Abstract
This review discusses the potential place of soluble adenylyl cyclase (sAC) in the framework of signaling in the cardiovascular system. cAMP has been studied as a critical and pleiotropic second messenger in cardiomyocytes, endothelial cells, and smooth muscle vascular cells for many years. It is involved in the transduction of signaling by catecholamines, prostaglandins, adenosine, and glucagon, just to name a few. These hormones can act via cAMP by binding to a G protein-coupled receptor on the plasma membrane with subsequent activation of a heterotrimeric G protein and its downstream effector, transmembrane adenylyl cyclase. This has long been the canonical standard for cAMP production in a cell. However, the relatively recent discovery of a unique source of cAMP, sAC, creates the potential for a shift in this signaling paradigm. In fact, sAC has been shown to play a role in apoptosis in coronary endothelial cells and cardiomyocytes. Additionally, it links nutrient utilization with ATP production in the liver and brain, which suggests one of many potential roles for sAC in cardiac function. The possibility of producing cAMP from a source distal to the plasma membrane provides a critical new building block for reconstructing the cellular signaling infrastructure.
Keywords: adenosine 3′,5′-cyclic monophosphate; compartmentalization; cardiomyocytes
this review article is part of a collection on G Protein Kinase A Signaling in Cardiovascular Physiology and Disease. Other articles appearing in this collection, as well as a full archive of all Review collections, can be found online at http://ajpheart.physiology.org/.
Background
The cardiovascular system is composed of multiple cell types, and vast amounts of work have been done in describing functional signaling pathways in cardiomyocytes, endothelial cells, and vascular smooth muscle cells (VSMCs). One important common intracellular signaling pathway is built around the second messenger adenosine 3′,5′-cyclic monophosphate (cAMP). cAMP is a critical and ubiquitous second messenger that plays a role in a diverse multitude of signaling pathways in virtually every type of cell. It was first described in 1958 by Sutherland and Rall (56) in experiments investigating how glycogen phosphorylase activity is upregulated following treatment with epinephrine and glucagon (45). This liver signaling pathway is now known to be widely expressed, including in the heart. However, this is by far not the only role of cAMP in the cardiovascular system. It has been shown that many signaling pathways involve cAMP as a second messenger in heart tissue (cardiomyocytes) and in the vasculature (endothelial cells and smooth muscle cells).
The stereotypical role of cAMP is the transduction of signaling that originates at the extracellular face of the plasma membrane. The binding of hormones to G protein-coupled receptors (GPCRs) leads to the activation of heterotrimeric G proteins, which subsequently activate transmembrane adenylyl cyclases (tmACs) to catalyze the formation of cAMP from ATP. Pioneering work by many investigators elucidated this system that governs how extracellular signals (“first messengers”) are transduced into intracellular responses (“second messengers”) via coupling with tmACs (40, 57). A large number of hormones signal through a change in cAMP levels in cardiomyocytes, each with a unique profile of downstream effects. This list includes epinephrine, prostaglandins E1 (PGE1) and E2, glucagon, glucagon-like peptide-1, adenosine, acetylcholine, endothelin, and urocortin.
Downstream, cAMP may act through four (currently known) effectors: protein kinase A (PKA), exchange protein activated by cAMP (EPAC), cyclic nucleotide regulated ion channels, and phosphodiesterases (PDEs). PDEs catabolize cAMP into 5′-AMP, terminating its ability to modulate these downstream effectors, but they also represent effectors given that a subset of cGMP-hydrolyzing PDEs, specifically PDE10A, can be inhibited by physiologically relevant concentrations of cAMP acting as either a competitive antagonist (21) or potentially as an allosteric regulator (24). The best studied of cAMP's effectors in the heart is PKA, a serine/threonine kinase made up of two regulatory subunits and two catalytic subunits. cAMP binds to the regulatory subunits, liberating the catalytic subunits to phosphorylate a host of downstream effector molecules, including enzymes (e.g., glycogen phosphorylase), transcription factors [cyclic-nucleotide regulatory element binding protein (CREB)], motor proteins (troponin I, myosin binding protein C), channels [L-type Ca2+ channels (LTCC), ryanodine receptor], and other signaling components (phosphatase inhibitor-1). Distinct signaling pathways and their functional consequences are defined by the particular array of PKA substrates that are affected.
Most cAMP research has focused on this traditional role of transducing ligand-receptor binding. Indeed, this is fitting as the majority of cAMP pathways described fit this archetype. However, cAMP also plays roles independent from GPCR mechanisms in a number of different systems (58). It remains to be seen whether any of these are also present in cells of the cardiovascular system, which would raise the question of where the cAMP comes from.
The story is not much different in VSMCs and endothelial cells, although the particular players and/or actions may vary. For example, in VSMCs epinephrine mediates its effects via β-adrenergic receptors and cAMP just as in cardiomyocytes; however, epinephrine increases contractility and chronotropy in cardiomyocytes while it regulates vascular tone in smooth muscle. Furthermore, just as in cardiomyocytes, there are cAMP pathways in VSMCs and endothelial cells that do not necessarily involve hormones or receptors. For example, work has shown that cytosolic cAMP production (by some as yet unidentified mechanism) is important in controlling endothelial barrier formation (47).
The sheer number of processes involving cAMP produces a conundrum: How can a single molecule mediate or modulate so many distinct responsibilities? The elucidation of individual signaling pathways has been pursued for many decades, but it was not until more recently that the issue of signal specificity has garnered paramount attention.
Compartmentalization
A paradox with the cAMP signaling paradigm was revealed by the observation that two different hormones could lead to similar increases in intracellular cAMP but subsequently result in very distinct effects. Keely (36, 37) was the first to report a difference between PGE1 and epinephrine in isolated rat heart; only epinephrine caused activation of glycogen phosphorylase. Brunton and coworkers extended this divergence, finding that the epinephrine analog isoproterenol, but not PGE1, led to changes in the rate of left ventricular pressure change (dP/dt), activation of phosphorylase kinase, inhibition of glycogen synthase (27, 28), and phosphorylation of troponin I (7). In these studies, it was well documented that the observed differences were in spite of the fact that both hormones elevated cAMP and activated PKA [reviewed in (53)].
Soon thereafter, evidence suggested that signal pathway compartmentalization could be the mechanism behind these findings. First, PKA activity was increased in the cytosolic fraction of perfused heart with both hormones, but PKA activity in the particulate fraction was enhanced only with isoproterenol and not with PGE1 (14, 26, 28). The Brunton group (10, 25) confirmed that this dichotomy was not due to cellular heterogeneity in heart tissue by repeating the findings with cultured or isolated myocytes. This idea of compartmentalization was rejuvenated by a set of elegant patch-clamp experiments by Jurevicius and Fischmeister (31) where a local application of isoproterenol caused the activation exclusively of nearby LTCCs, but the local application of forskolin, a plant diterpene that pharmacologically activates transmembrane isoforms of adenylyl cyclase, caused a widespread activation of LTCCs throughout the cell.
The existence of cAMP-signaling compartmentalization has become well established over the past decade as a concept for allowing the second messenger to regulate different pathways. Discovering the involved mechanisms for establishing distinct signaling microdomains is still an important and very active field. Efforts up to this point have unveiled a host of new building blocks with which to better understand how the cell achieves this compartmentalization. The primary areas of research in this field center on PDEs; A kinase-anchoring proteins (AKAPs), which are scaffolding proteins that assemble PKA into multiprotein signaling complexes; and physical compartmentalization of the plasma membrane, e.g., caveolae and T-tubules. This general topic has been extensively reviewed elsewhere (62), and these areas of research are also the subject of other reviews in this series (15).
Mechanisms such as AKAPs, PDEs, and membrane-delineated compartments have been used to explain how cAMP can modulate distinct cellular changes in response to different stimuli. However, there are still many gaps in the current conceptual model of signaling compartmentalization. Most notably, if cAMP diffusion is restricted by PDEs, how do you activate PKAs and EPACs that are localized distal to plasma membrane-bound sources of cAMP? For example, muscle-selective AKAP (mAKAP) is a 255-kDa protein that localizes PKA to the nuclear envelope, as well as to the sarcoplasmic reticulum in heart and skeletal muscle (35, 44). Multiple reports have pieced together the mAKAP complex to include PKA, PDE4D3, the ryanodine receptor, phosphatases, EPAC, and MAPK cascade components (16, 18, 33, 43). The number and variety of tethered proteins underscore mAKAP's ability to coordinate greater complexity in its associated signal transduction, including a built-in negative feedback loop with the cAMP catabolizing PDE4D3 (12, 17). This PDE is proposed to maintain a low level of resting cAMP, too low to activate the associated PKA. Upon stimulation, the rise in cAMP overpowers the PDE and initiates the signaling cascade. However, because nuclear membrane-localized mAKAP can be far from plasma membrane-bound tmACs, the source of cAMP that acts upon its signaling complex remains unclear. The second messenger could diffuse through the cytoplasm from tmACs on the plasma membrane, or it could originate from tmACs on internalized vesicles (11, 20). This latter idea has some support from a recent report finding tmAC type V (AC5) associated with mAKAP (34). However, another possibility for localized signal production has been recently discovered. Just as there are both particulate and soluble guanylyl cyclases, there exists, in distinction from tmACs, a soluble adenylyl cyclase (sAC).
Discovery of sAC
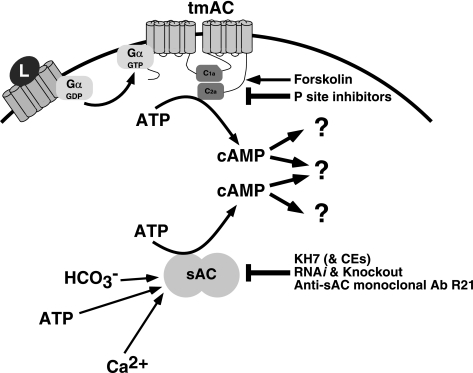
sAC is a novel class of adenylyl cyclase(s), distinct from tmACs, that occupies a unique niche in cAMP signaling (Fig. 1). In 1975, Braun and Dods (5) described an adenylyl cyclase activity purified from the cytosolic fraction of testis. Since tmACs would not be present in this fraction, this suggested the possibility of a novel source of cAMP. It was predicted to be molecularly distinct from the more widely studied tmACs because it was insensitive to heterotrimeric G proteins (6) and forskolin (54), both known activators of tmACs. The enzyme responsible for this cytosolic cAMP generation was identified in 1999. We purified cytosolic adenylyl cyclase activity from 950 rat testes using classical column chromatography (8). Tryptic digestion and sequencing of resultant peptides followed by degenerate PCR resulted in the isolation of two cDNA clones that represented splice variants of a single gene, dubbed sAC (Sacy; Adcy10). The NH2-terminus shared by both clones contains two domains that show homology to nucleotidyl cyclase catalytic domains. The amino acid sequence of these two domains confirmed sAC to be unlike all other known mammalian cyclases, being instead most closely related to cyclases from cyanobacteria (8). Heterologous expression and biochemical characterization revealed differences between sAC and tmACs, including the unique activation of sAC by bicarbonate (HCO3−) (13) and calcium (30, 42) and unique sensitivity to pharmacological and genetic modulators (4, 22, 29, 55, 59) (Fig. 2).
Fig. 1.
Two sources of cAMP in mammalian cells. Unique properties differentiate the 9 transmembrane adenylyl cyclases (tmACs) from the single family of soluble adenylyl cyclase (sAC) isoforms. These differences equip them to play distinct roles in cAMP signaling. C1a and C2a refer the 2 catalytic domains of tmACs.
Fig. 2.
Cyclase-specific reagents to study sources of cAMP in mammalian cells. Selective activators and inhibitors provide tools to distinguish the relative contributions of tmACs and sAC to cAMP signaling. L refers to ligand of G protein-coupled receptor; Gα-GDP and Gα-GTP refer to the inactive and active forms of the α-subunit of heterotrimeric G protein; C1a and C2a refer the two catalytic domains of tmACs; CE refers to catechol derivatives of estrogen, such as 2- or 4-hydroxyestradiol, which inhibit sAC (and related enzymes) by chelating the magnesium ion in the enzyme's active site (52). KH7 is a specific sAC inhibitor, which works via an unknown mechanism, identified in a small molecule screen (29).
Studies revealed that sAC plays a crucial role in sperm physiology (19, 29, 61) and is also involved in the signaling pathways of various somatic tissues, including the pancreas, brain, kidneys, and lungs (23, 46, 49, 55, 59). Furthermore, recent work by Acin-Perez et al. (3) shows that sAC regulates oxidative phosphorylation in mitochondria, potentially placing sAC within every cell type. In each of these systems, sAC's roles are based on its activation by HCO3− and/or calcium. In sperm, it is involved in the induction of hyperactivated motility upon encountering the bicarbonate-rich fluids of the male and female reproductive tracts. In mitochondria, sAC mediates the coupling of the electron transport chain with cellular respiration via levels of the metabolic waste product carbon dioxide (CO2), which is in instantaneous equilibrium with HCO3− due to the presence of carbonic anhydrases.
A key attribute of sAC that defines it as a new building block in cAMP physiology is its ability to be localized throughout the cytoplasm and within organelles (9, 63, 64). Whereas tmACs are restricted to the plasma membrane and are consequently well suited to transduce extracellular signals, sAC is uniquely poised to serve as a local cAMP signaling source inside the cell (60). To date, sAC has been demonstrated to be inside the nucleus and mitochondria, and at microtubules and centrioles, in a variety of mammalian cell lines: MDCK, HeLa, HEK293, Hepa 1–6, PC12, SH-SY5Y, TM4, NIH3T3, and primary fibroblasts (63). Of these, functional roles have been discovered in the nucleus where sAC was shown to define a cAMP microdomain that includes nuclear PKA and CREB (64), and in the mitochondria, which is discussed in greater detail below. Indeed, its unique set of modulators (HCO3−, calcium, and potentially ATP) are consistent with the possibility that sAC acts as a sensor for intracellular changes, a complement to tmACs' role as a sensor for extracellular signals (13, 32). However, it is narrow sighted to consider these categorizations as definitive. Babcock and coworkers (51) have shown that hormones and neurotransmitters may not only act by binding to receptors on the surface of cells, but they may also be transported directly into the cell for signal initiation (51). In fact, sAC was found to be essential for signal propagation of adenosine and isoproterenol in this unorthodox pathway (50). All of this leads to an obvious but important question: Could sAC also be present in cardiomyocytes, VSMCs, and endothelial cells and add another mechanism by which cAMP signals can be processed into varied downstream effects?
Roles for sAC in The Cardiovascular System
Recently, sAC was shown to play a number of functions in the cardiovascular system. Studies by the Ladilov group describe a role for sAC in an apoptotic signaling pathway in both coronary endothelial cells (39) and in cardiomyocytes (A. Appukuttan and Y. Ladilov, personal communication). Following ischemia, sAC-generated cAMP activates PKA to phosphorylate the proapoptotic Bcl-2-family member Bax. This causes the translocation of Bax to the mitochondria; interestingly, sAC is also found to translocate to the mitochondria. Upon reperfusion, the mitochondrial pathway of apoptosis is activated, with stereotypical radical oxygen species production, cytochrome-c release, and caspase-9/-3 cleavage. Pharmacological or genetic inhibition of sAC during ischemia, but not during the reperfusion phase of injury, suppressed these hallmarks of apoptosis. These researchers did not study the link between ischemia and sAC activation; however, as sAC has already been hypothesized to act as a metabolic sensor because of its unique regulation by HCO3− (4, 46, 65), sAC could be activated by the CO2/HCO3− buildup typical of the ischemic environment as vascular supply of oxygen and removal of CO2 is diminished. However, as we have already experienced with tmAC-cAMP signaling, things are not always simple. Another recent paper by the Ladilov group (38) paradoxically described that transport of HCO3− into coronary endothelial cells during ischemia suppressed, rather than induced, mitochondrial apoptosis. Consistently, work in corneal endothelial cells by Li et al. (41) found that HCO3−-activated sAC protected against mitochondrial-based apoptosis.
sAC also plays a nonapoptotic role in mitochondria. We recently showed that sAC was present in a unique signaling pathway wholly contained within the mitochondrial matrix (4). As the energy requirements of a cell increase, such as a cardiomyocyte contracting faster and harder in response to epinephrine, it requires increased production of ATP. Coupling ATP production via oxidative phosphorylation with nutrient utilization is important to prevent the buildup of radical oxygen species (3). We showed that CO2/HCO3− generated by the Krebs cycle stimulates intramitochondrially localized sAC. The resulting cAMP activates PKA,1 which increases the activity of the electron transport chain. Modulation of electron transport chain activity via phosphorylation had been proposed in the past, but it was not confirmed until Acin-Perez et al. (1) showed that PKA phosphorylated subunit IV of complex IV, disinhibiting it. Again, the theme repeats itself that sAC is uniquely poised to sense and respond to changes in CO2/HCO3−. This can be environmental, as in the case of ischemia, or it can be internal, as in the case of cellular respiration. Initially, this cascade was exclusively shown to exist in liver mitochondria, but it was recently shown to be more widely distributed (2). Work in our laboratory has confirmed existence of the CO2-sAC-PKA-complex IV signaling cascade in mitochondria from heart (K. C. Hess, L. R. Levin, and J. Buck, unpublished observation). Because cardiomyocytes are perhaps the most energy-demanding cells in the body, and thus metabolic activity and regulation are critical, we predict this cascade functions (at least) in cardiomyocytes.
Further work needs to be done to uncover additional roles of sAC in the cardiovascular system. Given what is known about the characteristics of the enzyme, its roles and regulation in other systems, and the research discussed above, the consistent themes that appear suggest potential areas where sAC may contribute to the framework of cAMP signaling within cardiomyocytes, endothelial cells, and VSMCs. We list here a few speculative roles.
Many processes are either known or suspected to be coupled to cellular respiration; it is reasonable to hypothesize that these are also coupled via sAC functioning as a tissue metabolic sensor. The main regulators of vascular tone are sympathetic innervation (norepinephrine), parasympathetic innervation (acetylcholine), circulating levels of hormones such as epinephrine, and local levels of metabolites. As a muscle works, it produces metabolic waste products, such as CO2, ADP, extracellular K+, and organic acids, that need to be cleared. These molecules can act directly on local arterioles to cause vasodilation, presumably to increase the flow of nutrients into the muscle and the flow of waste out of the muscle. The harder a muscle works, the more metabolites are produced, necessitating faster clearance to prevent toxic accumulation. The mechanism by which CO2 affects vascular tone is unknown. Due to the fairly ubiquitous presence of carbonic anhydrases, CO2 and HCO3− are constantly in equilibrium both inside and outside cells. Thus, HCO3− levels will rise proportionally to the rise in CO2, and since sAC is uniquely activated by HCO3−, it seems possible that sAC could mediate the CO2-induced vasoregulation.
In addition to sAC's regulation by HCO3−, its activity is also modulated by Ca2+ levels. In insulinoma cells, sAC activity is stimulated because of a rise in intracellular calcium entering through voltage gated calcium channels (46), and in the pheochromocytoma cell line, PC12, sAC activation due to neurotrophins is dependent on intracellular calcium (55). Fluctuations in calcium are an important signal in cardiovascular physiology, and it is possible that these changes may be coordinated to downstream effects via sAC activity. Could sAC, for example, be modulating the intrinsic beat frequency of isolated cardiomyocytes known to be dependent on Ca2+ influx from the sarcoplasmic reticulum or through LTCCs?
Another potential role that sAC may be playing in the cardiovascular system is regulation of endothelial barrier formation. There are distinct differences in the response to cAMP accumulation when it occurs at the plasma membrane versus in the cytoplasm of endothelial cells (48). cAMP pools produced near the plasma membrane increase the strength of the endothelial barrier. In contrast, cytosolic cAMP pools, as with Pseudomonas aeruginosa infection or introduction of artificial “soluble” tmAC chimeras, leads to increased permeability (47). It may be that these exogenous cyclases are exploiting an endogenous role of sAC in modulating endothelial barrier permeability.
Finally, as briefly discussed earlier, there is an interesting concept recently published that hormones can signal without binding to a cell surface receptor. Schuh et al. found that catecholamines and adenosine effect downstream changes in sperm via a sAC-dependent signaling pathway (50) that requires them to be transported directly into the cell (51). Catecholamines and adenosine have long been known to have multiple functions within the cardiovascular system. They have always been presumed to function via canonical GPCRs and tmACs; however, the observations in sperm suggest these hormones may also signal via a sAC-dependent, intracellular receptor-mediated cascade in the heart. Further work needs to be done to see whether this alternative pathway of hormone signal initiation occurs with other hormones and in other cell types.
Conclusion
In conclusion, sAC possesses two key characteristics that make it a potentially important new addition to the framework of cAMP compartmentalization in the cardiovascular system. First, its ability to be localized anywhere within a cell is in stark contrast to the obligate plasma membrane-bound nature of tmACs. Second, it is uniquely activated by HCO3−, a molecule that has metabolic or environmental significance in many physiological and pathological processes. We predict future studies will reveal multiple roles for sAC in cardiomyocytes, VSMCs, and endothelial cells.
GRANTS
This work was supported by funds from National Institutes of Health (NIH) Grants GM-62328 and HD-059913 (to L. R. Levin and J. Buck) and by NIH MSTP Grant GM-07739 (to J. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.C., L.R.L., and J.B. prepared figures; J.C., L.R.L., and J.B. drafted manuscript; J.C., L.R.L., and J.B. edited and revised manuscript; J.C., L.R.L., and J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Yuri Ladilov for communicating results before publication.
REFERENCES
- 1. Acin-Perez R, Gatti DL, Bai Y, Manfredi G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab 13: 712–719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Acin-Perez R, Russwurm M, Gunnewig K, Gertz M, Zoidl G, Ramos L, Buck J, Levin LR, Rassow J, Manfredi G, Steegborn C. A phosphodiesterase 2A isoform localized to mitochondria regulates respiration. J Biol Chem 286: 30423–30432, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Acin-Perez R, Salazar E, Brosel S, Yang H, Schon EA, Manfredi G. Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Mol Med 1: 392–406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab 9: 265–276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braun T, Dods RF. Development of a Mn2+-sensitive, “soluble” adenylate cyclase in rat testis. Proc Natl Acad Sci USA 72: 1097–1101, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braun T, Frank H, Dods R, Sepsenwol S. Mn2+-sensitive, soluble adenylate cyclase in rat testis. Differentiation from other testicular nucleotide cyclases. Biochim Biophys Acta 481: 227–235, 1977 [DOI] [PubMed] [Google Scholar]
- 7. Brunton LL, Hayes JS, Mayer SE. Hormonally specific phosphorylation of cardiac troponin I and activation of glycogen phosphorylase. Nature 280: 78–80, 1979 [DOI] [PubMed] [Google Scholar]
- 8. Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA 96: 79–84, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bundey RA, Insel PA. Discrete intracellular signaling domains of soluble adenylyl cyclase: camps of cAMP? Sci STKE 2004: pe19, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Buxton IL, Brunton LL. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem 258: 10233–10239, 1983 [PubMed] [Google Scholar]
- 11. Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol 7: e1000172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carlisle Michel JJ, Dodge KL, Wong W, Mayer NC, Langeberg LK, Scott JD. PKA-phosphorylation of PDE4D3 facilitates recruitment of the mAKAP signalling complex. Biochem J 381: 587–592, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Corbin JD, Sugden PH, Lincoln TM, Keely SL. Compartmentalization of adenosine 3′:5′-monophosphate and adenosine 3′:5′-monophosphate-dependent protein kinase in heart tissue. J Biol Chem 252: 3854–3861, 1977 [PubMed] [Google Scholar]
- 15. Diviani D, Dodge-Kafka KL, Li J, Kapiloff MS. A-kinase anchoring proteins-scaffolding proteins in the heart. Am J Physiol Heart Circ Physiol 301: H1742–H1753, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J 20: 1921–1930, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dodge-Kafka KL, Langeberg L, Scott JD. Compartmentation of cyclic nucleotide signaling in the heart: the role of A-kinase anchoring proteins. Circ Res 98: 993–1001, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 437: 574–578, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, Van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA 101: 2993–2998, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol 5: 734–742, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujishige K, Kotera J, Michibata H, Yuasa K, Takebayashi S, Okumura K, Omori K. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A). J Biol Chem 274: 18438–18445, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Gille A, Lushington GH, Mou TC, Doughty MB, Johnson RA, Seifert R. Differential inhibition of adenylyl cyclase isoforms and soluble guanylyl cyclase by purine and pyrimidine nucleotides. J Biol Chem 279: 19955–19969, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Hallows KR, Wang H, Edinger RS, Butterworth MB, Oyster NM, Li H, Buck J, Levin LR, Johnson JP, Pastor-Soler NM. Regulation of epithelial Na+ transport by soluble adenylyl cyclase in kidney collecting duct cells. J Biol Chem 284: 5774–5783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Handa N, Mizohata E, Kishishita S, Toyama M, Morita S, Uchikubo-Kamo T, Akasaka R, Omori K, Kotera J, Terada T, Shirouzu M, Yokoyama S. Crystal structure of the GAF-B domain from human phosphodiesterase 10A complexed with its ligand, cAMP. J Biol Chem 283: 19657–19664, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Hayes JS, Bowling N, King KL, Boder GB. Evidence for selective regulation of the phosphorylation of myocyte proteins by isoproterenol and prostaglandin E1. Biochim Biophys Acta 714: 136–142, 1982 [DOI] [PubMed] [Google Scholar]
- 26. Hayes JS, Brunton LL. Functional compartments in cyclic nucleotide action. J Cyclic Nucleotide Res 8: 1–16, 1982 [PubMed] [Google Scholar]
- 27. Hayes JS, Brunton LL, Brown JH, Reese JB, Mayer SE. Hormonally specific expression of cardiac protein kinase activity. Proc Natl Acad Sci USA 76: 1570–1574, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hayes JS, Brunton LL, Mayer SE. Selective activation of particulate cAMP-dependent protein kinase by isoproterenol and prostaglandin E1. J Biol Chem 255: 5113–5119, 1980 [PubMed] [Google Scholar]
- 29. Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Moss SB. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 9: 249–259, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci USA 100: 10676–10681, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jurevicius J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by beta-adrenergic agonists. Proc Natl Acad Sci USA 93: 295–299, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol 362: 623–639, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci 114: 3167–3176, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, Henson E, Efendiev R, Dessauer CW. An adenylyl cyclase-mAKAPbeta signaling complex regulates cAMP levels in cardiac myocytes. J Biol Chem 284: 23540–23546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kapiloff MS, Schillace RV, Westphal AM, Scott JD. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J Cell Sci 112: 2725–2736, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Keely SL. Activation of cAMP-dependent protein kinase without a corresponding increase in phosphorylase activity. Res Commun Chem Pathol Pharmacol 18: 283–290, 1977 [PubMed] [Google Scholar]
- 37. Keely SL. Prostaglandin E1 activation of heart cAMP-dependent protein kinase: apparent dissociation of protein kinase activation from increases in phosphorylase activity and contractile force. Mol Pharmacol 15: 235–245, 1979 [PubMed] [Google Scholar]
- 38. Kumar S, Flacke JP, Kostin S, Appukuttan A, Reusch HP, Ladilov Y. SLC4A7 sodium bicarbonate co-transporter controls mitochondrial apoptosis in ischaemic coronary endothelial cells. Cardiovasc Res 89: 392–400, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Kumar S, Kostin S, Flacke JP, Reusch HP, Ladilov Y. Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. J Biol Chem 284: 14760–14768, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lefkowitz RJ. Rodbell and Gilman win 1994 Nobel Prize for Physiology and Medicine. Trends Pharmacol Sci 15: 442–444, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Li S, Allen KT, Bonanno JA. Soluble adenylyl cyclase mediates bicarbonate-dependent corneal endothelial cell protection. Am J Physiol Cell Physiol 300: C368–C374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem 278: 15922–15926, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000 [DOI] [PubMed] [Google Scholar]
- 44. McCartney S, Little BM, Langeberg LK, Scott JD. Cloning and characterization of A-kinase anchor protein 100 (AKAP100). A protein that targets A-kinase to the sarcoplasmic reticulum. J Biol Chem 270: 9327–9333, 1995 [DOI] [PubMed] [Google Scholar]
- 45. Rall TW, Sutherland EW. Formation of a cyclic adenine ribonucleotide by tissue particles. J Biol Chem 232: 1065–1076, 1958 [PubMed] [Google Scholar]
- 46. Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J Gen Physiol 132: 329–338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sayner SL, Alexeyev M, Dessauer CW, Stevens T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res 98: 675–681, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res 95: 196–203, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Schmid A, Sutto Z, Nlend MC, Horvath G, Schmid N, Buck J, Levin LR, Conner GE, Fregien N, Salathe M. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J Gen Physiol 130: 99–109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schuh SM, Carlson AE, McKnight GS, Conti M, Hille B, Babcock DF. Signaling pathways for modulation of mouse sperm motility by adenosine and catecholamine agonists. Biol Reprod 74: 492–500, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Schuh SM, Hille B, Babcock DF. Adenosine and catecholamine agonists speed the flagellar beat of mammalian sperm by a non-receptor-mediated mechanism. Biol Reprod 77: 960–969, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Steegborn C, Litvin TN, Hess KC, Capper AB, Taussig R, Buck J, Levin LR, Wu H. A novel mechanism for adenylyl cyclase inhibition from the crystal structure of its complex with catechol estrogen. J Biol Chem 280: 31754–31759, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol 41: 751–773, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Stengel D, Guenet L, Desmier M, Insel P, Hanoune J. Forskolin requires more than the catalytic unit to activate adenylate cyclase. Mol Cell Endocrinol 28: 681–690, 1982 [DOI] [PubMed] [Google Scholar]
- 55. Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, Levin LR. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J Biol Chem 281: 17253–17258, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem 232: 1077–1091, 1958 [PubMed] [Google Scholar]
- 57. Taussig R, Gilman AG. Mammalian membrane-bound adenylyl cyclases. J Biol Chem 270: 1–4, 1995 [DOI] [PubMed] [Google Scholar]
- 58. Tresguerres M, Levin LR, Buck J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int 79: 1277–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu KY, Zippin JH, Huron DR, Kamenetsky M, Hengst U, Buck J, Levin LR, Jaffrey SR. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat Neurosci 9: 1257–1264, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xia Z, Refsdal CD, Merchant KM, Dorsa DM, Storm DR. Distribution of mRNA for the calmodulin-sensitive adenylate cyclase in rat brain: expression in areas associated with learning and memory. Neuron 6: 431–443, 1991 [DOI] [PubMed] [Google Scholar]
- 61. Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, van Duin M, Conti M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol 296: 353–362, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Zaccolo M. cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. Br J Pharmacol 158: 50–60, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J 17: 82–84, 2003 [DOI] [PubMed] [Google Scholar]
- 64. Zippin JH, Farrell J, Huron D, Kamenetsky M, Hess KC, Fischman DA, Levin LR, Buck J. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol 164: 527–534, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zippin JH, Levin LR, Buck J. CO2/HCO3−-responsive soluble adenylyl cyclase as a putative metabolic sensor. Trends Endocrinol Metab 12: 366–370, 2001 [DOI] [PubMed] [Google Scholar]