Abstract
Mineralocorticoid excess increases superoxide production by activating NADPH oxidase (NOX), and intracerebroventricular infusions of NADPH oxidase inhibitors attenuate aldosterone (Aldo)/salt-induced hypertension. It has been hypothesized that increased reactive oxygen species (ROS) in the brain may be a key mechanism in the development of hypertension. The present study investigated the brain regional specificity of NADPH oxidase and the role of NOX2 and NOX4 NADPH oxidase subunits in the hypothalamic paraventricular nucleus (PVN) in Aldo/salt-induced hypertension. PVN injections of adenoviral vectors expressing small interfering (si)RNA targeting NOX2 (AdsiRNA-NOX2) or NOX4 (AdsiRNA-NOX4) mRNAs were used to knock down NOX2 and NOX4 proteins. Three days later, delivery of Aldo (0.2 mg·kg−1·day−1 sc) via osmotic pump commenced and 1% NaCl was provided in place of water. PVN injections of either AdsiRNA-NOX2 or AdsiRNA-NOX4 significantly attenuated the development of Aldo/NaCl-induced hypertension. In an additional study, Aldo/salt-induced hypertension was also significantly attenuated in NOX2 (genomic) knockout mice compared with wild-type controls. When animals from both functional studies underwent ganglionic blockade, there was a reduced fall in blood pressure in the NOX2 and NOX4 knockdown/knockout mice. Western blot analyses of the PVN of siRNA-NOX2- or siRNA-NOX4-injected mice confirmed a marked reduction in the expression of NOX2 or NOX4 protein. In cultured PVN neurons, silencing either NOX2 or NOX4 protein production by culturing PVN cells with siRNA-NOX2 or siRNA-NOX4 attenuated Aldo-induced ROS. These data indicate that both NOX2 and NOX4 in the PVN contribute to elevated sympathetic activity and the hypertensivogenic actions induced by mineralocorticoid excess.
Keywords: blood pressure, reactive oxygen species, paraventricular nucleus, small interfering ribonucleic acid
in recent years aldosterone (Aldo) has come to be recognized as an even more important mediator of the effects of the renin-angiotensin system than previously assumed. Aldo is considered to be such a key signaling factor in the functional cascade of the renin-angiotensin system that the system is referred to by many as the renin-angiotensin-aldosterone system. Like angiotensin II (ANG II), Aldo is viewed as playing important roles in the pathophysiology of several cardiovascular diseases (8). A number of clinical studies indicate that blockade of the mineralocorticoid receptor (MR) is cardioprotective. Addition of the MR antagonist spironolactone has been demonstrated to reduce mortality in patients with severe heart failure who were receiving standard therapy, including angiotensin-converting enzyme inhibitors, ANG II receptor type 1 antagonists, and β-adrenergic receptor blockers (26). The increased therapeutic effect afforded by MR antagonist treatment may be because in many cases Aldo escapes from the control of ANG II so that plasma Aldo levels become elevated (29). Therefore, a better understanding of the mechanisms and sites of Aldo action is likely to lead to greater insights, resulting in improved treatment of patients with cardiovascular diseases.
Phagocytic NADPH oxidase (NOX) is a multisubunit enzyme that catalyzes the reduction of molecular oxygen to form reactive oxygen species (ROS). The subunits are composed of two essential membrane-bound components, gp91phox/NOX2 and p22phox, and four cytosolic components, p47phox, p67phox, p40phox, and Rac1 or 2 (5, 15). In nonphagocytic cells, the entire family of NOX proteins consists of NOX1, gp91phox/NOX2, NOX3, NOX4, NOX5, and dual oxidase 1 and 2 (3, 12, 20). In conjunction with p22phox, the NOX homologues are considered to be the catalytic component giving NADPH oxidase its functional capacity.
Numerous in vitro and in vivo studies (9, 25, 27) have demonstrated a direct role of Aldo in the development of cardiovascular disease via oxidative stress, and NADPH oxidase is suggested as a major source of ROS generated in response to Aldo administration. For example, Aldo/salt treatment induced hypertension and increased mRNA and protein expression of NOX2, NOX4, and p22phox in kidney and aortic rings of rats. These effects were inhibited by treatment with either an MR antagonist or the NADPH oxidase inhibitor apocynin (2, 22, 28). In cultured rat aortic endothelial cells, Aldo induced a time-dependent and dose-dependent increase in superoxide generation and Rac1. An MR antagonist, apocynin, or adenoviral gene transfer of dominant-negative Rac1 also abolished Aldo-induced superoxide generation (16). In NOX2-deficient mice, Aldo-induced activation of NADPH oxidase and NF-κB was prevented in the heart (17). These findings indicate that in the periphery, Aldo may act directly on specific subunit(s) of NADPH oxidase, thereby contributing to ROS generation and the progression of end-organ injury and hypertension. Aldo may have similar actions in the central nervous system (CNS). However, to date there have been no investigations on the source of ROS generation in the CNS during mineralocorticoid excess.
Over the past few decades, various sites of Aldo action within the CNS have been presumed on the basis of very little evidence. It has been proposed that central neurons activated by circulating Aldo should contain MR and the enzyme 11β-hydroxysteriod dehydrogenase type 2 (11β-HSD2). This particular enzyme allows access of Aldo to MR by metabolizing glucocorticoids that have greater affinity for MR and that are available in plasma in greater concentrations than Aldo (10). Although such neurons have been identified in the nucleus of the solitary tract, they do not seem likely to be the site of action upon which Aldo increases blood pressure (BP; 10). Previous studies from our laboratory (31) have shown that intracerebroventricular infusion of apocynin or tempol attenuated systemic Aldo/salt-induced hypertension. Zhang et al. (35) have reported that intracerebroventricular Aldo resulted in increased dihydroethidium staining (indicating oxidative stress) and Fra-like activity (indicating neuronal excitation) in neurons of the hypothalamic paraventricular nucleus (PVN) and that treatment with tempol minimized the increases in ROS and Fra-like activity and produced greater sympathoinhibition in Aldo-treated vs. vehicle-treated rats. These results suggest that Aldo may increase sympathetic nerve activity through increased oxidative stress in the hypothalamus. Additional recent studies from the same group demonstrated the presence of 11β-HSD2 mRNA in the PVN by RT-PCR analysis. Either intracerebroventricular or PVN microinjection of 11β-HSD2 inhibitors increase BP, heart rate (HR), and renal sympathetic nerve activity, suggesting that the PVN may be a prime forebrain site responsible for mediating central Aldo effects on sympathetic nerve activity and BP (34). The PVN is not only a major region implicated in central cardiovascular control but is involved also in sympathetic activation during hypertension and its development (4). With these considerations in mind, the present study focused on the Aldo-NADPH oxidase-ROS pathway in the PVN.
The studies just described demonstrate the general importance of NADPH oxidase in systemic and central Aldo signaling, such broad inhibition of enzyme actions (e.g., when studied by intracerebroventricular injections of apocynin or tempol) does not provide information about the molecular identity of the oxidase involved. Recently, Davisson and colleagues (15) compared the expression of NOX1, NOX2, and NOX4 in different regions of mouse brain using real-time PCR. NOX2 and NOX4 are the predominant homologues expressed in forebrain, including the PVN. These investigators also developed adenoviral vectors expressing small interfering (si)RNA to selectively silence NOX2 or NOX4 expression in the forebrain (24). In the present study, we tested the hypothesis that the NOX2- or NOX4-containing NADPH oxidase in the PVN is a key source of ROS, which contributes to the effects of Aldo on BP. We used PVN microinjections of siRNA-NOX2 and siRNA-NOX4 to induce localized, stable knockdown of NOX2 and NOX4, respectively, and then examined the effects of silencing these central NADPH oxidase subunits on Aldo-induced hypertension and on the autonomic contribution to arterial BP. Also in in vitro studies, we examined the effects of knocking down NOX2 or NOX4 in PVN cell cultures on Aldo-induced ROS production.
METHODS
Animals
Wild-type (WT; C57BL/6) male mice (12–16 wk old) were obtained from Harlan (Indianapolis, IN). NOX2 knockout mice were purchased from Jackson Laboratory. Mice were housed in temperature (22°C ± 0.2°C)- and light (12:12-h light-dark)-controlled animal quarters and were provided with mouse chow (7013 NIH-31 modified mouse diet, 0.25% NaCl) ad libitum. In Aldo-induced hypertension studies, the mice were randomly assigned to the following treatment groups: 1) PVN injection of adenoviral vectors expressing the control message enhanced green fluorescent protein (AdsiRNA-GFP; n = 8 i.e., as a control), 2) PVN AdsiRNA-NOX2 injection (n = 7), 3) PVN AdsiRNA-NOX4 injection (n = 8), 4) NOX2 knockout mice (n = 6), and 5) WT mice (n = 6). To induce hypertension with Aldo, the mice were infused subcutaneously with Aldo combined with 1% NaCl as the sole drinking fluid. During access to the saline solution, 1% NaCl intakes were measured daily. Control experiments were also conducted by giving animals 1% NaCl to drink without subcutaneous Aldo infusions.
For ROS imaging studies, neurons were collected from the PVN of 8-day-old rat pups who were from Sprague-Dawley mothers (Harlan). The cultured cells were divided into four groups: 1) incubation with vehicle (control), 2) incubation of AdsiRNA-GFP and Aldo, 3) incubation of AdsiRNA-NOX2 and Aldo, and 4) incubation of AdsiRNA-NOX4 and Aldo.
All experiments were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and approved by the University of Iowa Animal Care and Use Committee.
Adenoviral Vectors
AdsiRNA-Nox2, AdsiRNA-NOX4, or AdsiRNA-GFP was developed by Dr. Robin L Davisson and constructed and provided by the University of Iowa Gene Vector Core (24). In brief, 21-bp short hairpin RNAs representing sequences directed against NOX2, NOX4, or enhanced GFP were placed under the control of the mouse U6 promoter. A separate CMV promoter drives expression of a reporter gene (GFP for siNOX2- and siNOX4-expressing constructs and LacZ for the siGFP-expressing construct).
Surgical Procedures
Telemetry probe implantation.
Implantable mouse BP transmitters (TA11PA-C10; Data Sciences International) were used to chronically measure arterial BP. Mice were anesthetized with a ketamine-xylazine mixture. Through a ventral incision, the left carotid artery was accessed and isolated, and the catheter of a telemetry probe was inserted into the carotid and advanced into the aorta. Through the same incision, a subcutaneous tunnel was formed that passed across the right pectoral area and extended into the right flank where it was enlarged to form a pocket. The body of the transmitter was slipped into the pocket and secured with tissue adhesive. The ventral incision was then closed with suture.
PVN microinjection of adenovirus-siRNA and osmotic pump implantation.
After baseline BP and HR recordings were obtained, mice were again anesthetized with a ketamine-xylazine mixture. AdsiRNA-GFP, AdsiRNA-NOX2, or AdsiRNA-NOX4 (200 nl of 1.3 × 1012 genomic particles/ml, 30 s) was injected into the PVN bilaterally (anterior-posterior, −1.1 mm; medial-lateral, ± 0.6 mm; and dorsal-ventral, −5.2). Three days later, osmotic pumps (model 1002; ALZET) containing Aldo (0.2 mg·kg−1·day−1; Sigma) were implanted subcutaneously in the back, and tap water was changed to 1% NaCl. At the end of each experiment, animals were deeply anesthetized with pentobarbital and perfused transcardially with saline followed by 4% paraformaldehyde. The locations of the PVN injections in histological material were verified by visualization of expression of the reporter gene GFP using confocal microscopy. The animals with missed injections were excluded from analysis.
Western blotting analysis.
Protein samples were mixed with equal volumes of SDS-PAGE buffer and loaded on the 10% SDS-PAGE gel for electrophoresis and then transferred to a PVDF membrane (Bio-Rad Laboratories). The membrane was probed with mouse antibody for anti-gp91phox/NOX2 (1:1000; BD Biosceinces), rabbit antibody for anti-NOX4 (1:500; Abcam), or anti-β-actin (1:2,500; Cell Signaling), respectively. This was followed by horseradish peroxidase-labeled anti-mouse or anti-rabbit secondary antibody (Santa Cruz Biotechnology) and then treatment with an enhanced chemiluminesence reagent (Supersignal Substrate Western Blotting; Pierce Chemical). Band intensities were quantified with Imager (Bio-Rad) software and were normalized to β-actin.
PVN neuronal cultures.
Primary neuronal cultures were established from the PVN of preweaning pups (8 days old, 8–10 pups per culture). Cells were cultured for 4 days in DMEM:Ham's F-12 medium (1:1) supplemented with 10% FBS and 1% l-glutamine-penicillin-streptomycin.
Experimental Protocols
Measurement of BP and HR.
All mice were allowed 7 days to recover from transmitter implantation surgery before any measurements were made. Thereafter, BP and HR were telemetrically recorded and stored with the Dataquest ART data acquisition system (Data Sciences International).
In the control experiments, we tested if PVN injections of adenoviral vector or 1% NaCl as only drinking fluid had effects on basal BP and HR. Animals were also given PVN injections of siRNA-GFP and 1% NaCl to drink for 17 days without receiving Aldo treatments.
In Aldo-induced hypertension studies, we tested the BP and HR responses to PVN blockade of ROS production during Aldo infusions. Two hundred nanoliters of siRNA-GFP (control), siRNA-NOX2, or siRNA-NOX4 were injected bilaterally into the PVN. Three days later, Aldo was infused subcutaneously for 14 days and 1% NaCl replaced water as the drinking fluid.
Evaluation of BP responses to autonomic blockade.
The autonomic contribution to resting BP was assessed by administering the ganglionic blocker hexamethonium (30 mg/kg ip). Ganglionic blockade was repeated twice, once during baseline and then after 14 days of Aldo infusion. On the day of ganglionic blockade experiments, BP was recorded for 20 min both before and after hexamethonium injection. After hexamethonium injection, the largest decrease in BP occurred within 5 min. This nadir (2–3 min) was recorded as the maximum fall in BP.
NOX2 or NOX4 protein expression measurements.
Seventeen days after PVN injections of siRNA-GFP, siRNA-NOX2, or siRNA-NOX4, mice were deeply anesthetized with isoflurane. The brains were removed and stored at −80°C until assay. Brains were cut into consecutive 100-μm sections in a cryostat at −20°C, and micropunches (20-gauge needles) were made to collect the PVN. Total cellular protein was isolated from tissue punches of the PVN and analyzed for protein expression of NOX2 or NOX4 by Western blotting.
Measurement of ROS production in cultured PVN neurons.
After cells were incubated for 24 h under serum free conditions, the cultured neurons were treated with vehicle, AdsiRNA-GFP, AdsiRNA-NOX2, or AdsiRNA-NOX4 for another 24 h followed by overnight incubation with Aldo (10 μM). On the experimental day, the neurons were loaded with dihydroethidium (DHE; 5 μM; Invitrogen) for 30 min, and then ethidium fluorescence was imaged using confocal microscopy. Neurons are roundish, domed cells with one or two thin processes as verified by terminal experimental KCl-induced calcium flux. DHE fluorescence of neurons was quantified using Fluoview 5 (Olympus, Japan) analysis software and expressed relative to fluorescence of vehicle-treated neurons.
Data Analysis
Mean arterial pressure (MAP) and HR were collected for 5 baseline days and then for 14 consecutive days after Aldo pump implantation. MAP and HR are presented as mean daily values averaged from daytime and nighttime measurements. Difference scores for MAP and for HR were calculated for each animal based on the mean of the 5-day baseline values subtracted from the mean of the final 5 days of treatment. One-way ANOVAs for the experimental groups were then conducted on the means of calculated difference scores. After a significant ANOVA was established, follow-up t-tests were conducted to test for differences between pairs of mean change scores. To test differences in the mean of 5 days baseline vs. the mean of final 5 days of treatment, t-tests were performed.
For analysis of 1% NaCl intakes, mean intakes for each experimental group were averaged from daily measurements during the 14 days of treatment. A one-way ANOVA was conducted on these mean values.
Student t-tests were conducted to test for differences in NOX2 or NOX4 protein expression in siRNA-NOX2- or siRNA-NOX4-treated animals vs. siRNA-GFP-treated animals. The same statistical method was used to test the differences in Aldo-induced ROS production in neurons treated with AdsiRNA-GFP, AdsiRNA-NOX2, or AdsiRNA-NOX4.
All data are expressed as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
Effects of PVN Knockdown of NOX2 or NOX4 on Aldo/NaCl-Induced Hypertension
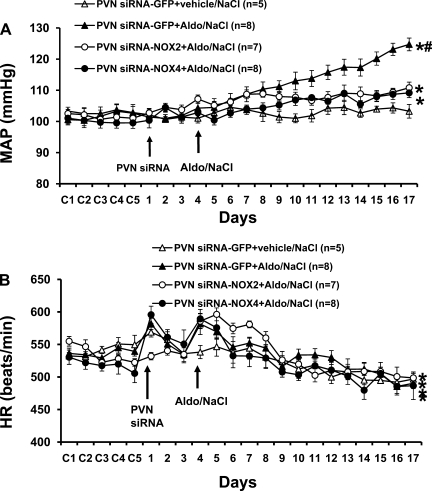
As presented in Fig. 1A, baseline MAP was unaltered (P > 0.05) after bilateral PVN injections of siRNA-GFP when the control (vehicle) for Aldo and 1% NaCl was given (Δ2.1 ± 2.6 mmHg; n = 5). In contrast, mice that received bilateral PVN injections of siRNA-GFP followed by Aldo plus 1% NaCl showed marked hypertension (Δ20.8 ± 3.4 mmHg; n = 8), as evidenced by a significant increase above their baseline BP and above that of the group that had received bilateral PVN injections of siRNA-GFP followed by systemic vehicle and 1% NaCl. Bilateral PVN injections of either siRNA-NOX2 or siRNA-NOX4 significantly attenuated the increase in MAP induced by Aldo/NaCl (Δ7.7 ± 2.0 and Δ7.8 ± 1.4, respectively; n = 8; P < 0.05) compared with that seen in mice with PVN siRNA-GFP injections plus systemic Aldo. Systemic Aldo infusions produced significant, comparable decreases in HR in all groups (Fig. 1B; PVN siRNA-GFP, −32.8 ± 2.3; siRNA-NOX2, −31.4 ± 5.9; and siRNA-NOX4, −30.8 ± 7.5 beats/min).
Fig. 1.
Effect of knocking down NADPH oxidase (NOX)2 or NOX4 in the hypothalamic paraventricular nucleus (PVN) on aldosterone (Aldo)/salt-induced hypertension in mice. Daily mean arterial pressures (MAP; A) and heart rates (HR; B) before and during systemic infusion of Aldo and 1% NaCl access in PVN small interfering (si)RNA-GFP-, siRNA-NOX2-, or siRNA-NOX4-injected mice. Control days (C) were followed by PVN injection of siRNA vectors and 14 days of systemic Aldo infusion. *P < 0.05, compared with baseline MAP and HR or 1% NaCl intake alone without Aldo treatment. #P < 0.05, compared with siRNA-NOX2- or NOX4-treated rats.
Effects of Global Knock Out of NOX2 on Aldo/NaCl-Induced Hypertension
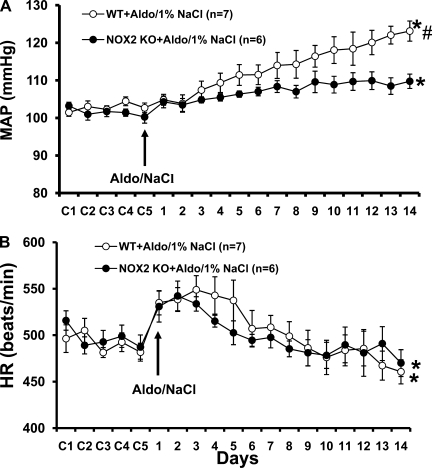
Fourteen days of Aldo infusion resulted in a 18.2 ± 2.6 mmHg (Fig. 2A; n = 7; P < 0.05) increase in MAP in WT mice, while in comparison NOX2 knockout mice showed a significant attenuation in the increase in MAP induced by Aldo/NaCl (Δ8.7 ± 2.5 mmHg; n = 6; P < 0.05).
Fig. 2.
Aldo/NaCl-induced hypertension in mice with genomic knockout of NOX2. Daily MAP (A) and HR (B) before and during systemic infusion of Aldo in wild-type (WT) and NOX2 knockout (KO) mice. Control days were followed by 14 days of systemic Aldo infusion. *P < 0.05, compared with baseline MAP and HR. #P < 0.05, compared with WT mice.
The chronic Aldo infusion produced a significant decrease in HR in both WT and NOX2 knockout mice (P < 0.05; Fig. 2B). The fall in HR during Aldo/NaCl treatment was similar between groups (WT, −28.7 ± 7.2; and NOX2 KO, −28.9 ± 5.8 beats/min; P > 0.05).
Effects of Aldo Infusion on 1% NaCl Intake
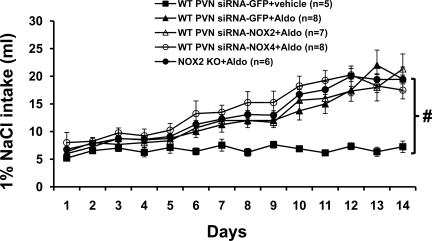
Average daily 1% NaCl intake was 6.7 ± 0.7 ml when the control (vehicle) for Aldo treatment was given for 14 days. Systemic infusion of Aldo significantly increased 1% NaCl intake in all groups of mice with or without NOX knockdown (P < 0.05). Also, there was no difference between groups (siRNA-GFP, 12.3 ± 0.9; siRNA-NOX2, 12.4 ± 0.6; siRNA-NOX4, 14.0 ± 1.0; and NOX2 knockout mice, 13.1 ± 0.6 ml/day; P > 0.05; Fig. 3).
Fig. 3.
Daily 1% NaCl intake during vehicle or Aldo infusions in PVN siRNA-GFP-, siRNA-NOX2-, or siRNA-NOX4-injected mice or NOX2 KO mice. #P < 0.05, compared with WT mice with infusion of vehicle for Aldo.
Localization and the Effects of Adenoviral Delivery of siRNA on NOX2 and NOX4 Proteins
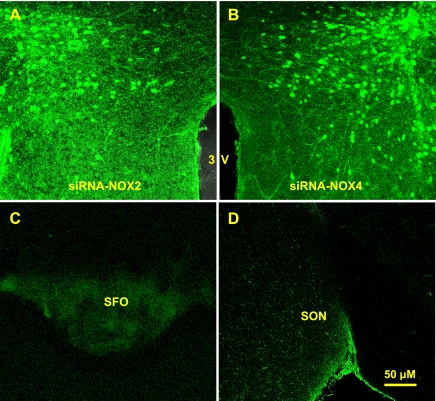
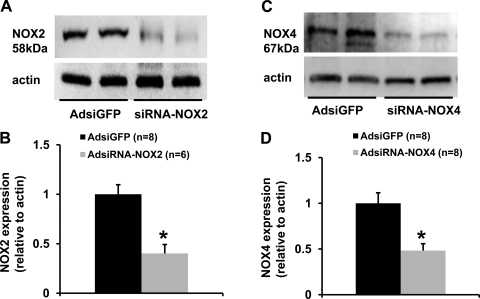
The effect of viral delivery of siRNA to knockdown NOX2 or NOX4 in the PVN was verified by Western blot analyses. AdsiRNA constructs contained a GFP construct that served as a marker indicating the site of delivery and the cells affected in the PVN. Seventeen days after PVN injections of siRNA-NOX2 (Fig. 4A) or NOX4 (Fig. 4B), highly robust GFP expression was present in the PVN but not in the subfornical organ (Fig. 4C) or the supraoptic nucleus (Fig. 4D). To confirm effective silencing of NOX2 or NOX4 in the PVN with these viruses, we performed Western blot analyses on the micropunches taken from the PVN. As shown in Fig. 5, NOX2 or NOX4 protein level was reduced by 50 to 60% in mice treated with siRNA-NOX2 or siRNA-NOX4 when compared with mice treated with the control vector siRNA-GFP.
Fig. 4.
Hypothalamic PVN viral delivery of the transgene for green fluorescent protein (GFP) results in robust transgene expression (GFP) localized to the PVN. Representative fluorescence microscope images of sections from siRNA-NOX2 (A)- or siRNA-NOX4 (B)- treated mice. GFP expression could not be detected in the subfornical organ (SFO; C) and supraoptic nucleus (SON; D) in the same animal, indicating that the injected Ad-siRNA did not leak into the ventricle.
Fig. 5.
Western blot analysis of NOX2 or NOX4 protein expression in the hypothalamic PVN from mice receiving PVN injections of siRNA-GFP, siRNA-NOX2, or siRNA-NOX4. A and C: representative Western blots of NOX2, NOX4, and β-actin. B and D: results of Western blot analyses showing the change in NOX2 or NOX4 protein expression, which was normalized with β-actin in the PVN. *P < 0.05, compared with siRNA-GFP treatment.
Effects of Autonomic Blockade on BP
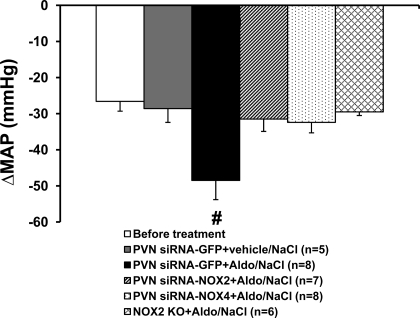
Figure 6 shows the decreases in BP with acute ganglionic blockade in all groups. Basal BP before hexamethonium injection was 101.6 ± 1.6 mmHg. The average reduction in the BP response to hexamethonium injection before central treatment and systemic infusion of Aldo was −26.6 ± 2.7 mmHg, which was not different from that seen in mice with bilateral PVN injections of siRNA-GFP when the control (vehicle) for Aldo and 1% NaCl was given (−28.6 ± 3.8 mmHg). Following 14 days of Aldo infusion, BPs were significantly increased in WT mice with PVN siRNA-GFP (126.6 ± 1.7 mmHg), PVN siRNA-NOX2 (110.8 ± 1.8 mmHg), or PVN siRNA-NOX4 (109.2 ± 1.5 mmHg) and in NOX2 knockout mice (109.5 ± 2.2 mmHg). Acute hexamethonium injection resulted in a greater reduction in BP in WT mice with PVN siRNA-GFP (−48.5 ± 5.3 mmHg; P < 0.05) compared with PVN siRNA-NOX2 (−31.5 ± 3.4 mmHg)- and PVN siRNA-NOX4 (−32.4 ± 2.9 mmHg)-treated mice or NOX2 knockout mice (−29.5 ± 4.0 mmHg; Fig. 6).
Fig. 6.
MAP in response to ganglionic blockade with hexamethonium before beginning Aldo infusions and on the last day of Aldo infusions in all groups. #P < 0.05, compared with NOX2 KO mice or WT mice receiving systemic infusions of vehicle for Aldo or hypothalamic PVN treatment with siRNA-NOX2 or siRNA-NOX4.
Effects of Knockdown NOX2 or NOX4 on Aldo-Induced ROS Production in Cultured PVN Neurons
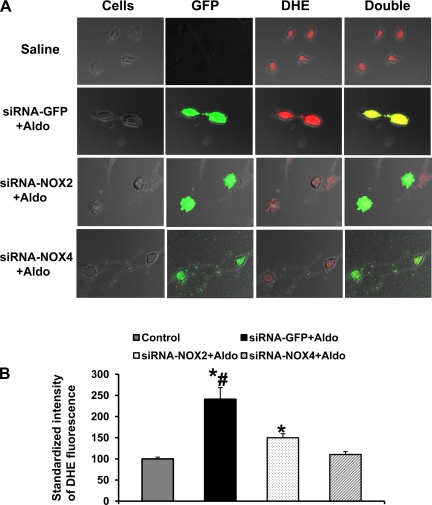
Figure 7A shows representative DHE fluorescent images of cultured PVN neurons with or without Aldo treatment. Overnight incubation of Aldo induced a 2.4-fold increase in ethidium intensity in the control siRNA-GFP-treated neurons (Fig. 7B; n = 21; P < 0.05) compared with that seen in PVN neurons with vehicle incubation (n = 115). Treatment with either siRNA-NOX2 or siRNA-NOX4 significantly inhibited or blocked this Aldo-induced increase in ROS production (NOX2, 1.5-fold, n = 94; and NOX4, 1.1-fold, n = 100).
Fig. 7.
Knockdown NOX2 or NOX4 prevents Aldo-induced reactive oxygen species (ROS) production in neurons cultured from the hypothalamic PVN. A: representative confocal images of dihydroethidium (DHE)-loaded neurons cultured from the PVN showing the effects of Aldo on ROS production in neurons treated with siRNA-GFP, siRNA-NOX2, or siRNA-NOX4. Green in the cytoplasm represents GFP, a marker for virus transfection. Red in the cytoplasm represents DHE, a marker for ROS production. B: summary of standardized emission intensity of DHE fluorescence in PVN neurons with or without overnight treatment of Aldo. Neurons were treated with Aldo 24 h after treatment with siRNA-GFP, siRNA-NOX2, or siRNA-NOX4. Change in ROS production was standardized by vehicle-treated neurons. *P < 0.05, compared with neurons without Aldo treatment. #P < 0.05 compared with neurons with siRNA-NOX2 or siRNA-NOX4 treatment.
DISCUSSION
The main findings of these studies are as follows: 1) acutely knocking down either NOX2 or NOX4 in the PVN by using viral delivery of siRNAs or chronically knocking out NOX2 by genomic manipulation significantly inhibits Aldo/NaCl-induced hypertension, 2) the attenuated BP effect on Aldo/NaCl-induced hypertension in mice produced by PVN blockade of ROS production is associated with decreased sympathetic tone, and 3) using siRNA to silence NOX2 or NOX4 blocks the generation of Aldo-induced ROS in cultured PVN neurons. Taken together, these results suggest that both NOX2 and NOX4 are necessary to generate functional NADPH oxidase in the PVN. Aldo acting in the CNS, especially PVN, results in the generation of ROS and oxidative stress to increase sympathetic drive and, in turn, elevate arterial BP to produce hypertension.
We (31) have previously reported that central MR antagonist, scavenging of ROS by tempol, or central blockade of NADPH oxidase attenuated Aldo-induced hypertension and that this attenuated effect of Aldo on the BP response involves a decrease in sympathetic tone. This is further supported by recent studies (33, 35) showing that increasing the central level of Aldo by direct intracerebroventricular infusion or by heart failure results in enhanced NADPH oxidase activity and ROS production in the PVN through activation of brain renin-angiotensin activity, thereby leading to increased neuronal activity and sympathetic drive. These observations suggest that cardiovascular-related cells of the CNS require ROS to carry out crucial functions in the control of BP and sympathetic activity during mineralocorticoid excess. In the periphery, an important role for NADPH oxidase is well established as the source of ROS in response to Aldo (2, 16, 22, 28). The present studies lend further insight into the role and mechanisms of the NADPH signaling pathway by implicating NOX2 and NOX4 in the CNS, more specifically in the PVN, in the regulation of an Aldo-mediated chronic pressor response.
In several models of hypertension including renovascular hypertension (23), phenol renal injury model of hypertension (1, 32) and obesity-induced hypertension (21) have been shown to increase oxidative stress in the hypothalamic PVN to contribute to the progression of hypertension through central sympathetic excitation. Enhanced NADPH oxidase activity associated with increased mRNA expression of several NADPH oxidase subunits including NOX2, NOX4, p22phox, and p47phox is a major source of increased oxidative stress. These findings are compatible with a recent study by Davisson and colleagues (15) showing that NOX2 and NOX4 are the predominant homologues expressed in forebrain of mice. Davisson and colleagues (24) also demonstrated that both NOX2 and NOX4 in the subfornical organ are required for the full vasopressor effects of brain ANG II. In the present study, we found that microinjections of either AdsiRNA-NOX2 or AdsiRNA-NOX4 into the PVN significantly attenuated Aldo-induced hypertension compared with AdsiRNA-GFP mice. The depressor effect of knocking down NOX2 in the PVN of adult mice on Aldo-induced hypertension was further confirmed in NOX2 knockout mice, which have a deficit of the NADPH oxidase subunit throughout the lifespan. Moreover, in Aldo/salt-treated mice, the fall in BP in response to ganglionic blockade was less in those with PVN injections of AdsiRNA-NOX2 or siRNA-NOX4 and in NOX2 knockouts compared with their respective controls. These observations add to the previously described studies by establishing the importance of both NOX2- and NOX4-containing NADPH oxidases in the actions of Aldo in the PVN. The consequence of activation of Aldo-NADPH oxidase-ROS pathway in the PVN is elevated sympathetic activity, which contributes to the development of Aldo-induced hypertension.
Assembly of NOX2 has been studied extensively. When p47phox is phosphorylated, it is recruited to the cytosolic tail of ph22phox, and p47phox then serves as a docking site for a p67phox/p40phox complex. The G-protein Rac is then bound to p67phox and is critical for oxidase activation (11). NOX4 also requires p22phox for activation. However, ROS production does not appear to require the interaction of cytoplasmic activator proteins such as p47phox, p67phox, or Rac1 (6, 30). Of the several subunits that constitute NADPH oxidase, p22phox, NOX2, NOX4, and p47phox have been shown to increase significantly during chronic Aldo infusion. Treatment with an MR antagonist, tempol, or apocynin has been shown to inhibit the rise in BP and the expression of these NADPH oxidase subunits (2, 22). However, these previous studies did not provide information about the molecular identity of the oxidase subunits involved because the pharmacological inhibitors of NADPH oxidase do not provide the specificity required to differentiate between the subunits to define those that are necessary (14). In the present study, we used adenovirus delivery of siRNA to selectively silence NOX2 or NOX4. In agreement with previous studies (24), PVN injections of siRNA induced significant but incomplete silencing of NOX2 or NOX4 within PVN tissue by 50–60%. Nevertheless, knockdown of these NADPH oxidase subunits resulted in significantly attenuating Aldo-induced hypertension, which confirms results produced by pharmacological antagonists on Aldo-induced increases in BP (31).
In imaging studies employing confocal microscopy and the fluorescent indicator DHE as an indicator of ROS production, we measured the changes in ROS production induced by Aldo in cultured PVN neurons. We found that PVN neurons showed a significant increase in ROS production after overnight incubation with Aldo. The increased ROS production was blocked by genetic silencing either NOX2 or NOX4 with siRNAs. These results provide further evidence to support the BP studies described above.
Aldo is implicated in the genesis of salt appetite (7). High salt intake itself also increases NADPH oxidase expression and oxidative stress (2, 13, 18, 19). It is possible that the synergistic action of Aldo and high salt intake could cause increases in ROS production, sympathetic activity, and BP. In the present study, systemic Aldo induced a significant increase in salt intake in all groups. However, PVN blockade of ROS production by silencing NOX2 or NOX4 reduced the BP responses to Aldo without reducing the Aldo-induced increase in saline intake. Moreover, our in vitro study demonstrated a direct role of Aldo in the activation of specific NADPH oxidase subunits such as NOX2 and NOX4 to increase ROS production. These results indicate that any oxidative stress induced by saline intake per se is unlikely to be necessary for producing Aldo-induced hypertension with the treatment parameters employed in the present studies.
In summary, the present results indicate that both NOX2 and NOX4 in the PVN are key sources of ROS production induced by Aldo, which contribute to elevated sympathetic activity and the pressor effects during mineralocorticoid excess.
GRANTS
This work was supported by the National Institute of Health Grants HL-14388, HL-98207, MH-80241.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.X. and A.K.J. conception and design of research; B.X., T.G.B., R.F.J., and F.G. performed experiments; B.X. analyzed data; B.X. interpreted results of experiments; B.X. prepared figures; B.X. drafted manuscript; B.X., M.H., and A.K.J. edited and revised manuscript; B.X., M.H., and A.K.J. approved final version of manuscript.
REFERENCES
- 1. Bai Y, Jabbari B, Ye S, Campese VM, Vaziri ND. Regional expression of NAD(P)H oxidase and superoxide dismutase in the brain of rats with neurogenic hypertension. Am J Nephrol 29: 483–492, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bayorh MA, Rollins-Hairston A, Adiyiah J, Lyn D, Eatman D. Eplerenone suppresses aldosterone/salt-induced expression of NOX-4. J Renin Angiotensin Aldosterone Syst 12: 195–201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65: 16–27, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Dworakowski R, Anilkumar N, Zhang M, Shah AM. Redox signalling involving NADPH oxidase-derived reactive oxygen species. Biochem Soc Trans 34: 960–964, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res 65: 495–504, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Fluharty SJ, Epstein AN. Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat: II. Synergistic interaction with systemic mineralocorticoids. Behav Neurosci 97: 746–758, 1983 [DOI] [PubMed] [Google Scholar]
- 8. Funder JW, Mihailidou AS. Aldosterone and mineralocorticoid receptors: clinical studies and basic biology. Mol Cell Endocrinol 301: 2–6, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Funder JW. Aldosterone, mineralocorticoid receptors and vascular inflammation. Mol Cell Endocrinol 217: 263–269, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Geerling JC, Loewy AD. Aldosterone in the brain. Am J Physiol Renal Physiol 297: F559–F576, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilbert KC, Brown NJ. Aldosterone and inflammation. Curr Opin Endocrinol Diabetes Obes 17: 199–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart 90: 491–493, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haque MZ, Majid DS. High salt intake delayed angiotensin II-induced hypertension in mice with a genetic variant of NADPH oxidase. Am J Hypertens 24: 114–118, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular reduced NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Infanger DW, Sharma RV, Davisson RL. NADPH oxidase of the brain: distribution, regulation, and function. Antioxid Redox Signal 8: 1583–1596, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology 149: 1009–1014, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 20: 1546–1548, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Koga Y, Hirooka Y, Araki S, Nozoe M, Kishi T, Sunagawa K. High salt intake enhances blood pressure increase during development of hypertension via oxidative stress in rostral ventrolateral medulla of spontaneously hypertensive rats. Hypertens Res 31: 2075–2083, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Kopkan L, Hess A, Husková Z, Cervenka L, Navar LG, Majid DS. High-salt intake enhances superoxide activity in eNOS knockout mice leading to the development of salt sensitivity. Am J Physiol Renal Physiol 299: F656–F6563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 119: 978–986, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 43: 841–848, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Oliveira-Sales EB, Nishi EE, Carillo BA, Boim MA, Dolnikoff MS, Bergamaschi CT, Campos RR. Oxidative stress in the sympathetic premotor neurons contributes to sympathetic activation in renovascular hypertension. Am J Hypertens 22: 484–492, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension 54: 1106–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep 8: 232–241, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Queisser N, Fazeli G, Schupp N. Superoxide anion and hydrogen peroxide-induced signaling and damage in angiotensin II and aldosterone action. Biol Chem 391: 1265–1279, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation 101: 1722–1728, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Stier CT, Jr, Rocha R, Chander PN. Effect of aldosterone and MR blockade on the brain and the kidney. Heart Fail Rev 10: 53–62, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Ueyama T, Geiszt M, Leto TL. Involvement of Rac1 in activation of multi-component Nox1 and Nox3-based NADPH oxidases. Mol Cell Biol 26: 2160–2174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 300: H555–H564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ye S, Zhong H, Yanamadala S, Campese VM. Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension 48: 309–315, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension 51: 727–733 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang ZH, Kang YM, Yu Y, Wei SG, Schmidt TJ, Johnson AK, Felder RB. 11β-hydroxysteroid dehydrogenase type 2 activity in hypothalamic paraventricular nucleus modulates sympathetic excitation. Hypertension 48: 127–133, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294: H1067–H1074, 2008 [DOI] [PubMed] [Google Scholar]