Abstract
Na+ current derived from expression of the cardiac isoform SCN5A is reduced by receptor-mediated or direct activation of protein kinase C (PKC). Previous work has suggested a possible role for loss of Na+ channels at the plasma membrane in this effect, but the results are controversial. In this study, we tested the hypothesis that PKC activation acutely modulates the intracellular distribution of SCN5A channels and that this effect can be visualized in living cells. In human embryonic kidney cells that stably expressed SCN5A with green fluorescent protein (GFP) fused to the channel COOH-terminus (SCN5A-GFP), Na+ currents were suppressed by an exposure to PKC activation. Using confocal microscopy, colocalization of SCN5A-GFP channels with the plasma membrane under control and stimulated conditions was quantified. A separate population of SCN5A channels containing an extracellular epitope was immunolabeled to permit temporally stable labeling of the plasma membrane. Our results demonstrated that Na+ channels were preferentially trafficked away from the plasma membrane by PKC activation, with a major contribution by Ca2+-sensitive or conventional PKC isoforms, whereas stimulation of protein kinase A (PKA) had the opposite effect. Removal of the conserved PKC site Ser1503 or exposure to the NADPH oxidase inhibitor apocynin eliminated the PKC-mediated effect to alter channel trafficking, indicating that both channel phosphorylation and ROS were required. Experiments using fluorescence recovery after photobleaching demonstrated that both PKC and PKA also modified channel mobility in a manner consistent with the dynamics of channel distribution. These results demonstrate that the activation of protein kinases can acutely regulate the intracellular distribution and molecular mobility of cardiac Na+ channels in living cells.
Keywords: sodium channels, membrane trafficking, protein kinase C
rapid inward current through voltage-gated Na+ channels is a critical determinant of electrical impulse conduction velocity in most areas of the heart (2, 26). A reduction in cardiac Na+ current (INa) slows conduction, which, in some cases, can enable reentrant circuits and promote the development of life-threatening ventricular arrhythmias (15, 28). This likely forms the basis for the proarrhythmia and excess mortality seen with Na+ channel-blocking drugs (14) and the propensity for sudden death in Brugada syndrome (32, 36). Thus, the factors that regulate the number of functional Na+ channels at the cell surface have important clinical implications.
Pathological states, such as cardiomyopathies, that increase the risk for sudden cardiac death are associated with neurohumoral activation (7). Adrenergic receptor stimulation of cellular signaling systems, and ultimately protein kinases, has been shown to modulate cardiac INa. In nearly all studies to date, the activation of PKC causes a generalized reduction in INa for both native and recombinant channels derived from the brain, skeletal muscle, and heart (16, 18, 26, 30). In previous experiments, we (27) have shown that direct and receptor-mediated activation of PKC suppresses INa derived from the human SCN5A channel. Our results specifically implicated conventional or Ca2+-sensitive (c)PKCs in this effect in addition to a possible role for loss of channels from the plasma membrane (31). Interestingly, mutations in the gene encoding glycerol-3-phosphate dehydrogenase 1-like protein (GPD1L), which cause Brugada syndrome and sudden infant death syndrome, have been shown to reduce SCN5A current (24, 34). Recent studies (24, 33) have implicated the downregulation of cell surface Na+ channels by PKC activation as the likely mechanism for this effect, although other data have suggested that the mechanism may be more complex (23). These data regarding GPD1L mutations further support a clinically important role for PKC-mediated regulation of cardiac INa in the generation of serious cardiac arrhythmias.
We (39) have previously generated an SCN5A channel with green fluorescent protein (GFP) attached to the COOH-terminus (SCN5A-GFP) and demonstrated that the resultant INa retains wild-type properties and responses to PKA activation. In this study, we used SCN5A-GFP to directly test the hypothesis that PKC activation acutely promotes the cellular redistribution of cardiac Na+ channels and that this effect can be visualized in living cells. Our results demonstrated a PKC-mediated effect to alter both the localization and mobility of SCN5A channels.
MATERIALS AND METHODS
Materials.
Reagent-grade chemicals, as well as 8-chlorophenylthio-cAMP, 3-isobutyl-1-methylxanthine, forskolin, PMA, and apocynin, were obtained from Sigma (St. Louis, MO). The PKC inhibitors Gö-6976 and Gö-6983 were purchased from Calbiochem (San Diego, CA), and the anti-hemaglutinin (HA) antibody and Alexa 564-conjugated secondary antibody were from Molecular Probes (Eugene, OR).
Na+ channel expression.
A human embryonic kidney (HEK)-293 cell line that stably expressed a fusion protein (SCN5A-GFP) with GFP attached to the COOH-terminus of SCN5A (SCN5A, GenBank Accession No. M77235) was generated as previously described (19). In a separate construct, three copies of the HA epitope were inserted into the initial, extracellular portion of the domain I S5-S6 loop of SCN5A (SCN5A-HA), with the construct subcloned into the pCR3 vector as previously described (19). In addition, we generated a mutant SCN5A-GFP channel in which the conserved PKC site at Ser1503 was eliminated by a mutation to alanine (SCN5A-S1503A-GFP). All constructs were sequenced to verify the mutation and to exclude inadvertent cloning errors. For the transient expression of SCN5A-HA, HEK-293 cells were transfected with 2 μg DNA using Lipofectamine. The medium was changed after 6 h, with cells harvested for study after 48 h. For the transient expression of SCN5A-S1503A-GFP and SCN5A-HA, 1 μg DNA was used for each construct.
Electrophysiology.
INa was recorded from HEK-293 cells that expressed SCN5A or SCN5A-GFP using the whole cell patch-clamp technique as previously described (37). In brief, the bath solution contained (in mmol/l) 145 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH 7.35, adjusted with NaOH). The pipette or intracellular solution contained (in mmol/l) 10 NaCl, 120 CsAsp, 10 CsCl, 2 EGTA, and 10 HEPES (pH 7.35, adjusted with CsOH). Osmolarity was adjusted to 310 mosM with sucrose for all solutions. Data acquisition was carried out with an Axopatch 200B patch-clamp amplifier and pCLAMP 8.2 software (Axon Instruments, Molecular Devices, Sunnyvale, CA), and data analysis was performed using pCLAMP. Electrode resistance ranged from 0.8 to 1.5 MΩ. From a holding potential of −120 mV, standard voltage-clamp protocols were used to examine the voltage dependence of channel activation, steady-state availability, and recovery from fast inactivation (37). For pharmacological experiments, PMA was freshly prepared before experiments from 100 μM stock solutions stored at −20°C. Currents were recorded at room temperature in the absence and presence of a 30-min exposure to PMA (10 nM) in the bath solution. In separate experiments, data were obtained during acute bath superfusion of PMA.
Confocal laser scanning microscopy.
Near-confluent (∼70%) HEK-293 cells expressing either SCN5A-GFP or SCN5A-S1503A-GFP and SCN5A-HA were imaged using a confocal microscope (LSM510) with a ×63/1.4 plan apochromat objective (Carl Zeiss). Cells were plated onto a Matek dish (Matek, Ashland, MA) and imaged after 48 h. The plasma membrane was labeled by immunostaining SCN5A-HA, with the HA epitope tag positioned in an extracellular location (see above). Cells stably expressing SCN5A-GFP were transfected with SCN5A-HA, whereas control HEK-293 cells were transfected with both SCN5A-S1503A-GFP and SCN5A-HA. After 48 h, cells were washed with DMEM (Sigma), exposed to a primary anti-HA antibody (1 h at 37°C), washed with DMEM, and incubated with an Alexa 594-conjugated secondary antibody (1 h at 37°C) followed by confocal imaging. Both GFP and Alexa 594 were excited at 543 nm with the LSM510 argon laser, with detection using a 585-nm bandpass filter. The confocal aperture was adjusted to restrict detection to optical sections of 1 μm. To control for cross talk between the detection of GFP and Alexa 594, single-labeled control cells (i.e., immunolabeled HEK-293 cells expressing SCN5A-HA or cells expressing SCN5A-GFP/SCN5A-S1503A-GFP in the absence of SCN5A-HA coexpression) were imaged under identical conditions as those used for dual-labeled probes to confirm the proper signal isolation of each channel. The initial data analysis was performed using LSM510 software. To minimize the effect of light exposure, images were obtained at 5-min intervals unless otherwise stated (19). Average single cell red fluorescence intensity was calculated by dividing the total red channel field intensity (above the background, on scale) by the number of red fluorescent cells.
Direct activation of PKC was achieved by the exposure of cells to PMA (10 nM), whereas receptor-mediated stimulation was achieved with histamine (10 μM) (35). The combination of 8-chlorophenylthio-cAMP (200 μM), 3-isobutyl-1-methylxanthine (1 mM), and forskolin (10 μM) was used for PKA stimulation (19).
Quantitative analysis of SCN5A trafficking.
The time-dependent spatial relationship of GFP-tagged SCN5A channels with the Alexa 594-labeled plasma membrane was measured using Metamorph image-analysis software (Universal Imaging, Downington, PA), as previously described (13, 19). The dynamic range for both the green (GFP) and red (Alexa 594) channels was maximized to achieve the highest precision and contrast in the image. The minimum threshold signal was set to the value of the background signal plus 5%, whereas the maximal threshold was set to the maximum minus 1 [or 254 arbitrary units (AU)], so that the signal measured was both above the background and on scale. For a field of cells, the pixel coordinates that contained both red and green signal were identified. Because Alexa 594 fluorescence was restricted to the plasma membrane, the green/red ratio (G/R; defined as the percentage of red-containing pixels that also contained green signal) reflected the colocalization of SCN5A-GFP/SCN5A-S1503A-GFP channels with the plasma membrane (within the resolution space, ∼0.1 μm3). Statistical analyses using general linear model repeated measures were performed using SPSS version 16.0 (SPSS, Chicago, IL), with the results expressed as means ± SE. P values of <0.05 were considered statistically significant.
Fluorescence recovery after photobleaching.
Using HEK-293 cells expressing SCN5A-GFP, a cytosolic region of interest (ROI) near the plasma membrane was photobleached by 40 iterations of 100% laser transmission. Pre- and postbleach images were acquired every 10 s for 20 scans (224 s). The average fluorescence intensity within the bleached ROI was normalized to prebleach intensity, and recovery kinetics were determined using an exponential function of the averaged data. The mobile fraction (Mf), a measure of channel protein mobility, was calculated as follows: Mf = (F∞ − F0)/(Fi − F0), where F∞ is the fluorescence intensity after the final recovery time point, Fi is the initial fluorescence intensity before bleach, and F0 is the immediate postbleach fluorescence intensity (6). Cumulative photobleaching outside of the ROI was typically ∼10–15% of the prebleach intensity.
RESULTS
Suppression of SCN5A-GFP currents by PKC activation.
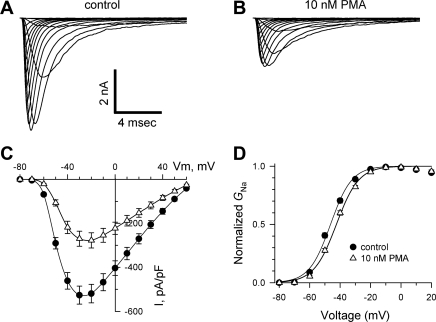
The effects of PKC activation on INa derived from SCN5A-GFP have not been previously investigated . Therefore, we recorded INa from HEK-293 cells that stably expressed this channel in the absence and presence of a 30-min exposure to PMA (10 nM), which directly activates PKC. Exposure to PMA caused a reduction in peak INa at all test potentials examined (from 519 ± 42 to 277 ± 35 pA/pF at −20 mV, or −47%; Fig. 1, A–C). This effect was associated with a small but significant shift in the voltage dependence of channel activation to more positive potentials [midpoints were −46 ± 1 and −42 ± 1 mV for control cells (n = 12) and PMA-treated cells (n = 14), respectively, P < 0.001; Fig. 1D]. Exposure to PMA had no effect on INa kinetics, the voltage dependence of steady-state availability [midpoints were −73 ± 2 and −72 ± 1 mV for control cells (n = 12) and PMA-treated cells (n = 14)], or the recovery from fast inactivation [τ1: 11 ± 1 and 11 ± 1 ms, τ2: 105 ± 15 and 91 ± 11 ms, A1: 84.7 ± 1.5% and 84.9 ± 3.8%, and A2: 15.3 ± 1.5% and 15.1 ± 3.8% for control cells (n = 12) and PMA-treated cells (n = 14), respectively; data not shown, where τ1 is the time constant of the fast component, τ2 is the time constant of the slow component, A1 is the amplitude of the fast component, and A2 is the amplitude of the slow component.]. Thus, the dominant effect of PMA was a reduction in INa. Similar findings were obtained after the expression of SCN5A lacking GFP: peak INa declined from 439 ± 38 to 250 ± 27 pA/pF at −20 mV (a 43% reduction). To determine whether PMA acutely blocks SCN5A channels, separate experiments were performed during which INa was recorded under control conditions and during bath superfusion of the PKC activator. Under these conditions, the magnitude of INa was unchanged [−3.2 ± 0.9% at −20 mV with PMA exposure for 5 min (n = 4); data not shown].
Fig. 1.
Effect of PKC stimulation on Na+ currents (INa) in human embryonic kidney (HEK)-293 cells stably expressing SCN5A channels with green fluorescent protein (GFP) fused to the channel COOH-terminus (SCN5A-GFP). A and B: using a holding potential of −120 mV, representative INa is shown for HEK-293 cells expressing SCN5A-GFP after a 30-min incubation in the absence (A) and presence (B) of the PKC activator PMA (10 nM). C: summary data with peak INa plotted as a function of the test potential. Activation of PKC caused a significant reduction in INa amplitude (between −60 and +40 mV, P < 0.001). Vm, membrane voltage. D: voltage dependence of channel activation was estimated by dividing INa at each test potential by the electrochemical driving force for Na+, with normalization to the maximum Na+ conductance (GNa). Activation of PKC caused a small but significant shift in the activation curve to more positive potentials (see text; P < 0.001), with no change in the slope factor [the slope factor was 6.9 ± 0.2 and 7.3 ± 0.3 mV for control cells (n = 12) and PMA-treated cells (n = 14), respectively].
Plasma membrane labeling using extracellular HA-tagged SCN5A channels.
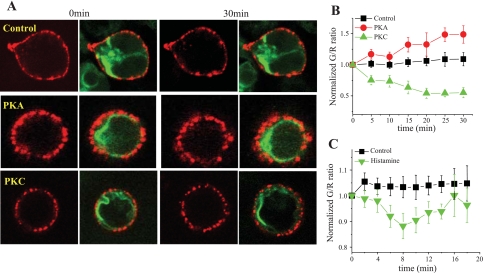
We hypothesized that membrane Na+ channels bound to an extracellular antibody would be rendered relatively immobile and thus provide quantifiably stable labeling of the plasma membrane for confocal imaging. HEK-293 cells were transfected with SCN5A-HA. After 48 h, SCN5A-HA channels were immunolabeled by an exposure to primary anti-HA and Alexa 594-conjugated secondary antibodies. As shown in Fig. 2A, top, immunolabeled SCN5A-HA channels generated a diffuse red signal at the plasma membrane. Moreover, coexpression of SCN5A-HA had no apparent effect on the intracellular distribution of SCN5A-GFP channels (data not shown). To assess the stability of membrane labeling using this experimental strategy, cells were imaged for 30 min, and the average single cell fluorescence intensity for the red channel was quantified. There were no observable changes in this measurement over time [118 ± 2 and 113 ± 3 AU at baseline and 30 min, respectively (n = 13)]. Thus, immunolabeled SCN5A-HA channels provided appropriate labeling of the plasma membrane in living cells over time during confocal imaging.
Fig. 2.
Differential effects of PKA and PKC stimulation on the cellular distribution of SCN5A-GFP channels relative to the plasma membrane. A: confocal imaging was performed using HEK-293 cells stably expressing SCN5A-GFP and transiently transfected with three copies of the hemaglutinin (HA) epitope inserted into the initial, extracellular portion of the domain I S5-S6 loop of SCN5A (SCN5A-HA), followed by immunolabeling using an anti-HA antibody and Alexa 594-conjugated secondary antibody. Representative images of immunolabeled SCN5A-HA channels (red) in HEK-293 cells that also expressed SCN5A-GFP (green) are shown at baseline (left) and after 30 min (right) under control, nonstimulated conditions (top) and after PKA (middle) or PKC (bottom) activation. B: summary data for the normalized green-to-red ratio (G/R) over time under control conditions and after kinase activation. The effects of both PKA and PKC stimulation on G/R were significant (P = 0.03 and P = 0.003, respectively). C: similar data for normalized G/R after an exposure to histamine (10 μM), with a transient but significant reduction that peaked at 8 min (P = 0.01).
Of note, the images shown in Fig. 2A demonstrated minimal yellow color at the plasma membrane, although INa can be readily recorded in these cells. Thus, visual interpretation of the merged images is not sufficiently sensitive to detect colocalization: only combinations of red and green that are similar in intensity will produce a color (yellow) that is easily visualized as overlapping. These data illustrate a well-established principle of confocal imaging: that colocalization must be measured and interpreted using numerical analysis of the digital image data (8–10, 22).
Effects of PKC activation on Na+ channel distribution.
To further explore the mechanism for PKC-mediated suppression of INa, we investigated the effects of PMA on the cellular distribution of SCN5A-GFP channels in living HEK-293 cells. For these experiments, we used a robust method to quantify the colocalization of GFP-tagged SCN5A channels (green) with the Alexa 594-immunolabeled plasma membrane (red) (13, 19). As detailed in the materials and methods, both green and red signals were optimized during and after data acquisition. For each microscopic field, the pixels containing green or red signals were identified. The fraction of pixels containing red signal (representing the membrane) that also contained green signal (representing SCN5A channels) was calculated (G/R). Importantly, this spatial measurement indicates the colocalization of Na+ channels with the membrane region, irrespective of the red and/or green signal intensity. As expected, under control, nonstimulated conditions, G/R was constant over time, with minimal changes during a 30-min imaging period [+9 ± 1% at 30 min (n = 5); Fig. 2B]. Exposure of the cells to PMA caused a gradual decline in G/R [−55 ± 1% at 30 min (n = 8); Fig. 2B], indicating a reduction in SCN5A channel density in the region of the cell membrane. On the other hand, stimulation of PKA increased this parameter [+48 ± 16% at 30 min (n = 9)], demonstrating increased colocalization of the channels with the cell surface. In addition, receptor-mediated activation of PKC using histamine produced a similar, albeit more modest, effect, with a transient but significant reduction in G/R [−16 ± 3.8% at 8 min (n = 9); Fig. 2C]. This self-limited effect is consistent with the transient time course of substrate phosphorylation previously demonstrated under these conditions (35). For PKA, these results support our previous findings (19) showing that kinase activation promotes the movement of Na+ channels toward the plasma membrane, whereas for PKC, they demonstrate redistribution away from the cell surface.
Effects of PKC are mediated by conventional PKC isoforms.
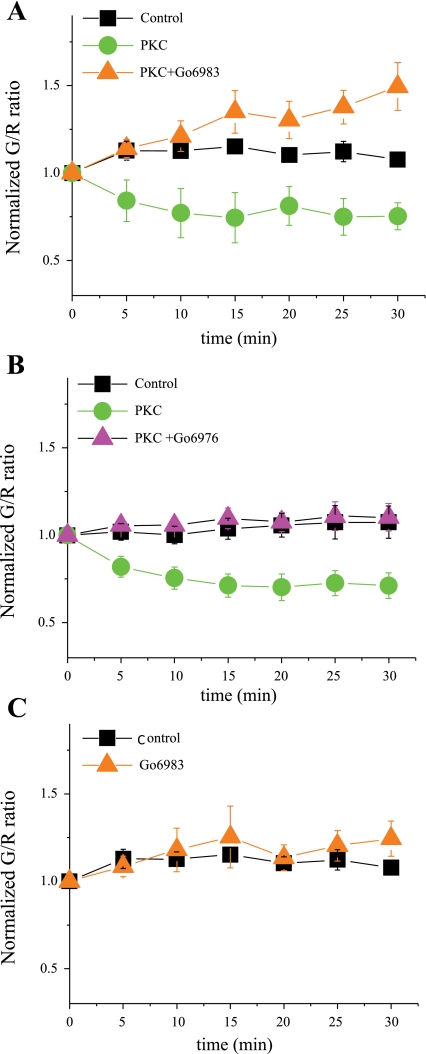
To further demonstrate that the effects of PMA shown in Fig. 2 were mediated by PKC activation, additional experiments were performed. Preincubation of cells with the pan PKC inhibitor Gö-6983 (1 μM; Fig. 3A) (17, 25, 35) or the cPKC inhibitor Gö-6976 (1 μM; Fig. 3B) (5, 17, 25) prevented the effects of PMA, confirming the participation of cPKC isoforms in the observed effect. In the absence of PKC activation, exposure of the cells to Gö-6983 had no effect, indicating that basal stimulation by the kinase was minimal (Fig. 3C).
Fig. 3.
PKC effects are mediated by conventional (c)PKC isoforms. A: the effect of PKC activation with PMA (10 nM) to reduce G/R compared with control conditions was prevented by a preincubation (for 30 min) with the pan PKC inhibitor Gö-6983 [1 μM (n = 7), P < 0.002]. B: similar data for the cPKC inhibitor Gö-6976 [1 μM (n = 7), P = 0.001]. C: PKC inhibition with Gö-6983 did not affect G/R under basal, nonstimulated conditions.
Mechanisms of altered SCN5A trafficking.
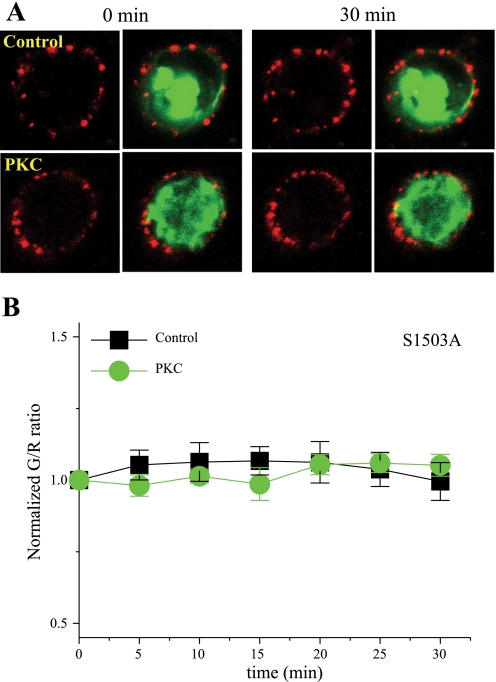
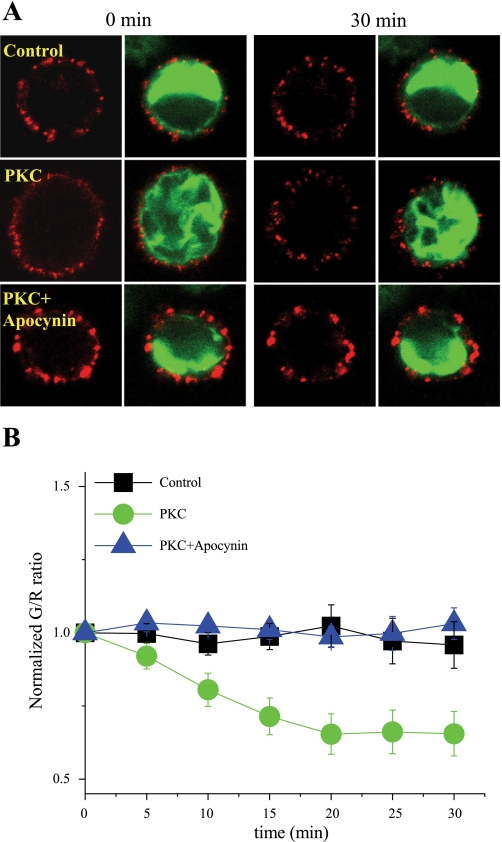
We (27) have previously shown that elimination of a conserved PKC site in SCN5A, Ser1503, inhibits PKC-mediated suppression of cardiac INa. To determine whether this site plays a similar role in PKC-mediated Na+ channel trafficking, Ser1503 was mutated to alanaine (S1503A) in SCN5A-GFP, and HEK-293 cells were cotransfected with SCN5A-S1503-GFP and SCN5A-HA for colocalization experiments. In the presence of S1503A, PKC-mediated effects to promote Na+ channel redistribution were eliminated, as shown in Fig. 4 (n = 10 for control cells and 20 for PMA-treated cells, respectively). These results are consistent with our previous electrophysiology data and demonstrate a critical role for SCN5A phosphorylation at this site. A recent study (23) has also demonstrated a role for ROS in PKC-related suppression of SCN5A current. Because NADPH oxidase is a major source of intracellular superoxide, additional experiments were conducted to examine the effects of PKC activation on Na+ channel trafficking in the presence of the apocynin (200 μM), an NADPH oxidase inhibitor. Under these conditions, PKC stimulation did not alter Na+ channel colocalization with the plasma membrane, as reflected by a stable G/R (n = 8 for control cells, 12 for PMA-treated cells, and 12 for PMA plus apocyanin-treated cells, respectively; Fig. 5). Taken together, these data indicate that both channel phosphorylation and ROS are required for the PKC-mediated effect to alter Na+ channel trafficking.
Fig. 4.
Effect of eliminating the conserved PKC site in SCN5A, Ser1503. A: representative images of immunolabeled SCN5A-HA channels (red) in HEK-293 cells that also expressed SCN5A-S1503A-GFP (green) at baseline (left) and after a 30-min exposure (right) to PKC activation. B: summary data for normalized G/R over time under control conditions and after PKC activation.
Fig. 5.
Effect of the NADPH oxidase inhibitor apocyanin on PKC-mediated SCN5A channel redistribution. A: representative images of immunolabeled SCN5A-HA channels (red) in HEK-293 cells that also expressed SCN5A-GFP (green) at baseline (left) and after a 30-min exposure (right) to PKC activation in the presence of apocynin. B: summary data for normalized G/R over time under control conditions and after PKC activation with apocyanin present.
Altered molecular mobility of SCN5A channels with protein kinase activation.
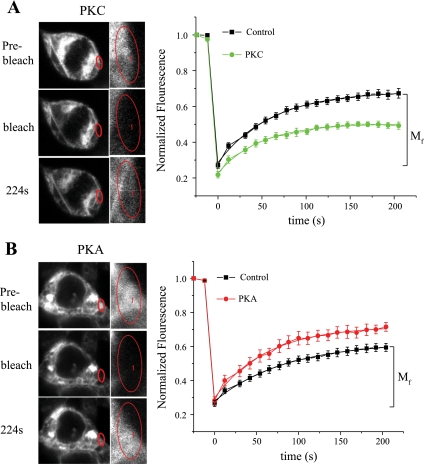
For a membrane protein such as SCN5A, the number of channels at the cell surface represents a balance between movement of newly synthesized/recycled channels into the membrane and internalization of membrane channels into the cytosol for degradation/recycling. We hypothesized that kinase activation would affect Na+ channel mobility in a manner consistent with the distribution dynamics found in the colocalization measurements. To test this hypothesis, experiments were performed using fluorescence recovery after photobleaching (FRAP) in HEK-293 cells expressing SCN5A-GFP. This technique is based on the principle that after the irreversible photobleaching of fluorescently labeled proteins, the recovery of fluorescence into the photobleached area reflects the influx or trafficking of unbleached molecules from adjacent regions in the cell. Figure 6, left, shows a cytosolic region near the plasma membrane subjected to photobleaching in the absence and presence of kinase stimulation. Under control conditions, the difference between the recovered and photobleached fluorescence intensity in this region (Fig. 6, right) reflects the mobile function (Mf) of intracellular SCN5A channels at baseline [49 ± 5% (n = 19)]. With PKC stimulation, Mf was decreased compared with control [35 ± 2% (n = 9); Fig. 6A], whereas PKA activation increased this parameter [60 ± 2% (n = 9); Fig. 6B]. The time course of this process was not altered by stimulation of either kinase [for PKA, the time constant was 69 ± 3 and 63 ± 4 ms for control cells (n = 14) and stimulated cells (n = 9), respectively; for PKC, the time constant was 69 ± 3 and 76 ± 4 ms for control cells (n = 19) and stimulated cells (n = 9), respectively]. These results demonstrate that changes in channel mobility with protein kinase activation are consistent with kinase-mediated SCN5A channel redistribution, further strengthening the evidence for a role for PKC and PKA in regulating intracellular Na+ channel localization. In addition, our findings provide evidence, albeit indirect, to support the reduced mobility of channels near (and likely toward) the plasma membrane with PKC stimulation.
Fig. 6.
Effect of kinase stimulation on SCN5A molecular mobility. A, left: confocal images demonstrating the photobleached region of interest (a cytoplasmic area near the plasma membrane) in the presence of PKC stimulation. The areas in the red circles were enlarged in the insets. Right, normalized fluorescence intensity showing the fluorescence recovery after photobleaching in the region of interest [mobile fraction (Mf) for control] in the absence and presence of PKC stimulation. B: similar results for the activation of PKA.
DISCUSSION
In this study, we tested the hypothesis that the activation of PKC can suppress SCN5A currents by acutely modifying the cellular trafficking of cardiac Na+ channels in living cells. Electrophysiology experiments demonstrated that the addition of GFP to the COOH-terminus of SCN5A did not alter the PKC-mediated reduction in INa previously shown. Using a confocal-based method that quantified the colocalization of GFP-tagged channels with the plasma membrane (13, 19), we observed that PKC promoted the redistribution of Na+ channels away from the cell surface in addition to the previously observed effect of PKA to increase channels in this region. Additional experiments demonstrated that both SCN5A phosphorylation and ROS were required for the PKC-mediated effects. Using FRAP, we made the novel observation that the stimulation of both protein kinases modifies the molecular mobility of cardiac Na+ channels, further supporting the concept of kinase-regulated intracellular trafficking. In addition, for PKC activation, they imply reduced channel movement to the plasma membrane in the PKC-mediated reduction of cardiac INa. Importantly, these results do not rule out a contribution of a functional, nontrafficking mechanism(s) to the PKC-mediated suppression of INa.
For some protein kinases, previous studies examining the effects of kinase activation on INa have provided conflicting results. However, PKC stimulation has been consistently shown to reduce INa in multiple tissues. As in the brain and skeletal muscle (16, 30), direct activation of PKC suppresses INa in cardiomyocytes (3, 29). We (27) previously found similar results using the recombinant human SCN5A channel, with experimental data supporting a principal role for cPKCs (31). In addition, when cells expressing SCN5A were preincubated with concanavalin A (which cross links extracellular carbohydrates and can inhibit the internalization of cell membrane proteins), PKC-mediated suppression of SCN5A current was prevented, implying a role for reduced cell membrane channel density in the observed effect (31). Thus, our findings in the present study demonstrating PKC-mediated redistribution of Na+ channels away from the plasma membrane are consistent with our electrophysiological results observed for both SCN5A and the GFP-tagged channel. A limitation of our study is that the experiments were performed after the heterologous expression of SCN5A channels in HEK-293 cells and, thus, they await further confirmation in cardiomyocytes.
For SCN5A, recent evidence has pointed to PKC-mediated suppression of cardiac INa as a cause for inherited arrhythmia syndromes. Mutations in GPD1L causing the Brugada syndrome and sudden infant death syndrome reduce SCN5A current through the activation of PKC (23, 33). However, studies to investigate the mechanism of INa suppression have not provided consistent results. In a study by Valdivia et al. (33), flow cytometry detecting FLAG-tagged SCN1B (the β1-subunit) was used to demonstrate evidence supporting reduced cell surface Na+ channels with GPD1L mutations. On the other hand, Liu and colleagues (23) found that GPD1L mutations increased intracellular NADH, and they proposed that SCN5A current was suppressed by PKC activation through excess NADH. Using confocal imaging and biotinylation, their results demonstrated that cell surface Na+ channel density was not affected by a brief exposure to PMA (10 min). Thus, the mechanism(s) by which PKC activation suppresses cardiac INa has remained controversial, and experiments to investigate the acute effects on kinase activation on trafficking of cardiac Na+ channels in living cells have not been previously undertaken. Our study used a robust, well-established confocal-based method (13, 19) that generates sensitive measurements of colocalization. By combining these results with data obtained using FRAP, our findings provide strong support for PKC-mediated effects to promote Na+ channel trafficking away from the plasma membrane, with a requisite role for channel phosphorylation and ROS in this effect. Thus, these results provide novel insights into the nature and mechanisms of PKC-mediated suppression of cardiac INa. The link to GPD1L mutations further validates the concept that PKC-mediated downregulation of SCN5A expression at the cell membrane can critically alter electrophysiological properties in a manner that could increase the susceptibility to life-threatening arrhythmias in humans.
It is increasingly recognized that the activation of protein kinases can regulate not only ion channel function but also channel density at the cell surface. For example, the activation of PKC has been shown to cause the internalization of voltage-gated Na+ channels in adrenal chromaffin cells (38) as well as the downregulation of membrane ATP-sensitive K+ channels (21). Stimulation of PKA promotes the trafficking of CFTR to the plasma membrane (1, 4), while insulin signaling redistributes glucose transporter 4 to the cell surface (20). In addition to protein kinases, other cellular mechanisms can alter membrane protein trafficking, including agonist/antagonist interactions (12). In immature neurons, persistent neurotoxin-mediated activation of Na+ channels can promote channel internalization (11).
In conclusion, our results provide direct evidence that PKC activation redistributes cardiac Na+ channels away from the plasma membrane in living mammalian cells, with an additional effect to modify the rate of intracellular channel trafficking. These observations add to the mounting body of evidence showing that physiological stimuli play an important role to modulate the density of Na+ channel proteins at the cell surface, in addition to their effects on molecular function.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HL-55665 and HL-071002. Confocal microscopy and image analysis were performed through the Vanderbilt Cell Imaging Shared Resource, which is supported by NIH Grants CA-68485, DK-20593, DK-58404, HD-15052, DK-59637, and EY-08126.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.H., A.L.G., K.S.W., and K.T.M. conception and design of research; H.H., D.W.W., J.D.K., A.L.G., K.S.W., and K.T.M. performed experiments; H.H., D.W.W., J.D.K., A.L.G., K.S.W., and K.T.M. analyzed data; H.H., D.W.W., A.L.G., K.S.W., and K.T.M. interpreted results of experiments; H.H. and D.W.W. prepared figures; H.H., A.L.G., and K.T.M. edited and revised manuscript; H.H., A.L.G., and K.T.M. approved final version of manuscript; K.T.M. drafted manuscript.
REFERENCES
- 1. Ameen NA, Martensson B, Bourguinon L, Marino C, Isenberg , McLaughlin GE. CFTR channel insertion to the apical surface in rat duodenal villus epithelial cells is upregulated by VIP in vivo. J Cell Sci 112: 887–894, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Balser JR. The cardiac sodium channel: gating, function, and molecular pharmacology. J Mol Cell Cardiol 33: 599–613, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Benz I, Herzig JW, Kohlhardt M. Opposite effects of angiotensin II and the protein kinase C activator OAG on cardiac Na+ channels. J Membr Biol 130: 183–190, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Bradbury NA, Bridges RJ. Role of membrane trafficking in plasma membrane solute transport. Am J Physiol Cell Physiol 267: C1–C24, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Bright R, Mochly-Rosen D. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke 36: 2781–2790, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Lagerholm BC, Yang B, Jacobson K. Methods to measure the lateral diffusion of membrane lipids and proteins. Methods 39: 147–153, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 819–823, 1984 [DOI] [PubMed] [Google Scholar]
- 8. Comeau JW, Costantino S, Wiseman PW. A guide to accurate fluorescence microscopy colocalization measurements. Biophys J 91: 4611–4622, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costantino S, Comeau JW, Kolin DL, Wiseman PW. Accuracy and dynamic range of spatial image correlation and cross-correlation spectroscopy. Biophys J 89: 1251–1260, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J 86: 3993–4003, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dargent B, Paillart C, Carlier E, Alcaraz G, Martin-Eauclaire MF, Couraud F. Sodium channel internalization in developing neurons. Neuron 13: 683–690, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Delisle BP, Anson BD, Rajamani S, January CT. Biology of cardiac arrhythmias: ion channel protein trafficking. Circ Res 94: 1418–1428, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol 300: C723–C742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 324: 781–788, 1991 [DOI] [PubMed] [Google Scholar]
- 15. El-Sherif N. Reentrant mechanisms in ventricular arrhythmias. In: Cardiac Electrophysiology: From Cell to Bedside, edited by Zipes DP, Jalife J. Philadelphia, PA: Saunders, 1995, p. 576–582 [Google Scholar]
- 16. Fozzard HA, Hanck DA. Structure and function of voltage-dependent sodium channels: comparison of brain II and cardiac isoforms. Physiol Rev 76: 887–926, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Gallegos LL, Kunkel MT, Newton AC. Targeting protein kinase C activity reporter to discrete intracellular regions reveals spatiotemporal differences in agonist-dependent signaling. J Biol Chem 281: 30947–30956, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Grant AO, Wendt DJ. Block and modulation of cardiac Na+ channels by antiarrhythmic drugs, neurotransmitters and hormones. Trends Pharmacol Sci 13: 352–358, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Hallaq H, Yang Z, Viswanathan PC, Fukuda K, Shen W, Wang DW, Wells KS, Zhou J, Yi J, Murray KT. Quantitation of protein kinase A-mediated trafficking of cardiac sodium channels in living cells. Cardiovasc Res 72: 250–261, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Holman GD, Kasuga M. From receptor to transporter: insulin signalling to glucose transport. Diabetologia 40: 991–1003, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron 38: 417–432, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Lachmanovich E, Shvartsman DE, Malka Y, Botvin C, Henis YI, Weiss AM. Co-localization analysis of complex formation among membrane proteins by computerized fluorescence microscopy: application to immunofluorescence co-patching studies. J Microsc 212: 122–131, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Liu M, Sanyal S, Gao G, Gurung IS, Zhu X, Gaconnet G, Kerchner LJ, Shang LL, Huang CL, Grace A, London B, Dudley SC., Jr Cardiac Na+ current regulation by pyridine nucleotides. Circ Res 105: 737–745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, Viswanathan PC, Pfahnl AE, Shang LL, Madhusudanan M, Baty CJ, Lagana S, Aleong R, Gutmann R, Ackerman MJ, McNamara DM, Weiss R, Dudley SC., Jr Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation 116: 2260–2268, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma HT, Lin WW, Zhao B, Wu WT, Huang W, Li Y, Jones NL, Kruth HS. Protein kinase C beta and delta isoenzymes mediate cholesterol accumulation in PMA-activated macrophages. Biochem Biophys Res Commun 349: 214–220, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Marban E, Yamagishi T, Tomaselli GF. Structure and function of voltage-gated sodium channels. J Physiol 508: 647–657, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murray KT, Hu NN, Daw JR, Shin HG, Watson MT, Mashburn AB, George AL., Jr Functional effects of protein kinase C activation on the human cardiac Na+ channel. Circ Res 80: 370–376., 1997 [DOI] [PubMed] [Google Scholar]
- 28. Poole JE, Bardy GH. Sudden cardiac death. In: Cardiac Electrophysiology: From Cell to Bedside, edited by Zipes DP, Jalife J. Philadelphia, PA: Saunders, 1995, p. 812–832 [Google Scholar]
- 29. Qu Y, Rogers J, Tanada T, Scheuer T, Catterall WA. Modulation of cardiac Na+ channels expressed in a mammalian cell line and in ventricular myocytes by protein kinase C. Proc Natl Acad Sci USA 91: 3289–3293, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schreibmayer W. Isoform diversity and modulation of sodium channels by protein kinases. Cell Physiol Biochem 9: 187–200, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Shin HG, Murray KT. Conventional protein kinase C isoforms and cross-activation of protein kinase A regulate cardiac Na+ current. FEBS Lett 495: 154–158, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Tan HL, Bezzina CR, Smits JP, Verkerk AO, Wilde AA. Genetic control of sodium channel function. Cardiovasc Res 57: 961–973, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Valdivia CR, Ueda K, Ackerman MJ, Makielski JC. GPD1L links redox state to cardiac excitability by PKC-dependent phosphorylation of the sodium channel SCN5A. Am J Physiol Heart Circ Physiol 297: H1446–H1452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Norstrand DW, Valdivia CR, Tester DJ, Ueda K, London B, Makielski JC, Ackerman MJ. Molecular and functional characterization of novel glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) mutations in sudden infant death syndrome. Circulation 116: 2253–2259, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol 161: 899–909, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viswanathan PC, Balser JR. Inherited sodium channelopathies: a continuum of channel dysfunction. Trends Cardiovasc Med 14: 28–35, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Wang DW, Crotti L, Shimizu W, Pedrazzini M, Cantu F, De Filippo P, Kishiki K, Miyazaki A, Ikeda T, Schwartz PJ, George AL., Jr Malignant perinatal variant of long-QT syndrome caused by a profoundly dysfunctional cardiac sodium channel. Circ Arrhythm Electrophysiol 1: 370–378, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yanagita T, Wada A, Yamamoto R, Kobayashi H, Yuhi T, Urabe M, Niina H. Protein kinase C-mediated down-regulation of voltage-dependent sodium channels in adrenal chromaffin cells. J Neurochem 66: 1249–1253, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Zhou J, Yi J, Hu N, George AL, Jr, Murray KT. Activation of protein kinase A modulates trafficking of the human cardiac sodium channel in Xenopus oocytes. Circ Res 87: 33–38, 2000 [DOI] [PubMed] [Google Scholar]