Abstract
Erythrocytes have been implicated as controllers of vascular caliber by virtue of their ability to release the vasodilator ATP in response to local physiological and pharmacological stimuli. The regulated release of ATP from erythrocytes requires activation of a signaling pathway involving G proteins (Gi or Gs), adenylyl cyclase, protein kinase A, and the cystic fibrosis transmembrane conductance regulator as well as a final conduit through which this highly charged anion exits the cell. Although pannexin 1 has been shown to be the final conduit for ATP release from human erythrocytes in response to reduced oxygen tension, it does not participate in transport of ATP following stimulation of the prostacyclin (IP) receptor in these cells, which suggests that an additional protein must be involved. Using antibodies directed against voltage-dependent anion channel (VDAC)1, we confirm that this protein is present in human erythrocyte membranes. To address the role of VDAC in ATP release, two structurally dissimilar VDAC inhibitors, Bcl-xL BH44–23 and TRO19622, were used. In response to the IP receptor agonists, iloprost and UT-15C, ATP release was inhibited by both VDAC inhibitors although neither iloprost-induced cAMP accumulation nor total intracellular ATP concentration were altered. Together, these findings support the hypothesis that VDAC is the ATP conduit in the IP receptor-mediated signaling pathway in human erythrocytes. In addition, neither the pannexin inhibitor carbenoxolone nor Bcl-xL BH44–23 attenuated ATP release in response to incubation of erythrocytes with the β-adrenergic receptor agonist isoproterenol, suggesting the presence of yet another channel for ATP release from human erythrocytes.
Keywords: iloprost, UT-15C, isoproterenol, carbenoxolone, Bcl-xL BH44–23, TRO19622, red blood cell
regulated release of atp plays a role in complex signaling pathways within different organ systems. Erythrocytes have been implicated as controllers of vascular caliber by virtue of their ability to release the vasodilator, ATP, in response to local physiological stimuli (46, 47, 49) as well as when exposed to pharmacological agents (29, 41). When released from erythrocytes, ATP can bind to purinergic receptors on the vascular endothelium inducing the local formation of endothelium-derived vasodilators including prostaglandins, nitric oxide, and endothelium-derived hyperpolarizing factors (16, 48, 49).
Erythrocytes release ATP in response to diverse stimuli including exposure to reduced oxygen (O2) tension (9, 13, 46), mechanical deformation (43, 44), β-adrenergic receptor agonists (4, 29), and prostacyclin (PGI2) analogs (41). It has been demonstrated previously that exposure of the erythrocyte to low O2 tension or mechanical deformation activates the heterotrimeric G protein Gi, whereas stimulation of erythrocyte β-adrenergic or PGI2 (IP) receptors activates the heterotrimeric G protein Gs (27–29). Stimulation of either G protein activates a signal transduction pathway that includes adenylyl cyclase, PKA, and the CFTR and, ultimately, results in release of ATP (40, 42, 43, 45, 50). Although these components of the signaling pathways for ATP release from erythrocytes have been well characterized, the identity of the final ATP conduit appears to depend on the initiating stimulus.
ATP can be released from cells via three primary mechanisms: exocytosis, channels or transporters, or via cell lysis (30). The erythrocyte lacks the protein machinery to form vesicles (52), and hemolysis does not serve as a regulated form of ATP release. Therefore, ATP release from the erythrocyte must occur through channels or transporters. Previously, it was demonstrated that pannexin 1, a protein known to form a channel capable of serving as an ATP conduit in other cell types, is involved in the release of ATP from erythrocytes in response to exposure of the cells to lowered oxygen tension (50). However, treating human erythrocytes with three structurally dissimilar inhibitors of pannexin 1 did not alter iloprost-induced ATP release (50). This finding suggested that a channel or transporter other than pannexin 1 must serve as a conduit for iloprost-induced ATP release from erythrocytes.
Here, we investigated the hypothesis that the voltage-dependent anion channel (VDAC) serves as the conduit for IP receptor-mediated ATP release. VDAC is a 30–35 kDa protein that is predominately found in the outer membrane of mitochondria and is known to be responsible for most of the metabolite flux across that membrane, including the movement of ATP (35, 39). In addition to being present in mitochondria, VDAC is also present in plasma membranes of mammalian cells, including erythrocytes (12, 39), and has been suggested to serve as a conduit for ATP release (26). In this study, we confirmed that VDAC is a component of human erythrocyte membranes. In addition, we determined that two dissimilar VDAC inhibitors, Bcl-xL BH44–23 and TRO19622, attenuate ATP release in response to IP receptor activation with either of two PGI2 analogs, iloprost or UT-15C. These studies support the role of VDAC as the conduit for ATP released from the erythrocyte in response to pharmacological activation of the IP receptor.
MATERIALS AND METHODS
Isolation of erythrocytes.
Human blood was obtained from healthy volunteers by venipuncture using a syringe containing heparin (500 units). Blood was collected from 14 females and 9 males with an average age of 34 ± 3 years (range 19 to 61 years). Rabbit blood was obtained from male New Zealand white rabbits anesthetized with ketamine (12.5 mg/kg) and xylazine (1.5 mg/kg) intramuscularly, followed by pentobarbital sodium (10 mg/kg) administered via a cannula placed in an ear vein. A catheter was subsequently placed in a carotid artery, and heparin (500 units) was administered. After 10 min, the animals were exsanguinated.
After collection, whole blood was centrifuged at 500 g at 4°C for 10 min and the plasma, buffy coat, and uppermost erythrocytes were removed by aspiration and discarded. The remaining erythrocytes were washed three times in wash buffer containing (in mM) 21.0 tris(hydroxymethyl)aminomethane, 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, and 5.5 glucose and 0.5% bovine albumin fraction V (final pH 7.4). Wright stains of erythrocytes prepared in this fashion revealed less than 1 leukocyte per 50 high power fields (∼8–10 leukocytes/mm3). Previous studies demonstrate that these erythrocyte preparations are also devoid of platelet contamination (17). Cells were prepared on the day of use. The protocols for blood removal from humans and rabbits were approved by the Institutional Review Board of Saint Louis University and the Institutional Animal Care and Use Committee, respectively. Human subjects gave written informed consent.
All studies evaluating IP receptor-mediated increases in cAMP and ATP release were conducted using erythrocytes from healthy humans. Erythrocytes from both healthy humans and rabbits were used in studies in which the presence of VDAC in cell membranes was investigated.
Measurement of ATP.
ATP was measured by the luciferin-luciferase technique (51). A 200 μl sample of erythrocyte suspension was injected into a cuvette containing 100 μl of firefly lantern extract (10 mg/ml, FLE 250; Sigma) and 100 μl of a solution of synthetic D-luciferin (50 mg/100 ml; Sigma). The light emitted was detected using a luminometer (Turner Designs). A standard curve was obtained for each experiment. Cell counts were obtained from the suspension of erythrocytes, and amounts of ATP measured were normalized to 4 × 108 cells/ml.
Measurement of total intracellular ATP of erythrocytes.
A known number of erythrocytes were lysed in distilled water and diluted 8,000-fold. ATP was measured as described above, and the values were normalized to ATP concentration per erythrocyte.
Measurement of free hemoglobin.
To exclude the presence of significant hemolysis in studies where the release of ATP was measured, samples were centrifuged at 500 g at 4°C for 10 min and the presence of free hemoglobin in the supernatant was determined by light absorption at a wavelength of 405 nm. If increases in free hemoglobin were detected, the studies were not included.
Purification of erythrocyte membranes and Western analysis.
Washed (human or rabbit) erythrocytes were diluted 1:100 with ice-cold hypotonic buffer containing (in mM) 5 Tris·HCl and 2 EDTA (pH 7.4) and stirred vigorously at 4°C for 20 min. The lysate was centrifuged at 23,000 g for 15 min at 4°C. The supernatant was removed and discarded. The pellet containing the erythrocyte membranes was washed two times with ice-cold buffer and centrifuged. The membranes were resuspended in ice cold buffer and frozen at −80°C. Membrane protein concentrations were determined using BCA Protein Assay (Pierce). Purified erythrocyte membranes were solubilized in SDS buffer of 0.277 M SDS, 60% glycerol, 0.25 M Tris·HCl (pH 6.8), 0.004% bromophenol blue, and 0.400 M dithiothreitol, boiled, loaded onto a precast gradient (4–20%) gel (Lonza), and subjected to electrophoresis. The proteins were transferred to a polyvinylidene difluoride membrane in buffer containing 25 mM Tris, 192 mM glycine, and 10% methanol. Membranes were blocked overnight with 5% nonfat dry milk in PBS containing 0.1% Tween 20 and then immunoblotted with one of three antibodies directed against VDAC. One antibody was directed against insect VDAC (Heliothis virescens), which has 66% sequence homology with human VDAC (generated and characterized at St. Louis University, rabbit polyclonal) (36). The other two mouse monoclonal VDAC antibodies used have been extensively characterized and are selective for human VDAC 1 (Calbiochem AB-1 and AB-4) (53). Positive controls for VDAC were present on all gels and were either homogenized H. virescens larva (36) or isolated mouse cardiac mitochondria. After a wash to remove unbound primary antibody, membranes were incubated with an appropriate secondary antibody in 1% nonfat dry milk and the proteins that were identified were visualized using enhanced chemiluminescence (GE Healthcare and Amersham).
Determination of ATP release from human erythrocytes in response to incubation with prostacyclin analogs in the absence and presence of inhibitors of the VDAC.
Isolated erythrocytes diluted to a 20% hematocrit were incubated with one of two chemically dissimilar prostacyclin analogs, iloprost (Ilo; 1 μM) or UT-15C (UT; 1 μM), or their vehicle, saline. The incubations were performed in the presence of a 25-min pretreatment with one of two VDAC inhibitors, Bcl-xL Bh44–23 (BCL; 5 × 10−2 μg/ml; Calbiochem) or TRO19622 (Tro) (10 μM; Tocris), or their vehicle dimethylformamide (DMF). The concentrations of BCL and Tro were based on previous studies (10, 39). ATP released from erythrocytes was determined in the absence of the prostacyclin analog (baseline) and at 5, 10, and 15 min after the addition of Ilo or UT. The maximal response to Ilo or UT is reported. The time for the peak release of ATP from erythrocytes exposed to PGI2 analogs alone was 11.3 ± 0.8 min. This was unchanged in studies in which erythrocytes were pretreated with either BCL or Tro where the peak ATP release occurs after 12.7 ± 0.6 and 12.5 ± 0.8 min, respectively. The time course for studies performed in the absence and presence of VDAC inhibitors was identical. We have determined that ATP release in response to PGI2 analogs is not different from baseline after 20 min.
Determination of Ilo-induced increases in cAMP in the absence and presence of the VDAC inhibitor BCL.
Isolated human erythrocytes were diluted to a 50% hematocrit (1 ml) and incubated with Ilo (1 μM) or its vehicle (saline) for 30 min in the presence of BCL (5 × 10−2 μg/ml) or its vehicle (DMF). The reaction was stopped with the addition of 4 ml ice-cold acidified ethanol containing 1 mM HCl per 1 ml of erythrocyte suspension. The erythrocyte-ethanol mixture was centrifuged at 14,000 g for 10 min at 4°C, to remove precipitated proteins. The supernatant was removed and stored overnight at −20°C. Samples were centrifuged a second time at 3,700 g for 10 min at 4°C, to remove cryoprecipitates. The supernatant was again removed and dried under vacuum centrifugation. Concentrations of cAMP were determined by EIA (GE Healthcare). Cell counts were obtained from the erythrocyte suspension before addition of acidified ethanol and cAMP values were corrected to 1 × 1010 erythrocytes/ml.
Determination of ATP release from erythrocytes in response to incubation with isoproterenol in the absence and presence of the VDAC inhibitor BCL or the pannexin-1 inhibitor carbenoxolone.
Isolated human erythrocytes diluted to a 20% hematocrit were incubated with isoproterenol (1 μM) or its vehicle (saline) in the presence of a 25-min pretreatment with either BCL (5 × 10−2 μg/ml) or carbenoxolone (Carb; 100 μM; Sigma) or their respective vehicles (DMF or saline). ATP released from erythrocytes was determined at baseline and 5, 10, and 15 min after the addition of isoproterenol. The maximal response to isoproterenol is reported. The time for the peak release of ATP from erythrocytes exposed to isoproterenol alone was 10.5 ± 1.3 min. This was unchanged in studies in which erythrocytes were pretreated with either BCL or CARB where the peak ATP release occurs after 10.8 ± 1.5 and 10.0 ± 1.6 min, respectively. The time course for studies performed in the absence and presence of the inhibitors was identical. We have determined that ATP release in response to isoproterenol is not different from baseline after 20 min.
Data analysis.
Statistical significance was determined using ANOVA. In the event that the F-ratio indicated a change had occurred, a Fisher's least-significant different test was performed to identify individual differences. Results are reported as means ± SE. In all studies, n refers to the number of different individuals (or rabbits for the Western blot studies) from which erythrocyte samples were obtained. For each set of experiments, no sample from an individual was used twice. However, some individuals were studied in more than one experimental protocol. The time for maximal ATP release was 11 ± 1, 12 ± 1, and 11 ± 1 min for Ilo, UT, and isoproterenol, respectively and these times were not altered by incubation with any inhibitors.
RESULTS
Determination of VDAC expression in human and rabbit erythrocyte membranes.
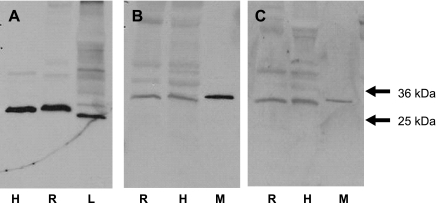
VDAC protein was identified as a component of purified erythrocyte membranes from healthy humans (n = 5) and rabbits (n = 5) (Fig. 1). The membranes were probed with three distinct antibodies directed against VDAC (larval antibody) or VDAC1 (AB1 and AB4). In all cases a single band was identified at a molecular mass of ∼31 kDa (Fig. 1). In addition, the H. virescens-derived antibody recognized a protein at this molecular mass in larval preparations, and the antibodies directed against human VDAC recognized the protein in mouse mitochondrial preparations (Fig. 1).
Fig. 1.
Identification of voltage-dependent anion channel (VDAC) in human and rabbit erythrocyte membranes. Solubilized erythrocyte membranes of humans (H) and rabbits (R) were resolved on precast gradient (4–20%) gels and probed with antibodies generated against human or H. virescens VDAC. Crude H. virescens larva preparations (L) and isolated mouse cardiac mitochondria (M) were used as positive controls. The gels pictured are representative of 5 human and 5 rabbit membrane preparations. Gel A (A), larval antibody; gel B (B), AB1; gel C (C), AB4.
Effect of BCL on IP receptor agonist-induced ATP release.
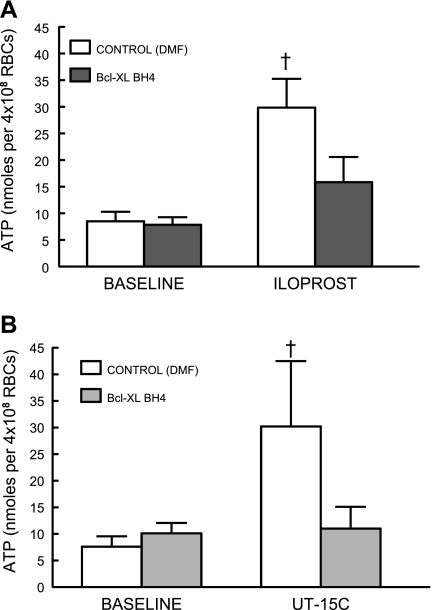
Isolated human erythrocytes release ATP when incubated with the IP receptor agonists Ilo (1 μM, n = 7; Fig. 2A) or UT (1 μM, n = 6; Fig. 2B). Preincubation with BCL (5 × 10−2 μg/ml) resulted in inhibition of ATP release stimulated by both agonists with no effect on baseline ATP levels. The decreases in ATP release were not accompanied by a reduction in total ATP content of the erythrocytes; therefore, the decreased release cannot be attributed to depletion of ATP within the cells (Table 1).
Fig. 2.
Effect of Bcl-xL BH44–23 (BCL; 5 × 10−2 μg/ml) on ATP release from human erythrocytes incubated with iloprost (1 μM, n = 7; A) or UT-15C (1 μM, n = 6; B). Erythrocytes were incubated with BCL or its vehicle, DMF, for 25 min before addition of the prostacyclin analog. Values are means ± SE. †Different from respective baseline and iloprost or UT-15C in the presence of BCL (P < 0.01); n, the number of different individuals studied. RBCs, red blood cells.
Table 1.
Effect of voltage-dependent anion channel inhibitors on intracellular ATP levels (in mM/cell) in human erythrocytes
| Bcl-xL Bh44–23 | TRO19622 | |
|---|---|---|
| n | 9 | 15 |
| Vehicle | 3.12 ± 0.31 | 3.35 ± 0.39 |
| Inhibitor | 3.16 ± 0.25 | 3.43 ± 0.39 |
Values are means ± SE.
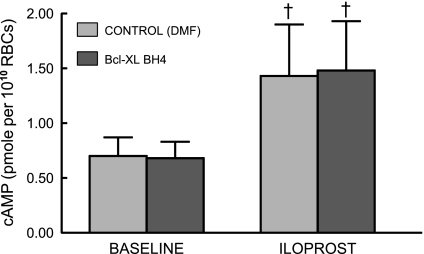
Effect of BCL on Ilo-induced increases in cAMP accumulation.
Increases in cAMP are required for Gs-coupled receptor-mediated ATP release from human erythrocytes (4, 41). To establish that BCL did not interfere with this component of the signaling pathway, we measured Ilo-induced increases in cAMP in the presence and absence of the inhibitor. BCL pretreatment was not associated with any change in cAMP levels produced by incubation of erythrocytes with 1 μM Ilo (n = 6; Fig. 3).
Fig. 3.
Effect of Bcl-xL BH44–23 (BCL; 5 × 10−2 μg/ml) on iloprost-induced increases in cAMP levels in human erythrocytes. Erythrocytes were incubated with BCL or its vehicle, DMF, for 30 min before addition of iloprost (1 μM, n = 6). The reaction was stopped 15 min after the addition of iloprost. Values are means ± SE. †Different from respective control (P < 0.01); n, the number of different individuals studied.
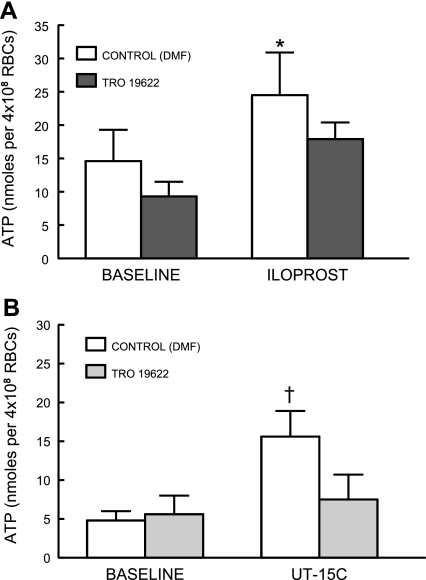
Effect of a second chemically dissimilar VDAC inhibitor, Tro, on IP receptor agonist-induced ATP release.
In studies identical to those using BCL, ATP release from human erythrocytes stimulated by Ilo (1 μM, n = 8; Fig. 4A) and UT (1 μM, n = 6; Fig. 4B) was attenuated by pretreatment with Tro (10 μM). Again total ATP content of erythrocytes was not different in Tro-treated cells (Table 1).
Fig. 4.
Effect of TRO19622 (Tro; 10 μM) on ATP release from human erythrocytes incubated with iloprost (1 μM, n = 7; A) or UT-15C (1 μM, n = 6; B). Erythrocytes were incubated with Tro or its vehicle, saline, for 30 min before addition of the prostacyclin analog. Values are means ± SE. *Different from respective baseline (P < 0.05); †different from respective baseline and UT15C in the presence of Tro (P < 0.01); n, the number of different individuals studied.
Effect of BCL and the pannexin 1 inhibitor, Carb, on isoproterenol-induced ATP release.
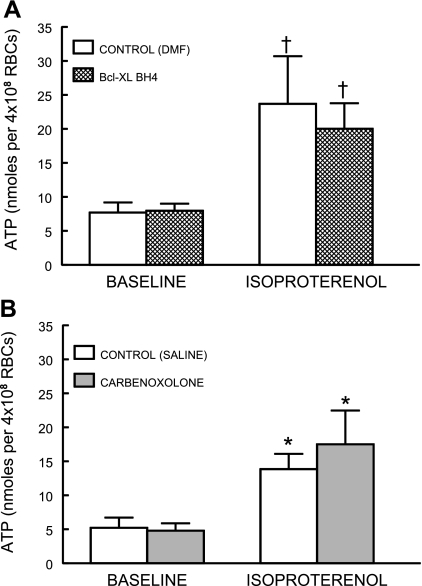
To determine whether pannexin 1 or VDAC is involved in β-adrenergic receptor-mediated ATP release from the erythrocyte, erythrocytes were pretreated with BCL or Carb followed by isoproterenol (1 μM). Preincubation of human erythrocytes with BCL did not alter ATP release in response to the addition of the β-adrenergic receptor agonist, isoproterenol (n = 6, Fig. 5A). In addition, isoproterenol-induced ATP release was not inhibited by the pannexin 1 inhibitor Carb (n = 5; Fig. 5B).
Fig. 5.
Effect of Bcl-xL Bh44–23 (BCL; 5 × 10−2 μg/ml, n = 6; A) or carbenoxolone (100 μM, n = 5; B) on ATP release from human erythrocytes incubated with isoproterenol. Erythrocytes were incubated with either the inhibitor or its respective vehicle, DMF or saline, for 25 min before addition of isoproterenol (1 μM). Values are means ± SE. *Different from respective baseline (P < 0.05); †different from respective baseline (P < 0.01); n, the number of different individuals studied.
DISCUSSION
The mechanisms responsible for regulated release of ATP from cells, including erythrocytes, have been the subject of much interest in recent years. Although mature erythrocytes lack a nucleus, mitochondria, and other intracellular organelles, they do contain the glycolytic machinery required to make ATP (21–22). In addition to being important for the maintenance of erythrocyte flexibility and ionic equilibrium, ATP is released from erythrocytes through regulated signaling pathways (24, 25, 49). It is well-established that erythrocytes release ATP in response to exposure to low oxygen (O2) tension (9), mechanical deformation (43, 45), β-adrenergic receptor agonists (29), or prostacyclin (IP) receptor agonists (41). Exposure of the erythrocyte to low O2 tension and mechanical deformation activates the heterotrimeric G protein Gi (27, 28), whereas β-adrenergic receptor and IP receptor stimulation activates the heterotrimeric G protein Gs (29, 41). Stimulation of either G protein activates signal transduction pathways that include adenylyl cyclase, PKA, and the CFTR (41–43, 45, 50). There have been a number of recent studies focused on identifying the conduits for ATP release from cells that are associated with specific signaling pathways. Some proteins that have been implicated as candidates for ATP conduits include gap junction proteins such as connexins and pannexins (8, 18, 31, 38), ATP binding cassette proteins such as MDR and MRP (1–3, 32, 33) maxi-anion channels (19), and VDAC (26).
We and others have reported that pannexin 1 is the conduit for ATP released in response to exposure of erythrocytes to reduced O2 tension (20, 50). However, it was also shown that the conduit for ATP release in response to activation of the IP receptor was not pannexin 1 (30a, 50). Here we demonstrate that ATP release from the erythrocyte in response to simulation of the IP receptor occurs via a VDAC.
VDAC was classically described as a protein only present in mitochondria where it makes up ∼20% of the outer membrane in eukaryotic cells (34). In conjunction with other proteins in the inner outer mitochondrial membrane, VDAC serves as the mitochondrial gatekeeper responsible for regulating the release of cytochrome c and the exchange of ATP and ADP (34). In addition to its mitochondrial localization, VDAC has been recently identified in the plasma membrane in many different cell types, including erythrocytes (12, 39). The role of the plasma membrane VDAC has been highly debated. In human umbilical vein endothelial cells, it was demonstrated that VDAC serves as a receptor for kringle 5 on the cell surface (15). Other studies have suggested that plasma membrane VDAC may play a role in redox regulation (6, 7). However, it has also been suggested that, similar to its role in the mitochondria, VDAC localized to the plasma membrane plays a role in regulating the release of ATP from cells (26). This hypothesis is supported by the finding that transfecting NIH 3T3 fibroblasts with the plasmalemmal form of VDAC 1 (pl-VDAC 1) led to increased ATP release from these cells in response to changing the culture medium, a technique thought to reflect mechanical stimulation of the cells (26). The same study also demonstrated that nasal and tracheal epithelial cells and fibroblasts isolated from VDAC 1 knockout mice released less ATP in response to hypotonic stress compared with cells isolated from the wild-type counterparts (26).
Although VDAC is present in the plasma membrane of erythrocytes, a physiological role for this protein remains uncharacterized. Here, we confirm that VDAC 1 is present in human erythrocyte membranes as well as membranes of rabbit erythrocytes. In addition, we demonstrate for the first time that treating human erythrocytes with BCL, a compound known to inhibit VDAC (39), attenuates IP receptor-mediated ATP release (Fig. 2). Importantly, treating erythrocytes with BCL did not decrease intracellular ATP (Table 1), demonstrating that the reduced ATP release cannot be attributed to attenuation of ATP synthesis. In addition, BCL did not inhibit Ilo-induced cAMP accumulation (Fig. 3), demonstrating that BCL does not inhibit a component of the ATP release signal transduction pathway upstream of the activation of adenylyl cyclase. To further support the hypothesis that VDAC serves as an ATP conduit, we pretreated cells with a second purported inhibitor of VDAC, Tro. Although BCL is a well-established inhibitor of VDAC activity (39), less is known about the specificity and mechanism of action of Tro. Tro is a lipid soluble compound with a cholesterol-like structure that binds to the peripheral benzodiazepine receptor (TSPO). TSPO is known to closely associate with VDAC in mitochondria as part of the mitochondrial permeability transition pore complex (mPTP) (54). Importantly, it has been demonstrated previously that TSPO is present in erythrocyte membranes (11). A previous study using motor neurons to investigate the effects of Tro on cell survival reported that Tro binds TSPO and, consequently, inhibits the activity of VDAC (10). In our studies, Tro attenuated both Ilo- and UT-induced ATP release from the erythrocyte further supporting the role of VDAC as the conduit for ATP release in response to IP activation.
In addition to receptor-mediated activation of Gs via stimulation of IP receptors, erythrocytes also release ATP in response to stimulation of Gs-coupled β-adrenergic receptors by agonists, including isoproterenol. To determine whether pannexin 1 or VDAC are involved in isoproterenol-induced ATP release, we examined the effect of Carb and BCL on isoproterenol-induced ATP release. Similar to the studies with Ilo (50), Carb did not attenuate isoproterenol-induced ATP release. Interestingly, BCL also had no effect on isoproterenol-induced ATP release. This finding demonstrates that BCL is not a nonselective inhibitor of ATP release from erythrocytes, and it suggests that ATP release in response to isoproterenol requires a conduit distinct from that involved in Ilo-induced ATP release. This interesting finding is not entirely unexpected in light of studies demonstrating that components of the Gs-mediated signal transduction pathway for ATP release from the erythrocyte are compartmentalized (4, 5); that is, it has been shown that activation of one pathway for ATP release from erythrocytes (IP receptor-mediated pathway) involves components that are independent of the components involved in another pathway (β-adrenergic pathway) (4, 5). Thus, although the stimulation of either IP or β-adrenergic receptors leads to the activation of Gs, the phosphodiesterases and protein kinases involved in regulating the cAMP levels of these respective pathways are different. Phosphodiesterase 3 and protein kinase A and C are involved in the ATP release pathway associated with the prostacyclin receptor, whereas phosphodiesterases 2 and 4 and protein kinase A are involved in the pathway associated with the β-adrenergic receptor (4, 5). Therefore, our finding that VDAC is involved in Ilo but not isoproterenol-induced ATP release is consistent with the selective compartmentalization involved in these signaling pathways within the erythrocyte. Recently, it was reported that ATP released from erythrocytes in response to isoproterenol, when augmented by the addition of forskolin and papaverine, was attenuated by carbenoxolone (23). However, under the conditions of that study, the nonselective activation of adenylyl cyclase by forskolin and inhibition of multiple phosphodiesterases with papaverine would result in the stimulation of multiple ATP release pathways in human erythrocytes. Thus the results of this study do not implicate pannexin 1 as an ATP conduit in the isoproterenol signaling pathway.
Although our work supports the hypothesis that VDAC is an ATP conduit in the IP receptor signaling pathway in human erythrocytes, an earlier study suggested that VDAC in the plasma membrane might not play such a role (37). It was reported that, using gene knockout and gene silencing techniques, VDAC did not serve as the maxi-anion channel that had been purported to be an ATP conduit in swelling-induced ATP release from mouse mammary C127 cells and swelling-, ischemia-, or hypoxia-induced ATP release from neonatal rat cardiomyocytes (37). It had long been assumed that the maxi-anion channel activity associated with ATP release in many cell types was due to the activity of VDAC based on the similarity of biophysical properties between these two channels. However, based on their studies, the authors concluded that the maxi-anion channel activity in mouse fibroblasts did not correlate with the presence of any of the three different isoforms of VDAC. These findings contradict the long held hypothesis that the maxi-anion channel represents a plasma membrane VDAC protein. Although this study did not support the hypothesis that the VDAC protein and the maxi-anion channel were one in the same, two points must be considered. The first is that although it provided evidence that the maxi-anion channel activity recorded could not be due to VDAC, the study did not suggest that VDAC could not also be an ATP conduit in cell membranes. Another important factor to consider is that the stimulus for ATP release was cell-swelling, a very different type of stimulus than receptor-mediated activation of a signaling pathway for ATP release. We demonstrated previously that in human erythrocytes, different stimuli for ATP release require different ATP conduits; therefore, the findings of Sabirov et al. (37) could also reflect the existence of additional yet to be identified conduits for ATP from these cells.
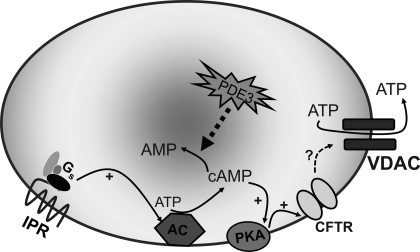
In conclusion, we confirm that VDAC 1 is present in human erythrocyte membranes. In addition we show that VDAC serves as the conduit for IP receptor agonist-induced ATP release from these cells (Fig. 6). In addition, we provide evidence suggesting the presence of a yet to be identified conduit for ATP release that is a component of a signaling pathway associated with activation of β-adrenergic receptors in human erythrocytes. The finding that different conduits for ATP release are components of discrete erythrocyte signaling pathways suggests that the selective activation of these pathways is important in vascular regulation. Moreover, the understanding of these pathways could make the erythrocyte a unique therapeutic target for the development of new strategies for the treatment of vascular disease.
Fig. 6.
Proposed signaling pathway for ATP release from erythrocytes in response to activation of the prostacyclin receptor. Exposure of human erythrocytes to prostacyclin (PGI2) analogs results in activation of Gs, leading to increases in cAMP that are regulated by phosphodiesterase 3 (PDE3) activity. Increases in cAMP activate PKA and, subsequently, CFTR. The final conduit for ATP release is VDAC. AC, adenylyl cyclase.
GRANTS
This work was supported by grants from the American Diabetes Association (BS-150), the National Heart, Lung, and Blood Institute (HL-089094), United Therapeutics (to R. S. Sprague), and the President's Research Fund of Saint Louis University (R. S. Sprague).
DISCLOSURES
Dr. Sprague has a grant from United Therapeutics, who manufacture UT-15C. The company had no input into experimental design, interpretation of results, or the conclusions of this work. United Therapeutis is acknowledged for grant support in the manuscript.
AUTHOR CONTRIBUTIONS
Author contributions: M.S., M.L.E., and R.S.S. conception and design of research; M.S., E.A.B., J.P.R., M.K., K.L.D., K.A.D., and R.S.S. performed experiments; M.S., E.A.B., J.P.R., M.K., K.L.D., K.A.D., and R.S.S. analyzed data; M.S., E.A.B., J.P.R., K.L.D., M.L.E., and R.S.S. interpreted results of experiments; M.S. and R.S.S. prepared figures; M.S., M.L.E., and R.S.S. drafted manuscript; M.S., E.A.B., J.P.R., M.K., K.L.D., K.A.D., A.H.S., M.L.E., and R.S.S. edited and revised manuscript; M.S., E.A.B., J.P.R., M.K., K.L.D., K.A.D., A.H.S., M.L.E., and R.S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank J. L. Sprague and V. Sridharan for inspiration, Dr. J. Ryerse for the kind gift of VDAC antibody, and Dr. T. M. Egan for advice and assistance.
REFERENCES
- 1. Abraham EH, Okunieff P, Scala S, Vos P, Oosterveld MJ, Chen AY, Shrivastav B. Cystic fibrosis transmembrane conductance regulator and adenosine triphosphate. Science 275: 1324–1326, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Abraham EH, Prat AG, Gerweck L, Seneveratne T, Arceci RJ, Kramer R, Guidotti G, Cantiello HF. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc Natl Acad Sci USA 90: 312–316, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abraham EH, Sterling KM, Kim RJ, Salikhova AY, Huffman HB, Crockett MA, Johnston N, Parker HW, Boyle WE, Jr, Hartov A, Demidenko E, Efird J, Kahn J, Grubman SA, Jefferson DM, Robson SC, Thakar JH, Lorico A, Rappa G, Sartorelli AC, Okunieff P. Erythrocyte membrane ATP binding cassette (ABC) proteins: MRP1 and CFTR as well as CD39 (ecto-apyrase) involved in RBC ATP transport and elevated blood plasma ATP of cystic fibrosis. Blood Cells Mol Dis 27: 165–180, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Adderley SP, Dufaux EA, Sridharan M, Bowles EA, Hanson MS, Stephenson AH, Ellsworth ML, Sprague RS. Iloprost- and isoproterenol-induced increases in cAMP are regulated by different phosphodiesterases in erythrocytes of both rabbits and humans. Am J Physiol Heart Circ Physiol 296: H1617–H1624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adderley SP, Sridharan M, Bowles EA, Stephenson AH, Ellsworth ML, Sprague RS. Protein kinases A and C regulate receptor-mediated increases in cAMP in rabbit erythrocytes. Am J Physiol Heart Circ Physiol 298: H587–H593, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker MA, Lane DJ, Ly JD, De Pinto V, Lawen A. VDAC1 is a transplasma membrane NADH-ferricyanide reductase. J Biol Chem 279: 4811–4819, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Baker MA, Ly JD, Lawen A. Characterization of VDAC1 as a plasma membrane NADH-oxidoreductase. Biofactors 21: 215–221, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 40–47, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Bordet T, Buisson B, Michaud M, Drouot C, Galea P, Delaage P, Akentieva NP, Evers AS, Covey DF, Ostuni MA, Lacapere JJ, Massaad C, Schumacher M, Steidl EM, Maux D, Delaage M, Henderson CE, Pruss RM. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J Pharmacol Exp Ther 322: 709–720, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Bouyer G, Cueff A, Egee S, Kmiecik J, Maksimova Y, Glogowska E, Gallagher PG, Thomas SL. Erythrocyte peripheral type benzodiazepine receptor/voltage-dependent anion channels are upregulated by Plasmodium falciparum. Blood 118: 2305–2312, 2011 [DOI] [PubMed] [Google Scholar]
- 12. De Pinto V, Messina A, Lane DJ, Lawen A. Voltage-dependent anion-selective channel (VDAC) in the plasma membrane. FEBS Lett 584: 1793–1799, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand 168: 551–559, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Gonzalez-Gronow M, Kalfa T, Johnson CE, Gawdi G, Pizzo SV. The voltage-dependent anion channel is a receptor for plasminogen kringle 5 on human endothelial cells. J Biol Chem 278: 27312–27318, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hammer LW, Overstreet CR, Choi J, Hester RL. ATP stimulates the release of prostacyclin from perfused veins isolated from the hamster hindlimb. Am J Physiol Regul Integr Comp Physiol 285: R193–R199, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Hanson MS, Stephenson AH, Bowles EA, Sridharan M, Adderley S, Sprague RS. Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost-induced increases in cAMP. Am J Physiol Heart Circ Physiol 295: H786–H793, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte hemichannels. J Neurosci 29: 7092–7097, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu HT, Toychiev AH, Takahashi N, Sabirov RZ, Okada Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res 18: 558–565, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 103: 7655–7659, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miseta A, Bogner P, Berenyi E, Kellermayer M, Galambos C, Wheatley DN, Cameron IL. Relationship between cellular ATP, potassium, sodium and magnesium concentrations in mammalian and avian erythrocytes. Biochim Biophys Acta 1175: 133–139, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Miseta A, Somoskeoy S, Galambos C, Kellermayer M, Wheatley DN, Cameron IL. Human and dog erythrocytes: relationship between cellular ATP levels, ATP consumption and potassium concentrations. Physiol Chem Phys Med NMR 24: 11–20, 1992 [PubMed] [Google Scholar]
- 23. Montalbetti N, LealDenis MF, Pignataro OP, Kobatake E, Lazarowski ER, Schwarzbaum PJ. Homeostasis of extracellular ATP in human erythrocytes. J Biol Chem 286: 38397–38407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakao M, Nakao T, Yamazoe S. Adenosine triphosphate and maintenance of shape of the human red cells. Nature 187: 945–946, 1960 [DOI] [PubMed] [Google Scholar]
- 25. Nakao M, Nakao T, Yamazoe S, Yoshikawa H. Adenosine triphosphate and shape of erythrocytes. J Biochem 49: 487–492, 1961 [DOI] [PubMed] [Google Scholar]
- 26. Okada SF, O'Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol 124: 513–526, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol Heart Circ Physiol 286: H940–H945, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. NO inhibits signal transduction pathway for ATP release from erythrocytes via its action on heterotrimeric G protein Gi. Am J Physiol Heart Circ Physiol 287: H748–H754, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Receptor-mediated activation of the heterotrimeric G-protein Gs results in ATP release from erythrocytes. Med Sci Monit 7: 669–674, 2001 [PubMed] [Google Scholar]
- 30. Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal 5: 433–446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a. Qiu F, Wang J, Spray DC, Scemes E, Dahl G. Two non-vesicular ATP release pathways in the mouse erythrocyte membrane. FEBS Lett 585: 3430–3435, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 41: 525–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roman RM, Lomri N, Braunstein G, Feranchak AP, Simeoni LA, Davison AK, Mechetner E, Schwiebert EM, Fitz JG. Evidence for multidrug resistance-1 P-glycoprotein-dependent regulation of cellular ATP permeability. J Membr Biol 183: 165–173, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Roman RM, Wang Y, Lidofsky SD, Feranchak AP, Lomri N, Scharschmidt BF, Fitz JG. Hepatocellular ATP-binding cassette protein expression enhances ATP release and autocrine regulation of cell volume. J Biol Chem 272: 21970–21976, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Rostovtseva T, Colombini M. VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys J 72: 1954–1962, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rostovtseva TK, Bezrukov SM. ATP transport through a single mitochondrial channel, VDAC, studied by current fluctuation analysis. Biophys J 74: 2365–2373, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ryerse J, Colombini M, Hagerty T, Nagel B, Liu TT. Isolation and characterization of the mitochondrial channel, VDAC, from the insect Heliothis virescens. Biochim Biophys Acta 1327: 193–203, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Sabirov RZ, Sheiko T, Liu H, Deng D, Okada Y, Craigen WJ. Genetic demonstration that the plasma membrane maxianion channel and voltage-dependent anion channels are unrelated proteins. J Biol Chem 281: 1897–1904, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol 3: 199–208, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shimizu S, Matsuoka Y, Shinohara Y, Yoneda Y, Tsujimoto Y. Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J Cell Biol 152: 237–250, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sprague R, Bowles E, Stumpf M, Ricketts G, Freidman A, Hou WH, Stephenson A, Lonigro A. Rabbit erythrocytes possess adenylyl cyclase type II that is activated by the heterotrimeric G proteins Gs and Gi. Pharmacol Rep 57, Suppl: 222–228, 2005 [PubMed] [Google Scholar]
- 41. Sprague RS, Bowles EA, Hanson MS, DuFaux EA, Sridharan M, Adderley S, Ellsworth ML, Stephenson AH. Prostacyclin analogs stimulate receptor-mediated cAMP synthesis and ATP release from rabbit and human erythrocytes. Microcirculation 15: 461–471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sprague RS, Bowles EA, Olearczyk JJ, Stephenson AH, Lonigro AJ. The role of G protein beta subunits in the release of ATP from human erythrocytes. J Physiol Pharmacol 53: 667–674, 2002 [PubMed] [Google Scholar]
- 43. Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275: H1726–H1732, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol Heart Circ Physiol 271: H2717–H2722, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281: C1158–C1164, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S, Procknow J, Ellsworth ML. Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep 61: 183–190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sprague RS, Stephenson AH, Dimmitt RA, Weintraub NL, Branch CA, McMurdo L, Lonigro AJ. Effect of l-NAME on pressure-flow relationships in isolated rabbit lungs: role of red blood cells. Am J Physiol Heart Circ Physiol 269: H1941–H1948, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Sprague RS, Stephenson AH, Dimmitt RA, Weintraub NL, Branch CA, McMurdo L, Lonigro AJ. Inhibition of nitric oxide synthesis results in a selective increase in arterial resistance in rabbit lungs. Pol J Pharmacol 46: 579–585, 1994 [PubMed] [Google Scholar]
- 49. Sprague RS, Stephenson AH, Ellsworth ML. Red not dead: signaling in and from erythrocytes. Trends Endocrinol Metab 18: 350–355, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol 299: H1146–H1152, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Strehler B, McElroy W. Assay of adenosine triphosphate. In: Methods in Ezymology, edited by Colowick S, Kaplan N. New York: Academic Press, 1957, p. 871–873 [Google Scholar]
- 52. Taraschi TF, O'Donnell M, Martinez S, Schneider T, Trelka D, Fowler VM, Tilley L, Moriyama Y. Generation of an erythrocyte vesicle transport system by Plasmodium falciparum malaria parasites. Blood 102: 3420–3426, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Thinnes FP, Reymann S. New findings concerning vertebrate porin. Naturwissenschaften 84: 480–498, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Veenman L, Shandalov Y, Gavish M. VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis. J Bioenerg Biomembr 40: 199–205, 2008 [DOI] [PubMed] [Google Scholar]