suggests that neither increases in Ca2+ spark activity nor rises in global intracellular Ca2+ significantly contribute to this signaling cascade, strongly suggesting that communication from VSM to the endothelium is mediated by VSMC IP3 diffusing through myoendothelial gap junctions. Moreover, the diffusion gradient between VSMCs and ECs for Ca2+ is likely to be very modest compared with the IP3 gradient. The idea that IP3 acts as the intercellular currency in this context is consistent with the dramatically lower mobility of Ca2+ (∼13 μm/s) compared with IP3 (∼280 μm/s), which, in turn, reflects the strong intracellular buffering of Ca2+ by Ca2+-binding proteins (1). Earlier reports (4, 16, 37) that stimulation of smooth muscle by either PE or activation of VDCCs by membrane depolarization with high K+ increased endothelial Ca2+ led to the suggestion that Ca2+ is the transcellular signaling entity. However, other studies (19, 22) have reported that activation of VDCCs has no effect on endothelial Ca2+. The consensus of recent studies (17, 22, 35) supports the concept that IP3 is the currency of communication after the stimulation of VSMC adrenergic receptors with PE. Our data reinforce the importance of adrenergic signaling in this mechanism and further define the nature of the EC Ca2+ signal, showing that NE transiently released from sympathetic nerves acts through VSMC α1-adrenergic receptor signaling pathways to increase EC Ca2+ pulsar activity. Furthermore, our data suggest that activation of EC IK channels should attenuate the NE-induced smooth muscle membrane potential depolarization.
Previous studies (4, 14, 16, 22, 35, 37) have examined the steady-state effects of continuous PE exposure on endothelial Ca2+. Here, we examined the effects of nerve stimulation for 5 s on EC Ca2+ signaling associated with endothelial projections to smooth muscle. We found that Ca2+ pulsar activity was elevated ∼3 s after the onset of EFS and remained elevated for ∼40 s (Fig. 1). Consistent with the idea that IP3 derived from stimulated smooth muscle causes a delayed activation of IP3-mediated Ca2+ pulsars in endothelial projections, Wier and colleagues (23) found that smooth muscle IP3-mediated Ca2+ waves remained elevated for 60 s after the cessation of EFS. Our data on nerve-evoked constrictions are also consistent with this view in that block of IK channels affected the duration, measured as the AUC, but not the initial, purinergic-mediated amplitude (Fig. 4). Ca2+ pulsars return to baseline within 1 min after stimulation, whereas the constriction lasts longer. This lag presumably reflects the time course of membrane repolarization, reduction in smooth muscle Ca2+, and ultimately force. Collectively, these findings are in accord with reports suggesting a role for IP3 rather than Ca2+ in VSM-to-endothelium communication.
The architectural features of endothelial projections through the elastic lamina create a microdomain that forms the basis for the signaling mechanism described here. These endothelial projections are home to evaginations of the endoplasmic reticulum containing IP3Rs (the source of Ca2+ pulsars) and make direct contact with VSMCs via myoendothelial gap junctions, which are composed of Cxs (Cx37 and Cx40), that allow the diffusion of small molecules between VSMCs and ECs (7, 30, 36). Even though endothelial NOS has been shown to colocalize at myoendothelial gap junctions (38), our data suggest that neither NOS nor COX activity plays a significant role in opposing sympathetic nerve-induced vasoconstriction in third-order mesenteric arteries (Fig. 4). Importantly, IK channels have been shown to localize to the myoendothelial projections (27, 36), placing them in a position to respond to local elevations of Ca2+ generated by Ca2+ pulsar activity. Our data suggest that EC IK channels tonically oppose pressure-induced, as well as nerve-induced, constrictions in small resistance arteries (Fig. 4), similar to their regulation of parenchymal arteriolar diameter and cortical cerebral blood flow (8). Interestingly, the increase in Ca2+ pulsar activity observed upon nerve stimulation reflected the recruitment of new Ca2+ pulsar sites in ECs rather than an increase in the activity of existing sites (Figs. 1 and 3). Although our experiments did not address this directly, this outcome is precisely what one would predict if the “goal” was to create a system with maximal gain, assuming that IK channels in the vicinity of existing pulsar sites are at least partially activated under basal conditions. The effect of EFS on pulsars was somewhat different from that of ACh, which predominantly recruited new pulsar sites but also increased the activity of existing sites (27). This difference could reflect the source of IP3 generation (endothelium vs. smooth muscle) and the degree to which IP3Rs are saturated with IP3 and Ca2+.
Taken together, our data elucidate a novel signaling mechanism in which the stimulation of sympathetic nerves activates VSMC adrenergic receptors to increase EC Ca2+ pulsar activity in endothelial projections, ultimately activating nearby IK channels to oppose nerve-induced vasoconstriction and regulate sympathetic tone. Thus, the endothelium actively responds to sympathetic nerve stimulation to regulate vessel diameter and sympathetic tone.
GRANTS
This work was supported by American Heart Association Postdoctoral Fellowship 10POST3870008 (to L. W. M. Nausch), National Heart, Lung, and Blood Institute Grants P01-HL-095488-01 and R01-HL-44455, and the Totman Trust for Medical Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.W.N. and M.T.N. conception and design of research; L.W.N. and A.D.B. performed experiments; L.W.N. and A.D.B. analyzed data; L.W.N., A.D.B., T.J.H., and M.T.N. interpreted results of experiments; L.W.N. and A.D.B. prepared figures; L.W.N. drafted manuscript; L.W.N., A.D.B., T.J.H., and M.T.N. edited and revised manuscript; L.W.N., A.D.B., T.J.H., Y.N.T., M.I.K., and M.T.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. David Hill-Eubanks, Dr. Bernhard Nausch, and Dr. Nuria Villalba for comments on the manuscript.
REFERENCES
- 1. Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258: 1812–1815, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, Edwards G. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol 135: 1133–1143, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Feletou M, Vanhoutte PM. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol 137: 1346–1354, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci USA 94: 6529–6534, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feletou M, Huang Y, Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol 164: 894–912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 117: 139–155, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol 291: H2047–H2056, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SKCa and IKCa channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 31: 1175–1186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heppner TJ, Layne JJ, Pearson JM, Sarkissian H, Nelson MT. Unique properties of muscularis mucosae smooth muscle in guinea pig urinary bladder. Am J Physiol Regul Integr Comp Physiol 301: R351–R362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT. Calcium Signaling in Smooth Muscle. Cold Spring Harb Perspect Biol 3: a004549, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill-Eubanks DC, Werner ME, Nelson MT. Local elementary purinergic-induced Ca2+ transients: from optical mapping of nerve activity to local Ca2+ signaling networks. J Gen Physiol 136: 149–154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iino M, Tsukioka M. Feedback control of inositol trisphosphate signalling bycalcium. Mol Cell Endocrinol 98: 141–146, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Imlach WL, Finch SC, Miller JH, Meredith AL, Dalziel JE. A role for BK channels in heart rate regulation in rodents. PLos One 5: e8698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson WF, Boerman EM, Lange EJ, Lundback SS, Cohen KD. Smooth muscle α1D-adrenoceptors mediate phenylephrine-induced vasoconstriction and increases in endothelial cell Ca2+ in hamster cremaster arterioles. Br J Pharmacol 155: 514–524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 279: C1528–C1539, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium 44: 135–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kapela A, Bezerianos A, Tsoukias NM. A mathematical model of vasoreactivity in rat mesenteric arterioles: I. Myoendothelial communication. Microcirculation 16: 694–713, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim M, Hennig GW, Smith TK, Perrino BA. Phospholamban knockout increases CaM kinase II activity and intracellular Ca2+ wave activity and alters contractile responses of murine gastric antrum. Am J Physiol Cell Physiol 294: C432–C441, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Knot HJ, Lounsbury KM, Brayden JE, Nelson MT. Gender differences in coronary artery diameter reflect changes in both endothelial Ca2+ and ecNOS activity. Am J Physiol Heart Circ Physiol 276: H961–H969, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Kohler R, Degenhardt C, Kuhn M, Runkel N, Paul M, Hoyer J. Expression and function of endothelial Ca2+-activated K+ channels in human mesenteric artery: a single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ Res 87: 496–503, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Kong JQ, Taylor DA, Fleming WW. Functional distribution and role of alpha-1 adrenoceptor subtypes in the mesenteric vasculature of the rat. J Pharmacol Exp Ther 268: 1153–1159, 1994 [PubMed] [Google Scholar]
- 22. Lamboley M, Pittet P, Koenigsberger M, Sauser R, Beny JL, Meister JJ. Evidence for signaling via gap junctions from smooth muscle to endothelial cells in rat mesenteric arteries: possible implication of a second messenger. Cell Calcium 37: 311–320, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Lamont C, Vainorius E, Wier WG. Purinergic and adrenergic Ca2+ transients during neurogenic contractions of rat mesenteric small arteries. J Physiol 549: 801–808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lamont C, Vial C, Evans RJ, Wier WG. P2X1 receptors mediate sympathetic postjunctional Ca2+ transients in mesenteric small arteries. Am J Physiol Heart Circ Physiol 291: H3106–H3113, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Lamont C, Wier WG. Evoked and spontaneous purinergic junctional Ca2+ transients (jCaTs) in rat small arteries. Circ Res 91: 454–456, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Ledoux J, Bonev AD, Nelson MT. Ca2+-activated K+ channels in murine endothelial cells: block by intracellular calcium and magnesium. J Gen Physiol 131: 125–135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–78, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Li G, Cheung DW. Effects of paxilline on K+ channels in rat mesenteric arterial cells. Eur J Pharmacol 372: 103–107, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res 97: 399–407, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Mauban JR, Lamont C, Balke CW, Wier WG. Adrenergic stimulation of rat resistance arteries affects Ca2+ sparks, Ca2+ waves, and Ca2+ oscillations. Am J Physiol Heart Circ Physiol 280: H2399–H2405, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147, Suppl 1: S193–S201, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rummery NM, Brock JA, Pakdeechote P, Ralevic V, Dunn WR. ATP is the predominant sympathetic neurotransmitter in rat mesenteric arteries at high pressure. J Physiol 582: 745–754, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandow SL, Grayson TH. Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol 297: H1–H7, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What's where and why at a vascular myoendothelial microdomain signalling complex. Clin Exp Pharmacol Physiol 36: 67–76, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KCa) and connexins: possible relationship to vasodilator function? J Anat 209: 689–698, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schuster A, Oishi H, Beny JL, Stergiopulos N, Meister JJ. Simultaneous arterial calcium dynamics and diameter measurements: application to myoendothelial communication. Am J Physiol Heart Circ Physiol 280: H1088–H1096, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Compartmentalized connexin 43 S-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler Thromb Vasc Biol 31: 399–407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res 101: 1300–1309, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res 93: 124–131, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Todorov LD, Mihaylova-Todorova ST, Bjur RA, Westfall DP. Differential cotransmission in sympathetic nerves: role of frequency of stimulation and prejunctional autoreceptors. J Pharmacol Exp Ther 290: 241–246, 1999 [PubMed] [Google Scholar]
- 42. Tuttle JL, Falcone JC. Nitric oxide release during α1-adrenoceptor-mediated constriction of arterioles. Am J Physiol Heart Circ Physiol 281: H873–H881, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Williams TJ, Clarke DE. Characterization of alpha 1-adrenoceptors mediating vasoconstriction to noradrenaline and nerve stimulation in the isolated perfused mesentery of rat. Br J Pharmacol 114: 531–536, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winter P, Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol 582: 335–347, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium 38: 397–407, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Zang WJ, Zacharia J, Lamont C, Wier WG. Sympathetically evoked Ca2+ signaling in arterial smooth muscle. Acta Pharmacol Sin 27: 1515–1525, 2006 [DOI] [PubMed] [Google Scholar]