Abstract
We have previously shown that ethanol microinjection into the rostral ventrolateral medulla (RVLM) elicits sympathoexcitation and hypertension in conscious spontaneously hypertensive rats (SHRs) but not in Wistar-Kyoto (WKY) rats. In this study, evidence was sought to implicate the oxidative breakdown of ethanol in this strain-dependent hypertensive action of ethanol. Biochemical experiments revealed significantly higher catalase activity and similar aldehyde dehydrogenase (ALDH) activity in the RVLM of SHRs compared with WKY rats. We also investigated the influence of pharmacological inhibition of catalase (3-aminotriazole) or ALDH (cyanamide) on the cardiovascular effects of intra-RVLM ethanol or its metabolic product acetaldehyde in conscious rats. Compared with vehicle, ethanol (10 μg/rat) elicited a significant increase in blood pressure in SHRs that lasted for the 60-min observation period but had no effect on blood pressure in WKY rats. The first oxidation product, acetaldehyde, played a critical role in ethanol-evoked hypertension because 1) catalase inhibition (3-aminotriazole treatment) virtually abolished the ethanol-evoked pressor response in SHRs, 2) intra-RVLM acetaldehyde (2 μg/rat) reproduced the strain-dependent hypertensive effect of intra-RVLM ethanol, and 3) ALDH inhibition (cyanamide treatment) uncovered a pressor response to intra-RVLM acetaldehyde in WKY rats similar to the response observed in SHRs. These findings support the hypothesis that local production of acetaldehyde, due to enhanced catalase activity, in the RVLM mediates the ethanol-evoked pressor response in SHRs.
Keywords: catalase, Wistar-Kyoto rats
previously reported experimental and epidemiological studies (39, 41) have shown that alcohol use positively correlates with the incidence of hypertension. Among several other mechanisms, sympathoexcitation has been implicated in the ethanol-evoked hypertension as exemplified by the rise in plasma norepinephrine levels (20) and efferent sympathetic neural activity (37). In a more recent report, we used microinjection and electrochemical protocols in rats to determine neuronal substrates in the brain stem that underlie the hypertensive action of ethanol. The rostral ventrolateral medulla (RVLM) was targeted because it is the brain stem pressor region from which bulbospinal sympathetic neurons descend to the intermediolateral cell column of the spinal cord (9) and is a major site for the sympathoexcitatory action of ethanol (29). Intra-RVLM ethanol produced dose-dependent increases in RVLM norepinephrine electrochemical signals and blood pressure (BP) in spontaneously hypertensive rats (SHRs), in contrast to no effects in Wistar-Kyoto (WKY) rats (26). Similarly, increases in BP and RVLM norepinephrine signals were observed after systemic ethanol administration in SHRs (29). Since the RVLM contains nerve terminals of noradrenergic neurons that originate from other medullary areas (9, 18) and the activity of these neurons is proportionally related to sympathetic neural activity (6, 29), the data signified a primary role for norepinephrine released from RVLM noradrenergic neurons in the sympathoexcitatory and pressor actions of ethanol in SHRs (26).
The metabolic breakdown of ethanol involves its oxidation into acetaldehyde via multiple enzymatic pathways, including alcohol dehydrogenase, catalase, or cytochrome P-450 2E1 (34). Biochemical, pharmacological, and molecular studies have shown that catalase is the principal enzyme responsible for the oxidative catabolism of ethanol into acetaldehyde in brain tissues (42). Alcohol dehydrogenase and cytochrome P-450 2E1 are more important for the hepatic metabolism of ethanol (27). Acetaldehyde is subsequently oxidized into acetate mostly through aldehyde dehydrogenase (ALDH) (36). Conflicting data are available concerning the role of acetaldehyde in the neurobiological effects of ethanol. Numerous studies (21, 25, 32) have implicated acetaldehyde in the behavioral, reinforcing, and neurotoxic effects of ethanol. Other studies (34, 35), however, have argued against the involvement of acetaldehyde in mediating the effects of ethanol or proposed that the two substances might elicit similar but mechanistically different responses.
Despite extensive knowledge concerning the role of acetaldehyde in the biological effects of ethanol, limited or no studies are available to date with regard to its potential involvement in the central strain-dependent cardiovascular effects of ethanol. Furthermore, previously reported findings on brain catalase activity in experimental models of genetic hypertension are limited and inconsistent (1, 19), and there are no reports on RVLM ALDH activity in SHRs versus WKY rats. In the present study, we tested the hypothesis that enhanced ethanol oxidation to acetaldehyde by local catalase underlies, at least partly, the exaggerated pressor response elicited by intra-RVLM ethanol in SHRs. To test this hypothesis, we conducted 1) neurochemical experiments to measure basal catalase and ALDH catalytic activities in the RVLM of SHRs and WKY rats, 2) integrative experiments to determine if intra-RVLM acetaldehyde produces a strain-dependent pressor response similar to that produced by ethanol, and 3) pharmacologic experiments to investigate the effects of prior inhibition of catalase or ALDH on the cardiovascular actions of ethanol or acetaldehyde. The integrative/pharmacological experiments were conducted in conscious SHRs and WKY rats preinstrumented with intravascular and intracranial (RVLM) cannulas.
MATERIALS AND METHODS
Male SHRs and WKY rats (12–13 wk old, Charles River, Raleigh, NC) were used. All experiments were approved by the Institutional Animal Care and Use Committee and carried out in accordance with the Declaration of Helsinki and with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cannulation
Intracranial cannulation.
The method described in our previous studies (26, 30) was used. Briefly, rats were anesthetized with intraperitoneal ketamine (90 mg/kg) and xylazine (10 mg/kg), with anesthesia supplemented as needed. Rats were mounted in the prone position in a cranial stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). A steel guide cannula (23 gauge, Small Parts, Miami, FL) was implanted intracranially so that its tip was positioned 2 mm above the RVLM using the interaural line as the reference and the following coordinates: posterior ± 2.8 mm, lateral ± 2.0 mm, and dorsoventral ± 0.5 mm. The cannula was secured in place with small metal screws and dental acrylic cement (Durelon, Thompson Dental Supply, Raleigh, NC). Each rat received intramuscular buprenorphine (30 μg/kg) and subcutaneous Durapen (penicillin G benzathine and penicillin G procaine in an aqueous suspension, 100,000 U/kg) and was housed in a separate cage.
Intravascular cannulation.
Three days after intracranial cannulation, catheters (each consisting of 5-cm polyethylene-10 tubing bonded to 15-cm polyethylene-50 tubing) filled with heparinized saline (100 U/ml) were placed in the abdominal aorta and vena cava via the left femoral vessels for the measurement of BP and intravenous injections, respectively, as in our previous studies (13–15).
Hemodynamic Measurements
Five days after intracranial cannulation, BP was measured by connecting the arterial catheter to a pressure transducer (Gould-Statham, Oxnard, CA), and BP was displayed on a PowerLab system (AD Instruments) as previously described (16, 30). Heart rate (HR) was computed from BP waveforms and displayed on another channel of the recording system. A 30-gauge microinjector, connected to a 0.5-μl Hamilton syringe, was inserted into the guide cannula with its tip located in the RVLM. Accurate placement of the injector was verified by chemical and histological means. Chemical identification was made at the beginning of the experiment by observing the pressor response after a microinjection of 1 nmol of l-glutamate (40 nl). Histologically, 40 nl of fast green dye (1%) was delivered at the end of the experiment to mark the injection site. Frozen coronal sections (40 μm) were cut and examined for the injection site using the atlas of Paxinos and Watson (33).
Measurements of RVLM Catalase and ALDH Activity
At the conclusion of the in vivo experiments, rats treated with 3-aminotriazole (ATZ), cyanamide, or the vehicle were euthanized with an overdose of pentobarbital sodium (100 mg/kg). The brains were rapidly removed and stored at −80°C until used. For the measurement of enzymatic activity, micropunches were taken from the RVLM with a stainless steel bore (inner diameter: 0.94 mm, Stoelting, Wood Dale, IL), as reported in previous studies, including ours (4, 30). Tissue was homogenized in 35 μl lysis buffer and centrifuged at 2,700 g for 4 min at 4°C. The supernatant was separated and assayed for protein content (Bradford assay, Bio-Rad). Catalase activity was determined colorimetrically in 15 μg protein using the Catalase Assay Kit (catalog no. CAT-100, Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions. ALDH activity was measured fluorometrically based on the oxidation of 6-methoxy-2-naphtaldehyde into fluorescent 6-methoxy-2-naphtoate (31). The reaction mixture consisted of 15 μl of supernatant, 3.96 μl of substrate (60 nmol/ml), 1.3 μl of 11.4 mM NAD, and 176.5 μl of 50 mM of sodium phosphate buffer (pH 8.5). The mixture also contained 2.3 μl of a 12 mM solution of 4-methylpyrazole as a specific inhibitor of alcohol dehydrogenase activity. Fluorescence was read at an excitation wavelength of 310 nm and an emission wavelength of 360 nm using an “Infinite M200” spectrofluorophotometer (TECAN Austria).
Protocols and Experimental Groups
Strain-dependent hemodynamic effects of intra-RVLM ethanol or acetaldehyde.
The main objective of this experiment was to determine whether intra-RVLM acetaldehyde replicates the strain (SHR)-dependent pressor response elicited by intra-RVLM ethanol under the same experimental conditions. Furthermore, the findings of this experiment were compared with the corresponding findings after pharmacological inhibition of catalase or ALDH (see below). Six groups of conscious rats (3 SHRs and 3 WKY rats, n = 4–7 each) were used in this experiment to investigate the hemodynamic effects of intra-RVLM administration of ethanol, acetaldehyde, or their vehicle. A period of at least 60 min was allowed at the beginning of each experiment for BP and HR stabilization. Subsequently, one group from each rat strain received one of the following RVLM microinjections: 1) ethanol (10 μg), 2) acetaldehyde (2 μg), or 3) artificial cerebrospinal fluid (aCSF; 80 nl, 123 mM NaCl, 0.86 mM CaCl2, 3 mM KCl, 0.89 mM MgCl2, 25 mM NaHCO3, 0.5 mM NaH2PO4, and 0.25 mM Na2HPO4; pH 7.4). The 10-μg dose of ethanol has been shown in our previous study (26) to elicit a pressor response in conscious SHRs, whereas the dose of acetaldehyde was selected based on reported relative potencies of acetaldehyde and ethanol in a neurobiological study (34). BP and HR were monitored for 60 min after ethanol, acetaldehyde, or aCSF administration.
Influence of catalase or ALDH inhibition on the cardiovascular effects of intra-RVLM ethanol or acetaldehyde.
In this experiment, we investigated the effects of inhibiting the catalytic conversion of ethanol into acetaldehyde (catalase) and degradation of acetaldehyde (ALDH) on the cardiovascular responses elicited by intra-RVLM ethanol or acetaldehyde. The doses and route of administration (intravenous) of the catalase inhibitor ATZ (0.5 g/kg) and the ALDH inhibitor cyanamide (10 mg/kg) were based on the use of such a regimen to investigate the impact interfering with ethanol metabolism on the behavioral effects of microinjected ethanol into discrete brain areas in reported studies (10, 22, 32, 34). Seven groups of conscious SHRs (n = 5–7 each) were used and allocated to receive one of the following regimens: 1) saline + aCSF, 2) ATZ + aCSF, 3) cyanamide + aCSF, 4) ATZ + ethanol, 5) ATZ + acetaldehyde, 6) cyanamide + ethanol, and 7) cyanamide + acetaldehyde. Ten minutes were allowed between successive treatments of each regimen. Hemodynamic monitoring continued for 60 min after the last treatment of each regimen (i.e., aCSF, ethanol, or acetaldehyde). Parallel experiments with ATZ were not conducted in WKY rats because the findings of experiment 1 showed that intra-RVLM ethanol or acetaldehyde elicited minimal BP effects in this strain of rats. However, in another experiment, three groups of WKY rats were used to investigate the effect of ALDH inhibition (cyanamide) on the cardiovascular effects of intra-RVLM acetaldehyde. Rats were treated with either 1) saline + aCSF, 2) cyanamide + aCSF, or 3) cyanamide + acetaldehyde. BP and HR were monitored for 60 min after the second treatments.
Drugs
Ketaject (ketamine) and Xyla-ject (xylazine) (Phoenix Pharmaceuticals, St Joseph, MI); buprenorphine (Rickitt & Colman, Richmond, VA); Durapen (Vedco, Overland Park, KS); cyanamide, ATZ, and acetaldehyde (Sigma Chemical); and ethanol (Midwest Grain Products, Weston, MO) were purchased from commercial vendors.
Statistical Analysis
Values are expressed as means ± SE. Mean arterial pressure (MAP) was computed via integration of the area under the pulse pressure curve using LabChart software. Student's t-test was used in the analysis of unpaired data (interstrain differences in baseline BP, HR, and enzymatic activity). ANOVA followed by a Newman-Keuls post hoc analysis was used for multiple comparisons (hemodynamic effects of ethanol or acetaldehyde in the absence and presence of ATZ or cyanamide), with the level of significance set at P < 0.05.
RESULTS
Baseline Data
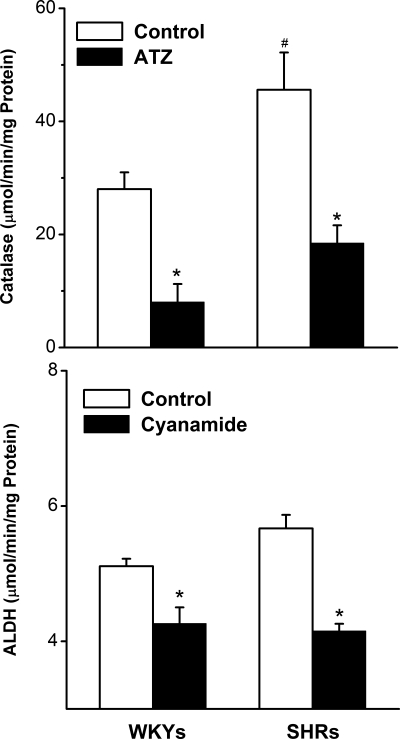
Pooled MAP data of all groups showed that compared with WKY rats, SHRs exhibited significantly higher baseline MAP (155 ± 3 vs. 120 ± 2 mmHg, unpaired Student's t-test, P < 0.05). On the other hand, there were no intergroup differences in baseline MAP within each rat strain before they received the different treatments (Table 1). RVLM neurons of SHRs exhibited approximately threefold (P < 0.05) higher catalase activity compared with the control (WKY rats) value (Fig. 1). On the other hand, similar activities for ALDH were observed in the RVLM of the two rat strains (Fig. 1). Furthermore, in both rat strains, intravenous ATZ or cyanamide (10 mg/kg each) significantly (P < 0.05) reduced the activity of catalase and ALDH, respectively, in the RVLM (Fig. 1).
Table 1.
Baseline values of mean arterial pressure and heart rate
| Group | Mean Arterial Pressure, mmHg | Heart Rate, beats/min |
|---|---|---|
| Spontaneously hypertensive rats | ||
| Saline + aCSF | 155 ± 11 | 361 ± 12 |
| Saline + ethanol | 158 ± 9 | 355 ± 15 |
| Saline + acetaldehyde | 164 ± 5 | 365 ± 18 |
| ATZ + aCSF | 154 ± 9 | 333 ± 20 |
| ATZ + ethanol | 159 ± 5 | 388 ± 14 |
| ATZ + acetaldehyde | 157 ± 9 | 348 ± 21 |
| Cyanamide | 149 ± 4 | 352 ± 10 |
| Cyanamide + ethanol | 144 ± 4 | 368 ± 9 |
| Cyanamide + acetaldehyde | 151 ± 7 | 378 ± 11 |
| Wistar-Kyoto rats | ||
| Saline + aCSF | 119 ± 4 | 327 ± 14 |
| Saline + ethanol | 114 ± 6 | 371 ± 9 |
| Saline + acetaldehyde | 129 ± 3 | 339 ± 16 |
| Cyanamide | 121 ± 5 | 353 ± 8 |
| Cyanamide + ethanol | 119 ± 3 | 341 ± 16 |
Values are means ± SE.
aCSF, artificial cerebrospinal fluid; ATZ, 3-aminotriazole.
Fig. 1.
Activity of catalase and aldehyde dehydrogenase (ALDH) measured in micropunched neuronal tissues from the rostral ventrolateral medulla (RVLM) of spontaneously hypertensive rats (SHR) and Wistar-Kyoto (WKY) rats in the absence or presence of catalase inhibitor [3-aminotriazole (ATZ)] or ALDH inhibitor (cyanamide) (10 mg/kg iv). Values are means ± SE of 5 observations. *P < 0.05 vs. the corresponding WKY values; #P < 0.05 vs. the respective WKY values.
Strain-Dependent Cardiovascular Effects of Intra-RVLM Ethanol or Acetaldehyde
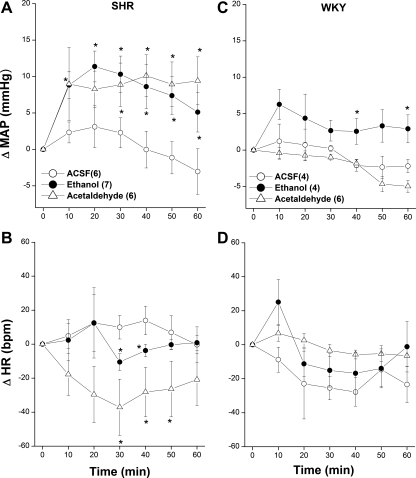
The cardiovascular effects caused by ethanol or acetaldehyde microinjection into the RVLM of SHRs and WKY rats are shown in Fig. 2. Compared with vehicle (aCSF), intra-RVLM ethanol (10 μg) produced an immediate and significant increase in MAP with a peak pressor effect of 12 ± 2 mmHg observed after ∼20 min (Fig. 2A). Afterward, the MAP of ethanol-treated SHRs showed a gradual decline but remained significantly higher than corresponding control (aCSF) values throughout the 60-min posttreatment period (Fig. 2A). Ethanol also caused slight but significant reductions in HR in SHRs at 30 and 40 min (Fig. 2B). Similarly, in SHRs, intra-RVLM acetaldehyde (2 μg) increased BP to levels that were similar in magnitude and duration to those caused by ethanol (Fig. 2A). The pressor action of acetaldehyde was associated with decreases in HR that were apparently greater and of longer duration than those caused by ethanol (Fig. 2B). In WKY rats, except for a modest pressor effect (∼ 5–6 mmHg) caused by ethanol during the last 20 min of the experiment, neither MAP (Fig. 2C) nor HR (Fig. 2D) were altered by the exposure to intra-RVLM ethanol or acetaldehyde.
Fig. 2.
Changes in mean arterial pressure (ΔMAP; A and C) and heart rate [ΔHR; in beats/min (bpm); B and D] caused by the intra-RVLM administration of ethanol (10 μg), acetaldehyde (2 μg), or an equal volume of artificial cerebrospinal fluid (aCSF; 80 nl) in conscious SHRs (left) and WKY rats (right). Values are means ± SE; numbers of rats in each group are shown in parentheses. *P < 0.05 vs. the respective time-course aCSF values.
Effect of Catalase or ALDH Inhibition on the Cardiovascular Responses Elicited by Intra-RVLM Ethanol or Acetaldehyde
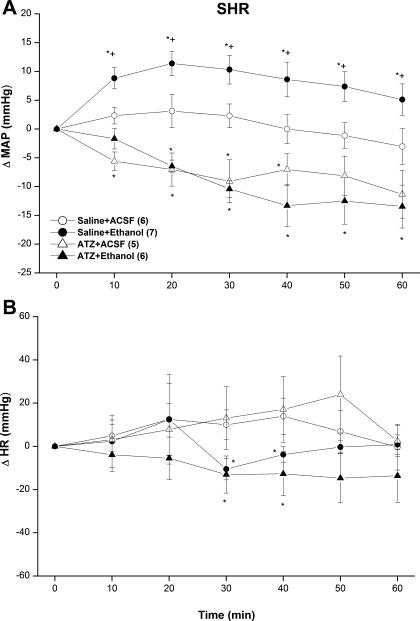
Figures 3 and 4 show the influence of catalase inhibition (ATZ) on MAP and HR responses elicited by intra-RVLM ethanol or acetaldehyde in SHRs. Compared with saline, the catalase inhibitor ATZ (10 mg/kg iv) had no effect on HR (Fig. 3B) but produced a gradual and sustained decrease in MAP of ∼10 mmHg (Fig. 3A). This hypotensive effect of ATZ was not altered by subsequent intra-RVLM ethanol (10 μg) or an equal volume of aCSF, which indicated abolition by ATZ of the pressor response caused by intra-RVLM ethanol (Fig. 3A). In contrast, the pressor response elicited by intra-RVLM acetaldehyde (2 μg) was still evident after ATZ pretreatment under the same conditions (Fig. 4A). The bradycardic action of ethanol (Fig. 3B) or acetaldehyde (Fig. 4B) was still evident in ATZ-treated SHRs.
Fig. 3.
ΔMAP (A) and ΔHR (B) caused by the intra-RVLM administration of ethanol (10 μg) or an equal volume of aCSF (80 nl) in the absence and presence of the catalase inhibitor ATZ (10 mg/kg iv) in conscious SHRs. Values are means ± SE; numbers of rats in each group are shown in parentheses. *P < 0.05 vs. the respective saline + aCSF values; +P < 0.05 vs. the respective ATZ + ethanol values.
Fig. 4.
ΔMAP (A) and ΔHR (B) caused by the intra-RVLM administration of acetaldehyde (2 μg) or an equal volume of aCSF (80 nl) in the absence and presence of the catalase inhibitor ATZ (10 mg/kg iv) in conscious SHRs. Values are means ± SE; numbers of rats in each group are shown in parentheses. *P < 0.05 vs. the respective saline + aCSF values; +P < 0.05 vs. the respective ATZ + acetaldehyde values; #P < 0.05 vs. the respective ATZ + aCSF values.
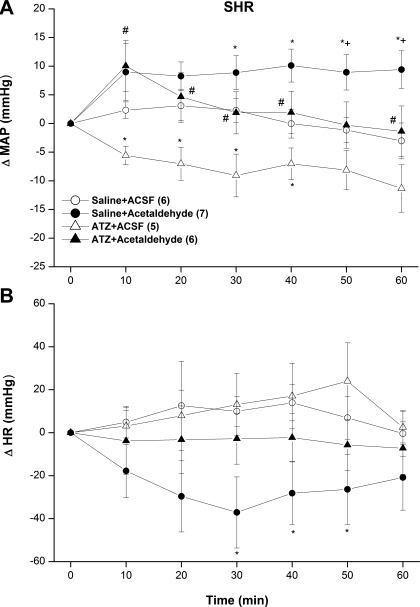
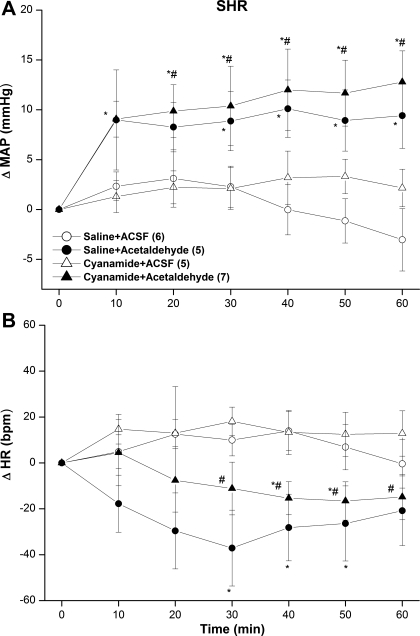
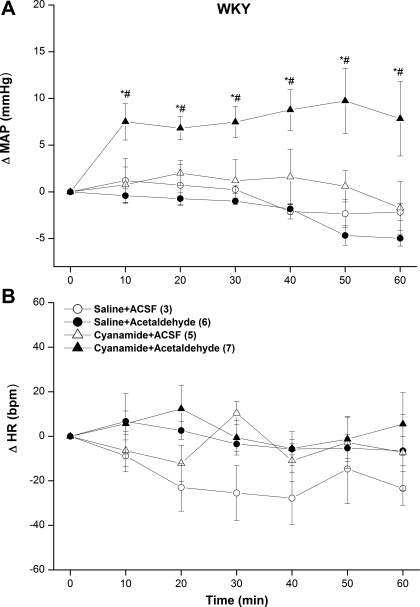
The inhibition of ALDH by cyanamide (10 mg/kg iv) caused no significant changes in baseline MAP (Figs. 5A and 6A) or HR (Figs. 5B and 6B) compared with the corresponding values in saline-treated SHRs or WKY rats. Furthermore, neither the pressor (Fig. 5A) nor bradycardic (Fig. 5B) responses elicited by intra-RVLM acetaldehyde was altered in cyanamide-pretreated SHRs. On the other hand, in cyanamide-pretreated WKY rats, intra-RVLM acetaldehyde produced abrupt and sustained increases in MAP (Fig. 6A), whereas HR remained unchanged (Fig. 6B). This latter finding is in marked contrast to the lack of a change in BP caused by intra-RVLM acetaldehyde in WKY rats in the absence of ALDH inhibition (Fig. 2C).
Fig. 5.
ΔMAP (A) and ΔHR (B) caused by the intra-RVLM administration of acetaldehyde (2 μg) or an equal volume of aCSF (80 nl) in the absence and presence of the ALDH inhibitor cyanamide (10 mg/kg iv) in conscious SHRs. Values are means ± SE; numbers of rats in each group are shown in parentheses. *P < 0.05 vs. the respective saline + aCSF values; #P < 0.05 vs. the respective cyanamide + aCSF values.
Fig. 6.
ΔMAP (A) and ΔHR (B) caused by the intra-RVLM administration of acetaldehyde (2 μg) or an equal volume of aCSF (80 nl) in the absence and presence of the ALDH inhibitor cyanamide (10 mg/kg iv) in conscious WKY rats. Values are means ± SE; numbers of rats in each group are shown in parentheses. *P < 0.05 vs. the respective saline + aCSF values; #P < 0.05 vs. the respective cyanamide + aCSF values.
DISCUSSION
The first experimental evidence of strain-dependent pressor response elicited by intra-RVLM ethanol in the SHR was established in our laboratory (26). Although the oxidative product acetaldehyde has been implicated in the behavioral effects of ethanol (25, 32), no attempt has been made to investigate whether acetaldehyde contributes to the ethanol-evoked pressor response and its strain specificity. The present investigation, which was performed in conscious freely moving rats, provides convincing evidence for the involvement of the oxidative product acetaldehyde in the stain-dependent hypertensive action of intra-RVLM ethanol. Our findings that RVLM neurons of SHRs exhibited higher catalase activity compared with WKY rats and that catalase inhibition (ATZ) abrogated the hypertensive action of ethanol in SHRs infer a critical role for one or more of the oxidative products of ethanol in the evoked pressor response. Subsequent experiments highlighted acetaldehyde as the most likely candidate because, like ethanol, intra-RVLM acetaldehyde increased BP in SHRs but not in WKY rats. Equally important, intra-RVLM acetaldehyde increased BP in WKY rats only after ALDH inhibition by cyanamide. Collectively, the present findings suggest a significant role for local (RVLM) ethanol metabolism in the strain-specific pressor response elicited by intra-RVLM ethanol in conscious SHRs.
We hypothesized that an imbalance between acetaldehyde generation, from ethanol by catalase, and its catabolism by ALDH, leads to the accumulation of acetaldehyde within the RVLM. This hypothesis was based on the premise that acetaldehyde induces oxidative stress in neuronal tissues (2), and oxidative stress within the RVLM leads to sympathoexcitation (7). Importantly, the pressor response caused by intra-RVLM ethanol in SHRs in this study replicates our previous finding, which implicated RVLM generated sympathoexcitation in such a response in the same model system (26). Therefore, considering the abundance and importance of catalase as the primary and major enzyme that catalyzes ethanol oxidation to acetaldehyde in the central nervous system (34, 42), we felt it important to determine if RVLM catalase activity is higher in the SHR and to evaluate the influence of catalase inhibition on the pressor response caused by intra-RVLM ethanol. Consistent with our hypothesis, RVLM catalase activity was higher in the SHR, and the pressor effect of ethanol was virtually abolished in SHRs pretreated with the catalase inhibitor ATZ. One additional approach was ised to support the premise that acetaldehyde is the primary mediator of the hypertensive action of ethanol. We showed that, similar to ethanol, acetaldehyde microinjection into the RVLM caused hypertension only in SHRs. Furthermore, the increase in BP caused by acetaldehyde in SHRs was similar, in duration and magnitude, to that caused by intra-RVLM ethanol. Interestingly, this is the first demonstration of a neuronally mediated effect of acetaldehyde in discrete areas of the brain stem that play a significant role in BP control (9). We considered the possibility that systemic administration of the catalase (ATZ) or ALDH (cyanamide) inhibitor might have confounded the interpretation of the data. For example, ATZ-evoked hypotension might have counterbalanced the pressor effect of intra-RVLM ethanol perhaps via physiological antagonism. This possibility seems highly unlikely because, under the same experimental conditions, systemic ATZ failed to alter the pressor response elicited by intra-RVLM acetaldehyde. The reason for ATZ-evoked hypotension is not clear; notably, variable ATZ effects on BP have been reported, including increases (40), decreases (8), or no change (24). It is important to note that the pharmacological paradigm adopted in the present study to inhibit ethanol or acetaldehyde metabolism within the RVLM has been used in previously reported studies (10, 22, 32, 34) on the behavioral effects of microinjected ethanol into discrete brain areas. Collectively, these findings suggest that acetaldehyde generation by the catalytic oxidation of ethanol in RVLM neurons is causally linked to its hypertensive effect in SHRs.
The other observation that supports the acetaldehyde hypothesis was obtained from experiments on ALDH inhibition in normotensive rats. Although we did not measure RVLM acetaldehyde levels, the pharmacological findings support our hypothesis because the lack of effect of intra-RVLM acetaldehyde on BP in WKY rats was transformed into a significant and sustained pressor response after ALDH inhibition (cyanamide) in the same rat strain. Therefore, it is conceivable to suggest that ethanol-derived acetaldehyde undergoes rapid ALDH-mediated catabolism in the RVLM of normotensive (WKY) rats. ALDH inhibition appeared to have created an environment conducive to acetaldehyde accumulation in the RVLM and permitted the full expression of its pressor effect in WKY rats. Albeit indirectly deduced, these findings argue against a significant role for acetate, the next step in the metabolism of ethanol (36), in the hypertensive action of ethanol or acetaldehyde. This conclusion is contrary to a contributory role for acetate in the neurobiological/behavioral effects of ethanol (11, 12).
The present neurochemical data revealed that while a substantially higher RVLM catalase activity was detected in SHRs compared with WKY rats, RVLM ALDH activity was similar in the two rat strains. This pattern is expected to lead to a higher local ethanol-derived formation/accumulation of acetaldehyde in the RVLM of the SHR compared with WKY rats. Notably, microinjection of the same amount of acetaldehyde into the RVLM caused a pressor response only in SHRs despite the similar RVLM ALDH activity in the two rat strains. These findings raise the interesting possibility that other intrinsic nonenzymatic mechanisms (34) may have contributed to the strain-dependent BP effect of acetaldehyde in SHRs and WKY rats in the present study. To our knowledge, this is the first demonstration of strain-dependent differences in catalase activity in RVLM neurons of rats. In fact, little and contradictory data are available concerning interstrain variability in catalase activity in other brain areas. For example, higher catalase activity has been detected in the striatum, whereas lower activity has been reported in the whole brain homogenate of SHRs compared with normotensive controls (1, 19).
The hypertensive response elicited by ethanol or acetaldehyde was associated with bradycardia. While the pressor effect of intra-RVLM ethanol was attributed to the enhancement of central sympathetic tone (26, 29) and the present findings infer a similar mechanism for acetaldehyde, the mechanism of the associated bradycardia has not been elucidated. It could be speculated that the bradycardia might be a baroreflex response to the BP elevation. Arguing against this assumption is the observation that acetaldehyde elevated BP in cyanamide-treated WKY rats but caused no bradycardia. Similarly, acetaldehyde increased BP in ATZ-treated SHRs without affecting HR. More studies are clearly needed to characterize the mechanism(s) of the bradycardic effect of intra-RVLM ethanol.
It is important to comment on the potential role of osmolality changes in the pressor effect of ethanol. Remarkably, hyperosmolality has been shown in clinical and experimental studies to produce sustained increases in sympathetic neural activity and BP through the stimulation of central osmoreceptors (38). These osmotically mediated effects have been attributed to the activation of vasopressinergic and glutamatergic pathways projecting to the RVLM sympathetic neurons (3, 5, 17). Given that ethanol is osmotically active and that osmolarity modulation accounts for the ethanol-evoked changes in secretory (23) and disease states (28), future studies are needed to determine if osmotic changes in RVLM neurons contribute to the sympathetic and BP responses elicited by ethanol in our model system.
In conclusion, this study provides important information regarding the role of oxidative catabolism in RVLM neurons in the strain-dependent pressor effect of ethanol in conscious rats. Acetaldehyde, the breakdown product of ethanol under catalase activity, appears to play a causal role in the pressor response produced by intra-RVLM ethanol in the SHR. This conclusion gains credence from the following observations: 1) compared with WKY rats, SHRs exhibited higher RVLM catalase activity; 2) catalase inhibition virtually abolished the pressor action of intra-RVLM ethanol in SHRs; 3) intra-RVLM acetaldehyde replicated the strain-dependent pressor effect of ethanol; and 4) ALDH inhibition (cyanamide) uncovered a significant pressor response to intra-RVLM acetaldehyde in WKY rats. The findings clearly highlight the importance of the balance between aldehyde-producing and -eliminating enzymes in the RVLM in guarding against the potential accumulation of potentially neurotoxic aldehyde levels in a brain stem area that controls sympathetic activity and BP. Specifically, tipping the balance toward higher production/accumulation of ethanol-derived acetaldehyde in the RVLM of the SHR can lead to an exacerbation of hypertension in this genetic model of hypertension.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant 2-R01-AA-07839-18.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.M.E.-M. and A.A.A.-R. conception and design of research; M.M.E.-M. and A.A.A.-R. analyzed data; M.M.E.-M. and A.A.A.-R. interpreted results of experiments; M.M.E.-M. prepared figures; M.M.E.-M. drafted manuscript; M.M.E.-M. and A.A.A.-R. approved final version of manuscript; A.A.A.-R. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Kui Sun, Jeannie Zhang, and Hoda Khafaga for technical assistance.
M. M. El-Mas is on a sabbatical leave from the Department of Pharmacology, Faculty of Pharmacy, Alexandria University, Alexandria 21526, Egypt (e-mail: mahelm@hotmail.com).
REFERENCES
- 1. Abilio VC, Silva RH, Carvalho RC, Grassl C, Calzavara MB, Registro S, D'Almeida V, Ribeiro Rde A, Frussa-Filho R. Important role of striatal catalase in aging- and reserpine-induced oral dyskinesia. Neuropharmacology 47: 263–272, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Amanvermez R, Agara E. Does ascorbate/l-Cys/l-Met mixture protect different parts of the rat brain against chronic alcohol toxicity? Adv Ther 23: 705–718, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Antunes VR, Yao ST, Pickering AE, Murphy D, Paton JF. A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation. J Physiol 576: 569–583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension 55: 284–290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brooks VL, Freeman KL, O'Donaughy TL. Acute and chronic increases in osmolality increase excitatory amino acid drive of the rostral ventrolateral medulla in rats. Am J Physiol Regul Integr Comp Physiol 287: R1359–R1368, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bruandet N, Rentero N, Debeer L, Quintin L. Catecholamine activation in the vasomotor center on emergence from anesthesia: the effects of α2 agonists. Anesth Analg 86: 240–245, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Campos RR, Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT. The role of oxidative stress in renovascular hypertension. Clin Exp Pharmacol Physiol 38: 144–152, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Cardoso LM, Colombari DS, Menani JV, Toney GM, Chianca DA, Jr, Colombari E. Cardiovascular responses to hydrogen peroxide into the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 297: R462–R469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chalmers J, Pilowsky P. Brainstem and bulbospinal neurotransmitter systems in the control of blood pressure. J Hypertens 9: 675–694, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Chen X, Patel K, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Acute antihypertensive action of tempol in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 293: H3246–H3253, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Correa M, Arizzi MN, Betz A, Mingote S, Salamone JD. Open field locomotor effects in rats after intraventricular injections of ethanol and the ethanol metabolites acetaldehyde and acetate. Brain Res Bull 62: 197–202, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Cullen N, Carlen PL. Electrophysiological actions of acetate, a metabolite of ethanol, on hippocampal dentate granule neurons: interactions with adenosine. Brain Res 588: 49–57, 1992 [DOI] [PubMed] [Google Scholar]
- 13. El-Mas MM, Abdel-Rahman AA. Direct evidence for selective involvement of aortic baroreceptors in ethanol-induced impairment of baroreflex control of heart rate. J Pharmacol Exp Ther 264: 1198–1205, 1993 [PubMed] [Google Scholar]
- 14. El-Mas MM, Abdel-Rahman AA. Estrogen-dependent hypotensive effects of ethanol in conscious female rats. Alcohol Clin Exp Res 23: 624–632, 1999 [PubMed] [Google Scholar]
- 15. El-Mas MM, Abdel-Rahman AA. Ovariectomy abolishes ethanol-induced impairment of baroreflex control of heart rate in conscious rats. Eur J Pharmacol 349: 253–261, 1998 [DOI] [PubMed] [Google Scholar]
- 16. El-Mas MM, El-Gowelli HM, Abd-Elrahman KS, Saad EI, Abdel-Galil AG, Abdel-Rahman AA. Pioglitazone abrogates cyclosporine-evoked hypertension via rectifying abnormalities in vascular endothelial function. Biochem Pharmacol 81: 526–533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farquhar WB, Wenner MM, Delaney EP, Prettyman AV, Stillabower ME. Sympathetic neural responses to increased osmolality in humans. Am J Physiol Heart Circ Physiol 291: H2181–H2186, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Guyenet PG, Schreihofer AM, Stornetta RL. Regulation of sympathetic tone and arterial pressure by the rostral ventrolateral medulla after depletion of C1 cells in rats. Ann NY Acad Sci 940: 259–269, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Hector Polizio A, Pena C. Effects of angiotensin II type 1 receptor blockade on the oxidative stress in spontaneously hypertensive rat tissues. Regul Pept 128: 1–5, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Ireland MA, Vandongen R, Davidson L, Beilin LJ, Rouse IL. Acute effects of moderate alcohol consumption on blood pressure and plasma catecholamines. Clin Sci 66: 643–648, 1984 [DOI] [PubMed] [Google Scholar]
- 21. Karahanian E, Quintanilla ME, Tampier L, Rivera-Meza M, Bustamante D, Gonzalez-Lira V, Morales P, Herrera-Marschitz M, Israel Y. Ethanol as a prodrug: brain metabolism of ethanol mediates its reinforcing effects. Alcohol Clin Exp Res 35: 606–612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kinoshita H, Jessop DS, Finn DP, Coventry TL, Roberts DJ, Ameno K, Jiri I, Harbuz MS. Acetaldehyde, a metabolite of ethanol, activates the hypothalamic-pituitary-adrenal axis in the rat. Alcohol Alcohol 36: 59–64, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Kucerová J, Strbák V. The osmotic component of ethanol and urea action is critical for their immediate stimulation of thyrotropin-releasing hormone (TRH) release from rat brain septum. Physiol Res 50: 309–314, 2001 [PubMed] [Google Scholar]
- 24. Lauar MR, Colombari DS, De Paula PM, Colombari E, Cardoso LM, De Luca LA, Jr, Menani JV. Inhibition of central angiotensin II-induced pressor responses by hydrogen peroxide. Neuroscience 171: 524–530, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Lee RD, An SM, Kim SS, Rhee GS, Kwack SJ, Seok JH, Chae SY, Park CH, Choi YW, Kim HS, Cho HY, Lee BM, Park KL. Neurotoxic effects of alcohol and acetaldehyde during embryonic development. J Toxicol Environ Health A 68: 2147–2162, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Li G, Wang X, Abdel-Rahman AA. Brainstem norepinephrine neurons mediate ethanol-evoked pressor response but not baroreflex dysfunction. Alcohol Clin Exp Res 29: 639–647, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta 257: 59–84, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Magner PO, Ethier JH, Kamel KS, Halperin ML. Interpretation of the urine osmolality: the role of ethanol and the rate of excretion of osmoles. Clin Invest Med 14: 355–358, 1991 [PubMed] [Google Scholar]
- 29. Mao L, Li G, Abdel-Rahman AA. Effect of ethanol on reductions in norepinephrine electrochemical signal in the rostral ventrolateral medulla and hypotension elicited by I1-receptor activation in spontaneously hypertensive rats. Alcohol Clin Exp Res 27: 1471–1480, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Nassar N, Li G, Strat A, Abdel-Rahman AA. Enhanced hemeoxygenase activity in the rostral ventrolateral medulla mediates the exaggerated hemin-evoked hypotension in the SHR. J Pharmacol Exp Ther 339: 267–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Orywal K, Jelski W, Zdrodowski M, Szmitkowski M. The activity of class I, II, III and IV alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in cervical cancer. Clin Biochem 24: 334–339, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pastor R, Aragon CM. Ethanol injected into the hypothalamic arcuate nucleus induces behavioral stimulation in rats: an effect prevented by catalase inhibition and naltrexone. Behav Pharmacol 19: 698–705, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1986 [DOI] [PubMed] [Google Scholar]
- 34. Quertemont E, Tambour S, Tirelli E. The role of acetaldehyde in the neurobehavioral effects of ethanol: a comprehensive review of animal studies. Prog Neurobiol 75: 247–274, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Quertemont E. Discriminative stimulus effects of ethanol with a conditioned taste aversion procedure: lack of acetaldehyde substitution. Behav Pharmacol 14: 343–350, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Ramchandani VA, Bosron WF, Li TK. Research advances in ethanol metabolism. Pathol Biol (Paris) 49: 676–682, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Russ R, Abdel-Rahman ARA, Wooles WR. Role of sympathetic nervous system in ethanol-induced hypertension in rats. Alcohol 8: 301–307, 1991 [DOI] [PubMed] [Google Scholar]
- 38. Stocker SD, Osborn JL, Carmichael SP. Forebrain osmotic regulation of the sympathetic nervous system. Clin Exp Pharmacol Physiol 35: 695–700, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Wakabayashi I, Hatake K. [Effects of ethanol on the nervous and vascular systems: the mechanisms of alcohol-induced hypertension]. Nihon Eiseigaku Zasshi 55: 607–617, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Yu Y, Zhong MK, Li J, Sun XL, Xie GQ, Wang W, Zhu GQ. Endogenous hydrogen peroxide in paraventricular nucleus mediating cardiac sympathetic afferent reflex and regulating sympathetic activity. Pflügers Arch 454: 551–557, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Zilkens RR, Burke V, Hodgson JM, Barden A, Beilin LJ, Puddey IB. Red wine and beer elevate blood pressure in normotensive men. Hypertension 45: 874–879, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Zimatkin SM, Pronko SP, Vasiliou V, Gonzalez FJ, Deitrich RA. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res 30: 1500–1505, 2006 [DOI] [PubMed] [Google Scholar]