Abstract
Our laboratory has shown that λ-carrageenan-induced peripheral inflammatory pain (CIP) can alter tight junction (TJ) protein expression and/or assembly leading to changes in blood-brain barrier xenobiotic permeability. However, the role of reactive oxygen species (ROS) and subsequent oxidative stress during CIP is unknown. ROS (i.e., superoxide) are known to cause cellular damage in response to pain/inflammation. Therefore, we examined oxidative stress-associated effects at the blood-brain barrier (BBB) in CIP rats. During CIP, increased staining of nitrosylated proteins was detected in hind paw tissue and enhanced presence of protein adducts containing 3-nitrotyrosine occurred at two molecular weights (i.e., 85 and 44 kDa) in brain microvessels. Tempol, a pharmacological ROS scavenger, attenuated formation of 3-nitrotyrosine-containing proteins in both the hind paw and in brain microvessels when administered 10 min before footpad injection of λ-carrageenan. Similarly, CIP increased 4-hydroxynoneal staining in brain microvessels and this effect was reduced by tempol. Brain permeability to [14C]sucrose and [3H]codeine was increased, and oligomeric assemblies of occludin, a critical TJ protein, were altered after 3 h CIP. Tempol attenuated both [14C]sucrose and [3H]codeine brain uptake as well as protected occludin oligomers from disruption in CIP animals, suggesting that ROS production/oxidative stress is involved in modulating BBB functional integrity during pain/inflammation. Interestingly, tempol administration reduced codeine analgesia in CIP animals, indicating that oxidative stress during pain/inflammation may affect opioid delivery to the brain and subsequent efficacy. Taken together, our data show for the first time that ROS pharmacological scavenging is a viable approach for maintaining BBB integrity and controlling central nervous system drug delivery during acute inflammatory pain.
Keywords: brain vascular permeability, tight junctions, oxidative stress
the blood-brain barrier (BBB) is the principal physical and metabolic barrier that separates the central nervous system (CNS) from the systemic circulation. The BBB has evolved to effectively restrict xenobiotic permeability in an effort to maintain CNS homeostasis. Brain microvascular endothelial cells are joined by tight junctions (TJs), dynamic protein complexes that restrict paracellular solute diffusion. TJs form a continuous, almost impermeable barrier that limits paracellular flux of xenobiotics with the exception of small, lipid-soluble molecules (1). The high BBB transendothelial resistance (∼1,800 Ωcm2) further restricts free flow of water and solutes (1).
BBB TJ complexes are formed by multiple transmembrane protein constituents including transmembrane proteins junctional adhesion molecules, claudins, and occludin (10). Occludin is specifically localized to the TJ at endothelial cell margins (18,21) and links with the cytoskeleton through interactions with accessory proteins [i.e., zonulae occluden (ZO)-1, -2, and -3; Refs. 4, 10]. It was previously suggested that while occludin is localized to the TJ, it is not essential for TJ assembly and has no direct “tightening” function (4). In contrast, recent studies (21, 22) by our group have clearly shown that occludin is a critical regulator of BBB permeability in vivo. This essential role is believed to occur via interaction between two extracellular loops on occludin monomers and homologous segments on occludin molecules localized to adjacent endothelial cells (8). This interaction creates a tight seal that restricts paracellular solute diffusion (8). Occludin also assembles into dimers and higher order oligomers at the TJ (22–24). Such occludin oligomeric assemblies are required for physiological function at the TJ, particularly as a restrictor of paracellular permeability (21).
BBB disruption contributes to pathogenesis of neurodegenerative diseases including Alzheimer's disease, Parkinson's disease, and multiple sclerosis (41). Our laboratory (6, 12–14, 21, 29) has also shown that peripheral pain/inflammation can alter BBB integrity via changes in occludin expression. BBB disruption during peripheral inflammatory pain is demarcated by enhanced paracellular permeability to vascular markers such as sucrose (6, 29) as well as opioid analgesic drugs morphine (32) and codeine (9). Our work (9) with codeine was particularly intriguing because increased brain uptake corresponded with enhanced antinociceptive profile. Codeine analgesia is centrally mediated, which implies that this opioid must accumulate within the brain to be an efficacious drug (9). Opioid pharmacotherapy is also associated with significant CNS side effects (i.e., respiratory depression, addiction, and tolerance), indicating that brain opioid concentrations must be precisely controlled. Therefore, the effects of peripheral pain/inflammation on brain microvascular permeability to opioids are crucial considerations in therapeutic drug dosing and/or potential adverse drug reactions.
Our laboratory (5, 6, 29) has identified various physiological and biochemical mechanisms that modulate BBB integrity under conditions of peripheral inflammatory pain; however, the role of reactive oxygen species (ROS) such as superoxide anion and subsequent oxidative stress in mediating these BBB changes during pain/inflammation have not been elucidated. Using the λ-carrageenan model of peripheral inflammatory pain, Wang et al. (36) demonstrated that the superoxide dismutase (SOD) mimetic M40403 attenuated both inflammation and hyperalgesia, indicating that ROS are critical components of peripheral inflammatory pain pathophysiology. Similar observations were obtained in the same peripheral pain model using the free radical scavenger and SOD mimetic 4-hydroxy-2, 2,6,6-tetramethylpiperidine-N-oxyl (tempol; Ref. 16). More recently, our laboratory (18) has shown that tempol administration prevents changes in occludin localization and structure in a rodent component model of global hypoxia. Taken together, these studies suggest that oxidative stress may play a prominent role in modulating BBB functional integrity during peripheral inflammatory pain.
In the present study, we investigated, in vivo, the effect of oxidative stress on 1) structural protein changes in occludin (i.e., disulfide bond modulation in occludin oligomeric assemblies); and 2) paracellular xenobiotic permeability by measuring brain uptake of [14C]sucrose and [3H]codeine using in situ brain perfusion. Both research objectives were evaluated using the λ-carrageenan model of peripheral inflammatory pain. In all of our studies, we used tempol to determine if ROS scavenging, a pharmacological mechanism that directly modulates oxidative stress responses, may be a viable therapeutic approach for maintaining BBB functional integrity during acute inflammatory pain.
MATERIALS AND METHODS
Materials.
Codeine was a generous gift from the Division of Neuroscience and Behavioral Research, National Institute on Drug Abuse, National Institutes of Health (Bethesda, MD) and was radiolabeled with tritium (18.2 Ci/mmol) by RTI International (Research Triangle Park, NC). [14C]sucrose (0.0096 Ci/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO). Rabbit polyclonal antibodies directed against occludin, 3-nitrotyrosine, and platelet-endothelial cell adhesion molecule-1 (PECAM-1) were purchased from Invitrogen Life Technologies (Carlsbad, CA). The rabbit polyclonal antibody against 4-hydroxynoneal (4-HNE) was purchased from Abcam (Cambridge, MA). Tempol was purchased from Arcos Organics (Geel, Belgium). All other antibodies and reagents, unless otherwise noted, were purchased from Sigma-Aldrich (St. Louis, MO).
Animals and treatment.
All animal experiments were approved by the Institutional Animal Care and Use Committee at the College of Medicine, University of Arizona and conform to National Institutes of Health's guidelines. Adult female Sprague-Dawley rats (230–280 g) were purchased from Harlan (Indianapolis, IN), housed under standard 12-h:12-h light-dark conditions, and provided with food and water ad libitum. Female rats were selected to enable comparison with previous work (6,18, 21, 29). Animals were randomly assigned to each treatment group. Rats were injected (100 μl sc) with 3% λ-carrageenan (m/vol in 0.9% saline) or 0.9% saline (m/vol) into the plantar surface of the right hind paw. For experiments designed to examine effects of ROS scavenging and/or oxidative stress on BBB permeability and occludin disulfide bond formation, tempol [200 mg/kg (1.0 ml/kg) ip] or vehicle [0.9% saline (1.0 ml/kg ip)] was injected 10 min before footpad injection. The dose of tempol was selected based upon a previous study, which showed that 200 mg/kg was the lowest dose that both effectively scavenged ROS and did not elicit any adverse side effects in experimental animals (18). For experiments designed to study effects of peripheral inflammatory pain in the presence and absence of tempol on codeine analgesia, codeine [7.0 mg/kg (1.0 ml/kg) ip] or vehicle [0.9% saline (1.0 ml/kg ip)] was injected 15 min after footpad injection. After 3-h exposure to λ-carrageenan or saline, animals were anesthetized with pentobarbital sodium [64.8 mg/kg (1.0 ml/kg) ip] and prepared for in situ brain perfusion, microvessel isolation, and/or hind paw harvesting.
In situ brain perfusion.
The in situ brain perfusion technique was performed as previously described by our laboratory (18, 29). Three hours after paw injection, animals were anesthetized and heparinized [10,000 U/kg (1.0 ml/kg) ip]. Body temperature was maintained at 37°C using a heating pad. The common carotid arteries were cannulated with silicone tubing connected to a perfusion circuit. The perfusate was erythrocyte-free modified mammalian Ringer's solution consisting of the following: 117 mM NaCl, 4.7 mM KCl, 0.8 mM MgSO4, 24.9 mM NaHCO3, 1.2 mM KH2PO4, 2.5 mM CaCl2, 10 mM d-glucose, 3.9% dextran (molecular weight 60,000), and 1.0 g/l BSA (type IV) pH 7.4, warmed to 37°C, and oxygenated with 95% O2-5% CO2. Evan's blue dye (55 mg/l) was added to the perfusate to serve as a visual marker of BBB integrity. Perfusion pressure and flow rate were maintained at 95–105 mmHg and 3.1 ml/min, respectively. Both jugular veins were severed to allow for perfusate drainage. With the use of a slow-drive syringe pump (Harvard Apparatus, Holliston, MA), [14C]sucrose (0.5 μCi/ml; 0.052 mM total concentration) or [3H]codeine (0.5 μCi/ml; 2.75 × 10−5 mM total concentration) was added to inflowing perfusion solution at a rate of 0.5 ml/min per cerebral hemisphere. After a 20-min perfusion followed by a 1-min washout (i.e., inflowing perfusate consisted of modified Ringer's solution without addition of radiolabeled tracer), the rat was decapitated and the brain was removed. The meninges and choroid plexus were excised and the cerebral hemispheres were sectioned. TS2 tissue solubilizer (1.0 ml; Research Products International, Mount Prospect, IL) was added to tissue samples, which were allowed to solubilize for 2 days at room temperature. To eliminate chemiluminescence, 100 μl of 30% glacial acetic acid were added, along with 2.0 ml Optiphase SuperMix liquid scintillation cocktail (PerkinElmer, Boston, MA). Radioactivity was measured using a model 1450 Liquid Scintillation and Luminescence Counter (PerkinElmer). Results were reported as the ratio of radioactivity in the brain to that in the perfusate (RBr), which is equal to the total amount of radioisotope in the brain (CBrain, dpm/g tissue) divided by the amount of radioisotope in the perfusate (CPerfusate, dpm/ml).
Edema formation and paw withdrawal latency.
Hind paw edema and paw withdrawal latency in response to a thermal stimulus were determined as previously described (6, 29). Briefly, hind paw edema was measured using a plethysmometer (model 7141; Ugo Basile, Comeiro, Varese, Italy). Thermal allodynia through paw withdrawal latency was determined using a Plantar Analgesia Instrument (model 7375; Ugo Basile) that used an infrared heat stimulus. To ensure uniformity of testing, rats were prehabituated to the experimental apparatus for 20 min before thermal allodynia measurements. Paw withdrawal latencies are defined as the time (sec) for the rat to remove its paw from the heat source. Three consecutive measurements were performed on the right hind paw with 5-min intervals between measurements. Data were also collected from the left hind paw (i.e., contralateral, noninjected paw) to confirm that edema and thermal allodynia were localized to the injection site.
Microvessel isolation and fractionation.
Three hours after paw injection, rats were anesthetized and decapitated, and brains were removed. Whole brains were placed in 4.0 ml of microvessel isolation buffer [103 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 15 mM HEPES, 25 mM NaHCO3, 10 mM d-glucose, 1 mM sodium pyruvate, and 1% dextran (MW 64,000) pH 7.4] containing protease and phosphatase inhibitors (i.e., 2 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 mM sodium fluoride, and 1 mM sodium pyrophosphate; Roche EDTA-free complete protease inhibitor, Roche, Indianapolis, IN). The homogenates were then centrifuged at 5,800 g for 10 min at 4°C in ice-cold microvessel isolation buffer containing 26% dextran. The supernatant was aspirated, and the pellets were resuspended in microvessel isolation buffer and centrifuged at 5,800 g for 10 min at 4°C in ice-cold buffer containing 26% dextran. Pellets were then resuspended in microvessel isolation buffer and passed through a 100-μm filter. The filtrate was collected and then passed through a 40-μm filter. Microvessels remaining on the 40-μm filter were collected in microvessel isolation buffer and centrifuged at 1,500 g for 10 min at 4°C. Aliquots of whole microvessels were then collected for Western blot analysis of protein nitrosylation. The protein concentration of each sample was determined using the Coomassie Plus Better Bradford Assay kit (Pierce Biotechnology, Rockford, IL).
Experiments to study occludin oligomers in λ-carrageenan-induced peripheral inflammatory pain (CIP) animals or in saline control animals administered tempol were performed on fractionated whole microvessels as previously described (22). Briefly, pellets enriched in whole microvessels were resuspended in fractionation buffer (20 mM Tris·HCl, 250 mM sucrose, 1 mM CaCl2, and 1 mM MgCl2 pH 7.8) containing protease inhibitors, drawn 20 times through a 21-gauge needle, and gently homogenized using a Dounce homogenizer. Equal volume quantities of microvessel homogenates were then mixed with 50% OptiPrep (Accurate Chemical, Westbury, NY) in fractionation buffer. This resultant 25% OptiPrep-microvessel homogenate suspension was layered beneath a discontinuous 0/5/10/15/20% Optiprep gradient (prepared in fractionation buffer without ions) and then centrifuged at 52,000 g for 90 min at 4°C. Microvessel fractions (1.0 ml) were collected from the top of the gradient, and aliquots of each fraction were stored at −20°C until later use. The present study was focused on microvessel fractions 7 and 8 because these fractions are associated with plasma membrane lipid raft domains that are enriched in oligomeric isoforms of occludin (22).
Western blot analysis.
For Western blotting, equal protein aliquots of whole microvessels or equal protein aliquots of microvessel fractions 7 and 8 were mixed in 4× XT sample loading buffer with or without XT reducing agent [i.e., Tris(2-carboxyethyl)phosphine hydrochloride (TCEP)] and heated for 10 min at 70°C. The samples were resolved on 10% sodium dodecylsulfate-polyacrylamide gels (Bis-Tris Criterion XT; Bio-Rad, Hercules, CA). Gels were electrotransferred onto a PVDF membrane. Membranes were blocked in 5% (m/vol) nonfat milk in TBS (15 mM Tris·HCl and 150 mM NaCl pH 7.6) containing 0.05% (vol/vol) Tween-20 (TBS-T) for 1 h at room temperature. After six washes (5 min each) with TBS-T, membranes from SDS-PAGE of microvessel fractions 7 and 8 were incubated overnight at 4°C with polyclonal rabbit anti-occludin antibody (1:1,000 dilution). Similarly, membranes from SDS-PAGE of whole microvessel aliquots were incubated with polyclonal rabbit anti-nitrotyrosine antibody (1:1,000 dilution) overnight at 4°C. After a second wash, membranes were incubated 1 to 2 h with anti-rabbit horseradish-peroxidase-conjugated secondary antibody in TBS-T at room temperature. Protein bands were detected by enhanced chemiluminescence. The membranes were stained for total protein with Coomassie, and the optical density (OD) of each band was normalized to the total protein in each sample (i.e., loading control) according to a previously published method (2). Bands were quantitated and corrected for background using ImageJ densitometric software (Wayne Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, MD).
Immunofluorescence and confocal microscopy.
Immunofluorescence was performed as previously described (18). Briefly, brain microvessels were heat fixed on glass slides at 95°C for 10 min, followed by fixation in ice-cold ethanol for 10 min. The slides were blocked in 2% goat serum and 1% BSA before incubation in rabbit anti-4-HNE antibody (1:250) and in mouse monoclonal antibody directed against the endothelial-specific marker PECAM-1 (1:1,000). Slides were incubated in the presence of both primary antibodies overnight at 4°C. All slides were then incubated with appropriate Alexa Fluor-conjugated secondary antibodies (i.e., Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 514 goat anti-mouse IgG; Invitrogen) for 90 min at room temperature in the dark. All slides from control and treated rats were collected and processed in parallel. Primary antibody was omitted from slides in each treatment group as a negative control.
All slides were imaged on a Zeiss LSM 510 meta-NLO confocal microscope (Zeiss, Oberkochen, Germany) with filters appropriately set to avoid bleed through. Only microvessels positive for PECAM-1 were used for analysis.
Immunohistochemistry.
Hind paws were harvested from anesthetized rats and snap frozen 3 h after injection with saline or λ-carrageenan. Frozen sections (30-μm thick) were placed on glass slides and allowed to air dry before fixation in 3.7% formaldehyde for 10 min at room temperature. Endogenous peroxidase was quenched, and sections were blocked with 3% goat serum and permeabilized with 0.1% Triton X-100 for 30 min at room temperature. Slides were incubated in polyclonal rabbit anti-nitrotyrosine antibody (1:1,000) and then were gently agitated overnight in the antibody amplifier (Prohisto, Columbia, SC) at 4°C. The Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) was used for the rest of the procedure according to manufacturer's instructions. The slides were developed with nickel-conjugated 3,3′-diaminobenzidine and mounted in Clearmount mounting solution (Invitrogen).
Statistical analysis.
Paw edema, paw withdrawal latency, and densitometry analysis data were standardized to percentage of control and are reported as means ± SE from three separate experiments. In this regard, “control” refers to animals injected with 0.9% saline both in the hind paw and intraperitoneally. In our densitometric analyses, each treatment group consists of pooled microvessels from three animals. In situ brain perfusion data are reported as means ± SE from six individual animals per treatment group. To determine statistical significance between treatments in Western blot experiments, Student's t-test was used for unpaired experimental data. To determine significance of brain [14C]sucrose or [3H]codeine accumulation, one-way ANOVA and the post hoc multiple-comparison Bonferroni t-test was used. A value of P < 0.05 was accepted as statistically significant.
RESULTS
Measurement of paw edema and thermal hyperalgesia.
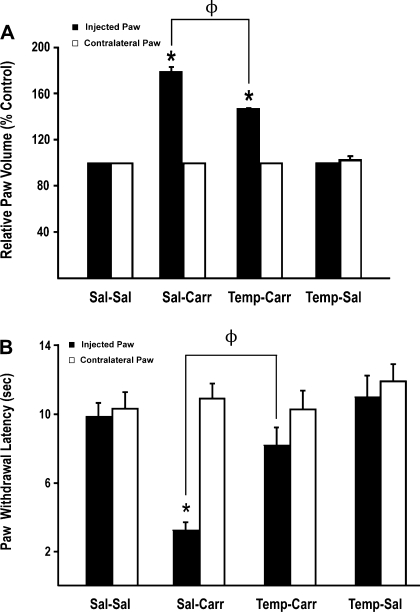
Previous studies (6, 29) in our laboratory have demonstrated that CIP enhances paw swelling as well as the animal's response to noxious thermal stimuli. To determine if pharmacological scavenging of ROS affected peripheral inflammatory pain, we measured both of these parameters in the presence and absence of the SOD mimetic tempol. Three hours after paw injection, administration of λ-carrageenan induced a significant (P < 0.05) right hind paw edema (Fig. 1A). Administration of tempol before CIP attenuated paw edema. Tempol had no effect on paw edema in saline control animals. For comparison purposes, the contralateral (i.e., left) hind paw was measured and showed no volume changes among any of the treatment groups studied.
Fig. 1.
Effect of λ-carrageenan-induced peripheral inflammatory pain (CIP) on paw edema and thermal allodynia and the effect of tempol administration. A: edema formation in the injured paw (i.e., injected) and in the contralateral paw measured as the relative paw volume 3 h after injection with saline or λ-carrageenan in rats in the presence and absence of tempol. Results are expressed as means ± SE of 6 animals per treatment group. Temp-Sal, tempol-saline. *P < 0.05, for comparison between treatment groups and control animals [i.e., saline-saline (Sal-Sal)]. ΦP < 0.05 for comparison between saline-carrageenan (Sal-Carr) and tempol-carrageenan (Temp-Carr) treatment groups. B: thermal allodynia based on paw withdrawal latency from an infrared heat source. Paw withdrawal latency was measured 3 h after injection with saline or λ-carrageenan in rats in the presence and absence of tempol. Results are expressed as means ± SE of six animals per treatment group. *P < 0.05, for comparison between treatment groups and control animals (i.e., Sal-Sal). ΦP < 0.05, for comparison between Sal-Carr and Temp-Carr treatment groups.
Thermal hyperalgesia during CIP was determined based on paw withdrawal latency from an infrared heat source. The response to this thermal stimulation was significantly increased (P < 0.05) in animals subjected to pain/inflammation compared with saline controls (Fig. 1B). Injection of tempol before footpad injection significantly attenuated this response in λ-carrageenan-treated animals. Taken together, these results suggest that tempol administration can greatly decrease the peripheral inflammatory pain response in rats subjected to λ-carrageenan-induced peripheral inflammatory pain.
Nitrotyrosine immunoreactivity in the paw.
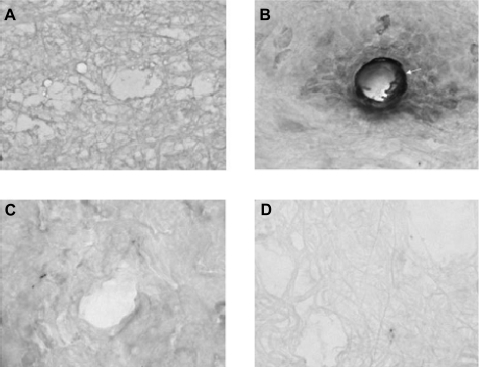
Excess production of ROS and/or oxidative stress is directly associated with formation of protein adducts containing 3-nitrotyrosine (26, 27). Nitrosylation of proteins occurs when tyrosine residues react with peroxynitrite, a free radical that is produced by the reaction between superoxide and nitric oxide. It has been previously shown that protein modifications demarcated by increased formation of 3-nitrotyrosinylated proteins are produced in rodents following injection with λ-carrageenan (36). Therefore, we investigated if tempol could attenuate protein nitrosylation at the site of injury (i.e., hind paw injected with λ-carrageenan) via immunohistochemistry. In control (i.e., saline treated) animals, we did not detect any staining for 3-nitrotyrosine in the injected hindpaw (Fig. 2A). In contrast, 3-nitrotyrosine immunoreactivity was observed in the hind paw following injection of λ-carrageenan (Fig. 2B), which suggests induction of oxidative stress in the periphery. This increase in immunoreactivity was primarily detected in both vascular and perivascular tissues. In animals administered tempol 10 min before paw injection, no nitrotyrosine immunoreactivity was observed in animals subjected to CIP (Fig. 2C) or in saline control animals (Fig. 2D). These data indicate that the CIP-associated oxidative stress response in the inflamed paw is attenuated by administration of tempol.
Fig. 2.
Immunohistochemical analysis examining 3-nitrotyrosine immunoreactivity in the hind paw during CIP in the presence and absence of tempol. Rats were injected (ip) with saline or tempol 10 min before paw injection with either saline or λ-carrageenan. Injected paws were sectioned and immunostained for 3-nitrotyrosine. A: saline + saline. B: saline + λ-carrageenan. C: tempol + λ-carrageenan. D: tempol + saline. Images were taken at ×400 magnification and represent 3 animals per treatment group. Arrow corresponds to vascular tissue that shows intense 3-nitrotyrosine immunoreactivity.
Expression of 3-nitrotyrosine in rat brain microvessels during CIP.
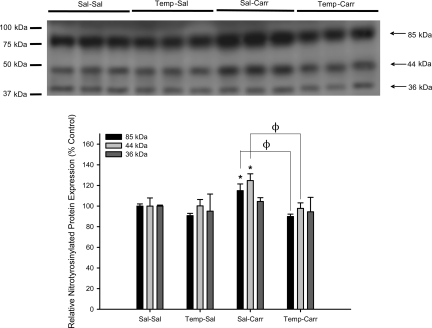
Similar to the periphery, formation of protein adducts containing 3-nitrotyrosine can occur in the brain in response to ROS production and/or oxidative stress (26, 27). Therefore, we measured protein nitrosylation in microvessels isolated from rats subjected to 3 h CIP. Western blot analysis detected prominent bands corresponding to 3-nitrotyrosine at 85, 44, and 36 kDa (Fig. 3). CIP increased formation of protein adducts containing 3-nitrotyrosine at 85 kDa (1.2-fold) and 44 kDa (1.3-fold) compared with saline controls (Fig. 3). Interestingly, administration of tempol before CIP induction prevented the increase in formation of both of these nitrotyrosinylated proteins. Taken together, these data indicate that acute inflammatory pain induces oxidative stress-associated damage in brain microvessels, which can be reversed by exogenous administration of a pharmacological ROS scavenger such as tempol.
Fig. 3.
Expression of proteins containing 3-nitrotyrosine in brain microvessels during CIP in the presence and absence of tempol. Western blot analysis of microvessels isolated from rats treated with saline or λ-carrageenan in the presence and absence of tempol, a SOD mimetic and reactive oxygen species (ROS) scavenger. Whole brain microvessel preparations (10 μg) were resolved on a 10% sodium dodecyl-sulfate-polyacrylamide gel and transferred to a PVDF membrane. Samples were analyzed for expression of 3-nitrotyrosine containing proteins using the rabbit polyclonal anti-3-nitrotyrosine antibody (1:1,000 dilution). Relative levels of protein adducts containing 3-nitrotyrosine were determined by densitometric analysis. Results are expressed as means ± SE of 3 separate experiments. *P < 0.05, for comparison between treatment groups and control animals (i.e., Sal-Sal). ΦP < 0.05, for comparison between Sal-Carr and Temp-Carr treatment groups.
Localization of 4-HNE to rat brain microvessels during CIP.
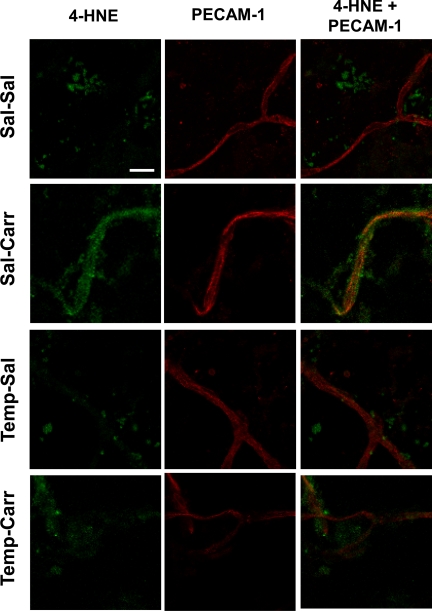
To further examine effects of oxidative stress during CIP on rat brain microvessels, we examined endothelial localization of 4-HNE, a well-established marker of oxidative stress. We observed marked changes in isolated brain microvessels labeled for 4-HNE and vascular endothelial cells labeled with PECAM-1 in animals subjected to CIP in the presence and absence of tempol (Fig. 4). In control (i.e., saline-treated) animals, vessels were clearly demarcated by staining with the endothelial-specific marker PECAM-1 but displayed no detectable staining for 4-HNE. After CIP (3 h), an increase in 4-HNE staining was observed along the entire length of the microvessel, which is indicative of an oxidative stress response. Of particular significance, tempol dramatically reduced, but did not completely abolish, localization of 4-HNE staining to brain microvessels under conditions of CIP. Tempol had no effect on 4-HNE staining in brain microvessels in animals injected with saline. Overall, these data further demonstrate that acute inflammatory pain induces oxidative-stress-associated damage in brain microvessels. Furthermore, oxidative damage to brain microvasculature can be attenuated by administration of an antioxidant drug such as tempol.
Fig. 4.
Localization of 4-hydroxynoneal (4-HNE) in brain microvessels during CIP in the presence and absence of tempol. Immunofluorescence of 4-HNE (green) and the endothelial-specific marker platelet-endothelial cell adhesion molecule 1 (PECAM-1; red) in brain microvessels from animals with saline or λ-carrageenan in the presence and absence of tempol, a SOD mimetic and ROS scavenger. Images represent 6 rats per treatment group (n = 6). Scale bar = 20 μm.
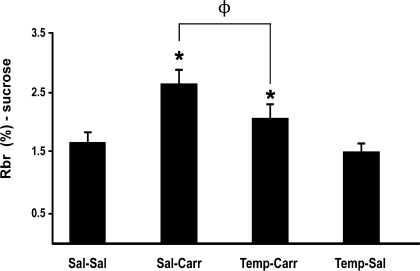
BBB permeability to [14C]sucrose.
To investigate if ROS/oxidative stress are involved in regulation of BBB permeability during acute inflammatory pain, we measured brain uptake of [14C]sucrose in the presence and absence of tempol in saline-treated and CIP animals. Because tempol attenuated oxidative stress-associated molecular changes in brain microvessels, we postulated that this SOD mimetic may also prevent the increased BBB permeability that is known to occur in response to peripheral inflammatory pain (6, 14, 29). In control (i.e., saline-treated) animals, the RBr was 1.67 ± 0.16% (Fig. 5). Three hours after CIP, the RBr for [14C]sucrose was significantly increased (2.65 ± 0.21%; P < 0.05), suggesting an increase in BBB permeability associated with inflammatory pain. Administration of 200 mg/kg tempol 10 min before footpad injection significantly reduced the RBr for [14C]sucrose in animals treated with λ-carrageenan (2.08 ± 0.21%; P < 0.05). Pretreatment with tempol in saline-treated (i.e., control) rats did not significantly alter the RBr for [14C]sucrose (1.51 ± 0.14%). Overall, these data suggest that ROS-induced oxidative stress is prominently involved in the regulation of BBB permeability during peripheral inflammatory pain. A visual assessment of the brain parenchyma after in situ perfusion showed no Evan's blue-albumin staining, suggesting that the BBB was morphologically intact.
Fig. 5.
Changes in paracellular permeability to [14C]sucrose during peripheral inflammatory pain in the presence and absence of tempol. Graph shows the percentage of radioactivity detected in the brain compared with that in the perfusate (RBr %) for the 4 treatment groups 3 h after injection of 3% λ-carrageenan or 0.9% saline into the plantar surface of the right hind paw. Tempol (200 mg/kg) or vehicle (0.9% saline) were injected intraperitoneally 10 min before footpad injection. Results are expressed as means ± SE of 6 animals per treatment group. *P < 0.05, for comparison between treatment groups and control animals (i.e., Sal-Sal). ΦP < 0.05, for comparison between Sal-Carr and Temp-Carr treatment groups.
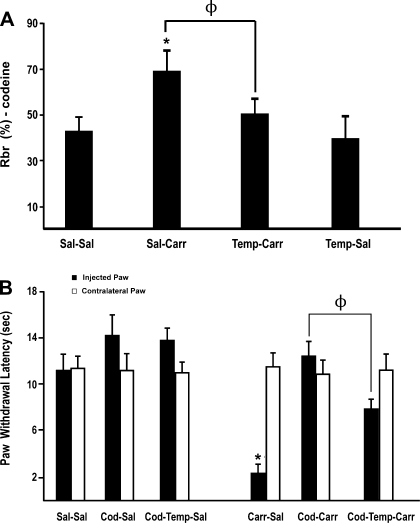
BBB permeability to [3H]codeine.
Our sucrose data indicate that brain microvascular permeability to circulating xenobiotics is enhanced in response to CIP. Furthermore, our results also imply that tempol, via its known pharmacological activity as a ROS scavenging drug, can attenuate these changes in BBB paracellular permeability. Therefore, we sought to determine if these alterations in microvascular permeability affected CNS uptake of an analgesic drug commonly used in treatment regimens for management of inflammatory pain. We focused our analyses on codeine, an opioid analgesic that is known to access brain parenchyma via the paracellular route and is widely prescribed for pain relief (9). In saline control animals, the RBr was 42.4 ± 6.9% (Fig. 6A). Three hours after CIP, the RBr for [3H]codeine was significantly increased (68.9 ± 9.4%; P < 0.05), suggesting an increase in BBB permeability associated with inflammatory pain. Tempol treatment before footpad injection attenuated this increase in the RBr for [3H]codeine in CIP animals (49.8 ± 7.0%; P < 0.05). Administration of tempol in saline-treated (i.e., control) rats did not significantly alter the RBr for [3H]codeine (40.7 ± 8.8%). These results suggest that ROS-induced oxidative stress is prominently involved in regulation of BBB permeability during peripheral inflammatory pain. A visual assessment of brain tissue immediately following in situ perfusion showed no Evan's blue-albumin staining, which implies that the BBB was morphologically intact.
Fig. 6.
Effect of peripheral inflammatory pain on codeine brain uptake and analgesia in the presence and absence of tempol. A: graph shows the percentage of radioactivity for [3H]codeine detected in the brain compared with that in the perfusate (RBr %) for the 4 treatment groups 3 h after injection of 3% λ-carrageenan or 0.9% saline into the plantar surface of the right hind paw. Tempol (200 mg/kg) or vehicle (0.9% saline) were injected intraperitoneally 10 min before footpad injection. Results are expressed as means ± SE of 6 animals per treatment group. *P < 0.05, for comparison between treatment groups and control animals (i.e., Sal-Sal). ΦP < 0.05, for comparison between Sal-Carr and Temp-Carr treatment groups. B: thermal allodynia based on paw withdrawal latency from an infrared heat source for animals subjected to CIP and administered codeine (Cod; 7.0 mg/kg) or Cod (7.0 mg/kg) and tempol (200 mg/kg). Paw withdrawal latency was measured 3 h after injection with saline or λ-carrageenan in rats. Results are expressed as means ± SE of 6 animals per treatment group. *P < 0.05, for comparison between treatment groups and control animals (i.e., Sal-Sal). ΦP < 0.05, for comparison between Cod-Carr and Cod-Temp-Carr treatment groups.
Codeine analgesia following CIP.
Codeine analgesia in animals treated with saline or λ-carrageenan for 3 h in presence and absence of tempol was determined based on paw withdrawal latency from an infrared heat source. Codeine (7.0 mg/kg) significantly enhanced (P < 0.05) paw withdrawal latency in animals subjected to CIP, which suggests an increased analgesic effect by codeine (Fig. 6B). Interestingly, this codeine-associated enhancement in paw withdrawal latency was reduced by administration of tempol. This observation correlates well with our in situ perfusion data, which show that tempol reduces CNS codeine uptake under CIP conditions. Tempol had no effect on codeine analgesia in saline-treated animals. Taken together, these data suggest that tempol reduces the pharmacological effect of codeine (i.e., analgesia) under CIP conditions.
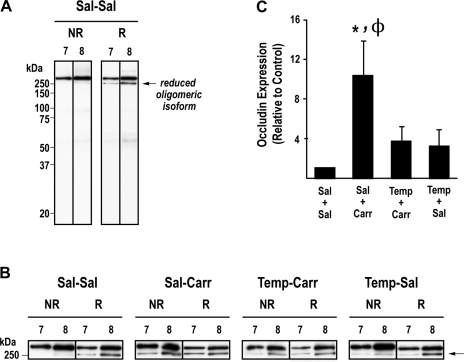
Expression of occludin oligomers.
Our laboratory (21) has previously demonstrated that assembly of occludin into oligomeric structures at the BBB is modulated by CIP. To determine if ROS production/oxidative stress had an effect on occludin oligomeric assemblies, we performed Western blot analysis on fractionated brain microvessels isolated from control (i.e., saline-treated) and CIP animals that were administered tempol or vehicle (i.e., 0.9% saline). Enriched microvessel preparations were subjected to density gradient subcellular fractionation to analyze TJ-associated plasma membrane lipid raft domains (fraction 7 and 8) containing oligomeric assemblies of occludin, as previously shown by our laboratory (22). Equal protein aliquots of fractions 7 and 8 were examined by SDS-PAGE/Western blot analysis under nonreducing and reducing conditions to identify changes in occludin oligomeric integrity due to disulfide-bond reduction. Our data show the banding pattern of occludin under noreducing and reducing conditions in fractions 7 and 8 in control animals (Fig. 7A). Under nonreducing conditions, occludin is expressed almost exclusively as a single high molecular weight oligomer in these fractions. Upon exposure to TCEP, TJ-associated occludin is detected as a doublet with the lower band (i.e., 250 kDa) representing a reduced occludin isoform. Hydrophilic reducing agents (i.e., TCEP) are unable to access the hydrophobic core of occludin oligomeric assemblies, which causes only partial reduction of higher order protein complexes and presence of bands corresponding to occludin oligomers under reducing SDS-PAGE conditions. A greater amount of reduced oligomeric isoform of occludin is present in the higher density fraction 8, which contains occludin oligomers that are not as tightly packed and, therefore, more susceptible to reduction by TCEP. Figure 7B shows representative Western blots of occludin oligomers electrophoresed under nonreducing and reducing conditions in all treatment groups. Given that appearance of reduced occludin oligomeric isoforms at a molecular mass of 250 kDa is indicative of disulfide-bond reduction, relative changes in the measured OD of this band in fraction 8 (under noreducing conditions) were used to compare effects of different treatments on occludin oligomeric integrity (Fig. 7C). Samples prepared from animals treated with λ-carrageenan revealed, under noreducing conditions only, significant increases (P < 0.05) in OD of the reduced occludin isoform, suggesting that inflammatory pain promotes cleavage of disulfide bonds within occludin oligomeric assemblies at the TJ. Tempol significantly decreased the OD of the reduced oligomeric occludin isoform, providing evidence that ROS induces disulfide bond breakage of occludin oligomers during inflammatory pain. As protein complexes with a structural role, alterations in disulfide bonding of occludin oligomeric assemblies may lead to changes in functional integrity of BBB TJs. The OD of occludin oligomers electrophoresed under noreducing conditions in the saline-carrageenan-treated group was significantly increased compared with the other treatment groups (P < 0.01 vs. saline-saline; P < 0.05 vs. tempol-carrageenan and tempol-saline).
Fig. 7.
Effect of peripheral inflammatory pain on occludin oligomeric assemblies in the presence and absence of tempol. Rats were given an intraperitoneal injection of saline or tempol (200 mg/kg) 10 min before interplantar injection of saline or 3% λ-carrageenan. A: banding pattern of occludin in nonreduced (NR) and reduced (R) conditions in tight junction (TJ) fractions 7 and 8 in control animals. B: representative bands of TJ associated occludin oligomers under nonreducing and reducing conditions in all treatment groups. C: optical density of the reduced TJ occludin oligomeric isoform. Results are expressed as means ± SE of 3 separate experiments. *P < 0.05, for comparison between treatment groups and control animals (i.e., Sal-Sal). ΦP < 0.05, for comparison between Sal-Carr and Temp-Carr treatment groups.
DISCUSSION
The BBB is a highly regulated interface between brain parenchyma and systemic circulation (1, 10). It is characterized by the presence of precisely maintained TJ protein complexes between adjacent endothelial cells that limit paracellular uptake of xenobiotics into brain extracellular milieu. Our laboratory (6, 12–14, 21, 29) has previously demonstrated reorganization of BBB TJ complexes in response to acute peripheral inflammatory pain. Such changes were directly correlated with increased CNS uptake of circulating solutes including sucrose (6, 9, 13, 29) and codeine (9). Recently, we have begun to delineate specific biochemical mechanisms that enable peripheral pain/inflammation to “transmit” upstream signals and alter TJ protein expression and/or BBB permeability. For example, Brooks et al. (5) administered diclofenac, a commonly prescribed nonsteroidal anti-inflammatory drug, to rats subjected to CIP and demonstrated that this therapeutic compound attenuated the increase in BBB permeability to sucrose observed in CIP animals (5). The results of this study and others (7) have clearly shown that cyclooxygenase activity is a critical regulator of BBB functional integrity. Additionally, we reported that transforming growth factor-β (TGF-β) signaling is involved in modulating substrate permeability and TJ protein expression at the BBB during acute peripheral inflammatory pain (29), an observation that further emphasizes the complexity of BBB regulation in response to pathological stressors. Of paramount significance, we showed that the cyclooxygenase inhibitor diclofenac interacts with the TGF-β pathway by preventing both decreases in serum TGF-β and reduced microvascular expression of activin receptor-like kinase (ALK) 1/ALK5 in animals subjected to CIP, providing evidence for cross-talk between these two regulatory pathways (30).
In addition to inflammatory mechanisms, it is essential to consider that other pathophysiological processes (i.e., oxidative stress) may occur simultaneously. Production of highly potent ROS such as superoxide anion is a well-established component of both pain and inflammation (16, 31, 36). Our data corroborate this phenomenon because we showed that paw edema and thermal allodynia elicited by CIP are both attenuated by administration of tempol, a pharmacological ROS scavenger and SOD mimetic. Biological activity of superoxide anion, a by product of normal physiological processes, is tightly controlled by SOD enzymes. During acute inflammation, superoxide is produced at such high levels that the ability of SODs to metabolize it is overwhelmed (31). In this context, increased serum levels of superoxide result in oxidative stress and subsequent injury to the BBB endothelium (4, 40). One mechanism of cellular damage involves peroxynitrite, a potent cytotoxic and proinflammatory molecule formed by conjugation of superoxide with nitric oxide. Peroxynitrite is well known to induce cellular damage by its ability to nitrosylate tyrosine residues, leading to functional modifications of critical proteins (31). Therefore, we postulated that CIP could induce oxidative stress-associated cellular damage at both the site of injury (i.e., hind paw injected with λ-carrageenan) and at the BBB. Indeed, we showed increased 3-nitrotyrosine immunostaining in the injected hind paw and enhanced formation of nitrotyrosine-conjugated proteins in whole brain microvessels isolated from CIP rats. Our Western blots of 3-nitrotyrosine protein adduct formation are particularly intriguing as we report increased presence of distinct bands at molecular masses (85 and 44 kDa) that have been reported to correspond to major 3-nitrotyrosine protein adducts in vivo (11). Although the magnitude of these changes were small, they were statistically significant. These data may indicate that we have detected an early phase of microvessel damage in response to oxidative stress. Immunostaining for 3-nitrotyrosine and the presence of 3-nitrotyrosinylated proteins were not altered in CIP animals that were administered tempol, which demonstrates that this ROS scavenger can protect against oxidative stress-associated cellular damage at the BBB during peripheral inflammatory pain. Evidence of oxidative stress-associated microvessel damage is further shown by increased localization of 4-HNE, a well-known marker of oxidative stress, in brain microvessels isolated from CIP rats. Tempol also reduced 4-HNE-associated fluorescence, an observation that points to tempol's ability to protect the BBB from oxidative damage. Tempol is an amphilite nitroxide mimetic of SOD that is membrane permeable and a potent metabolizer of superoxide anion and several other ROS (38). In fact, this mechanism of action has enabled use of tempol for the study of superoxide-associated oxidative stress effects in several models of brain pathologies including angiotensin II cerebral microvascular inflammation (40) and CNS injury associated with hypoxia/reoxygenation (18). Our present study is the first to demonstrate this relationship in the context of peripheral pain/inflammation.
Based on the above results, we hypothesized that oxidative stress-associated damage may be a critical event that contributes to enhance xenobiotic permeability at the BBB. We tested this hypothesis by in situ brain perfusion using [14C]sucrose. The BBB is slightly permeable to sucrose under normal physiological conditions (3). In the present study, λ-carrageenan treatment enhanced brain [14C]sucrose uptake within 3 h, suggesting that BBB permeability is altered in response to acute peripheral inflammatory pain. Involvement of oxidative stress on regulation of BBB permeability during CIP was evaluated by incorporating tempol into our in situ perfusion studies. Interestingly, pretreatment with tempol before induction of CIP led to an increase in [14C]sucrose brain uptake compared with saline controls; however, this increase was not as large in magnitude as that observed in CIP animals, which indicates that tempol can only partially protect against BBB permeability changes that are characteristic of peripheral inflammatory pain. By scavenging superoxide anion, tempol restores biological activity of nitric oxide; however, elevated levels of nitric oxide, as are known to occur during the cellular response to oxidative stress, can increase BBB permeability to circulating solutes (20). Therefore, tempol can protect against peroxynitrite-associated cellular injury but may increase circulating levels of nitric oxide, an effect that can explain the incomplete attenuation of permeability changes observed in our tempol-CIP treatment group.
Our studies with [14C]sucrose implied that altered BBB paracellular permeability (i.e., leak) may have a significant impact on CNS drug delivery. Indeed, we demonstrated that opening of the paracellular route to circulating solutes does lead to increased drug uptake of specific therapeutics. One such drug is the moderate μ-opioid receptor agonist codeine. The therapeutic effects of codeine are centrally mediated thus indicating that this opioid analgesic must accumulate within the CNS to exert its analgesic and antitussive properties (9). Furthermore, physicochemical properties of codeine dictate that BBB transport primarily occurs via passive diffusion and is highly dependent on BBB transcellular permeability and blood flow (39). Therefore, any pathophysiological stimulus that alters BBB integrity will likely affect CNS codeine uptake. Using the λ-carrageenan model of peripheral inflammatory pain, Hau et al. (9) showed enhanced brain uptake of codeine at 3 and 48 h postinjection of λ-carrageenan compared with saline controls. Furthermore, antinociception studies (i.e., radiant-heat tail flick measurement) demonstrated that increased brain uptake of codeine led to an enhanced antinociceptive profile (9). In the present study, we show that tempol administration attenuates CNS uptake of codeine and enhances paw withdrawal latency in animals subjected to CIP. These data are critical for pharmacotherapy, particularly with respect to therapeutic drug dosing and/or adverse reactions that are commonly associated with opioid analgesics (i.e., neurotoxicity, addiction, and acute tolerance). Such adverse events are the result of increased opioid concentrations within the CNS (28) and may occur due to increased BBB leak induced by pain/inflammation in the periphery. Elevated opioid concentrations can also magnify adverse events by opioid-induced glial activation, which is mediated by Toll-like receptor 4 (TLR4) at the glial cell surface (37). Activation of glial TLR4 receptors is involved in neuropathic pain and directly counteracts opioid analgesic efficacy (37). Although opioid-mediated activation of glial TLR4 signaling can be inhibited by (+)-naloxone or (+)-naltrexone (15), it remains critical that opioid concentrations in the brain be maintained precisely to ensure efficacious management of pain and to limit adverse drug reactions. In this context, our current data suggest that tempol may be a useful adjuvant to opioid therapy by preventing BBB changes associated with peripheral inflammatory pain. This is highly clinically significant as coadministration of tempol with an opioid for management of pain/inflammation in the periphery may enable more accurate control of CNS opioid concentrations and a reduction in incidence of adverse drug reactions.
It has been previously indicated that changes in paracellular permeability induced by peripheral inflammatory pain are not sufficient to enable CNS penetration of small molecule therapeutics (19). Specifically, Lu et al. (19) utilized Evan's blue dye as a model of a small molecule and, therefore, as an indicator of BBB integrity. In the interpretation of these studies, it is important to consider that use of Evan's blue dye is inappropriate in this context. Evan's blue dye, when unconjugated to plasma proteins, has a molecular mass of 960.8 Da. It is well established that Evan's blue dye irreversibly binds to serum albumin in vivo. This leads to formation of a very large, solute-protein complex (i.e., in excess of 60,000 Da) that can only traverse the BBB under considerable pathological stress (i.e., leak) such as that observed during ischemic stroke (33). In the context of peripheral inflammatory pain, changes in BBB permeability are not sufficient to allow leakage of large molecules such as Evan's blue-albumin from the systemic circulation. In fact, we (6, 9, 13, 29, 32) have shown that altered BBB functional integrity in response to peripheral inflammatory pain affects brain microvascular permeability only to small xenobiotics such as sucrose, codeine, and morphine. In all of these studies, we measured CNS uptake of Evan's blue-albumin and did not observe or detect any measurable uptake of this extremely large protein complex, further suggesting that BBB opening during peripheral inflammatory pain will only affect paracellular uptake of small molecule solutes such as opioid analgesic drugs.
TJs are responsible for maintaining low BBB paracellular permeability to xenobiotics. One of the principal components of TJ protein complexes is occludin (8). At the BBB, occludin exists in lipid rafts almost exclusively as high molecular mass (>250 kDa) oligomeric assemblies (22). Using a newly developed, detergent-free, density gradient centrifugation and subcellular fractionation of enriched brain microvessel preparations, we (21, 23) have previously observed structural alterations in occludin assemblies at the TJ during CIP and hypoxia/reoxygenation. Furthermore, administration of tempol to animals subjected to global hypoxia attenuated disruption of occludin oligomeric assemblies, suggesting that TJ protein complexes are sensitive to modulation by ROS and/or oxidative stress (18). Our present study showed that CIP induced no changes in relative expression of occludin oligomers when microvessel samples were subjected to SDS-PAGE and Western blot analysis under standard reducing conditions. Interestingly, when the same samples were subjected to SDS-PAGE under nonreducing conditions, expression of a lower molecular weight, reduced oligomeric isoform of occludin was shown to be significantly increased by peripheral inflammatory pain. This suggests that CIP caused cleavage of disulfide bonds within occludin oligomeric assemblies. It has been suggested that the C terminal of occludin is involved in oligomerization in a redox-sensitive manner through formation of disulfide bonds (34, 35). Conformational changes in occludin oligomers at the TJ via disulfide bond cleavage can lead to increased paracellular permeability (21). In animals administered tempol before injection with λ-carrageenan, the increase in expression of the reduced occludin oligomeric isoform was significantly attenuated, which implies that TJ integrity is modulated by ROS/oxidative stress in animals subjected to CIP. This observation directly relates to tempol's ability to scavenge ROS because superoxide has been shown to be a potent reducing agent that can reduce disulfide bonds and affect cellular function (25).
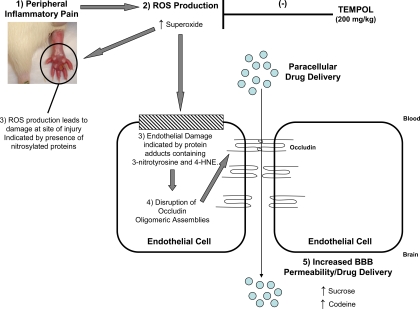
In summary, this study describes altered BBB functional integrity in an in vivo model of inflammatory pain. Our data provide evidence for involvement of ROS production and subsequent oxidative stress in regulation of BBB permeability and assembly of the critical TJ protein occludin into higher order structures during an inflammatory pain response triggered by λ-carrageenan (Fig. 8). Our results also provide evidence that the SOD mimetic tempol can attenuate permeability changes to xenobiotics (i.e., sucrose, codeine) and protect occludin oligomeric assemblies from disruption during CIP. Overall, these observations suggest that pharmacological scavenging of ROS is a viable therapeutic approach for maintaining BBB functional integrity during acute inflammatory pain.
Fig. 8.
Proposed involvement of ROS production and subsequent oxidative stress during acute peripheral inflammatory pain. Acute CIP, characterized by an increase in paw edema and thermal allodynia (1), contributes to production of ROS such as superoxide (2). In turn, this leads to tissue damage at the site of injury (i.e., hind paw injected with λ-carrageenan) demarcated by increased 3-nitrotyrosine immunostaining (3). Additionally, ROS production also causes endothelial cell damage characterized by increased formation of protein adducts containing 3-nitrotyrosine in brain microvessels (3). Increased presence of 3-nitrotysine protein adducts is indicative of oxidative stress, which leads to disruption of TJ protein complexes. Specifically, occludin oligomeric assemblies are modulated (4), which then leads to increased paracellular permeability and enhanced access of the brain extracellular milieu to therapeutic agents such as codeine (5). Administration of SOD mimetics such as tempol prevents endothelial cell damage and protects the blood-brain barrier from pathological changes associated with pain/inflammation (i.e., increased permeability to circulating solutes and disruption of TJ protein complexes). This occurs by tempol's ability to scavenge superoxide anions, which reduces conjugation with nitric oxide to form peroxynitrite and thereby prevents cellular damage associated with protein nitrosylation.
GRANTS
This work was supported by National Institutes of Health Grants R01-NS-42652 and R01-DA-11271 (to T. P. Davis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.J.L., T.P.D., and P.T.R. conception and design of research; J.J.L., G.M., L.S.-C., J.D.F., K.M.D., C.E.Q., and P.T.R. performed experiments; J.J.L., G.M., T.P.D., and P.T.R. analyzed data; J.J.L., G.M., T.P.D., and P.T.R. interpreted results of experiments; J.J.L., G.M., T.P.D., and P.T.R. prepared figures; J.J.L., T.P.D., and P.T.R. drafted manuscript; T.P.D. and P.T.R. edited and revised manuscript; T.P.D. and P.T.R. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address for J. J. Lochhead: School of Pharmacy, University of Wisconsin, Madison, WI.
REFERENCES
- 1. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 37: 13–25, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods 172: 250–254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhattacharjee AK, Nagashima T, Kondoh T, Tamaki N. Quantification of early blood-brain barrier disruption by in situ brain perfusion technique. Brain Res Brain Res Protoc 8: 126–131, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Blasig IE, Bellmann C, Cording J, del Vecchio G, Zwanziger D, Huber O, Haseloff RF. Occludin protein family: oxidative stress and reducing conditions. Antioxid Redox Signal 15: 1195–1219, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Brooks TA, Nametz N, Charles R, Davis TP. Diclofenac attenuates the regional effect of lambda-carrageenan on blood-brain barrier function and cytoarchitecture. J Pharmacol Exp Ther 325: 665–673, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Campos CR, Ocheltree SM, Hom S, Egleton RD, Davis TP. Nociceptive inhibition prevents inflammatory pain induced changes in the blood-brain barrier. Brain Res 1221: 6–13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, Fiebich BL, Rosenberg GA. Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J Pharmacol Exp Ther 323: 488–498, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev 57: 883–917, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Hau VS, Huber JD, Campos CR, Davis RT, Davis TP. Effect of lambda-carrageenan-induced inflammatory pain on brain uptake of codeine and antinociception. Brain Res 1018: 257–264, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57: 173–185, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Hinson JA, Michael SL, Ault SG, Pumford NR. Western blot analysis for nitrotyrosine protein adducts in livers of saline-treated and acetaminophen-treated mice. Toxicol Sci 53: 467–473, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Huber JD, Campos CR, Mark KS, Davis TP. Alterations in blood-brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol 290: H732–H740, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huber JD, Hau VS, Borg L, Campos CR, Egleton RD, Davis TP. Blood-brain barrier tight junctions are altered during a 72-h exposure to λ-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol 283: H1531–H1537, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol 280: H1241–H1248, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of Toll-like receptor 4 (TLR4). Eur J Neurosci 28: 20–29, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khattab MM. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur J Pharmacol 548: 167–173, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain 133: 9–17, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, Davis TP. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab 30: 1625–1636, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu P, Gonzales C, Chen Y, Adedoyin A, Hummel M, Kennedy JD, Whiteside GT. CNS penetration of small molecules following local inflammation, widespread systemic inflammation or direct injury to the nervous system. Life Sci 85: 450–456, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Mark KS, Burroughs AR, Brown RC, Huber JD, Davis TP. Nitric oxide mediates hypoxia-induced changes in paracellular permeability of cerebral microvasculature. Am J Physiol Heart Circ Physiol 286: H174–H180, 2004 [DOI] [PubMed] [Google Scholar]
- 21. McCaffrey G, Seelbach MJ, Staatz WD, Nametz N, Quigley C, Campos CR, Brooks TA, Davis TP. Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia. J Neurochem 106: 2395–2409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD, Davis TP. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem 103: 2540–2555, 2007 [DOI] [PubMed] [Google Scholar]
- 23. McCaffrey G, Willis CL, Staatz WD, Nametz N, Quigley CA, Hom S, Lochhead JJ, Davis TP. Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem 110: 58–71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci 109: 2287–2298, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Peterson DA, Archer SL, Weir EK. Superoxide reduction of a disulfide: a model of intracellular redox modulation? Biochem Biophys Res Commun 200: 1586–1591, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Phares TW, Fabis MJ, Brimer CM, Kean RB, Hooper DC. A peroxynitrite-dependent pathway is responsible for blood-brain barrier permeability changes during a central nervous system inflammatory response: TNF-alpha is neither necessary nor sufficient. J Immunol 178: 7334–7343, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Readnower RD, Chavko M, Adeeb S, Conroy MD, Pauly JR, McCarron RM, Sullivan PG. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J Neurosci Res 88: 3530–3539, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ronaldson PT, Davis TP. Targeting blood-brain barrier changes during inflammatory pain: an opportunity for optimizing CNS drug delivery. Ther Deliv 2: 1015–1041, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ronaldson PT, DeMarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb Blood Flow Metab 29: 1084–1098, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ronaldson PT, Finch JD, DeMarco KM, Quigley CE, Davis TP. Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier. J Pharmacol Exp Ther 336: 827–839, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans 34: 965–970, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Seelbach MJ, Brooks TA, Egleton RD, Davis TP. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem 102: 1677–1690, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Strbian D, Durukan A, Pitkonen M, Marinkovic I, Tatlisumak E, Pedrono E, Abo-Ramadan U, Tatlisumak T. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience 153: 175–181, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Walter JK, Castro V, Voss M, Gast K, Rueckert C, Piontek J, Blasig IE. Redox-sensitivity of the dimerization of occludin. Cell Mol Life Sci 66: 3655–3662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walter JK, Rueckert C, Voss M, Mueller SL, Piontek J, Gast K, Blasig IE. The oligomerization of the coiled coil-domain of occludin is redox sensitive. Ann NY Acad Sci 1165: 19–27, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther 309: 869–878, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci 30: 581–591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of Tempol and other nitroxides. Pharmacol Rev 60: 418–469, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xie R, Hammarlund-Udenaes M. Blood-brain barrier equilibration of codeine in rats studied with microdialysis. Pharm Res 15: 570–575, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience 171: 852–858, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57: 178–201, 2008 [DOI] [PubMed] [Google Scholar]