Abstract
We recently reported translocation and activation of Akt in cardiac mitochondria. This study was to determine whether activation of Akt in mitochondria could inhibit apoptosis of cardiac muscle cells. Insulin stimulation induced translocation of phosphorylated Akt to the mitochondria in primary cardiomyocytes. A mitochondria-targeted constitutively active Akt was overexpressed via adenoviral vector and inhibited efflux of cytochrome c and apoptosis-inducing factor from mitochondria to cytosol and partially prevented loss of mitochondria cross-membrane electrochemical gradient. Activation of caspase 3 was suppressed in the cardiomyocytes transduced with mitochondria-targeted active Akt, whereas a mitochondria-targeted dominant negative Akt enhanced activation of caspase 3. Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling assay showed that mitochondrial activation of Akt significantly reduced the number of apoptotic cells. When the endogenous Akt was abolished by LY294002, the antiapoptotic actions of mitochondrial Akt remained effective. These experiments suggested that mitochondrial Akt suppressed apoptosis signaling independent of cytosolic Akt in cardiac muscle cells.
Keywords: caspase 3, cytochrome c
mitochondria are the most abundant organelles in cardiac muscle and play leading roles in energy production, oxidative stress, and regulation of apoptosis (21). We (26) recently reported that insulin acutely stimulated translocation and activation of Akt to myocardial mitochondria and modulated mitochondrial oxidative phosphorylation. Moreover, insulin-stimulated Akt translocation to mitochondria was significantly diminished in insulin-resistant myocardium (26). Phosphocreatine-to-ATP ratios were reduced in heart failure patients (15), and dysregulation of myocardial bioenergetics may play a role in the development of diabetic cardiomyopathy (1). The energy produced from electron transport chain helps pump protons out of the inner membrane to maintain an electrochemical gradient (ΔΨm) across mitochondria membranes (21). Maintenance of adequate ΔΨm prevents mitochondria membrane depolarization and is essential to allow ATP production and prevent induction of apoptosis (21). Apoptosis of cardiac muscle cells contributes to development of cardiomyopathy and heart failure (9, 23). Understanding the mechanisms through which hormonal signaling modulates apoptosis may offer new opportunities to prevent loss of cardiac functioning unit.
Activation of the phosphatidylinositol 3-kinase (PI3-kinase)-Akt pathway negatively modulates mitochondrial apoptosis signaling in the cardiomyocytes and other cell types (8, 19). We (24) have previously shown that a plasma membrane-targeted constitutive active PI3-kinase inhibited mitochondria-mediated apoptosis in cardiomyocytes, at least in part through activation of Akt. Insulin receptor signaling sequentially activates PI3-kinase and production of Ptdlns(3,4,5)P3/P2, PDK1, and Akt/PKB (12, 19). Akt may phosphorylate Bad and sequester Bad from acting on mitochondria to promote cell survival (16). Although previous studies (25) have shown that activation of Akt pathway could suppress efflux of cytochrome c from mitochondria and activation of caspases in cardiac muscle cells, our knowledge on Akt/PKB actions is largely focused on the cytosolic compartment and nucleus. Whether activation of Akt in mitochondria can modulate apoptosis of cardiac muscle cells is not known. Insulin-induced Akt translocation to mitochondria is a novel paradigm underlying dysregulation of myocardial bioenergetics in animal models of diabetes (26). Since Akt can be translocated and activated in mitochondria, it is necessary to determine whether activation of Akt in mitochondria alone can modulate apoptosis in cardiomyocytes. We therefore investigated the role of mitochondrial Akt activation, and the results indicated that mitochondrial activation of Akt could independently inhibit cardiac apoptosis signaling.
RESEARCH DESIGN AND METHODS
Materials.
FBS and cell culture medium were purchased from Irvine Scientific (Santa Ana, CA). Immobilon-P membranes were from Millipore (Bedford, MA). Anti-cytochrome c, anti-β-actin, anti-poly(ADP-ribose) polymerase-1, anti-α-tubulin, and anti-Akt antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against the active fragment of caspase 3 and apoptosis-inducing factor (AIF) were from Cell Signaling Technology (Danvers, MA). Anti-porin antibody was obtained from MitoSciences (Eugene, Oregon, CA). Peroxidase-conjugated secondary antibodies were from Santa Cruz Biotechnology or GeneTex (Irvine, CA). Akt activity assay kit was purchased from BioVision (Mountain View, CA). JC-1 and terminal deoxynucleotidyltransferase-mediated nick-end labeling (TUNEL) imaging assay reagents were from Invitrogen (Carlsbad, CA). BSA was purchased from Fisher Scientific (Fairlawn, NJ). LY294002 was from Biomol (Plymouth Meeting, PA). All other chemicals were purchased from Sigma or Fisher Scientific.
Cell culture and transduction of viral constructs.
Primary cultures of neonatal cardiomyocytes were prepared from Sprague-Dawley rats according to a protocol we previously described (24). The animal experimental procedures were approved by the Institutional Animal Care and Use Committee at University of California, Irvine. Cardiomyocytes were plated in 100-mm Petri dishes (∼80–85% density) or 8-chamberslides and maintained with DMEM containing 10% FBS and 1% penicillin/streptomycin and incubated at 37°C, 5% CO2. To transduce adenoviral or lentiviral vectors, cardiomyocytes were incubated with equal amounts of viral vectors in DMEM containing 10% FBS for 72–96 h. Two recombinant adenoviruses were used in this study. An adenoviral vector expressed a mitochondria-targeting constitutively active mitochondrial-targeted Akt (Ad-Mito-Akt). Constitutively active Akt was created by mutating Thr308 and Ser473 to aspartic acid residues, which mimics phosphorylation and resulted in an active Akt construct (2). Mitochondrial targeting was achieved by fusing a mitochondria targeting sequence (MSVLTPLLLRGLTGSARRLPVPRAKIHSL) to the N terminal. The control adenovirus expressed green-fluorescent protein (Ad-GFP). A His-tagged dominant negative Akt (substitutions at K179A, T308A, and S473A) with mitochondria targeting sequence at the N terminus was subcloned into a Tet-on inducible lentiviral vector with and without GFP.
For induction of apoptosis, after overnight serum deprivation, cardiomyocytes were incubated with doxorubicin (1 μM) or H2O2 (100 μM) in serum-free DMEM for the indicated time period as described previously (22). When indicated, LY294002 was added to inhibit endogenous Akt signaling, 20 min before doxorubicin or H2O2 treatment.
Preparation of mitochondria, nuclear, and cytosolic proteins.
For preparation of mitochondria fractions, cells were harvested in mitochondria isolation buffer (20 mM HEPES-KOH pH 7.2, 10 mM KCl, 1.5 mM MgCl2, 1.0 mM sodium EDTA, 1.0 mM sodium EGTA, 1.0 mM dithiothreitol, and 250 mM sucrose), supplemented with protease/phosphatase inhibitors (3 μg/ml aprotinin, 3 μg/ml leupeptin, 2 mM phenylmethylsulfonyl fluoride, 20 mM NaF, 10 mM NaPP, and 2 mM Na3VO4). After incubating on ice for 30 min, the cells were homogenized with 20 strokes of loose pestle and 50 strokes of tight pestle with a homogenizer. The nuclei and cell debris were removed by centrifugation at 1,000 g for 15 min, 4°C. The supernatants were collected for centrifugation (10,000 g, 15 min at 4°C), and the resulting mitochondrial fractions were resuspended with mitochondria isolation buffer. The supernatants that contained the cytosolic fractions were further centrifuged at 100,000 g for 1 h at 4°C to collect cytosolic proteins. The cytosolic and mitochondrial fractions were stored at −80°C until further analysis. The protein concentrations were determined by the Bradford method.
To isolate the nuclear fractions, cells were harvested by scraping with ice-cold hypotonic buffer (10 mM HEPES pH 7.9, 1.5 mM MgCl2, and 10 mM KCl) with protease/phosphatase inhibitors. After incubating on ice for 10 min, the cells were homogenized with a homogenizer. After centrifugation at 3,300 g for 15 min, the pellets were resuspended with a low-salt buffer (20 mM HEPES, pH 7.9, 25% glycerol, 1.5 mM MgCl2, 20 mM KCl, and 0.2 mM EDTA) in the presence of protease/phosphatase inhibitors. Then, a high-salt buffer (20 mM HEPES pH 7.9, 25% glycerol, 1.5 mM MgCl2, 1.2 M KCl, and 0.2 mM EDTA) was added drop wise, and the nuclear proteins were extracted by continuous gentle mixing for 30 min. The mixtures were cleared by centrifugation at 25,000 g for 30 min, and the nuclear extracts in the supernatants were collected for further experiments.
Western blots.
Equal amounts of proteins from each sample were separated by SDS-PAGE, transferred to Immobilon-P membrane, and incubated with a blocking buffer (3% BSA in 20 mM Tris·HCl pH 7.5, 137 mM NaCl, and 0.1% Tween 20) for 1 h at room temperature. The membranes were incubated sequentially with primary antibodies overnight at 4°C, washed three times with TBS-T (20 mM Tris·HCl pH 7.5, 137 mM NaCl, and 0.1% Tween 20), and incubated with respective horseradish peroxidase-conjugated secondary antibodies (1:5,000 to 1:20,000 dilution in TBS-T) for 1 h at room temperature. The membranes were washed three times with TBS-T and incubated with West Pico Chemiluminescent Substrate to visualize the target proteins (Thermo Scientific, Pittsburgh, PA).
Akt activity assay.
The Akt activity assay kit used Akt-specific antibody for immunoprecipitation of Akt, and the activity of the immunoprecipitated kinase was measured in vitro using exogenously added recombinant GSK3α as the substrate. The resulting phosphorylated GSK3α is determined by immunoblotting. Equal amounts of proteins (250 μg) were immunoprecipitated with anti-Akt monoclonal antibody, and the Akt kinase assay was performed as per the manufacturer's instructions. Phospho-specific (Ser-21) antibodies to GSK3α were used for immunoblotting.
Immunofluorescence staining.
To locate the expressed Mito-Akt proteins in cardiomyocytes, the cells were infected with Ad-Mito-Akt for 48 h and fixed with 4% formaldehyde for 30 min at room temperature. After being washed three times with PBS, cells were treated with 0.05% saponin in ddH2O for 20 min and blocked with 10% normal sera for 30 min. The fixed cells were incubated with rabbit-anti-Mn-SOD (Upstate) to locate mitochondria and mouse-anti-HA antibodies to locate the expressed HA-tagged Mito-Akt, overnight at 4°C. The cells were incubated with conjugated secondary antibodies for analysis. For other immunofluorescence analysis, the cells were fixed with 4% paraformaldehyde for 15 min at room temperature and incubated with a blocking buffer(10% serum in PBS with 0.1% Triton X-100) for 30 min. Primary antibodies (1:500 dilutions) were added and incubated overnight at 4°C. After being washed, the cells were incubated with secondary antibodies conjugated with TexRed (1:400 dilutions) for 1 h and counterstained with DAPI and analyzed with Eclipse Ti fluorescence microscope (Nikon).
Analysis of the mitochondrial cross-membrane electrochemical gradient (δΨm).
JC-1 (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine iodide) was used to define the changes of mitochondria membrane potential. When mitochondria membrane depolarized, emission of JC-1 shifts from red to green (8). Cardiomyocytes were washed twice with PBS and incubated with a buffer containing JC-1(10 μM) for 20 min at 37°C under 5%CO2. The cardiomyocytes were immediately analyzed with Eclipse Ti fluorescence microscope (Nikon) and the images were recorded with a Nikon digital camera.
TUNEL assay.
TUNEL assays were performed to detect apoptotic cardiomyocytes using the Click-i TEdUTP TUNEL assay kit according to the manufacturer's procedure (Invitrogen). Cardiomyocytes were fixed with 4% paraformaldehyde and incubated with terminal deoxynucleotidyltransferase in reaction buffer containing EdUTP overnight in room temperature. Positive controls were treated with DNase I. Negative controls were incubated in a reaction buffer without terminal deoxynucleotidyltransferase. The cardiomyocytes were counterstained with DAPI. TUNEL-positive nuclei were counted under Eclipse Ti fluorescence microscope (Nikon) and expressed as percentage of the total number of cardiomyocyte nuclei (DAPI). Eight microscopic fields (×20 objective) were selected randomly from each sample and analyzed for TUNEL-positive cells.
Statistical analysis.
The data are presented as means ± SE. The intensity of bands from Western blots was scanned with densitometry and digitally analyzed. Statistical significance was tested with Student's t-test or ANOVA with post hoc analysis when appropriate. P < 0.05 was considered statistically significant.
RESULTS
Overexpression of mitochondria-targeted constitutively active Akt in primary cardiomyocytes did not alter cytosolic or nuclear Akt.
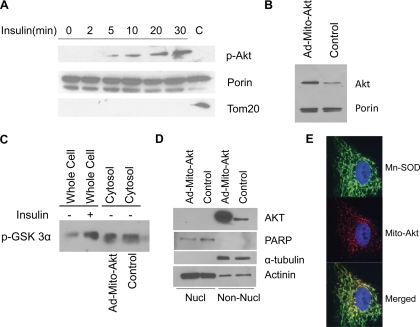
Primary cardiomyocytes were used as a model for this study. In vivo injection of insulin acutely increased Akt phosphorylation and translocation to the mitochondria in myocardium (26). Similar to the in vivo models, insulin increased accumulation of phosphorylated Akt in the mitochondria in primary cardiomyocytes (Fig. 1A). To study the effect of mitochondrial Akt activation, a mitochondria-targeted constitutively active Akt was overexpressed in the primary cardiomyocytes with adenoviral vector (Ad-Mito-Akt; Fig. 1B). To ensure expression of constitutive active Akt did not affect Akt activity in the cytosolic compartment, we analyzed Akt kinase activities, using recombinant GSK3α as substrates, in the cytosolic fractions isolated from the cardiomyocytes transduced with Ad-Mito-Akt or Ad-GFP (control). As shown in Fig. 1C, insulin increased Akt kinase activities in the untransduced cells but overexpressing Mito-Akt in the mitochondria did not increase Akt kinase activity in the cytosolic fraction. In this experiment, the kinase activity in the whole cell lysates could not be directly compared with the activity in cytosolic fraction (crude whole cell lysates vs. subfractionated cytosolic proteins). Since endogenous Akt could be translocated to the cell nucleus, we also analyzed the abundance of Akt proteins in the nuclear fraction (Fig. 1D). Forced expression of mitochondria-targeted active Akt did not increase Akt protein in the nuclear fraction; poly(ADP-ribose) polymerase and tubulin were used as specific markers for the nuclear and the nonnuclear fractions while actinin was present in both fractions. To confirm mitochondria targeting was achieved with Ad-Mito-Akt, the cardiomyocytes expressing Mito-Akt were analyzed with immunofluorescence staining (Fig. 1E), and the results showed that Mito-Akt colocalized with the mitochondria marker Mn-SOD.
Fig. 1.
Overexpression of mitochondria-targeted active Akt did not modulate nuclear cytosolic Akt kinase activity or enhance nuclear translocation of Akt in cardiomyocytes. A: insulin induced mitochondrial Akt signaling in cardiomyocytes. Primary cardiomyocytes were serum deprived overnight and stimulated with insulin (10−7 M) at indicated time intervals. Equal protein amounts of mitochondria lysates were resolved with SDS-PAGE and immunoblotted with specific antibodies. Cardiomyocyte mitochondria preps were treated with proteinase K to remove cytosolic protein contamination; the control lane (C) represents untreated mitochondria preps from unstimulated cells. Tom20 is an outer membrane protein that can be digested by proteinase K. B: Overexpression of a constitutively active Akt in the mitochondria of cardiomyocytes. Cardiomyocytes were infected with Ad-Mito-Akt or Ad-GFP and the mitochondria were isolated and immunoblotted with anti-Akt antibodies. Ad-Mito-Akt was constructed with a mitochondria-targeting sequence and a constitutively active Akt (see research design and methods). Abundance of mitochondria Akt was significantly increased in the cells infected with Ad-Mito-Akt. C: cytosolic Akt kinase activity was not increased in the cells transduced with Ad-Mito-Akt. Cardiomyocytes were transduced with Ad-Mito-Akt or Ad-GFP (Control), and the cytosolic fraction was isolated for kinase assay with recombinant GSK3α. Akt was immunoprecipitated from whole cell lysates or cytosolic fractions and the in vitro assay performed using GSK3α as substrate, the phosphorylation of GSK3α was measured by Western blot. Whole cell lysates served as positive control for the assay. D: nuclear localization of Akt. Nuclear (Nucl) and nonnuclear (Non-Nucl) compartments were subfractionated and immunoblotted with specific antibodies. Control cells were transduced with Ad-GFP. poly(ADP-ribose) polymerase (PARP) is a nuclear protein and tubulin is a cytosolic protein. E: immunofluorescence localization of expressed Mito-Akt in cardiomyocytes. Cardiomyocytes were infected with Ad-Mito-Akt for 48 h. Mito-Akt protein was identified by its HA tag (red), and mitochondria were localized with anti-Mn-SOD antibodies (green). Nucleus was counterstained with DAPI (blue).
Activation of mitochondrial Akt inhibited apoptosis signaling.
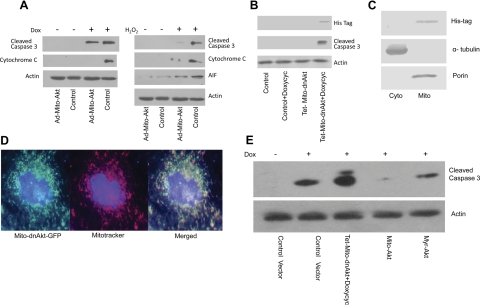
This series of experiments was designed to determine whether activating mitochondrial Akt can modulate mitochondrial apoptosis signaling. Cardiomyocytes were transduced with Ad-Mito-Akt or Ad-GFP (control), and apoptosis was induced by the addition of doxorubicin or H2O2 to culture media. The cells were subfractionated, and the cytosolic fractions were used to analyze activation of caspase 3 and release of cytochrome c. Figure 2A showed that Mito-Akt inhibited activation of caspase 3 and release of cytochrome c in the doxorubicin-treated cells. Similarly, Mito-Akt inhibited activation of caspase 3 and efflux of cytochrome c and AIF to the cytosolic compartment in the cells treated with H2O2. Cytochrome c and AIF release are considered key steps of mitochondrial apoptosis signaling. To further confirm the effect of mitochondrial Akt on apoptosis signaling, we investigated whether inhibition of mitochondrial Akt could activate caspase 3. To this end, the cardiomyocytes were transduced with a mitochondria-targeted dominant negative Akt via a Tet-inducible lentiviral vector (Lenti-Tet-Mito-dnAkt). As shown in Fig. 2B, mitochondria-targeted dominant negative Akt induced activation of caspase 3 in cardiomyocytes. These experiments provided additional evidence that mitochondrial Akt activity could modulate apoptosis signaling in cardiomyocytes. To confirm localization of the dnAkt in mitochondria, cardiomyocytes were subfractionated into cytosolic and mitochondria fractions and immunoblotted with anti-His-tag antibodies (Fig. 2C), the results confirmed the presence of dnAkt in the mitochondria fraction. To further visualize the distribution of Mito-dnAkt in cardiomyocytes, we infected cardiomyocytes with Mito-dnAkt and GFP. The results are presented in Fig. 2D, the majority of Mito-dnAkt colocalized with mitochondria in cardiomyocytes. Since previous studies have shown that cytosolic Akt signaling could inhibit cardiac apoptosis, we next compared the effects of membrane-targeted Akt signaling with mitochondria-targeted Akt signaling. To this end, a plasma-membrane-targeted constitutively active Akt (myristoylated-Akt, Myr-Akt) was overexpressed with adenoviral vector. Cardiomyocytes were infected with the same viral concentrations of Ad-Mito-Akt or Ad-Myr-Akt, and apoptosis was induced by doxorubicin treatment (Fig. 2E). Both Mito-Akt and Myr-Akt inhibited activation of caspase 3, but the effect of Mito-Akt was better than Myr-Akt. This experiment suggested that cytosolic Akt signaling and mitochondrial Akt signaling independently suppressed activation of caspase 3.
Fig. 2.
Activation of mitochondrial Akt modulated mitochondria apoptosis signaling. A: inhibition of cytochrome c/apoptosis-inducing factor (AIF) efflux and caspase 3 cleavage. Primary cardiomyocytes transduced with Ad-Mito-Akt or Ad-GFP (Control) were incubated with doxorubicin (Dox; 1 μM) or H2O2 (100 μM) to induce apoptosis. Cytosolic fractions were isolated from the cells and equal amounts of cytosolic proteins were assayed with Western blots. Abundance of cleaved caspase 3, cytochrome c, and AIF was significantly lowered in the cardiomyocytes transduced with Ad-Mito-Akt. B: inhibition of mitochondrial Akt suppressed caspase 3 activation in cardiomyocytes. A mitochondria-targeting dominant negative Akt was expressed via inducible lentiviral vector (Lenti-Tet-Mito-dnAkt). Expression of Mito-dnAkt was induced by doxycycline (Doxycyc) treatment for 48 h and verified by the presence of His tag in mitochondria prep. Activation of caspase 3 was analyzed by Western blot using equal amounts of cytosolic proteins. Expression of Mito-dnAkt amplified activation of caspase 3. C: expression of mitochondria-targeted dominant negative Akt in mitochondrial fraction. Cardiomyocytes were infected with Lenti-tet-Mito-dnAkt for 48 h and then incubated with doxycycline for 48 h to induce Mito-dnAkt expression, and the cells were fractionated into the cytosolic (Cyto) and mitochondria (Mito) fractions as described in RESEARCH design and methods, and the proteins were resolved with SDS-PAGE for immunoblotting. Cardiomyocytes infected with Lenti-tet-Mito-dnAkt showed increased expression of Mito-dnAkt (identified with anti-His-tag antibodies) in the mitochondria fraction. D: subcellular localization of Mito-dnAkt in cardiomyocytes. Mito-dnAkt was tagged with GFP and overexpressed in the cardiomyocytes and analyzed with fluorescence microscope. Mitochondria were labeled with MitoTracker staining (red). E: independent effects of mitochondria-targeted Akt and plasma-membrane targeted Akt on caspase 3. Cardiomyocytes were transduced with equal amounts of Ad-Mito-Akt, Ad-myr-Akt, or control vector. Constitutively active myristoylated-Akt (Ad-myr-Akt) targets the plasma membrane. For comparison, the effect of Mito-dnAkt was also included in this experiment. Cardiomyocytes were incubated with Dox to induce apoptosis when indicated. Mitochondria-targeted Akt and the plasma-membrane-targeted Akt independently suppressed activation of caspase 3.
Activation of mitochondrial Akt modulated mitochondria electrochemical gradient and cell death.
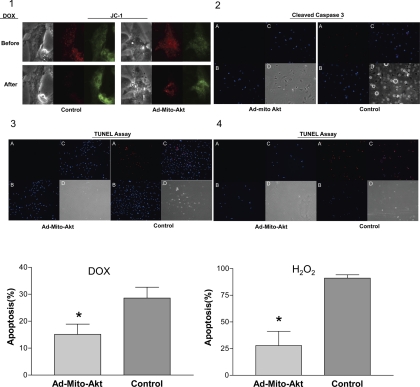
Collapse of mitochondria cross-membrance electrochemical gradient (ΔΨm) lead to release of apoptosis-triggering molecules, such as cytochrome c and AIF, from mitochondria to the cytosolic compartment to execute apoptosis. To determine whether cross-membrane electrochemical gradient was modulated by activation of mitochondrial Akt, we used JC-1 to characterize the gradient (Fig. 3). In the control cardiomyocytes transduced with control adenovirus, the gradient was lost after doxorubicin treatment (Fig. 3–1), whereas overexpressing mitochondria-targeted active Akt suppressed the loss of gradient (attenuated loss of red fluorescence). We also analyzed the presence of activated caspase 3 in cardiomyocytes in vivo. Figure 3–2 shows that activated caspase 3 was detected in the cardiomyocytes after doxorubicin treatment and overexpression of Mito-Akt inhibited activation of caspase 3 in vivo. To detect cell death, we analyzed these cells with TUNEL assay; overexpression of Mito-Akt significantly inhibited the occurrence of cell death in cardiomyocytes (Fig. 3–3 and 3–4). Overerxpression of Mito-Akt reduced the number of apoptotic cardiomyocytes (doxorubicin treatment: 16 vs. 27%, P < 0.01; H2O2 treatment: 23 vs. 92%, P < 0.001). Together with the results on apoptosis signaling, these data indicated that activation of mitochondrial Akt increased cardiomyocytes survival by suppressing activation of mitochondrial apoptosis signaling.
Fig. 3.
Effects of mitochondrial Akt activation on mitochondria cross-membrane electrochemical gradient and apoptosis in vivo. Cardiomyocytes were transduced with Ad-Mito-Akt or control adenovirus and apoptosis was induced by Dox. 3–1: Analysis of electrochemical gradient with JC-1 staining. Dox (1 uM) was added to the medium when indicated to induce apoptosis. Cardiomyocytes were washed twice with PBS buffer and incubated with a buffer containing JC-1 (10 uM) for 20 min at 37°C under 5% CO2. Cardiomyocytes were immediately analyzed under fluorescence microscope. Loss of red emission (maximum 590 nm) indicated loss of electrochemical gradient. JC-1 staining shifted to green emission (527 nm) when electrochemical gradient collapsed. Control cells were transduced with an empty adenoviral vector. 3–2: Immunostaining of cleaved caspase 3 in cardiomyocytes. Cardiomyocytes were incubated with Dox (1 uM) when indicated to induce apoptosis, fixed, stained, and analyzed with fluorescence microscopy. Control cells were transduced with an empty adenoviral vector. A: cleaved caspase 3 (red). B: DAPI counterstain (blue). C: cleaved caspase 3 and DAPI merged. D: bright field. 3–3: Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assay to determine Dox-induced apoptosis. A: TUNEL staining (red). B: DAPI counterstain (blue). C: TUNEL and DAPI merged. D: bright field. Cells were incubated with Dox to induce apoptosis. Control cells were transduced with an empty adenoviral vector. 3–4: TUNEL assay to determine H2O2-induced apoptosis. A: TUNEL staining. B: DAPI counterstain. C: TUNEL and DAPI merged. D: bright field. Cardiomyocytes were incubated with H2O2 to induce apoptosis. Bar graphs represent the data summarized from 3- to 4 independent experiments of TUNEL assay; these data were normalized to the number of cells plated in each well. Results are presented as percentage of TUNEL-positive cells in each well. Statistical analysis was determined with one-way ANOVA. *P < 0.05 vs. control.
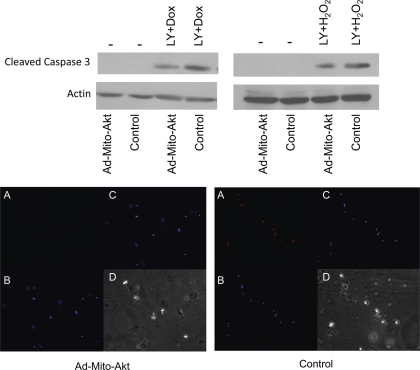
To determine whether the effect of mitochondrial Akt was independent of the actions of cytosolic Akt, we investigated the effect of Mito-Akt on caspase 3 when endogenous Akt had been inactivated. To this end, LY294002 was added to the media to inhibit endogenous Akt (Fig. 4, top). The results showed that while endogenous Akt was inhibited by LY294002, Mito-Akt could still suppress activation of caspase 3 in cardiomyocytes. These results render evidence that mitochondrial Akt can inhibit caspase 3 signaling independent of cytosolic Akt. Further experiments with TUNEL assay indicated that the antiapoptotic effect of Mito-Akt when endogenous Akt signaling was inhibited by LY294002, as the number of TUNEL-positive cardiomyocytes was significantly reduced in the cells transduced with Ad-Mito-Akt (Fig. 4, bottom). We thus concluded that activation of mitochondria Akt could inhibit cardiomyocytes apoptosis independent of cytosolic Akt.
Fig. 4.
Effects of Mito-Akt on caspase 3 were not affected by inhibition of endogenous Akt. Cardiomyocytes were transduced with Ad-Mito-Akt or Ad-GFP (control) and endogenous Akt activity was inhibited by the addition of LY294002 (20 uM) to the culture medium. Apoptosis was induced with Dox or H2O2. Caspase 3 activity was analyzed with cleaved caspase 3 immunoblots (top). Actin immunoblots served as loading controls. TUNEL assay was analyzed to assess the occurrence of apoptosis (bottom). Cardiomyocytes were infected with Ad-Mito-Akt or control viral vector for 3 days, and apoptosis was induced by incubation with Dox overnight. Cells were fixed, immunostained, and analyzed. A: TUNEL staining (red). B: DAPI counterstain. C: TUNEL and DAPI merged. D: bright field.
DISCUSSION
Our study showed that activation of mitochondrial Akt signaling directly inhibited apoptosis of cardiomyocytes. Apoptosis in cardiac muscle could be induced by diabetes (7), ischemia (13), reperfusion injury (3), viral infection (28), and cardiotoxins (22) and contributed to the development of cardiomyopathy. Inhibiting apoptosis signaling in cardiac muscle minimized myocardial injuries and improved myocardial function in experimental models of cardiomyopathy (6, 27). The central role of mitochondria in apoptosis is well established in invertebrate and vertebral animal cells. Mitochondria-mediated apoptosis has been observed across key model systems, from nematodes, flies, to mammalian cells. It is believed that various death signaling (intrinsic and extrinsic) converges on mitochondria and triggers cytochrome c release (18). The opening of mitochondrial permeability transition pores leads to matrix swelling, depolarization of the membrane potential (cross-membrane electrochemical gradient), and release of cytochrome c and other intermembrane space proteins to activate caspase cascades (5,18). Cytochrome c shuttles electrons between oxidative phosphorylation complexes and plays a role in respiration. However, its role of amplifying apoptosis connects mitochondria to caspase cascades.
Release of cytochrome c and activation of caspase 3 have been reported in human cardiomyopathy and are considered key regulators of cell death in cardiomyopathy (14). Activation of PI3-kinase and Akt pathway could protect cardiac muscle against cell injuries and preserve mitochondria function. Our laboratory (24) has shown that a plasma membrane-targeted constitutive active PI3-kinase inhibited apoptosis signaling induced by doxorubicin in cardiomyocytes (24). Ischemia- and reperfusion-induced cell death was also decreased by activation of PI3-kinase (5). Transducing myocardial with constitutive active Akt could minimize infarct size and improve myocardial function in animal models of myocardial ischemia (4). Infecting cardiomyocytes with constitutively active Akt inhibited induction of apoptosis whereas dominant negative Akt promoted apoptosis (20). However, whether Akt directly modulated mitochondria-mediated apoptosis in cardiomyocytes was not entirely clear. In this study, our data indicate that activation of Akt in mitochondria suppressed apoptosis in cardiomyotes by stabilizing mitochondria cross-membrane electrochemical gradient, inhibiting cytochrome c efflux, and suppressing caspase 3 activation.
There is evidence suggests the ability of Akt to inhibit apoptosis requires glucose metabolism (10, 17). Majewski et al. (11) has shown that dissociation of hexokinase from mitochondria inhibited the antiapoptotic effect of a plasma membrane-targeted constitutive active Akt in fibroblasts, but the direct effect of Akt activation in mitochondria could not be assessed in such experimental system because the membrane-targeted constitutively active Akt used could not be translocated into mitochondria. We have shown that activation of Akt in mitochondria promoted respiration when oxidative phosphorylation was assayed using pyruvate as substrates (26). Whether there is a relationship between the effects of mitochondrial Akt on glycolytic respiration and on apoptosis signaling is not yet clear and will require further investigation.
Akt/PKB, a serine/threonine kinase, is a critical signaling node within mammalian cells and plays significant role in the signaling regulation of human physiology and diseases (12). Most previous studies focused on signaling downstream of Akt in the cytosolic compartment and, to a lesser extent, in the nucleus. Interestingly, we have shown that insulin-stimulated Akt translocation to mitochondria was impaired in animals models of diabetic cardiomyopathy, accompanied by reduced oxidative phosphorylation (26). Together with the antiapoptotic effect of mitochondrial Akt, these data suggest that mitochondrial Akt signaling may play important pathophysiological roles in normal and diabetic myocardium. Recognition of mitochondrial Akt signaling underlies a complex Akt signaling network span through cytosol, nucleus, and mitochondria. Future investigations that identify direct phosphorylation targets of the kinase in mitochondria and its cellular functions will help define the role of mitochondrial Akt signaling in the Akt signaling network.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.-C.S., J.-Y.Y., H.-B.L., and Y.C. performed experiments; C.-C.S., H.-B.L., Y.C., and P.H.W. analyzed data; C.-C.S., J.-Y.Y., and P.H.W. interpreted results of experiments; C.-C.S. and H.-B.L. prepared figures; C.-C.S. and P.H.W. drafted manuscript; C.-C.S. and P.H.W. edited and revised manuscript; C.-C.S., J.-Y.Y., H.-B.L., Y.C., and P.H.W. approved final version of manuscript; P.H.W. conceptualized and designed research.
ACKNOWLEDGMENTS
This work is supported in part by National Heart, Lung, and Blood Institute Grant R01-HL-096987 (to P.-H. Wang).
REFERENCES
- 1. Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res 90: 234–242, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551, 1996 [PMC free article] [PubMed] [Google Scholar]
- 3. Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, Lefer AM. Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci USA 92: 8031–8035, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101: 660–667, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res 77: 334–343, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW, II, Armstrong RC, Kitsis RN. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation 108: 3036–3041, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes 50: 1414–1424, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Lai H, Liu J, Ting C, Sharma P, Wang PH. Insulin-like growth factor-1 prevents loss of electrochemical gradient in cardiac muscle mitochondria via activation of PI 3 kinase/Akt pathway. Mol Cell Endocrinol 205: 99–106, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Lee WL, Chen JW, Ting CT, Ishiwata T, Lin SJ, Korc M, Wang PH. Insulin-like growth factor I improves cardiovascular function and suppresses apoptosis of cardiomyocytes in dilated cardiomyopathy. Endocrinology 140: 4831–4840, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Majewski N, Nogueira V, Robey RB, Hay N. Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Mol Cell Biol 24: 730–740, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell 16: 819–830, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation 100: 2373–2379, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A, Dec GW, Israels S, Ballester M, Virmani R, Saxena S, Kharbanda S. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA 96: 8144–8149, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 96: 2190–2196, 1997 [DOI] [PubMed] [Google Scholar]
- 16. del Peso L, González-García M, Page C, Herrera R, Nuñez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278: 687–689, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Plas DR, Talapatra S, Edinger A, Rathmell J, Thompson C. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem 276: 12041–12048, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11: 621–632, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7: 85–96, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Uchiyama T, Engelman RM, Maulik N, Das DK. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation 109: 3042–3049, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang L, Ma W, Markovich R, Chen J, Wang PH. Regulation of cardiomyocyte apoptotic signaling by insulin-like growth factor I. Circ Res 83: 516–522, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72: 19–44, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Wu W, Lee W, Wu Y, Chen D, Liu T, Sharma PM, Wang PH. Expression of constitutively active phosphatidylinositol 3 kinase inhibits activation of caspase 3 and apoptosis of cardiac muscle cells. J Biol Chem 275: 40113–40119, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275: 1129–1132, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Yang J, Yeh H, Lin K, Wang PH. Insulin stimulates Akt translocation to mitochondria: implications on dysregulation of mitochondrial oxidative phosphorylation in diabetic myocardium. J Mol Cell Cardiol 46: 919–926, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yaoita H, Ogawa K, Maehara K, Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation 97: 276–281, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Zhang HM, Yanagawa B, Cheung P, Luo H, Yuan J, Chau D, Wang A, Bohunek L, Wilson JE, McManus BM, Yang D. Nip21 gene expression reduces coxsackievirus B3 replication by promoting apoptotic cell death via a mitochondria-dependent pathway. Circ Res 90: 1251–1258, 2002 [DOI] [PubMed] [Google Scholar]