Abstract
Nitric oxide (NO) mediates a major portion of arteriolar endothelium-dependent dilation in adults, but indirect evidence has suggested that NO contributes minimally to these responses in the young. Isolated segments of arterioles were studied in vitro to verify this age-related increase in NO release and investigate the mechanism by which it occurs. Directly measured NO release induced by ACh or the Ca2+ ionophore A-23187 was five- to sixfold higher in gracilis muscle arterioles from 42- to 46-day-old (juvenile) rats than in those from 25- to 28-day-old (weanling) rats. There were no differences between groups in arteriolar endothelial NO synthase (eNOS) expression or tetrahydrobiopterin levels, and arteriolar l-arginine levels were lower in juvenile vessels than in weanling vessels (104 ± 6 vs.126 ± 3 pmol/mg). In contrast, agonist-induced eNOS Thr495 dephosphorylation and eNOS Ser1177 phosphorylation (events required for maximal activity) were up to 30% and 65% greater, respectively, in juvenile vessels. Juvenile vessels did not show increased expression of enzymes that mediate these events [protein phosphatases 1 and 2A and PKA and PKB (Akt)] or heat shock protein 90, which facilitates Ser1177 phosphorylation. However, agonist-induced colocalization of heat shock protein 90 with eNOS was 34–66% greater in juvenile vessels than in weanling vessels, and abolition of this difference with geldanamycin also abolished the difference in Ser1177 phosphorylation between groups. These findings suggest that growth-related increases in arteriolar NO bioavailability may be due at least partially to changes in the regulation of eNOS phosphorylation and increased signaling activity, with no change in the abundance of eNOS signaling proteins.
Keywords: postnatal growth, microcirculation, nitric oxide, endothelial nitric oxide synthase, heat shock protein 90, phosphorylation
in recent years, there has been a dramatic increase in the incidence of childhood obesity, dyslipidemia, insulin resistance, and type 2 diabetes (1, 32, 34). Among the vascular deficits that develop in children with these conditions is endothelial dysfunction, which can contribute to the onset of overt cardiovascular disease later in adulthood (17, 19, 25, 33). To identify the mechanisms that underlie the progression of this dysfunction during juvenile growth, it is first necessary to understand the normal growth-related changes in endothelial function on which the pathological changes are superimposed. Based on the use of pharmacological inhibitors, studies from our laboratory and others (2, 30, 31, 35, 36, 38) suggest that nitric oxide (NO) plays little or no role in the endothelium-dependent dilation of arterioles in the young, whereas it mediates a large portion of these responses in juveniles and adults. There is no evidence of growth-related changes in oxidant production/antioxidant defense that might influence the rate of NO breakdown in these vessels (30), and the responsiveness of arteriolar smooth muscle to NO also does not change with growth (22, 30, 35). Collectively, these observations suggest that juvenile growth is most likely accompanied by an increase in endothelial NO production. The mechanisms responsible for this transition of the endothelium to a NO-releasing phenotype are unknown.
The activity of endothelial NO synthase (eNOS) is precisely regulated through changes in the phosphorylation status of key threonine and serine residues. In quiescent endothelial cells, eNOS activity is inhibited by its interaction with membrane-associated caveolin-1 (24). Under these conditions, eNOS is strongly phosphorylated at Thr495 in the Ca2+/calmodulin-binding domain (14, 21). In response to agonist or shear stress stimulation, dephosphorylation of Thr495 by protein phosphatases (PP)1 and PP2A allows for Ca2+/calmodulin binding to eNOS and disruption of the enzyme's interaction with caveolin-1, leading to eNOS activation (13, 24, 27). The activity of eNOS is then further increased by phosphorylation of specific serine residues, such as Ser1177 (27). PKA and PKB (Akt) are important kinases for phosphorylation of this and other serine residues (6, 13, 26). The binding of heat shock protein (Hsp)90 to eNOS greatly enhances Akt-mediated phosphorylation of Ser1177 (7, 27). In this context, Hsp90 acts as a scaffolding protein to bring eNOS and Akt into close proximity (15, 21).
The aims of the present study were twofold: 1) to directly verify that juvenile growth is accompanied by an increase in endothelial NO production at the arteriolar level and 2) to define the cellular/molecular events that are responsible for this increase, focusing on possible changes in the regulation of eNOS phosphorylation. We tested the hypothesis that signaling pathways for increasing the activity of eNOS become more effective during growth due to an increased capacity for dephosphorylation of eNOS at Thr495 and/or phosphorylation of eNOS at Ser1177.
MATERIALS AND METHODS
Animals.
All surgical and experimental procedures were approved by the Animal Care and Use Committee of West Virginia University. Experiments were performed on arterioles isolated from the gracilis muscle of male Sprague-Dawley rats (Harlan, Indianapolis, IN) aged 25–28 days (“weanling”) or 45–48 days (“juvenile”). Animals were housed at 23°C with a 12:12-h light-dark cycle and provided water and rat chow ad libitum. For the excision of arterioles, rats were anesthetized by an intraperitoneal injection of pentobarbital sodium (50 mg/kg body wt) with supplemental anesthetic (10% of the original dose) administered if needed.
Measurement of arteriolar NO production.
NO production from isolated arterioles was measured as previously described (28). Briefly, the left and right gracilis muscles were removed and placed in a dissecting dish containing Ca2+-free Dulbecco's PBS chilled to 4°C. Samples were typically pooled from five to six animals to achieve sufficient vessel mass. Microvessels were excised, lightly homogenized, and then briefly centrifuged to form a loose pellet. The pellet was then placed in a four-port biosensing chamber containing 2 ml Dulbecco's PBS with Ca2+ (3.6 mM), l-arginine (l-Arg; 0.2 mM), and tetrahydrobiopterin (BH4; 4 μM). In previous studies, we found that 0.2 mM l-Arg is the minimum bath concentration that will support eNOS activity and NO production under these conditions. Two chamber ports were fitted with ISO-NOP NO sensors (World Precision Instruments, Sarasota, FL), the third port was fitted with a temperature probe, and the last port was reserved for the addition of pharmacological agents. Sensors were connected to an Apollo 4000 Free Radical Analyzer (World Precision Instruments) to make direct electrochemical NO measurements in real time (11). Before each use, sensors were calibrated via S-nitroso-N-acetyl-d,l-penicillamine decomposition to NO in a copper catalyst solution [cuprous chloride (0.1 M)]. Data were collected at a rate of 10 samples/s, and measurements were made only during steady-state responses that were at least 30 s in duration. The mass of tissue from which NO was measured averaged 11.2 ± 1.2 mg for weanling arterioles and 13.7 ± 1.3 mg for juvenile arterioles. All NO measurements were normalized to tissue mass (nM NO/mg tissue).
Measurement of arteriolar l-Arg and BH4 levels.
Concentrations of l-Arg, the physiological substrate for NO synthesis, and BH4, a key eNOS cofactor, were measured in the arteriolar wall using the HPLC method as previously described (29). For each sample, arterioles from both gracilis muscles of four weanling or two juvenile rats (i.e., 8 or 4 muscles, respectively) were pooled to obtain enough tissue for analysis. For l-Arg analysis, vessels (10–15 μg) were homogenized with 0.2 ml of 1.5 M HClO4, with acidic or basic iodine. Homogenates were centrifuged at 10,000 g for 1 min, and an aliquot (0.2 ml) of the supernatant was used for the sample determination of l-Arg content (37). For BH4 analysis, arterioles (10–15 μg) were homogenized in 0.1 ml of 0.1 M phosphoric acid containing 5 mM DTT (an antioxidant), to which 17.5 μl of 2 M trichloroacetic acid were added. Samples were incubated in the dark for 1 h. Excess iodine was removed by the addition of ascorbic acid (final concentration: 0.1 M). The final solution was then analyzed on a C18 reversed-phase column using fluorescence detection and authentic biopterin as a standard. Values for BH4 and l-Arg concentrations were normalized to tissue weight.
Stimulation of vessels for phospho-eNOS or eNOS-Hsp90 analysis.
Arterioles from both gracilis muscles of each animal were excised and pooled to obtain sufficient protein. Vessels were immediately placed in PSS containing vehicle, 10 μM ACh, or 100 pM VEGF and incubated for 3 min at 37°C. For experiments examining Hsp90 and Akt inhibition, vessels were treated with geldanamycin (1 μM) or Akt inhibitor IV (1 μM), respectively, before stimulation with ACh (10 μM). After the incubation, PSS was removed, and vessels were immediately snap frozen until later use.
Immunoblot analysis.
Immunoblot analysis was performed as previously described (20). Briefly, after excision from the muscle, arterioles were dissected, immediately snap frozen, and stored at −80°C until use. To obtain sufficient protein, vessels from multiple animals were pooled. Vessels were lysed in 1× sample buffer [62.5 mM Tris (pH 6.8), 2% SDS, 6 M urea, 160 mM DTT, and 0.1% bromophenol blue], and protein concentrations were determined using the NanoOrange Protein Quantification Kit (Invitrogen, Carlsbad, CA). Samples (10 μg total protein) were subjected to SDS-PAGe and then transferred to polyvinylidene difluoride membranes. Membranes were blocked for 1 h at room temperature (5% nonfat dry milk in Tris-buffered saline + 0.1% Tween 20) and then incubated overnight at 4°C with antibodies against eNOS (1:1,000), PP1 (1:500), PP2A (1:10,000), PKA (1:2,000), Akt (1:2,000), or Hsp90 (1:2,000). Antibodies against PP1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against Akt were from Cell Signaling Technology (Danvers, MA). All other antibodies were from BD Transduction Laboratories (Franklin Lakes, NJ). After being washed, membranes were incubated with the respective horseradish peroxide-conjugated secondary antibody (Cell Signaling) for 1 h at room temperature. Peroxidase activity was determined using ECL Advance (GE Healthcare, Waukesha, WI), and images were analyzed using ImageJ software (National Institutes of Health). Loading differences were normalized by expressing all data as relative densitometric units of the protein of interest versus β-actin (1:2,500, Cell Signaling). For the examination of phospho-Ser1177 and phospho-Thr495 eNOS (1:500, BD Transduction Laboratories), vessels were stimulated as described above, and 20 μg total protein was subjected to gel electrophoresis. When possible, blots were cut into strips and probed, or stripped and reprobed, to minimize the number of samples required for analysis.
Coimmunoprecipitation of eNOS and Hsp90.
Isolated arterioles were incubated with vehicle, 10−5 M ACh, or 10−10 M VEGF, as described above. To obtain sufficient protein for each sample, vessels were pooled from four weanling or two juvenile animals. Samples were homogenized on ice in lysis buffer [20 mM Tris·HCl (pH 7.4), 2.5 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 100 mM NaCl, and 10 μg/ml protease inhibitors (3)]. Protein concentrations were determined using the Pierce BCA protein assay. eNOS antibody (2 μg) was preincubated with Dynabeads Protein G (Invitrogen) for 2 h at room temperature and then incubated with 500 μg vessel lysate overnight at 4°C. Samples were immunoprecipitated according to the manufacturer's instructions and immunoblotted using antibodies for eNOS and Hsp90, as described above.
Data and statistical analysis.
All data are presented as means ± SE. Differences between groups were analyzed by an unpaired Student's t-test or ANOVA with Neuman-Keuls post hoc analysis. P values of <0.05 were considered to be statistically significant.
RESULTS
The inclusive range of ages examined in this study (from 25 to 46 days) represents a period of rapid growth in the rat, with body weight increasing nearly threefold during this time (61 ± 3 g for weanling rats vs. 178 ± 7 g for juvenile rats, P < 0.05). Although individual vessel diameters were not measured in this study, our previous studies (30, 31) on rats of these ages indicated that both the active and passive diameters of gracilis muscle arterioles are 40–50% greater in juvenile rats than in weanling rats.
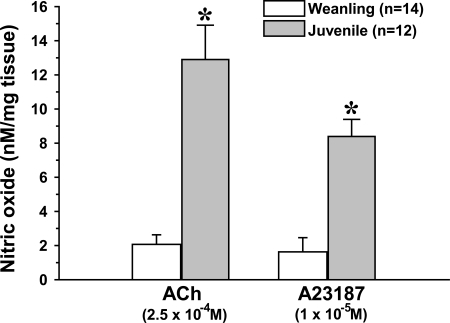
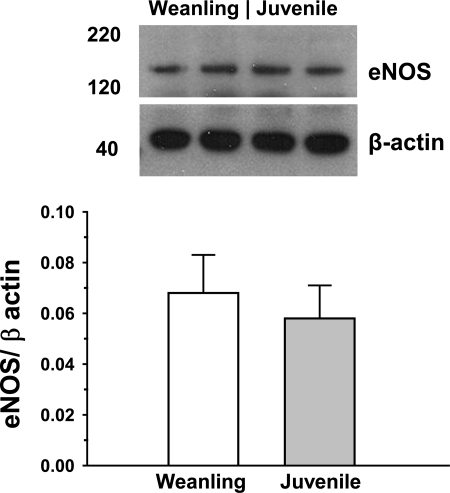
Acute exposure to ACh (2.5 × 10−4 M) stimulated NO production in isolated arterioles from both weanling and juvenile rats. In the presence of ACh, the steady-state NO concentration in the immediate vicinity of the arterioles averaged 19.2 ± 5.5 nM for weanling vessels versus 185.5 ± 37.5 nM for juvenile vessels. When normalized to tissue mass, the amount of NO released from juvenile arterioles (12.9 ± 2.0 nM/mg tissue) was more than sixfold greater than that released from weanling arterioles (2.1 ± 0.5 nM/mg tissue; Fig. 1). To determine if this difference could reflect an age-related change in postreceptor signaling, we also assessed arteriolar NO release in response to stimulation with a receptor-independent agonist, the Ca2+ ionophore A-23187 (1 × 10−5 M). As with ACh, A-23187 triggered much greater NO release from juvenile arterioles than from weanling arterioles, with steady-state NO concentrations averaging 112.3 ± 14.8 nM for juvenile vessels and 12.4 ± 3.8 nM for weanling vessels. Normalized values were 8.4 ± 1.0 nM/mg tissue for juvenile vessels versus 1.6 ± 0.8 nM/mg tissue for weanling vessels. In contrast to this profound difference in NO release, we found no differences in eNOS protein expression between weanling and juvenile arterioles (Fig. 2).
Fig. 1.
Nitric oxide (NO) production from isolated arterioles in response to ACh or the Ca2+ ionophore A-23187. n, Number of samples. *P < 0.05 vs. the weanling value.
Fig. 2.
Protein levels of endothelial NO synthase (eNOS) in gracilis muscle arterioles. Values are arbitrary densitometric units normalized to β-actin. n = 5 samples/group.
As shown in Table 1, there were no significant differences between age groups in arteriolar levels of BH4, a cofactor required by eNOS for the conversion of l-Arg to NO, or in total biopterin levels. In contrast, arteriolar l-Arg levels decreased by almost 20% from the weanling to juvenile stages.
Table 1.
Arteriolar wall tetrahydrobiopterin and l-arginine
| Weanling | Juvenile | |
|---|---|---|
| Total biopterin, fmol/mg | 169 ± 49 | 137 ± 42 |
| Tetrahydrobiopterin, fmol/mg | 146 ± 50 | 128 ± 41 |
| l-Arginine, pmol/mg | 126 ± 3 | 104 ± 6* |
Values are means ± SE.
P < 0.05 vs. the weanling value.
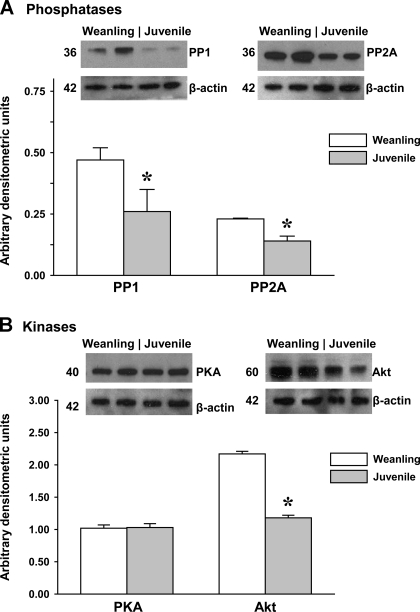
Levels of arteriolar protein expression for the key enzymes that regulate eNOS phosphorylation are shown in Fig. 3. Protein levels for both major phosphatases (PP1 and PP2A) as well as the kinase Akt significantly decreased with growth. In contrast, there were no differences in PKA protein levels between groups.
Fig. 3.
Protein levels of key phosphatases (A) and kinases (B) for the regulation of eNOS phosphorylation in gracilis muscle arterioles. Representative blots are shown above. Values are arbitrary densitometric units normalized to β-actin. PP, protein phosphatase. n = 5–6 samples/group. *P < 0.05 vs. weanling values.
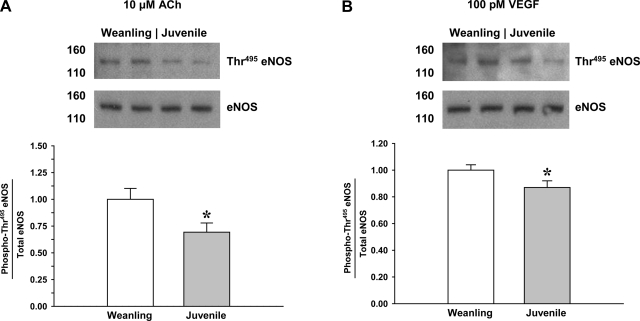
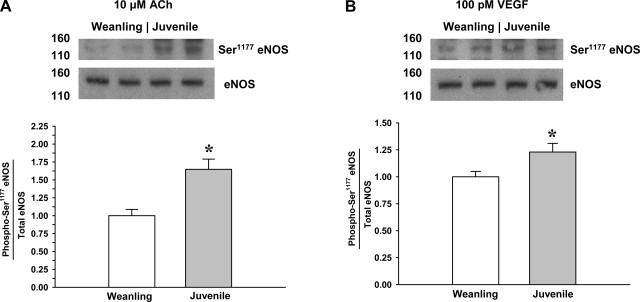
The level of eNOS Thr495 phosphorylation during exposure to ACh or VEGF is shown in Fig. 4. Compared with weanling arterioles, Thr495 phosphorylation in juvenile arterioles was 30% lower for ACh stimulation and 13% lower for VEGF stimulation. Conversely, as shown in Fig. 5, the level of eNOS Ser1177 phosphorylation after ACh or VEGF was higher in juvenile arterioles than in weanling arterioles (65% higher for ACh and 23% higher for VEGF).
Fig. 4.
Phosphorylation of eNOS at Thr495 in arterioles from weanling and juvenile rats after exposure to 10−5 M ACh (n = 6 samples/group; A) or 10−10 M VEGF (n = 5 samples/group; B). Representative blots show differences between age groups in the relative abundance of phospho-eNOS after agonist exposure. Data are expressed as the ratio of phospho-eNOS to total eNOS. *P < 0.05 vs. weanling values.
Fig. 5.
Phosphorylation of eNOS at Ser1177 in arterioles from weanling and juvenile rats after exposure to 10−5 M ACh (n = 9 samples/group; A) or 10−10 M VEGF (n = 6 samples/group; B). Representative blots show differences between age groups in the relative abundance of phospho-eNOS after agonist exposure. Data are expressed as the ratio of phospho-eNOS to total eNOS. Control blots for eNOS are identical to those shown in Fig. 4, as the same samples were probed for both Ser1177 and Thr495. *P < 0.05 vs. weanling values.
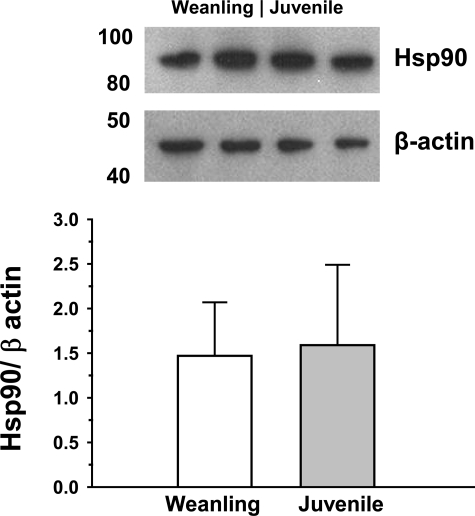
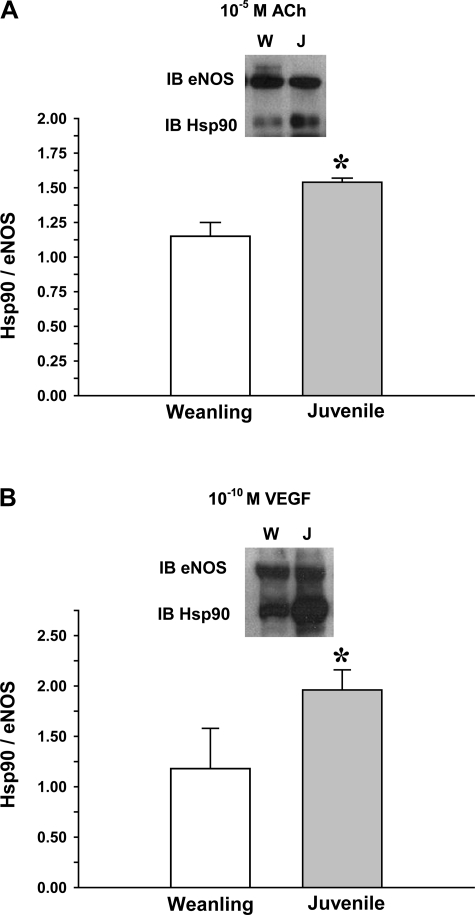
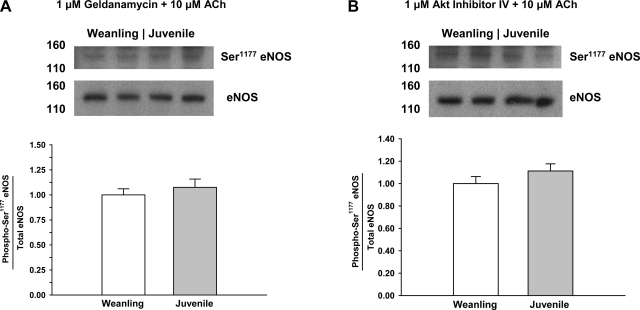
As shown in Fig. 6, there were no significant differences between weanling and juvenile arterioles in protein levels of the chaperone protein Hsp90. Exposure to ACh or VEGF increased the colocalization of Hsp90 with eNOS in both weanling and juvenile arterioles. However, despite similar levels of eNOS and Hsp90 protein in both groups (Figs. 2 and 6), the extent of eNOS-Hsp90 colocalization was greater in juvenile arterioles (34% greater for ACh and 66% greater for VEGF; Fig. 7). However, after inhibition of Hsp90-eNOS binding or Akt inhibiiton, there were no significant differences between age groups in eNOS Ser1177 phosphorylation (Fig. 8).
Fig. 6.
Protein levels of heat shock protein (Hsp)90 in gracilis muscle arterioles. Values are arbitrary densitometric units normalized to β-actin. n = 5 samples/group.
Fig. 7.
Association of Hsp90 with eNOS in arterioles from weanling (W) and juvenile (J) rats after exposure to 10−5 M ACh (n = 3 samples/group; A) or 10−10 M VEGF (n = 3 samples/group; B). Samples were immunoprecipitated for eNOS and then immunoblotted (IB) for Hsp90 and eNOS. *P < 0.05 vs. weanling values.
Fig. 8.
Phosphorylation of eNOS at Ser1177 in arterioles from weanling and juvenile rats after exposure to 10−5 M ACh in the presence of geldanamycin (n = 6 samples/group; A) or Akt inhibitor IV (n = 6 samples/group; B). Data are expressed as the ratio of phospho-eNOS to total eNOS.
DISCUSSION
Endothelium-derived NO is well established as a principal mediator of vasodilation and blood flow regulation in many adult vascular beds (4, 5, 12, 18, 20). However, results from studies in which l-Arg analogs have been used as NOS inhibitors indirectly suggest that NO plays a minimal to no role in mediating endothelium-dependent dilation in the microcirculation of young animals (2, 30, 31, 35, 36, 38). This may also be the case in humans, as although there are currently no data in microvessels, NG-monomethyl-l-arginine has no effect on endothelium-dependent relaxation to ACh in infant vertebral arteries but greatly attenuates these responses in adult vertebral arteries (8). The purposes of the present study were to verify this apparent growth-related increase in NO production through direct measurement of NO and to define the mechanism(s) through which this occurs. Specifically, we sought to determine whether signaling pathways for eNOS activation at the arteriolar level are altered during growth due to changes in the regulation of eNOS phosphorylation.
Based on the use of Nω-nitro-l-arginine methyl ester, we previously found no role for NO in endothelium-dependent dilation to ACh, VEGF, simvastatin, or A-23187 in gracilis muscle arterioles from weanling rats, whereas these responses appeared to be largely mediated by NO in juvenile rats (30, 31). Those conclusions are supported in the present study by our direct measurements indicating that arteriolar NO production in response to both receptor-dependent and -independent stimuli is minimal in weanling rats and increases dramatically with juvenile growth (Fig. 1). This growth-related increase in NO production occurs despite no increases in protein levels of either eNOS (Fig. 2) or several key proteins involved in the eNOS activation pathway (Figs. 3 and 6). Previous findings in rat pulmonary resistance vessels have also suggested a growth-related increase in NO production without an increase in eNOS protein expression, although those studies were conducted on younger animals (age: 7–14 days) than in the present study (1, 9). We also found no increase in arteriolar l-Arg or BH4 levels during growth (Table 1), suggesting that changes in eNOS cofactor and/or substrate availability do not contribute to increased NO production in the juvenile microcirculation. Instead, the results of this study suggest that changes in eNOS phosphorylation may contribute to the increase in NO production and that this transition likely involves a growth-related adaptation in the interaction between Hsp90 and eNOS.
eNOS activity is tightly regulated through the activation and deactivation of a series of phosphatases and kinases. In the inactive state, eNOS is phosphorylated at Thr495 (14). In response to endothelium-dependent stimuli, dephosphorylation of this residue by PP1 or PP2A renders eNOS activated and available for subsequent phosphorylation at Ser1177 or Ser633 by PKA and Akt (14, 27). In the present study, we found that stimulation with either ACh or VEGF induced greater dephosphorylation of eNOS at Thr495 in juvenile arterioles than in weanling arterioles (Fig. 4). As shown in Fig. 5, agonist-induced phosphorylation of Ser1177 was also greater in juvenile arterioles. Together, these data suggest that signaling pathways for the activation of eNOS become operational during growth due at least in part to changes in the regulation of eNOS phosphorylation. To our knowledge, there has been only one previous study (3) examining developmental changes in eNOS phosphorylation at the level of the resistance vessels. In that study (3), ACh-stimulated Ser1177 phosphorylation did not change between 2 and 12 days of age in porcine pulmonary resistance arteries, but the contribution of NO to the dilations did increase over that period. The difference between these findings and the present findings could reflect differences among vascular beds, species, or even the ages studied. Our study provides the first evidence demonstrating that differences in eNOS phosphorylation correlate with growth-related changes in NO production that occur in the skeletal muscle resistance vasculature.
Despite the greater agonist-induced dephosphorylation of Thr495 in juvenile arterioles, our immunoblot analyses revealed that PP1 and PP2A levels in these vessels were actually lower than those found in weanling arterioles (Fig. 3). Furthermore, despite greater Ser1177 phosphorylation in juvenile vessels, levels of Akt and PKA decreased with age or did not change, respectively. This raises the possibility that the age-related differences we observed in agonist-stimulated eNOS dephosphorylation or phosphorylation (Figs. 4 and 5) could be due in part to differences in phosphatase or kinase activity rather than in absolute protein content.
With respect to the age-related differences in Ser1177 phosphorylation, our findings lend more direct support to another possible cause. Hsp90 is a critical player in the eNOS activation cascade, and a study (16) has suggested that efficient NO production is contingent on the binding of Hsp90 to eNOS. Hsp90 acts as a chaperone protein, effectively facilitating the phosphorylation of Ser1177 by Akt (27). We found that Hsp90 protein content did not change during juvenile growth (Fig. 6), which is similar to a previous report (3) showing that there are no changes in either Hsp90 or eNOS protein levels in pulmonary resistance vessels during early postnatal growth. However, our findings do indicate there is a change in the interaction between Hsp90 and eNOS in the microcirculation during juvenile growth. Our measurements of coimmunoprecipitation of eNOS with Hsp90 demonstrate an increase in the agonist-induced eNOS-Hsp90 interaction with juvenile growth (Fig. 7), which is consistent with the increase in Ser1177 phosphorylation and endothelial NO release with juvenile growth. Furthermore, inhibition of Hsp90, as well as Akt, eliminates age-related differences in Ser1177 phosphorylation (Fig. 8), confirming the role of Hsp90 in mediating growth-related changes in Ser1177 phosphorylation and, most likely, eNOS activity. These findings are in contrast to a report by Mata-Greenwood et al. (23) showing that there are no differences in stimulated Hsp90-eNOS interactions in endothelial cells from fetal versus 4-wk old sheep. However, our findings are similar to those of Aschner et al. (3) indicating that agonist-induced Hsp90-eNOS interaction increases from 2 to 12 days of age in porcine resistance arteries. Our study is more closely aligned with the latter study in that we also studied intact resistance vessels (as opposed to isolated cells) and focused on postnatal growth (as opposed to the fetal-postnatal transition).
In conclusion, our data demonstrate an increased capacity for arteriolar NO release that develops with juvenile growth, due at least in part to an increased ability for eNOS to interact with scaffolding proteins such as Hsp90, leading to increased Ser1177 phosphorylation to further enhance eNOS activity. Gaining a clearer understanding of the mechanisms that regulate normal changes in NO signaling during growth may lead to the improved identification of pathological changes in NO signaling that can occur over this period in individuals with cardiovascular risk factors. This, in turn, could lead to the development of novel therapeutic agents aimed at treating endothelial dysfunction in the young (10).
GRANTS
This work was funded by American Heart Association Grants 0755264B and 09GRNT2250298 (to M. A. Boegehold) and 10GRNT4480020 (to G. Wu) as well as National Institute of Environmental Health Sciences Grants ES-018274 and ES-015022 (to T. R. Nurkiewicz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.S.K., T.R.N., and M.A.B. conception and design of research; L.S.K., T.R.N., and G.W. performed experiments; L.S.K., T.R.N., G.W., and M.A.B. analyzed data; L.S.K., T.R.N., G.W., and M.A.B. interpreted results of experiments; L.S.K. and M.A.B. prepared figures; L.S.K. and M.A.B. drafted manuscript; L.S.K. and M.A.B. edited and revised manuscript; L.S.K. and M.A.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Kim Wix and Carroll McBride for the excellent technical assistance.
REFERENCES
- 1. Amed S, Daneman D, Mahmud FH, Hamilton J. Type 2 diabetes in children and adolescents. Expert Rev Cardiovasc Ther 8: 393–406, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Armstead WM, Zuckerman SL, Shibata M, Parfenova H, Leffler CW. Different pial arteriolar responses to acetylcholine in the newborn and juvenile pig. J Cereb Blood Flow Metab 14: 1088–1095, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Aschner JL, Zeng H, Kaplowitz MR, Zhang Y, Slaughter JC, Fike CD. Heat shock protein 90-eNOS interactions mature with postnatal age in the pulmonary circulation of the piglet. Am J Physiol Lung Cell Mol Physiol 296: L555–L564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boegehold MA. Flow-dependent arteriolar dilation in normotensive rats fed low- or high-salt diets. Am J Physiol Heart Circ Physiol 269: H1407–H1414, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Bohlen HG, Nase GP. Dependence of intestinal arteriolar regulation on flow-mediated nitric oxide formation. Am J Physiol Heart Circ Physiol 279: H2249–H2258, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol 285: C499–C508, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Brouet A, Sonveaux P, Dessy C, Balligand JL, Feron O. Hsp90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase in vascular endothelial growth factor-exposed endothelial cells. J Biol Chem 276: 32663–32669, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Charpie JR, Schreur KD, Papadopoulos SM, Webb RC. Endothelium dependency of contractile activity differs in infant and adult vertebral arteries. J Clin Invest 93: 1339–1343, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chicoine LG, Paffett ML, Girton MR, Metropoulus MJ, Joshi MS, Bauer JA, Nelin LD, Resta TC, Walker BR. Maturational changes in the regulation of pulmonary vascular tone by nitric oxide in neonatal rats. Am J Physiol Lung Cell Mol Physiol 293: L1261–L1270, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics 122: 198–208, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Davies IR, Zhang X. Nitric oxide selective electrodes. Methods Enzymol 436: 63–95, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Dua AK, Dua N, Murrant CL. Skeletal muscle contraction-induced vasodilator complement production is dependent on stimulus and contraction frequency. Am J Physiol Heart Circ Physiol 297: H433–H442, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res 75: 247–260, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res 90: 866–873, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392: 821–824, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Glowinska-Olszewska B, Tolwinska J, Urban M. Relationship between endothelial dysfunction, carotid artery intima media thickness and circulating markers of vascular inflammation in obese hypertensive children and adolescents. J Pediatr Endocrinol Metab 20: 1125–1136, 2007 [PubMed] [Google Scholar]
- 18. Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol 77: 1288–1293, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Jarvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, Lehtimaki T, Ronnemaa T, Viikari J, Raitakari OT. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation 109: 1750–1755, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. Am J Physiol Heart Circ Physiol 297: H1087–H1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kupatt C, Dessy C, Hinkel R, Raake P, Daneau G, Bouzin C, Boekstegers P, Feron O. Heat shock protein 90 transfection reduces ischemia-reperfusion-induced myocardial dysfunction via reciprocal endothelial NO synthase serine 1177 phosphorylation and threonine 495 dephosphorylation. Arterioscler Thromb Vasc Biol 24: 1435–1441, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Linderman JR, Boegehold MA. Growth-related changes in the influence of nitric oxide on arteriolar tone. Am J Physiol Heart Circ Physiol 277: H1570–H1578, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Mata-Greenwood E, Jenkins C, Farrow KN, Konduri GG, Russell JA, Lakshminrusimha S, Black SM, Steinhorn RH. eNOS function is developmentally regulated: uncoupling of eNOS occurs postnatally. Am J Physiol Lung Cell Mol Physiol 290: L232–L241, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. J Biol Chem 272: 25907–25912, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Mimoun E, Aggoun Y, Pousset M, Dubern B, Bougle D, Girardet JP, Basdevant A, Bonnet D, Tounian P. Association of arterial stiffness and endothelial dysfunction with metabolic syndrome in obese children. J Pediatr 153: 65–70, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser1179. J Biol Chem 276: 30392–30398, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42: 271–279, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Chen BT, Frazer DG, Boegehold MA, Castranova V. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol Sci 110: 191–203, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nurkiewicz TR, Wu G, Li P, Boegehold MA. Decreased arteriolar tetrahydrobiopterin is linked to superoxide generation from nitric oxide synthase in mice fed high salt. Microcirculation 17: 147–157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Samora JB, Frisbee JC, Boegehold MA. Growth-dependent changes in endothelial factors regulating arteriolar tone. Am J Physiol Heart Circ Physiol 292: H207–H214, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Samora JB, Frisbee JC, Boegehold MA. Hydrogen peroxide emerges as a regulator of tone in skeletal muscle arterioles during juvenile growth. Microcirculation 15: 151–161, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Short KR, Blackett PR, Gardner AW, Copeland KC. Vascular health in children and adolescents: effects of obesity and diabetes. Vasc Health Risk Manag 5: 973–990, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest 93: 50–55, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension 40: 441–447, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Willis AP, Leffler CW. Endothelial NO and prostanoid involvement in newborn and juvenile pig pial arteriolar vasomotor responses. Am J Physiol Heart Circ Physiol 281: H2366–H2377, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Willis AP, Leffler CW. NO and prostanoids: age dependence of hypercapniaand histamine-induced dilations of pig pial arterioles. Am J Physiol Heart Circ Physiol 277: H299–H307, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Wu G, Meininger CJ. Impaired arginine metabolism and NO synthesis in coronary endothelial cells of the spontaneously diabetic BB rat. Am J Physiol Heart Circ Physiol 269: H1312–H1318, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Zuckerman SL, Armstead WM, Hsu P, Shibata M, Leffler CW. Age dependence of cerebrovascular response mechanisms in domestic pigs. Am J Physiol Heart Circ Physiol 271: H535–H540, 1996 [DOI] [PubMed] [Google Scholar]