Abstract
In human coronary arterioles (HCAs) from patients with coronary artery disease, flow-induced dilation is mediated by a unique mechanism involving the release of H2O2 from the mitochondria of endothelial cells (ECs). How flow activates ECs to elicit the mitochondrial release of H2O2 remains unclear. Here, we examined the role of the transient receptor potential vanilloid type 4 (TRPV4) channel, a mechanosensitive Ca2+-permeable cation channel, in mediating ROS formation and flow-induced dilation in HCAs. Using RT-PCR, Western blot analysis, and immunohistochemical analysis, we detected the mRNA and protein expression of TRPV4 channels in ECs of HCAs and cultured human coronary artery ECs (HCAECs). In HCAECs, 4α-phorbol-12,13-didecanoate (4α-PDD), a selective TRPV4 agonist, markedly increased (via Ca2+ influx) intracellular Ca2+ concentration. In isolated HCAs, activation of TRPV4 channels by 4α-PDD resulted in a potent concentration-dependent dilation, and the dilation was inhibited by removal of the endothelium and by catalase, a H2O2-metabolizing enzyme. Fluorescence ROS assays showed that 4α-PDD increased the production of mitochondrial superoxide in HCAECs. 4α-PDD also enhanced the production of H2O2 and superoxide in HCAs. Finally, we found that flow-induced dilation of HCAs was markedly inhibited by different TRPV4 antagonists and TRPV4-specific small interfering RNA. In conclusion, the endothelial TRPV4 channel is critically involved in flow-mediated dilation of HCAs. TRPV4-mediated Ca2+ entry may be an important signaling event leading to the flow-induced release of mitochondrial ROS in HCAs. Elucidation of this novel TRPV4-ROS pathway may improve our understanding of the pathogenesis of coronary artery disease and/or other cardiovascular disorders.
Keywords: transient receptor potential, transient receptor potential vanilloid type 4 channel, shear stress, coronary, mitochondria, free radicals, reactive oxygen species
shear stress generated by blood flow is an important physiological regulator of vascular tone. Flow or shear induces vasodilation in virtually every vascular bed studied, in both conduit arteries and resistance arteries (6, 15, 17, 18). Flow-induced dilation typically involves the release of the endothelial relaxing factors nitric oxide, prostacyclin, and/or cytochrome P-450 metabolites of arachidonic acid [e.g., epoxyeicosatrienoic acids (EETs)] (3). Our recent studies (20, 22, 26) indicated that, in human coronary arterioles (HCAs) from patients with coronary artery disease (CAD), flow induces vasodilation through a unique mechanism requiring the endothelial production of ROS, specifically H2O2 originating from the mitochondrial electron transport chain. The underlying mechanisms by which flow stimulates the release of mitochondrial H2O2 in coronary endothelial cells (ECs) remain largely unsolved.
The transient receptor potential (TRP) vanilloid type 4 (TRPV)4 channel, a Ca2+-permeable cation channel and a member of the TRP channel superfamily, is activated by chemical agonists, including the synthetic phorbol derivative 4α-phorbol-12,13-didecanoate (4α-PDD) (35) and arachidonic acid metabolites (e.g., EETs) (34, 36), as well as by mechanical stimuli, such as hypotonic cell swelling (19, 29), moderate warmth (>27°C) (11, 37), and membrane stretch (31). Recently, accumulating evidence has indicated that the TRPV4 channel is expressed in ECs and contributes to the regulation of vascular tone (41). In particular, activation of endothelial TRPV4 channels has been implicated in flow-mediated dilation in several animal vascular beds (12, 16, 23, 24). It is yet to be determined whether the TRPV4 channel plays a role in endothelium-dependent vasodilator responses in human vessels.
Given that an increase in intracellular Ca2+ concentration ([Ca2+]i) stimulates mitochondrial ROS generation in ECs, as well as in other mammalian cells (10, 40), we hypothesized that endothelial TRPV4-mediated Ca2+ entry may be a potential mechanism responsible for flow-induced mitochondrial ROS generation and vasodilation of HCAs. In this study, we examined TRPV4 expression and TRPV4-mediated Ca2+ responses in coronary ECs, TRPV4-mediated vasodilation and ROS production in HCAs, and the effect of TRPV4 blockade/knockdown on flow-induced dilation of HCAs. We also assessed mitochondrial ROS generation in coronary ECs in response to TRPV4 activation.
MATERIALS AND METHODS
Tissue acquisition.
Fresh human right atrial appendages were obtained as discarded surgical specimens from patients undergoing cardiopulmonary bypass procedures, as previously described (25). After surgical removal, atrial tissues were immersed in an ice-cold cardioplegia solution and immediately transported to the laboratory. Demographic data were obtained from hospital records at the time of surgery and are shown in Table 1. All protocols were approved by the appropriate local Institutional Review Boards on the use of human subjects in research.
Table 1.
Patient demographics
| Number of Patients | Percentage of Patients | |
|---|---|---|
| Sex | ||
| Male | 34 | 57 |
| Female | 26 | 43 |
| Age, yr (mean ± SD) | 64 ± 14 | |
| Surgical procedure | ||
| Myocardial revascularization | 29 | 48 |
| Coronary artery bypass graft | 4 | 7 |
| Mitral valve replacement | 23 | 38 |
| Aortic valve replacement | 18 | 30 |
| Tricuspid valve replacement | 11 | 18 |
| Other | 1 | 2 |
| Underlying disease | ||
| Coronary artery disease | 38 | 63 |
| Hypertension | 33 | 55 |
| Hyperlipidemia | 27 | 45 |
| Diabetes mellitus | 11 | 18 |
| Atrial fibrillation | 17 | 28 |
| Congestive heart failure | 8 | 13 |
| Myocardial infarction | 6 | 10 |
| Tobacco use | 13 | 22 |
| None of the above | 6 | 10 |
A total of 60 patients were studied.
Cell culture.
Human coronary artery ECs (HCAECs) and human coronary artery smooth muscle cells (SMCs) were obtained from Lonza (Walkersville, MD) and maintained in full growth medium (EGM-2 MV and SmGM-2 for ECs and SMCs, respectively) according to the manufacturer's instructions. Cells between passages 4 and 6 were used for experiments.
RNA extraction and RT-PCR.
Total RNA from cultured cells was extracted with TRIzol, and cDNA was synthesized using the iScript cDNA Synthesis kit (Bio-Rad). For some experiments, ECs and SMCs were enzymatically dissociated from isolated arterioles and selectively collected using a glass pipette, followed by cell disruption and cDNA synthesis as previously described (24). Gene-specific fragments were amplified by PCR with the following primers: TRPV4, forward 5′-ACAACAGCAAGATTGAGAACC-3′ and reverse 5′-ACCAGGACAGAGTAGATGAAG-3′ [for a 364-bp fragment (NM_021625)]; platelet-EC adhesion molecule (PECAM)-1, forward 5′-AGACAACCCCACTGAAGAC-3′ and reverse 5′-TCCAGACTCCACCACCTTAC-3′ [for a 154-bp fragment (NM_000442)]; and GAPDH, forward 5′-ATGGCAAATTCCATGGCACCGT-3′ and reverse 5′-TGCAGGAGGCATTGCTGATGAT-3′ [for a 300-bp fragment (NM_002046)].
Western blot analysis.
Vascular tissues or cells were homogenized in ice-cold lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% deoxycholic acid, 0.1% SDS, and 0.5% Nonidet P-40] supplemented with a protease inhibitor cocktail (Roche) and centrifuged at 12,000 g for 10 min at 4°C. Protein samples (10–20 μg) were subjected to 10% SDS-PAGE and transferred to nitrocellulose or polyvinylidene difluoride membranes. Membranes were blotted with a polyclonal antibody against the human TRPV4 channel (1:1,000 dilution, LS-A8583, MBL) or a monoclonal antibody against β-actin (1:10,000 dilution, ab6276, Abcam AC-15 clone) followed by peroxidase-conjugated secondary antibodies (1:5,000 dilution). Immunoreactive complexes were visualized using an ECL chemiluminescence detection system (Amersham).
Immunohistochemistry.
Freshly dissected coronary arteries and arterioles were fixed with 10% buffered zinc formalin, embedded into paraffin blocks, and cut into 4-μm sections. Tissue sections were deparaffinized in xylene and rehydrated through a graded series of ethanol and water. Sections were then treated with 3% H2O2 in PBS to block endogenous peroxidase followed by antigen retrieval by heating slides in citrate buffer (pH 6.0). After an additional block with 3% BSA in PBS, tissue sections were probed with a polyclonal antibody against the human TRPV4 channel (1:400 dilution, LS-A8583, MBL). For immunodetection, sections were incubated with a polyclonal goat anti-rabbit horseradish peroxidase-conjugated antibody (1:000 dilution, DAKO) and then with a peroxidase-substrate solution (DAKO Liquid DAB-Substrate Chromogen System, DAKO). Samples were then rinsed, counterstained with the nuclear dye hematoxylin (DAKO), and mounted. Negative controls were obtained by omitting the primary antibody.
Small interfering RNA transfection.
Two TRPV4-specific small interfering (si)RNA duplexes were designed and synthesized by Qiagen using the following target sequences: 1) 5′-CTGGGATTTGCCGGTGCTCAA-3′ [nucleotides 3194–3214 (NM_021625)] and 2) 5′-CTGGAGTGAATTCTCTCTTCA-3′ [nucleotides 1702–1722 (NM_021625)]. A nonsilencing scrambled siRNA was used as a control. The scrambled and TRPV4 siRNA duplexes were first tested on HCAECs for their efficacy and specificity. The two TRPV4 siRNA duplexes were found to be equally effective. For isolated vessels, only siRNA 1 was used. Coronary arterioles were transfected with the scrambled or TRPV4 siRNA duplex (100 nM) complexed with Lipofectamine 2000 (0.5 μg/ml, Invitrogen) for 4–6 h according to the manufacturer's instructions. Arterioles were incubated for additional 24–36 h in fresh medium at 37°C and assessed for TRPV4 protein knockdown using Western blot analysis. Transfected arterioles were examined for vasodilator responses as described below.
Measurement of [Ca2+]i.
ECs were plated onto 35-mm glass-bottom petri dishes and grown to 60–70% confluence. Cells were loaded with fura-2 AM (5 μM, Molecular Probes) at room temperature for 30–60 min in HEPES buffer containing (in mM) 140 NaCl, 4.7 KCl, 1.6 CaCl2, 1.17 MgSO4, 1.18 NaH2PO4, 5.5 glucose, and 10 HEPES (pH 7.4). Cytosolic Ca2+ changes were measured using a fluorescence imaging system as we have previously reported (24). Fura-2 signals were calibrated, and [Ca2+]i was calculated as previously described (39). All experiments were performed at 37°C.
Videomicroscopy.
Coronary arterioles (≈100–200 μm) were cannulated with two glass micropipettes for the continuous videomicroscopic measurement of internal diameters as previously described (20, 22). Vessels were pressurized to 60 mmHg and equilibrated for 1 h at 37°C in Krebs-physiological salt solution gassed with 21% O2-5% CO2. Vessels were preconstricted with endothelin-1 (5 × 1010–10−9 M) to 30–50% of the baseline internal diameter if the spontaneous myogenic tone was not sufficient to achieve the target reduction in diameter. Concentration-dependent responses to 4α-PDD (5 × 10−8–5 × 10−6 M), a selective TRPV4 activator, were examined in the absence or presence of catalase (500 U/ml). 4α-PDD was applied intraluminally with minimal flow generated under a 10-cmH2O pressure gradient, and catalase was given both intraluminally and extraluminally for 15 min before 4α-PDD. Flow was produced by changing the heights of two syringe reservoirs in equal and opposite directions. Flow-induced dilation (20–100 cmH2O) was examined before and after treatment with the TRPV4 blocker ruthenium red (1 μM), gadolinium III (100 μM) (33), or RN-1734 (20 μM) (32) or after transfection with scrambled or TRPV4 siRNA. Responses to bradykinin (10−10–10−6 M), an endothelium-dependent receptor agonist, or papaverine (10−4 M), an endothelium-independent vasodilator, were recorded as controls. In some vessels, the endothelium was removed with an air bolus. Disruption of endothelial function was confirmed by a markedly reduced dilation (<10%) to bradykinin (10−6 M). Vasodilator responses are expressed as percent relaxation relative to spontaneous tone or endothelin-1 preconstriction, with 100% relaxation representing the passive baseline diameter.
Detection of vascular ROS.
Production of H2O2 and superoxide in coronary arterioles was assessed by fluorescence microscopy using 2′,7′-dichlorodihydrofluorescein (DCFH) diacetate and dihydroethidium (DHE), respectively (21, 22). Briefly, two vessels dissected from each atrial tissue were perfused with vehicle or 4α-PDD (1 μM) under 10-cmH2O pressure gradient. Another vessel was incubated with polyethylene glycol (PEG)-SOD (150 U/ml) and PEG-catalase (500 U/ml) to assess nonspecific fluorescence. Vessels were then incubated with DCFH (5 μM) and DHE (5 μM) in a light-protected chamber for 30 min at 37°C. Vessel segments were then rinsed and examined under a fluorescence microscope. Samples were excited at 488 and 585 nm, and emission was measured at 526 and 620 nm, respectively. Images were analyzed for fluorescence intensity within a user-defined region. Artifactual autofluorescent regions were manually excluded from analysis. Relative average fluorescence intensity was normalized for surface area and compared between control and experimental vessels.
Measurement of mitochondrial ROS.
Mitochondrial ROS production in ECs was measured using MitoSOX, a fluorescent probe specific for mitochondrial superoxide. Cells were incubated in HEPES buffer containing vehicle, 4α-PDD (2 μM), 4α-PDD plus ruthenium red (1 μM), or 4α-PDD plus Mito Vitamin E (1 μM, a mitochondria-targeted antioxidant) (5). MitoSOX (5 μM, Molecular Probes) was added and incubated for 30 min at 37°C. Cellular fluorescence was examined under a fluorescence microscope at an excitation/emission of 510/580 nm according to the manufacturer's instructions. Results were expressed as relative fluorescence intensity normalized to controls.
Data analysis.
Data are presented as mean ± SE. Significant differences between mean values were evaluated by a Student t-test or repeated-measures ANOVA followed by the Student-Newman-Keuls multiple-comparison test. P values of <0.05 were considered statistically significant.
RESULTS
TRPV4 expression in human coronary vascular cells.
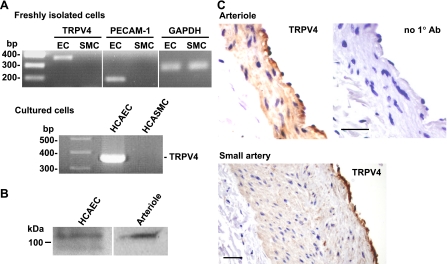
ECs and SMCs were freshly isolated from HCAs and examined for TRPV4 mRNA expression by RT-PCR analysis. As shown in Fig. 1A, TRPV4 transcripts were detected in ECs but not in SMCs. As parallel controls, a PCR product for PECAM-1, an endothelial marker, was detected in ECs but not in SMCs, whereas amplification of GAPDH transcripts was found in both cell types. Similarly, TRPV4 transcripts were detected in primary cultures of HCAECs but not human coronary artery SMCs. Furthermore, TRPV4 protein was detected in HCAECs and HCAs by Western blot analysis (Fig. 1B). The TRPV4 antibody detected one primary band of ∼110 kDa in HCAECs and HCAs and a second band of ∼98 kDa in HCAECs and less frequently in HCAs (data not shown). The 110-kDa band presumably represents the glycosylated form of TRPV4 protein (1), whereas the 98-kDa band matches with the unprocessed precursor of human TRPV4 protein with a calculated molecular weight of 98 kDa. Cell type-specific expression of TRPV4 protein was also examined in coronary arterioles and small coronary arteries using immunohistochemistry (Fig. 1C). In tissue sections of both types of vessels, the endothelium demonstrated a strong staining for TRPV4. There was much less immunoreactivity in the underlying smooth muscle, especially for small coronary arteries, and virtually no staining in the adventitial layer. The control section without the primary antibody showed negative staining. Similar to small coronary arteries or arterioles, we also detected prominent staining for TRPV4 in the endothelium of large human coronary arteries (data not shown).
Fig. 1.
Transient receptor potential vanilloid type 4 (TRPV4) expression in human coronary vascular cells. A: RT-PCR analysis of TRPV4 mRNA expression in endothelial cells (ECs) and smooth muscle cells (SMCs) freshly isolated from human coronary arterioles (HCAs; top) as well as in cultured human coronary artery ECs (HCAECs) and human coronary artery (HCASMCs; bottom). Amplification of platelet-EC adhesion molecule (PECAM)-1, an EC marker, and GAPDH, a ubiquitous housekeeping protein, were performed in parallel as controls. B: Western blot analysis of TRPV4 protein in HCAECs and HCAs. C: immunohistochemical staining for TRPV4 in HCAs and human coronary small arteries. Control sections were similarly processed except that the primary antibody was omitted (no 1° Ab). Scale bar = 10 μm. All data are representative of 3–4 separate experiments.
TRPV4-mediated endothelial Ca2+ entry.
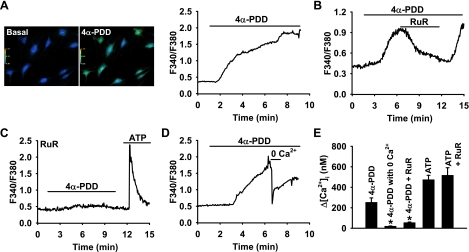
To examine whether TRPV4 channels are functional in coronary ECs, we examined the TRPV4-mediated Ca2+ response using a fura-2 assay. As shown in Fig. 2A, the addition of 4α-PDD (2 μM), a specific TRPV4 channel activator, elicited a marked increase in [Ca2+]i in HCAECs (change in [Ca2+]i: 253 ± 42 nM). This response was rapidly reversed by ruthenium red (1 μM, 55 ± 5 nM; Fig. 2B), a TRPV4 channel blocker, or by removing extracellular Ca2+ (20 ± 2 nM; Fig. 2D). Preincubation of cells with ruthenium red prevented 4α-PDD-induced Ca2+ responses but did not significantly affect the peak Ca2+ transient in response to ATP (1 mM), a receptor agonist that induces inositol 1,4,5-trisphosphate-mediated Ca2+ release from intracellular stores (Fig. 2C). Summary data are shown in Fig. 2E. ECs were more sensitive to the TRPV4 activator 4α-PDD at physiological (37°C) temperature compared with room temperature (data not shown), which is in accordance with the thermosensitive characteristics of TRPV4 channels.
Fig. 2.
TRPV4-mediated Ca2+ responses in coronary ECs. A: representative images (left) of HCAECs loaded with the fluorescent Ca2+ indicator fura-2. 4α-Phorbol-12,13-didecanoate (4α-PDD; 2 μM), a specific TRPV4 agonist, elicited a marked increase in intracellular Ca2+ concentration ([Ca2+]i). Right, a typical trace of the 4α-PDD-induced [Ca2+]i increase. F340/F380, ratio of fluorescence at 340 nm to that at 380 nm. B–E: other representative traces. B: the 4α-PDD-induced [Ca2+]i increase was rapidly reversed by ruthenium red (RuR; 1 μM), a TRPV4 channel blocker. C: preincubation of ECs with RuR blocked the Ca2+ response to 4α-PDD but not to ATP (1 mM), a receptor agonist that induces an inositol 1,4,5-trisphosphate-mediated Ca2+ release from intracellular stores. D: the 4α-PDD-induced [Ca2+]i increase was immediately reversed by removing extracellular Ca2+. E: summarized data. n = 3–6 experiments (10–20 cells/each). *P < 0.05 vs. 4α-PDD.
TRPV4-mediated dilation and ROS production.
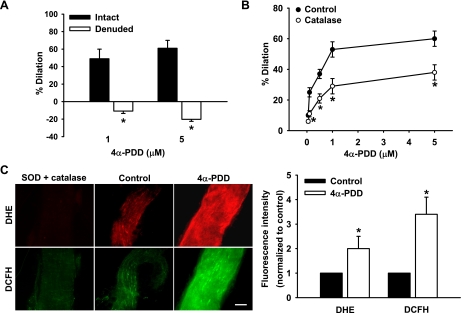
To determine whether TRPV4 activation affects vascular tone, HCAs were preconstricted with endothelin-1, and 4α-PDD (1 and 5 μM) was applied by intraluminal perfusion under a 10-cmH2O pressure gradient. The relative low flow generated under this pressure gradient alone did not produce significant vasodilation. Perfusion of arterioles with 4α-PDD elicited a potent endothelium-dependent dilation in a concentration-related manner. The dilation to 5 μM 4α-PDD was 61 ± 9% and −20 ± 2% for endothelium-intact and -denuded vessels, respectively (Fig. 3A).
Fig. 3.
TRPV4-mediated vasodilation and ROS generation in coronary arterioles. A: the TRPV4 agonist 4α-PDD (1 and 5 μM) dilated HCAs in an endothelium-dependent manner. Arterioles with an intact endothelium (intact) or after endothelial removal (denuded) were cannulated, and 4α-PDD was perfused into the lumen of arterioles with relatively low flow (10-cmH2O pressure gradient), which itself did not produce significant dilation. n = 5. *P < 0.05 vs. control. B: 4α-PDD-induced dilation was reduced after pretreatment of vessels with catalase (500 U/ml, intraluminal and extraluminal). n = 6. *P < 0.05 vs. control. C: 4α-PDD (1 μM) increased vascular superoxide and H2O2 production, as indicated by the enhanced fluorescence intensities of oxidative products of dihydroethidium (DHE) and 2′,7′-dichlorodihydrofluorescein (DCFH) compared with controls. Arterioles treated with polyethylene glycol (PEG)-SOD (150 U/ml) and PEG-catalase (500 U/ml) were used to assess nonspecific fluorescence. Scale bar = 100 μm. Left, representative images; right, summary of fluorescence intensity normalized to controls. n = 8. *P < 0.05 vs. control.
Our previous studies (20, 22, 26) have demonstrated that H2O2 is the primary relaxing factor released in HCAs in response to shear stress. Therefore, we next examined whether H2O2 is also involved in 4α-PDD-induced dilation. As shown in Fig. 3B, incubation of HCAs with catalase (500 U/ml) inhibited vasodilator responses to 4α-PDD (dilation at 5 μM: 38 ± 5%). To directly assess whether TRPV4 activation induces vascular ROS generation, we performed semiquantitative analysis of H2O2 and superoxide production using the fluorescent probes DCFH and DHE, respectively. Perfusion of coronary arterioles with 4α-PDD (1 μM) markedly increased the production of both H2O2 and superoxide compared with controls, with normalized fluorescence intensities of 2.0 ± 0.5 and 3.4 ± 0.7, respectively (Fig. 3C).
TRPV4 channels in flow-induced dilation of HCAs.
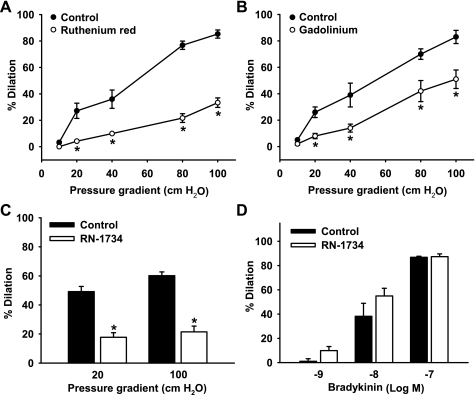
Fluid flow generated under 20- to 100-cmH2O pressure gradients induced a potent graded dilation of HCAs (dilation at 100 cmH2O: 85 ± 3%, n = 6; Fig. 4A). This flow-induced dilation was markedly inhibited (33 ± 4%, n = 6, P < 0.05 vs. control) by the TRPV4 antagonist ruthenium red (1 μM). Flow-mediated dilation of HCAs was also reduced by pretreatment of arterioles with 100 μM gadolinium III (51 ± 7% vs. 83 ± 5% of controls, n = 5, P < 0.05; Fig. 4B), another commonly used TRPV4 antagonist, or with 20 μM RN-1734 (21 ± 4% vs. 60 ± 3% of controls, n = 5, P < 0.05; Fig. 4C), a recently developed TRPV4 selective antagonist (32). In contrast, bradykinin-induced dilation was not affected by the TRPV4 antagonist RN-1734 (20 μM; Fig. 4D). As shown in Table 2, no significant effect on baseline vascular tone was observed for ruthenium red, gadolinium, or RN-1734. In addition, similar dilation to the smooth muscle relaxant papaverine (100 μM) was found in arterioles with or without pretreatment with these TRPV4 inhibitors (data not shown).
Fig. 4.
Effect of TRPV4 inhibitors on flow-induced dilation of coronary arterioles. Incubation of coronary arterioles with the TRPV4 inhibitors RuR (1 μM; A), gadolinium III (100 μM; B), or RN-1734 (20 μM; C) attenuated dilatory responses to flow. RN-1734 (20 μM; D) had no significant effect on bradykinin-induced dilation. Luminal flow was generated by pressure gradients of 10–100 cmH2O (A and B) or of 20–100 cmH2O (C). n = 6 (A), 4 (B), 4 (C), and 4 (D). *P < 0.05 vs. control.
Table 2.
Characteristics of isolated coronary arterioles
| Internal Diameter, μm |
|||||
|---|---|---|---|---|---|
| Passive | Active (without inhibitor) | Active (with inhibitor) | Endothelin-1 | Number of Coronary Arterioles | |
| Control | 141 ± 5 | 133 ± 4 | 90 ± 4 | 6 | |
| Ruthenium red | 141 ± 5 | 133 ± 4 | 91 ± 3 | 6 | |
| Control | 131 ± 16 | 126 ± 17 | 85 ± 10 | 5 | |
| Gadolinium | 131 ± 16 | 125 ± 9 | 88 ± 10 | 5 | |
| Control | 135 ± 18 | 134 ± 17 | 83 ± 9 | 4 | |
| RN-1734 | 135 ± 18 | 131 ± 20 | 79 ± 12 | 4 | |
| Scrambled | 148 ± 11 | 133 ± 6 | 97 ± 5 | 5 | |
| Transient receptor potential vanilloid type 4 small interfering RNA | 153 ± 9 | 140 ± 9 | 86 ± 11 | 5 | |
Values are means ± SE of flow-mediated dilation.
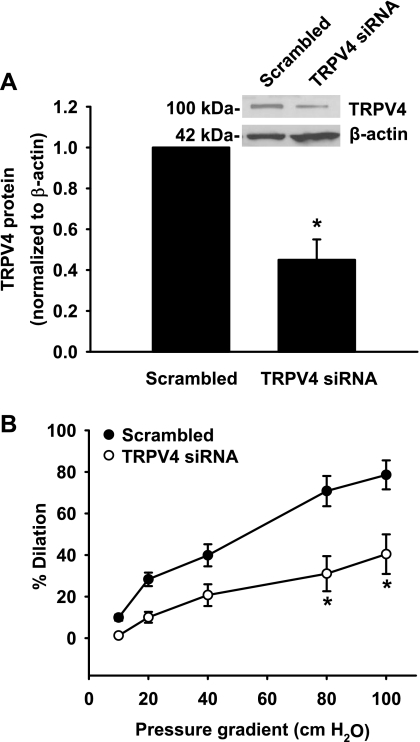
Since the above TRPV4 antagonists, especially ruthenium red and gadolinium, inhibit other TRP channels with varying efficacy (32, 33), we used the siRNA approach to further evaluate the role of TRPV4 channels in flow-induced dilation. Compared with the scrambled control, TRPV4 siRNA reduced the expression of TRPV4 protein in coronary arterioles to 45 ± 10% of control 24–36 h after transfection (Fig. 5A). A similar or greater reduction in the expression of TRPV4 protein and mRNA was observed in HCAECs, whereas TRPV4 siRNA has no significant effect on the expression of PECAM-1 or β-actin (24).
Fig. 5.
Effect of TRPV4 small interfering (si)RNA on flow-induced dilation of coronary arterioles. A: TRPV4-specific siRNA (100 nM) markedly reduced TRPV4 protein expression in coronary arterioles compared with scrambled siRNA (100 nM). The band density of TRPV4 protein was normalized to β-actin (an internal loading control) and the scrambled siRNA. n = 3–4. *P < 0.05 vs. control. The inset shows a representative immunoblot. B: TRPV4 siRNA inhibited flow-induced dilation of coronary arterioles. Luminal flow was generated by pressure gradients of 10–100 cmH2O. n = 5. *P < 0.05 vs. control.
Coronary arterioles transfected with TRPV4 siRNA had a blunted vasodilatory response to flow (dilation at 100 cmH2O: 40 ± 10% vs. 79 ± 7% of scrambled controls, n = 5, P < 0.05; Fig. 5B). Incubation of arterioles with TRPV4 siRNA had no significant effect on 100 μM papaverine-induced dilation (100 ± 1% vs. 99 ± 2% of controls, n = 4 for both). In separate experiments, we confirmed that 24–36 h of organ culture did not significantly change the magnitude of flow-induced dilation compared with freshly isolated arterioles (dilation at 20 and 100 cmH2O: 27 ± 4% and 82 ± 4% vs. 22 ± 4% and 79 ± 2% of controls, n = 5 and 4, respectively).
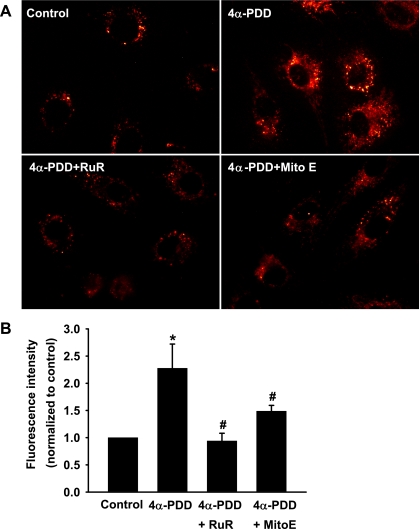
TRPV4 agonist-induced mitochondrial ROS generation.
As mentioned previously, flow-induced dilation in HCAs involves the release of H2O2, an endothelial relaxing factor primarily generated from the mitochondrial electron transport chain (22). We examined whether Ca2+ influx secondary to TRPV4 opening affects mitochondrial ROS production. The mitochondrial ROS production was examined using MitoSOX, a mitochondria-targeted fluorescent superoxide indicator. In preliminary experiments, we confirmed that MitoSOX fluorescence was localized in mitochondria by its colocalization with that of the mitochondria-specific probe MitoTracker green. As shown in Fig. 6, the TRPV4 agonist 4α-PDD (2 μM) increased mitochondrial ROS production in HCAECs. This increase was inhibited by ruthenium red (1 μM) and by Mito Vitamin E (1 μM), a mitochondria-targeted antioxidant, with the normalized fluorescence intensity was reduced from 2.3 ± 0.4 of 4α-PDD-treated cells to 0.9 ± 0.1 and 1.5 ± 0.1, respectively.
Fig. 6.
TRPV4 agonist-induced mitochondrial ROS production in coronary ECs. A: representative images of ECs loaded with MitoSOX, a fluorescent probe for mitochondrial superoxide. Activation of TRPV4 channels by 4α-PDD (2 μM) stimulated mitochondrial superoxide production, as indicated by the increased fluorescence intensity within the mitochondria compared with control cells. This response was markedly inhibited by RuR (1 μM), a TRPV4 blocker, and by Mito Vitamin E (MitoE; 1 μM), a mitochondria-targeted antioxidant. B: summary of fluorescence intensity normalized to controls. n = 5 experiments (10–20 cells/each). *P < 0.05 vs. control; #P < 0.05 vs. 4α-PDD.
DISCUSSION
This is the first study, to our knowledge, to examine the role of TRPV4 in the regulation of vascular tone in human vessels. Our findings indicate that TRPV4 channels play an essential role in mediating flow-induced dilation of HCAs. Furthermore, TRPV4-mediated Ca2+ influx may serve as a potential mechanism linking flow signal and mitochondrial ROS production in coronary ECs.
Previous studies have shown that flow increases [Ca2+]i (via Ca2+ entry and/or Ca2+ release from internal stores) in vascular ECs (7, 13, 14) and that this Ca2+ increase is critical for the flow-induced synthesis and release of endothelial relaxing factors (2, 4). However, the molecular identity of the responsible Ca2+ entry channel(s) remains elusive. In a recent study, Kohler et al. (16) provided the first pharmacological evidence that TRPV4 channels are involved in flow-induced dilation of rat carotid arteries and skeletal muscle arterioles. Using two independently generated knockout mouse lines, several laboratories, including ours, have subsequently showed that TRPV4 activation contributes to flow-induced dilation in mouse carotid and small mesenteric arteries (12, 23, 24). In the present study, we found that flow-induced dilation in human coronary microvessels also requires the activation of endothelial TRPV4 channels. Collectively, these findings indicate that the activation of TRPV4 channels may serve as a conserved mechanism transducing flow or a shear stimulus to the endothelial Ca2+ response and subsequent vasodilation. In addition to ECs, TRPV4-mediated Ca2+ entry in response to flow has been shown in other tissues and cells, such as TRPV4-transfected HEK-293 cells (9, 24) and renal tubular ECs (30, 38).
In this study, TRPV4 activation increases mitochondrial ROS production in coronary ECs and induces ROS-dependent vasodilation in coronary arterioles. Therefore, TRPV4 channel-mediated Ca2+ influx may be a potential mechanistic link between the flow signal and mitochondrial ROS production in coronary ECs. This hypothesis is in line with a growing body of literature suggesting that mitochondria act as signaling organelles participating in various cellular functions, e.g., Ca2+ and ROS signaling, through proximity to other signaling machinery (8, 10, 40). Of note, we (21) have recently reported that endothelial cytoskeletal elements also play an important role in shear-induced mitochondrial ROS production and subsequent dilation of coronary arterioles. It is thus tempting to speculate that plasmalemmal TRPV4 channels are in close apposition with mitochondria in ECs, an association facilitated by the cytoskeleton, so that TRPV4-mediated Ca2+ influx may be selectively targeted to mitochondria, leading to subsequent mitochondrial ROS production. How the TRPV4-mediated Ca2+ increase is coupled to mitochondrial ROS production remains unclear. It is possible that in coronary ECs, an increase in mitochondrial Ca2+ concentration stimulates ROS generation in the mitochondria (8, 10, 40). Although uptake of Ca2+ or other cations depolarizes mitochondrial membrane potential, which can lead to reduced ROS formation, Ca2+ may increase mitochondrial ROS formation by other mechanisms, such as enhancement of electron flow into the respiratory chain, stimulation of nitric oxide production and consequent inhibition of complex IV, and activation of other ROS-generating enzymes, such as α-ketoglutarate dehydrogenase (8, 10, 40). Alternatively, a ROS-induced ROS release mechanism involving other ROS sources, such as NADPH oxidase (42), may also play a role in TRPV4-induced ROS production in ECs. Future studies are required to explore these possibilities.
Although not pursued in this study, the mechanisms by which flow activates endothelial TRPV4 channels in HCAs may involve endogenous activators such as arachidonic acid and/or its cytochrome P-450 metabolites (e.g., EETs) (34, 36), as has been suggested for flow-induced dilation of mouse carotid arteries (12, 23). In isolated HCAs, both arachidonic acid and EETs are potent vasodilators (27), and inhibition of cytochrome P-450 enzyme markedly reduces flow-induced vasodilation (28). It remains to be determined in future studies whether flow induces the release of arachidonic acid and/or its metabolites and whether these endogenous compounds activate endothelial TRPV4, leading to flow-induced dilation of HCAs.
TRPV4 channels have been found to be expressed in vascular SMCs of several vascular beds, such as rat cerebral and pulmonary arteries (41). Using immunohistochemical analysis, we also found positive staining of TRPV4 protein in SMCs of human coronary arteries and HCAs, although at a much lower level than that in ECs. These results seem not to be in accordance with RT-PCR data showing that TRPV4 mRNA transcripts are absent in freshly isolated or cultured coronary SMCs. Arniges et al. (1) recently identified five splice variants of human TRPV4 channels and grouped them into two classes: group I (TRPV4-A and TRPV4-D) and group II (TRPV4-B, TRPV4-C, and TRPV4-E). We could not detect PCR amplification products of TRPV4 mRNA in coronary SMCs when the primers as shown in Fig. 1 were selective for group I isoforms. However, PCR amplification products were detected when primers for all isoforms were used (data not shown). These results suggest that human coronary SMCs express other isoforms of TRPV4, most likely group II TRPV4 channels. Intriguingly, group II TRPV4 channels, which lack parts of the ankyrin domains, are unable to oligomerize and thus are retained intracellularly, mainly in the endoplasmic reticulum (1). Therefore, the functional significance of TRPV4 channels in coronary SMCs remains to be characterized. Since no significant dilatory responses (small constriction instead) to the TRPV4 agonist 4α-PDD were observed in denuded coronary arterioles (Fig. 3A), the potential contribution of smooth muscle TRPV4 to the overall flow-mediated dilation would be minimal in this vascular bed.
Due to technique difficulties, we were unable to culture ECs from HCAs. For those experiments shown in Figs. 2 and 6, we instead used macrovascular ECs (i.e., HCAECs), which were derived from large coronary arteries and were typically from donors free of cardiovascular diseases. Therefore, we cannot exclude the possibility that observations made in large coronary ECs may differ from those in arteriolar ECs or that ECs from healthy subjects respond differently from those cells from patients who, as in this study, are not completely free of cardiovascular diseases. However, a previous study (21) has shown that, similar to that observed in HCAs, shear induces mitochondrial ROS production in HCAECs.
In summary, the present study provides evidence that the endothelial TRPV4 channel is importantly involved in flow-mediated dilation of HCAs. TRPV4 channels may represent a critical but previously unidentified component of the redox signaling pathway linking the flow stimulus with the mitochondrial release of ROS and subsequent dilation of HCAs. Flow-induced dilation in the human coronary microcirculation is mediated by the release of H2O2 from the mitochondria of ECs, a unique mechanism of vasodilation that is more prominent in the presence of CAD and other cardiovascular diseases or risk factors (20, 22, 26). Therefore, studies on TRPV4-mediated redox signaling (especially in the human heart) will not only enhance our understanding of coronary blood flow regulation in health and disease but also help to identify new therapeutic options for the treatment of CAD and/or other cardiovascular pathologies.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-096647 (to D. X. Zhang) and R01-HL-080704 and R01-HL-094971 (to D. D. Gutterman) and by American Heart Association Grant 0830042N (to D. X. Zhang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.H.B., S.A.M., X.Z., N.S.Z., and R.L. performed experiments; A.H.B., S.A.M., X.Z., and N.S.Z. analyzed data; A.H.B., S.A.M., X.Z., N.S.Z., R.L., D.D.G., and D.X.Z. edited and revised manuscript; A.H.B., S.A.M., X.Z., N.S.Z., R.L., D.D.G., and D.X.Z. approved final version of manuscript; D.D.G. and D.X.Z. conception and design of research; D.D.G. and D.X.Z. interpreted results of experiments; D.X.Z. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Chunrong Li for technical assistance with immunohistochemistry. The authors also thank the Division of Cardiothoracic Surgery at the Medical College of Wisconsin and the Veterans Affairs Medical Center, the Cardiothoracic Surgery Group of Milwaukee, the Cardiovascular Surgery Associates of Milwaukee, the Midwest Heart Surgery Institute, the Wisconsin Heart Group, Froedtert Memorial Lutheran Hospital, and the Aurora St. Luke's Medical Center for providing surgical specimens.
REFERENCES
- 1. Arniges M, Fernández-Fernández JM, Albrecht N, Schaefer M, Valverde MA. Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J Biol Chem 281: 1580–1586, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension 17: 187–193, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Busse R, Fleming I. Regulation of endothelium-derived vasoactive autacoid production by hemodynamic forces. Trends Pharmacol Sci 24: 24–29, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Cooke JP, Rossitch E, Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest 88: 1663–1671, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, Joseph J, Kalyanaraman B. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J Biol Chem 279: 37575–37587, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation 100: 1951–1957, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Falcone JC, Kuo L, Meininger GA. Endothelial cell calcium increases during flow-induced dilation in isolated arterioles. Am J Physiol Heart Circ Physiol 264: H653–H659, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci 14: 1197–1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao X, Wu L, O'Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem 278: 27129–27137, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Graier WF, Frieden M, Malli R. Mitochondria Ca2+ signaling: old guests, new functions. Pflügers Arch 455: 375–396, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22: 6408–6414, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Köhler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLos One 2: e827, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helmlinger G, Berk B, Nerem RM. Pulsatile and steady flow-induced calcium oscillations in single cultured endothelial cell. J Vasc Res 33: 360–369, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Hoyer J, Kohler R, Distler A. Mechanosensitive Ca2+ oscillations and STOC activation in endothelial cells. FASEB J 12: 359–366, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Köhler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26: 1495–1502, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Koller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ Res 72: 1276–1284, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol 259: H1063–H1070, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res 108: 566–573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Li H, Bubolz AH, Zhang DX, Gutterman DD. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Med Biol Eng Comput 46: 469–478, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res 80: 445–452, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 298: H466–H476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller FJ, Jr, Dellsperger KC, Gutterman DD. Pharmacologic activation of the human coronary microcirculation in vitro: endothelium-dependent dilation and differential responses to acetylcholine. Cardiovasc Res 38: 744–750, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: e31–e40, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Miura H, Gutterman DD. Human coronary arteriolar dilation to arachidonic acid depends on cytochrome P-450 monooxygenase and Ca2+-activated K+ channels. Circ Res 83: 501–507, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channels. Circulation 103: 1992–1998, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2: 695–702, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Taniguchi J, Tsuruoka S, Mizuno A, Sato J, Fujimura A, Suzuki M. TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. Am J Physiol Renal Physiol 292: F667–F673, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 104: 1123–1130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MA. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun 389: 490–494, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol 75: 1262–1279, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2+ permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 97: 908–915, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277: 13569–13577, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277: 47044–47051, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Wu L, Gao X, Brown RC, Heller S, O'Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol 293: F1699–F1713, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Yu JZ, Zhang DX, Zou AP, Campbell WB, Li PL. Nitric oxide inhibits Ca2+ mobilization through cADP-ribose signaling in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 279: H873–H881, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292: H2023–H2031, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Zhang DX, Gutterman DD. TRP channel activation and endothelium-dependent dilation in the systemic circulation. J Cardiovasc Pharmacol 57: 133–139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol 301: H647–H653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]