Abstract
Following myocardial infarction (MI) inflammatory responses transform cardiac fibroblasts to myofibroblasts, which in vitro studies show form heterocellular gap junctions with cardiac myocytes via Connexin43 (Cx43). The ability to form heterocellular junctions in the intact heart and the impact of these junctions on propagation is unclear. We used a canine model of MI and characterized the distribution and quantity of myofibroblasts in surviving epicardial cells [epicardial border zone (EBZ)]. We found a significant increase in myofibroblasts within the EBZ and no gap junction plaques between myofibroblasts and myocytes. Because myofibroblasts produce IL-1β, which downregulates Cx43, we asked whether myofibroblast proliferation causes loss of Cx43 near myofibroblast clusters. In vitro studies showed that IL-1β caused loss of Cx43 and reduced coupling. Western blot showed a significant increase of IL-1β in the EBZ, and immunohistochemistry showed a loss of Cx43 in regions of myofibroblasts in the intact heart. Additionally, dye studies in intact heart showed no coupling between myocytes and myofibroblasts. To quantify the effect of myofibroblasts on propagation we used a two-dimensional subcellular computer model of the EBZ, which showed that heterogeneities in myofibroblast density lead to conduction abnormalities. In conclusion, an increase of myofibroblasts in the infarcted heart causes heterogeneous Cx43 levels, possibly as a result of the release of IL-1β and decreased cell-cell communication, which leads to conduction abnormalities following MI.
Keywords: arrhythmia, gap junction, myocardial infarction
normal propagation through the ventricular myocardium is dependent, in large part, on uninterrupted gap junctional coupling between myocytes. This coupling allows for a continuous low resistance pathway for conduction in the normal heart (16). Following myocardial infarction (MI) myocytes have been shown to lose much of the Connexin43 (Cx43), which forms ventricular gap junctions (5, 15). In addition, inflammatory responses to MI initiate transformation of resident cardiac fibroblasts to myofibroblasts, which are involved in the normal immune response to inflammation and tissue repair (23). This transformation includes rapid cellular division of myofibroblasts, which forms regions of cellular heterogeneity within the ischemic and infarcted myocardium. Based on in vitro studies, which have shown Cx43-based cell-cell coupling between myofibroblasts and neonatal cardiac myocytes (20), it has been hypothesized that heterocellular coupling may occur in the postinfarct heart. However, whether myofibroblasts (as opposed to fibroblasts) form functional gap junctions with cardiac myocytes and how myofibroblasts impact propagation in vivo is unclear.
In cardiac tissue, electrical coupling between myocytes and fibroblasts has been demonstrated in the right atrium of rats (17). Spread of Lucifer Yellow has also been shown between myocytes and fibroblasts in rabbit sinoatrial node (SAN) tissue (6). Although fibroblasts transfected to express K+ channels affect myocyte effective refractory period, suggesting electrical interaction between myocytes and fibroblasts (31), there is no evidence of direct coupling in either normal or diseased ventricles. Additionally, as opposed to fibroblasts, whose main function is to produce and maintain the extracellular matrix (18), myofibroblasts have multiple functions including the production and release of inflammatory mediators such as the proinflammatory cytokine IL-1β (23), which we and others have previously shown downregulates Cx43 in an in vitro assay (10, 14). This suggests that myofibroblasts in the postinfarct heart may play an active role in the loss of Cx43, which is seen as early as 30 min post-MI (15).
Given the importance of Cx43-mediated cell-to-cell communication on propagation (3), we hypothesized that the reduction of Cx43 by myofibroblasts, possibly as a result of IL-1β production, causes conduction abnormalities of the cardiac impulse. To determine whether heterocellular coupling between cardiac myocytes and myofibroblasts occurs in the postinfarct heart we quantified myofibroblast proliferation in epicardial border zone (EBZ) of the 5-day-old canine infarcted heart. Using immunohistochemical analysis and electron microscopy, we identified myofibroblasts in both the central common pathway (CCP) and outer pathway (OP) of re-entrant ventricular tachycardia circuits, which form the figure of eight pattern of VT activation on the surface of the EBZ. Utilizing in vitro assays we determined the effect of IL-1β on Cx43 localization at cell membranes. To quantify the effect of myofibroblasts on propagation we used a two-dimensional subcellular computer model of the EBZ.
METHODS
Canine experiments.
All procedures were done in compliance with the U.S. Animal Welfare Act and approved by Columbia University Institute for Animal Care and Use Committee (Protocol No. AAAA6210). MI was produced in five mongrel dogs weighing 25–30 kg by a two-stage ligation of the left anterior descending coronary artery near its origin (15). Following 5 days of recovery, the animals were reanesthetized and the chest was opened by midline thoracotomy. After electrical mapping of the EBZ to identify the CCP and OP of re-entrant circuits, the heart was rapidly removed and tissue was obtained by shaving off a 2- to 3-mm-thick sample with a fine razor from the surface of the infarcted region (3, 5, 15). Five normal dogs were used as controls (19, 30).
Electron microscopy.
A 1 cm × 0.7 cm × 0.5 cm piece of the EBZ was cut and immediately immersed in cold 2.5% gluteraldehyde in 0.1 mol/l phosphate buffer (pH 7.35). Tissue blocks were trimmed to 1 × 2 mm size and further fixed for 4 h at 4°C. The tissue was then rinsed overnight in cold 0.1 mol/l phosphate buffer (pH 7.35), postfixed in cold 1.5% osmium tetroxide in 0.1 mol/l phosphate buffer (pH 7.35) for 1 h, and rinsed in three changes of 0.1 M phosphate buffer (pH 7.35) followed by three changes of 0.1 mol/l sodium acetate buffer (pH 6.0). En bloc staining in 1% uranyl acetate in 0.1 mol/l sodium acetate buffer (pH 6.0) for 1 h (cold) was followed by dehydration through ascending concentrations of ethanol to 100%. Two brief changes of propylene oxide were followed by gradual infiltration in propylene oxide/epoxy (1:1) for 1 h and propylene oxide/epoxy (1:3) for 1.5 h. The tissue was placed in complete epoxy and left overnight on an Orbit Shaker (Labline Instruments, Melrose Park, IL). The tissue was embedded in fresh complete epoxy in flat silicon molds and polymerized at 60°C for 4 days. Thick sections (1 to 2 μm) were prepared on glass slides and stained for assessment and orientation with Epoxy Tissue Stain (EMS, Fort Washington, PA). Block faces were trimmed to include longitudinally oriented fibers. Ultrathin sections (600 A°) were mounted on uncoated 200 mesh copper grids and poststained sequentially with cold saturated uranyl acetate in 25% ethanol, followed by lead citrate. The sections were examined in a JEOL Jem 1200 EX electron microscope.
Cell culture.
Rat neonatal myocytes were isolated from P0 rat pups as described in the Abcam published protocol (http://www.abcam.com/ps/pdf/protocols/neonatalratcardiomyocyteharvest.pdf). Mardin Darby Canine Kidney (MDCK) cells were used as a model system due to the fact that they contain high levels of Cx43 but unlike myocytes are able to form confluent monolayers, which aid in determination of gap junction function in vitro. MDCK cells and rat neonatal myocytes were plated on glass coverslips for immunohistochemistry. Cells were treated with IL-1β overnight (0, 0.025, 0.05, and 1 pM) and then fixed in 4% formaldehyde and stained for Cx43 as described below for tissue samples.
Western blot.
Frozen epicardial samples were prepared as described previously (15). Tissue samples were lysed in complete lysis buffer containing 50 mmol/l Tris·HCl (pH 7.4), 0.25 mmol/l Na-deoxycholate, 150 mmol/l NaCl, 2 mM EGTA, 0.1 mmol/l Na3VO4, 10 mmol/l NaF, 1 mmol/l PMSF, 1% Triton-X 100, and 1/2 tablet of Complete Protease Inhibitor (Roche Biochemicals, Indianapolis, IN). Lysates were sonicated for 30 s, maintained on ice for 30 min, and then triturated and spun at 10,000 rpm for 10 min. Following removal of the pellet protein levels were tested using bicinchoninic acid protein assay kit (Bio-Rad). Matched levels of total protein were mixed with loading buffer (2× laemini buffer + DTT), then run for Western blots using 10% SDS-PAGE gels. Gels were Commassie blue stained as loading control (28), and proteins were then transferred to nitrocellulose membranes and probed for Cx43 (Sigma). Bands were analyzed by densitometry (Cx43/Commassie blue) using National Institutes of Health (NIH) Scion Image.
Immunohistochemistry.
Rapidly frozen heart samples from noninfarcted epicardium and from the EBZ were sectioned (15 μm) using a Leica 3050S cryostat. Sections were fixed in 4% formaldehyde for 30 min at RT then incubated in 50 mM NH4Cl for 30 min to quench autofluorescence. Following quench, sections were blocked (PBS + 10% goat serum + 0.4% Triton-X 100) for 1 h at RT and then incubated with primary antibodies directed against Cx43 (Sigma) and smooth muscle actin (SMA; Abcam) at concentrations of 1:1,000 at 4°C overnight. Following 30-min rinse (3 × 10 min, PBS + 0.4% Triton-X 100) slices were incubated with secondary antibodies (anti-mouse 488 and anti-rabbit 595; Molecular Probes Alexa Fluor) at 1:2,000 for 1 h at RT. Slices were rinsed for 50 min (5 × 10 min), mounted on glass microscope slides with Vectashield anti-fade agent (Vector Laboratories, Burlingame, CA), and examined using a Zeiss Axiophott 200M equipped with both FITC and Texas Red filters.
Scrape loading.
Scrape loading was done as described previously (9). Briefly, MDCK cells were grown in 35-mm dishes in a medium of DMEM (American Type Culture Collection) supplemented with 10% fetal bovine serum (Sigma) and 1% penicillin-streptomycin (Cellgro) and then incubated overnight in a 1 μM concentration of IL-1β (Sigma) or a 0.1 μM concentration of IL-1β plus 3 μM concentration of the IL-1 receptor antagonist (R&D Systems). After incubation media was removed and cells were scraped with a razorblade and incubated for 5 min in 0.5% Rhodamine Dextran plus 2.5% Lucifer Yellow in 150 mM lithium chloride. Following PBS rinses (3 × 10 min) cells were fixed in 4% formaldehyde for 15 min and then examined on a Leica 5500 inverted microscope. Scrape loading was done on five dishes for each condition. The distance that dye spread was quantified using NIH ImageJ by the following method. Images were measured from the scrape line to the farthest edge of dye in three separate places on each image (at center and at 25% from both top and bottom of image). These measurements were averaged for each image. Each dish had at least two regions photographed.
Dye spread in intact heart.
Hearts were rapidly removed at either 48 h or 5 day postcoronary occlusion, and the left ventricle was dissected free and placed in warm oxygenated saline, epicardial side up. Dye spread was done as described previously (13, 24). With a 26-gauge needle a pinhole was made and 2.5% Lucifer Yellow in 150 mM lithium chloride was pipetted onto the surface. Dye that was not taken into the pinhole was wiped away to avoid entry of dye into the cut edges of the tissue. Tissue was incubated with dye for 15 min and then rapidly frozen in liquid nitrogen, placed in tissue embedding optimum cutting temperature, and sectioned to 14 μm. Sections were placed on glass coverslips and stained for α-SMA as described above and then examined on a Leica 5500 inverted microscope. α-SMA positive cells were then counted and identified as dye inclusive or dye exclusive.
Computer simulations.
We performed all simulations in a monodomain model with governing equation: ∇ ((1/(SvRiCm)) ∇Vm) = (Iion/Cm) + ∂tVm, where Vm is the transmembrane potential (in mV), Iion is the ionic current (in μA/cm2), Ri is the resistivity of the intracellular space, Sv is the surface-to-volume ratio (2,000 cm−1), and Cm is the specific capacitance (1 μF/cm2). Neumann (nonflow) boundary conditions were used. Membrane dynamics (Iion) were formulated by an ionic model of the action potential of the EBZ (IZ cell) (4). Cells were discretized with a space step of 10 μm in both longitudinal and transverse directions. We used the tissue architecture proposed by Spach and Heidlage, (29), which incorporates an experimentally based representation of cell size and shape as well as gap junction localization. Resistivity of the cytoplasm was 150 Ωcm. The governing equation was integrated using the semi-implicit Crank-Nicholson method with a time step of 0.5 μs. We ran all simulations in preparations that had a size of 5 mm in the longitudinal direction (500 nodes) and 2.5 mm (250 nodes) in the direction transverse to the fiber orientation. Flat propagating waves were initiated at the boundary of the preparation by electrical stimulation (2 × diastolic threshold). Myofibroblasts had a size of 20 μm × 20 μm and were distributed randomly in the preparation. Myofibroblasts had no electrical connections to myocytes.
RESULTS
Cellular composition of the EBZ.
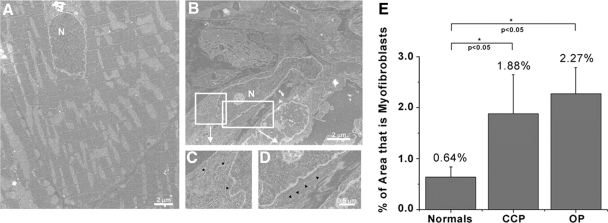
To examine the cellular composition of the regions found within the re-entrant circuit we used electron microscopy and counted the number of myofibroblasts within sections of normal epicardium (Fig. 1A) as well as within both the CCP and the OP of re-entrant circuits in the left ventricle post-coronary occlusion (Fig. 1, B–D). Myofibroblasts were identified by their highly intricate cell membranes, large nuclei, extensive rough endoplasmic reticulum, and stress fibers. Quantification of the percentage of myofibroblast area in total tissue (Fig. 1E) showed that less than 1% of the epicardial tissue examined from normal hearts had cells that had the morphology of myofibroblasts (0.64%). Following 5 days of coronary occlusion myofibroblasts were found in equal proportions in both the CCP and OP (1.88% and 2.27%, respectively). These data indicate that myofibroblasts infiltration occurs within days of coronary occlusion.
Fig. 1.
Electron microscopy of myofibroblasts in infarcted hearts. Normal myocardium (A) has almost no identifiable myofibroblasts, but following 5 days of coronary occlusion (B) myofibroblasts are readily identifiable by their large nuclei (N) as well as by their extensive endoplasmic reticulum (inset C) and the presence of stress fibers (inset D). Quantification of myofibroblasts (E) in different regions of the re-entrant circuits [common pathway (CCP) and outer pathway (OP)] showed that both regions had significant increases in the levels of myofibroblasts when compared with normal (0.64% in normal, 1.88% in CCP, and 2.27% in OP), but there was no difference in myofibroblasts levels in the CCP when compared with the OP, indicating that the electrical pathway does not affect myofibroblasts localization (n = 5).
Cell-to-cell junctions in the EBZ.
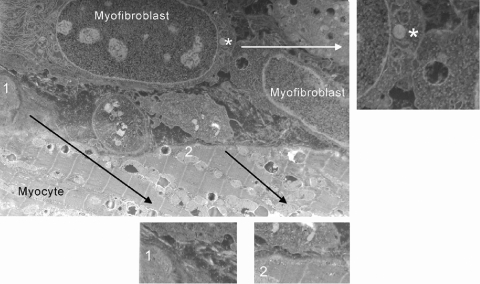
Examination of cell membrane interfaces (1,000 μm) between cells in the EBZ (Fig. 2) revealed abundant myofibroblast-myofibroblast gap junctional plaques (Fig. 2, inset), which were identifiable as regions of close membrane apposition with no membrane fusion. In contrast, examination of heterotypic cell interfaces showed no gap junctional plaques between myocytes and myofibroblasts (Fig. 2, insets 1 and 2, and Fig. 3). Although this does not preclude the presence of very small gap junctions or individual connexons (docked hemichannels) between these two cell types, it is indicative of very low probability of cell coupling between myocytes and myofibroblasts in the postinfarct heart.
Fig. 2.
Examination of cell-to-cell contacts between myofibroblasts and myocytes show that although gap junctions are identifiable between myofibroblasts (inset, *), there were no myocyte-to-myofibroblasts gap junctions (insets 1 and 2) in any section examined (1,050 μm of contact region membrane examined).
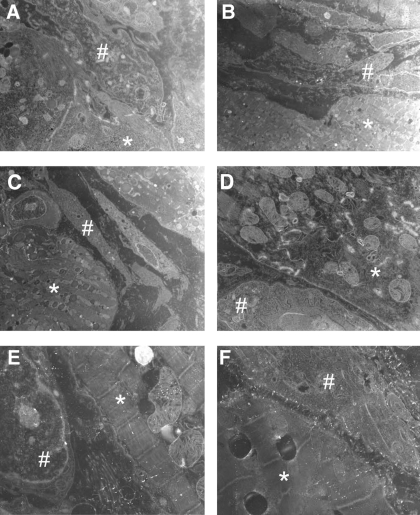
Fig. 3.
Electron micrographs of 5-day infarcted epicardial border zone (EBZ) with myocytes (*) and myofibroblasts (#) (A–F). Several examples of myocytes next of myofibroblasts showed no gap junctional plaques between these 2 cell types, even at the longitudinal ends of the myocytes (for example, see C).
Effect of IL-1β on Cx43 localization and function.
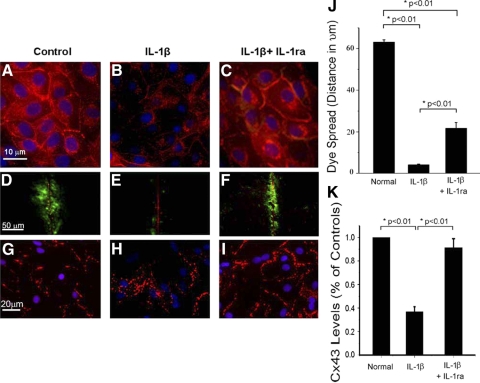
Our previous studies have indicated that IL-1β causes internalization of Cx43 in astrocytes (10). To examine whether this is a ubiquitous response to IL-1β, we examined the effect of IL-1β on Cx43 in MDCK cells (Fig. 4). These cells express Cx43 at cell membranes at sites of cell-cell contacts (Fig. 4A). Incubation of these cells with physiological levels of IL-1β caused an internalization of Cx43 starting at 0.05 μM concentrations (Fig. 4B). Incubation of cells with IL-1β in the presence of the specific IL-1β receptor antagonist IL-1ra inhibited the changes in Cx43 localization (Fig. 4C). These data show that IL-1β acts to regulate Cx43 via the canonical IL-1β signal transduction pathway. To determine whether the loss of Cx43 at cell membranes is sufficient to cause a loss of coupling within a population of cells we performed scrape loading experiments on the MDCK cells. Normal MDCK cells pass Lucifer Yellow dye through gap junctions (Fig. 4D), but incubation of cells with IL-1β causes almost complete loss of dye spread (Fig. 4E). Inhibition of IL-1β receptors with IL-1βra significantly inhibits the loss of cell coupling (Fig. 4F), indicating that IL-1β closes gap junction channels through the canonical IL-1β signal transduction pathway. Quantification of dye spread (Fig. 4J) shows that IL-1β causes more than a 10-fold decrease in coupling. Interestingly, the blockade of the receptor does not cause a complete recovery of coupling (only 30% of control). This may be due to incomplete blockade of the receptor by the IL-1ra or it may be that IL-1β causes internalization and closure of Cx43 but that an IL-1β receptor-independent mechanism is also responsible for channel closure. Thus when IL-1ra is present Cx43 remains at the cell membranes but the mechanisms for at least partial channel closure are still present; thus fewer channels are open to provide intercellular coupling. Although it is beyond the scope of these studies to distinguish between these two options, these findings merit further study. Although an IL-1β-stimulated change in Cx43 in MDCK cells may be of general interest, we asked the question as to whether cardiac myocytes also exhibited loss of cell coupling in the presence of IL-1β. We treated rat neonatal myocytes with 0.1 μM IL-1β and found that when compared with controls (Fig. 4G), treated myocytes showed internalization of Cx43 (Fig. 4H), which was inhibited by the presence of the receptor antagonist IL-1ra (Fig. 4I). Figure 4K shows quantification of the level of Cx43 in cultured rat neonatal myocytes. These data suggest that the effect of IL-1β on Cx43 is cell type independent.
Fig. 4.
A: Mardin Darby Canine Kidney (MDCK) cells contain Connexin43 (Cx43), which is found at sites of cell-cell contact (B). Treatment of 0.1 μM of IL-1β caused an internalization of Cx43, which was blocked by the IL-1β receptor antagonist IL-1ra (C). Dye spread studies showed that although control MDCK cells are highly coupled (D), IL-1β treatment caused a complete loss cell coupling (E). Inhibition of the IL-1β receptor with IL-1ra inhibited both the changes in Cx43 localization (C) and function (F). Treatment of rat neonatal myocytes (G) with 0.1 μM IL-1β (H) caused the same internalization of Cx43 as was seen in the MDCK cells that was also reversed by addition of the IL-1ra (I), suggesting that this effect of IL-1β is not cell type specific. J: quantification of the dye-spread studies (n = 3). Quantification of the level of Cx43 in cultured rat neonatal myocytes is seen in K. Values are reported as a percentage of normal.
Localization of myofibroblasts and their relationship to heterogeneous Cx43 in the EBZ.
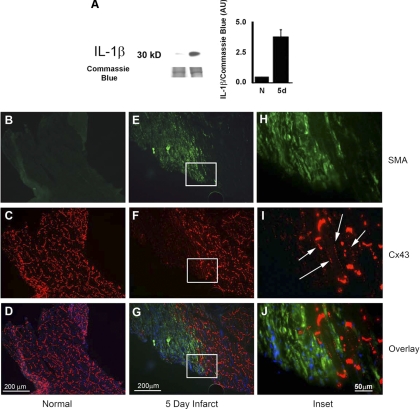
Examination of IL-1β levels in the EBZ shows that at 5 days postinfarct IL-1β is significantly increased (Fig. 5A). Myofibroblasts produce IL-1β and are the likely source of IL-1β found in the infarcted myocardium (1). To determine whether myofibroblasts are associated with regions of heterogeneous Cx43 localization, we immunostained sections of EBZ for Cx43 and SMA, which labels myofibroblasts (and smooth muscle cells of vessels; Fig. 5) (1). In normal heart we found very low levels of SMA (Fig. 5B). As expected, Cx43 was abundant in all areas and was primarily found at the intercalated disks of cardiac myocytes (Fig. 5C). Overlay of these images is seen in Fig. 5D. In contrast, in the 5-day infarcted heart we see extensive regions of SMA staining, indicating the presence of large clusters of myofibroblasts (Fig. 5E). Examination of the Cx43 in the myofibroblast-containing regions showed that regions of high SMA staining were devoid of Cx43, whereas areas with little or no SMA staining showed normal Cx43 staining (Fig. 5F). Overlay of these images shows that regions with myofibroblasts are lacking in Cx43, whereas regions clear of myofibroblasts have Cx43 in normal localization (Fig. 5G). Interestingly, if you look at the interface region, where the SMA staining ends (Fig. 5H), Cx43 on myocytes is found on the lateral myocyte membranes (Fig. 5I, arrows). In the overlay of the insets it can clearly be seen that the normal Cx43 localization ends at the areas in which SMA staining begins (Fig. 5J). These data suggest that heterogeneous loss of Cx43 following MI is related to the presence of myofibroblasts.
Fig. 5.
Western blot analysis shows an increase in IL-1β in the EBZ of 5-day (5d) infarcted heart (A). Immunohistochemical studies indicate that normal heart has very few myofibroblasts (B) but has abundant Cx43, which is homogeneously expressed in the epicardium at the myocyte intercalated disks (C). Overlay images can be seen in D. In contrast, epicardial tissue from the 5-day infarct shows large myofibroblast clusters (E). Cx43 is found in a heterogeneous distribution in the same tissue section (F) with positive staining only seen in regions devoid of α-smooth muscle actin (SMA) staining. The overlay image shows that regions of myocytes intermixed with myofibroblasts have very low levels of Cx43, whereas regions in which myofibroblasts are missing still contain Cx43 (G). Higher magnification images of the border region of α-SMA staining (H) clearly shows that the myocytes have lateralized Cx43 (I). Overlay image shows the intercalation of a myocyte with lateralized Cx43 at the border of the α-SMA positive region (J). AU, arbitrary units.
Lucifer Yellow dye spread in intact heart shows no coupling between myocytes and myofibroblasts.
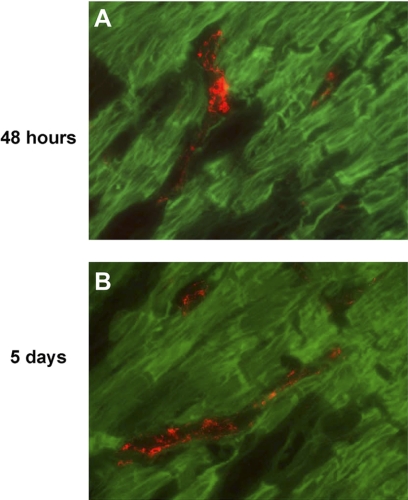
To determine whether cardiac myocytes of the EBZ were coupled to myofibroblasts by gap junctions too small to be detected by electron microscopy, we did as in situ dye assay to determine whether the gap junction permeant dye Lucifer Yellow was able to pass from cardiac myocytes to myofibroblasts. Following dye spread in myocytes we sectioned the tissue and stained for α-SMA to identify myofibroblasts (Fig. 6). We did two time points to insure that we did not miss earlier stage coupling between these two cell types. At 48 h post-coronary occlusion, dye spread assay showed no Lucifer Yellow (green) inside myofibroblasts (Fig. 6A, red; n = 3 animals). Additionally, when this experiment was done on 5-day infarct hearts we also saw no dye spread between myocytes and myofibroblasts (Fig. 6B; n = 3 animals). Being that the myofibroblasts in these pictures are completely surrounded by myocytes, if these two cell types were coupled the myofibroblasts should contain some Lucifer yellow dye. Thus these data suggest that if there is coupling between these two cell types in an intact heart, the levels of coupling are vanishingly small although the presence of low levels of electrical coupling is not ruled out by these studies.
Fig. 6.
Dye spread assay in intact canine infarcted heart. A: Lucifer Yellow (green) in myocytes of the EBZ of a 48-h infarct did not pass into myofibroblasts (red), indicating that these 2 cells types are not coupled in the intact heart after 48 h of infarction. B: myofibroblasts (red) were also not coupled directly to myocytes (green) in EBZ of a 5-day infarct.
Functional effect of myofibroblasts on propagation in the EBZ.
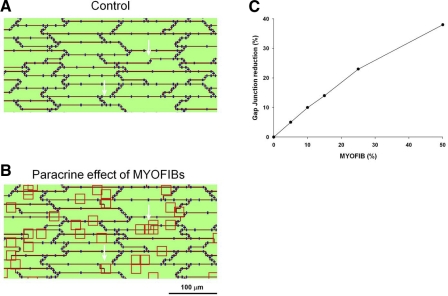
We simulated the paracrine effect of myofibroblast on myocytes as illustrated in Fig. 7. Figure 7A shows an area of the computational preparation in the absence of myofibroblasts. The red lines identify the boundaries of myocytes. Gap junctions are shown in blue. Green represents the cytoplasm. The only electrical connection between myocytes is through gap junctions. Figure 7B shows the effect of myofibroblasts with an average density of ∼25%. If myofibroblast clusters (red squares) are located on top of nodes that contain gap junctions (arrows), the gap junctions are eliminated to simulate the experimental finding that in the presence of myofibroblasts Cx43 is reduced. If the clusters are located on top of nodes that do not contain gap junctions, myofibroblasts do not have any effect. Given the relative concentration of gap junctions at the intercalated disks, most myofibroblast clusters are not located in an area containing gap junctions, and therefore do not have an effect in the immediate vicinity. In the computer model one-fourth of the nodes contain gap junctions. In the example in Fig. 7B, even though the myofibroblasts cover 25% of the preparation, only myofibroblasts covering about 6% of the preparation actually have an effect on gap junctions (that is, they are located close to gap junctions). Figure 7C shows the reduction in gap junctions for different myofibroblast densities. For example, a myofibroblast density of 25% results in a 23% reduction in gap junctions. Note that because in the computer model about one-fourth of the nodes contain gap junctions, for any given myofibroblast density, only about one-fourth of the myofibroblasts will have an effect on gap junctions.
Fig. 7.
Simulation of the paracrine effect of myofibroblasts (Myofib) on myocytes in the computer model. A: region of the simulated tissue in the absence of myofibroblasts. Red lines identify the boundaries of myocytes. Gap junctions are shown in blue. B: effect of myofibroblasts with an average density of ∼25% on the same tissue as in A. Note that if myofibroblast clusters (red squares) are located on top of nodes, which contain gap junctions (see white arrows for example), gap junctions are eliminated to simulate the experimental finding that in the presence of myofibroblasts Cx43 is reduced. C: reduction in gap junctions for different myofibroblast densities.
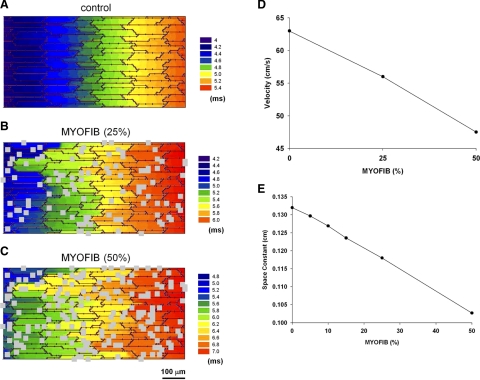
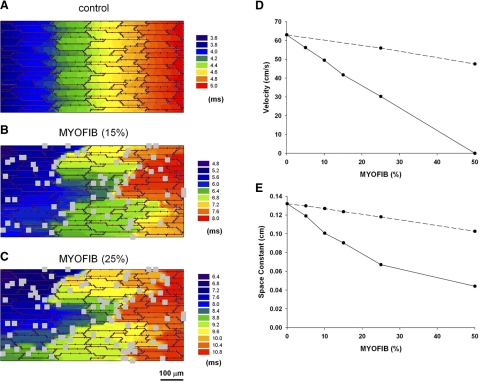
Figures 8 and 9 show the effect of myofibroblasts on propagation when myofibroblasts are located on top of myocytes and exert a purely paracrine effect on myocytes by reducing gap junctions and cell-to-cell conductance. Myofibroblast proliferation causes a decrease in conduction velocity. Figure 8 shows activation maps during longitudinal propagation (from left to right) in the absence of myofibroblast (Fig. 8A), for a density of 25% (Fig. 8B) and for a density of 50% (Fig. 8C) for a basic cycle length of 250 ms. Figure 8D shows the values of the velocities for the different densities. Although in the absence of myofibroblast propagation is essentially planar, the presence of myofibroblast results in more tortuous pathways of propagation, which result in a slower conduction velocity. The slower conduction velocity correlates with a decreased space constant with increasing myofibroblast densities (Fig. 8E).
Fig. 8.
Effect of myofibroblast proliferation on conduction velocity when myofibroblasts are located on top of myocytes and exert a purely paracrine effect on myocytes by reducing gap junctions and cell-to-cell conductance. Activation maps during longitudinal propagation (from left to right) in the absence of myofibroblasts (A), for a density of 25% (B) and for a density of 50% (C) for a basic cycle length of 250 ms are shown. D: longitudinal conduction velocity for different densities of myofibroblasts. E: space constant in the longitudinal direction for different densities of myofibroblasts.
Fig. 9.
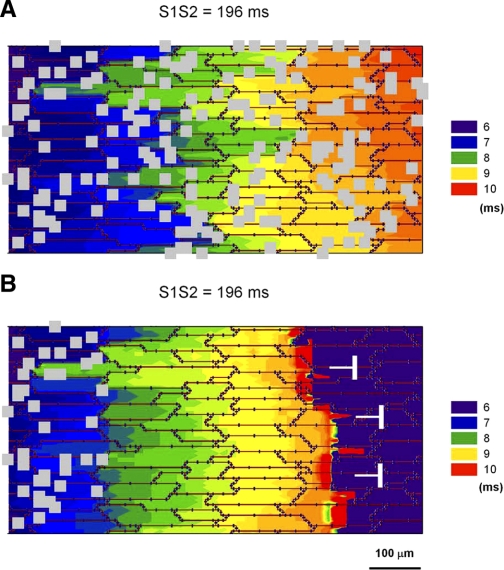
Effect of heterogeneities in myofibroblast (Myofib) density on propagation when myofibroblasts are located on top of myocytes and exert a purely paracrine effect on myocytes by reducing gap junctions and cell-to-cell conductance. A: propagation of a premature impulse (S1S2 = 196 ms) in a preparation with a myofibroblast density of ∼50%. The propagating impulse does not block. B: block of a premature impulse (S1S2 = 196 ms) at the boundary between an area with myofibroblasts (density 50%) and an area without myofibroblasts.
As illustrated by the experimental results, distribution of myofibroblast clusters is heterogeneous, and areas with a high density of myofibroblasts are located adjacent to areas with a lower density or without myofibroblasts. To investigate the effect of those heterogeneities on propagation, we performed the simulations in Fig. 9. Figure 9A shows propagation of a premature impulse (S1S2 = 196 ms) for a preparation with a myofibroblast density of ∼50%. Despite the slow conduction velocity resulting from prematurity and the presence of myofibroblast clusters there is no conduction block. However, in the presence of heterogeneities in myofibroblast density the propagating impulse can block. Figure 9B shows propagation of a premature impulse at the boundary between an area with a high density of myofibroblast (50%) and an area without myofibroblasts. The impulse propagates while in the area with high density (as in Fig. 9A) but blocks soon after the transition. Conduction failure is the result of impedance mismatch at the transition from low-coupled regions (shorter space constants) to highly coupled regions (longer space constants). The discontinuity does not cause block for a nonpremature impulse (S1S1 = 250 ms) (not shown).
Figures 10 and 11 show the effect of myofibroblast proliferation when myofibroblasts displace myocytes and are interspersed between myocytes. As in the simulations shown in Figs. 8 and 9, there are no electrical connections between myofibroblasts and myocytes. Figure 10 shows activation maps during longitudinal propagation (from left to right) in the absence of myofibroblasts (Fig. 10A), for a density of 15% (Fig. 10B), and for a density of 25% (Fig. 10C) for a basic cycle length of 250 ms. Figure 10D (solid line) shows a reduction in conduction velocity with increasing myofibroblast density. Figure 10D also shows for comparison the conduction velocities when myofibroblasts are located on top of myocytes (dashed line; same as Fig. 8D). The slower conduction velocity correlates with a decreased space constant with increasing myofibroblast densities (Fig. 10E). Shown in Fig. 10E (dashed line) also are the space constants when myofibroblasts are located on top of myocytes (same as Fig. 8E). Our results show that the effect of myofibroblasts on conduction velocity and space constant is more pronounced when myofibroblasts displace myocytes than when myofibroblasts are located on top of myocytes.
Fig. 10.
Effect of myofibroblast proliferation on conduction velocity when myofibroblasts displace myocytes. Activation maps during longitudinal propagation (from left to right) in the absence of myofibroblasts (A), for a density of 15% (B) and for a density of 25% (C) for a basic cycle length of 250 ms. D: longitudinal conduction velocity for different densities of myofibroblasts when myofibroblasts displace myocytes (solid line) and when myocytes are located on top of myocytes (dashed line). E: space constant in the longitudinal direction for different densities of myofibroblasts when myofibroblasts displace myocytes (solid line) and when myocytes are located on top of myocytes (dashed line).
Fig. 11.
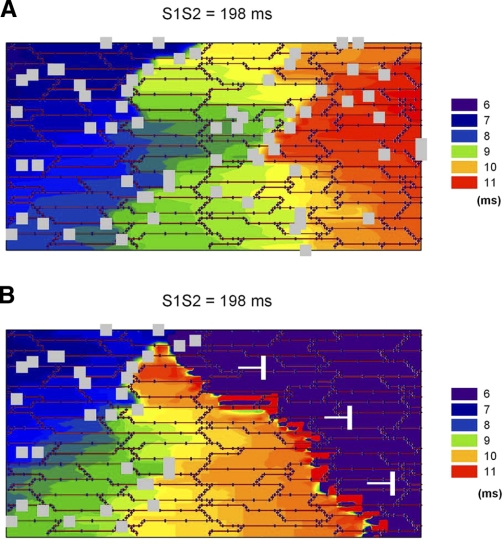
Effect of heterogeneities in myofibroblast (Myofib) density on propagation when myofibroblasts displace myocytes. A: propagation of a premature impulse (S1S2 = 198 ms) in a preparation with a myofibroblast density of ∼15%. The propagating impulse does not block. B: block of a premature impulse (S1S2 = 198 ms) at the boundary between an area with myofibroblasts (density 15%) and an area without myofibroblasts.
To investigate the effect of heterogeneities of myofibroblast distribution on propagation when myofibroblasts displace myocytes, we performed the simulations in Fig. 11. Figure 11A shows propagation of a premature impulse (S1S2 = 198 ms) for a preparation with a myofibroblast density of ∼15%. Despite the slow conduction velocity resulting from prematurity and the presence of myofibroblast clusters, there is no conduction block. However, in the presence of heterogeneities in myofibroblast density the propagating impulse can block. Figure 11B shows propagation of a premature impulse at the boundary between an area with a high density of myofibroblast (15%) and an area without myofibroblasts. The impulse propagates while in the area with high density (as in Fig. 11A) but blocks soon after the transition. The discontinuity does not cause block for a nonpremature impulse (S1S1 = 250 ms) (not shown). This results suggest that when myofibroblasts displace myocytes smaller heterogeneities in myofibroblast density can lead to block of premature impulses that when myofibroblasts are located on top of myocytes.
DISCUSSION
Based primarily on data from in vitro studies, it has been hypothesized that myocyte/fibroblast coupling may occur in the normal and infarcted heart and may affect propagation of the cardiac impulse (26). Little direct evidence has been generated demonstrating heterocellular coupling in normal or infarcted hearts in vivo. In the studies presented here, we show that in the EBZ of infarcted hearts gap junction plaques do not form between myofibroblasts and cardiac myocytes. Additionally, in infarcted hearts myofibroblasts may inhibit both myofibroblast-myocyte and myocyte-myocyte coupling by downregulating Cx43 expression via the IL-1β signal transduction pathway. Using computer simulations we show that myofibroblast proliferation in the EBZ causes slowed conduction and block.
Heterocellular coupling in vitro.
Multiple studies have convincingly shown that cells termed as fibroblasts form functional gap junctions with cardiac myocytes in the cell culture environment. There are at least two possible reasons why this occurs. First, in the vast majority of the in vitro studies the cardiac myocytes were derived from neonatal hearts, primarily from rats during the first 3 days of life, and the ability for these cells to undergo heterologous coupling may differ from that of adult cells. Specifically, it has been shown that during the neonatal period Cx43 is not yet localized to the intercalated disk. Instead, the cells are still somewhat rounded with Cx43 surrounding the sarcolemma, making cell-cell contacts on all sides (2). As development progresses cells elongate, form structural intercalated disks (ID) with cadherins and desmosomal proteins first localizing to the ID region, presumably to provide a structurally stable region with close cell contact preferentially with the cell, which neighbors in the longitudinal axis. This stable ID then becomes a platform for gap junction formation. Cells in culture do not undergo a complete transformation to the adult phenotype and do not form stable IDs. Thus the Cx43 remains in the developmental profile surrounding the myocyte and making indiscriminant junctions with neighboring cells, regardless of their phenotype. This phenomenon, known as promiscuous coupling, has been shown to occur between neonatal cells in culture from other systems (11, 27) when they do not, in fact, occur in the native tissue (25). In studies that examine coupling of adult myocytes and myofibroblasts (7, 21), it has been shown that coupling in vitro occurs, although under these conditions adult myocytes can dedifferentiate from being cells with a more mature phenotype to cells with a re-emergence of embryonic and neonatal cellular phenotypes. This again is likely to allow for promiscuous coupling in vitro, which is not seen in the adult infarcted heart.
In addition to the developmental stage of the cells, the typical culture conditions do not include mediators of the inflammatory response. Initial transformation of fibroblasts to myofibroblasts is due in part to IL-1β release by macrophages that hone to the site of injury following an MI. These macrophages are absent in culture dishes; thus although cultured fibroblasts all begin transformation to myofibroblasts, they may not have a fully differentiated myofibroblast phenotype, and thus they may not produce IL-1β. It may be that the fibroblast phenotype in culture is coupling competent but if macrophages were added to the milieu, the heterocellular coupling between the fibroblasts and the myocytes might be inhibited. Additional experiments examining the effect of inflammatory mediators on heterocellular coupling in vitro may add to our understanding of disparity between the in vitro and the in vivo studies.
Heterocellular coupling in vivo.
In contrast with the in vitro data, very few studies have shown coupling between fibroblasts and cardiac myocytes in vivo. Evidence for electrotonic interaction between these two cells types was initially described in the SAN in an isolated rat atrial preparation (17). At that time information on the isoform of connexin that might make these junctions was not yet available, but subsequent studies by the same group indicate that heterocellular junctions in the SAN were likely formed by Cx45 (6), at least in the rabbit model in which they used for these studies. The studies described here indicate that in the infarcted canine left ventricle following 5 days of coronary occlusion, heterocellular coupling does not appear to be present between cardiac myocytes and myofibroblasts. Although these studies do not directly prove that IL-1β regulates Cx43 in the intact heart, based on our data showing that IL-1β is present in the EBZ (Fig. 5A) and that it can regulate Cx43 in myocytes we propose that the lack of coupling is due, at least in part, to the inhibition of Cx43 by IL-1β.
Effect of myofibroblasts on impulse propagation.
Based on the results of the computer simulations we propose that myofibroblast proliferation results in a slowing of conduction velocity (Fig. 8) as a result of a reduction of Cx43. It is well established that a reduction of Cx43 leads to conduction velocity slowing in vivo (12). Myofibroblasts also slowed conduction in cell cultures (20), where myofibroblasts and myocytes are electrically coupled. In those experiments, the mechanism of slow conduction resulted from partial depolarization of myocytes by neighboring myofibroblasts, which caused a reduction in INa and the consequent slowing in conduction velocity. In contrast, in the simulations presented here, myofibroblasts and myocytes are not electrically coupled, and the slowing of conduction velocity results from the reduction in the space constant (Fig. 8E) that follows the paracrine of effect of myofibroblasts on Cx43.
We also found that heterogeneities in myofibroblast local densities result in conduction block of premature impulses in a computer model of the EBZ as a result of Cx43 heterogeneities (possibly the result of local inhibition of Cx43 by IL-1β). It has been shown experimentally that ventricular tachycardia can be initiated by premature stimulation in the EBZ of the canine infarcted heart, 4 to 5 days after ligation of the left anterior descending coronary artery (5). Tachycardia follows the formation of a line of unidirectional block. In this study we have not correlated the location of the lines of block leading to tachycardia with myofibroblast proliferation. But, based on the results of the computer simulations, we can speculate that block of premature impulses can occur at the boundary between regions with high myofibroblast density and regions with low myofibroblast density (Fig. 9). Because our experimental results (Fig. 5) show that the distribution of myofibroblasts clusters in the EBZ is heterogeneous, it is possible that heterogeneities in myofibroblast proliferation could be a factor contributing to the formation of lines of block in the EBZ. Several reports have linked Cx43 heterogeneities to an arrhythmogenic substrate. Heterogeneities in cell-to-cell gap junction coupling and Cx43 distribution exist in the EBZ, in regions where re-entrant circuits are originated and stabilize (4, 5). Heterogeneities in Cx43 expression have also been linked to lethal arrhythmias in conditional Cx43 knockout mice (8). Arrhythmogenic substrates resulting from heterogeneities in Cx43 expression have also been reported in experimental models of heart failure (1, 22).
On average, the increase in myofibroblast density in the EBZ is ∼2%. However, as we show in Fig. 5, distribution of myofibroblast clusters is heterogeneous, and regions with myofibroblast density above and below the average are likely to occur. Assuming a local paracrine effect of myofibroblasts on Cx43 and cell-to-cell coupling, such that myofibroblasts only affect the tissue below (Fig. 7), areas with myofibroblast densities of ∼50% are necessary to cause block of premature impulses (Fig. 9). However, if we assume that myofibroblasts can have an effect on Cx43 in a region around the myofibroblast clusters, perhaps as a result of diffusion of IL-1β ligands, the myofibroblast densities needed for block would be considerably reduced. It is unknown how far IL-1β ligands can diffuse, but our computer simulations using different myofibroblast densities can give us some insight into the functional consequences of IL-1β diffusion. For example, if IL-1β ligands diffuse ∼40 μm from myofibroblasts, then a myofibroblast cluster of size 20 μm × 20 μm could have a paracrine effect in an area ∼100 μm × 100 μm (∼25× larger than the cluster itself), and a ∼2% myofibroblast density would affect ∼50% of the preparation (effective area exposed to IL-1β ligands).
It is also possible that some of the myofibroblasts displace myocytes and cause a reduction in myocyte size. In that case, their effect on conduction is more pronounced than when myofibroblasts just lay on top of myocytes and exert a pure paracrine effect. This is reflected in the shorter space constants and slower conduction velocities for equivalent myofibroblast densities (Fig. 10). When myofibroblasts displace myocytes, substrates leading to block of premature impulses could occur at lower myofibroblast densities (Fig. 11).
Summary.
In contrast with several studies that show heterocellular coupling between fibroblasts and cardiac myocytes in vitro we find no evidence of heterocellular coupling in vivo in the EBZ of the post-MI canine heart. Based on our data if any coupling does occur between these two cell types it is likely to occur at very low levels. Additionally, using computer simulations, we show that the presence of myofibroblast clusters, which results in heterogeneous Cx43 reduction, leads to slow conduction and block. Together these data suggest that the presence of myofibroblasts in the postinfarct heart is one more component that aids in formation of the arrhythmogenic substrate.
GRANTS
This work was supported in part by American Heart Association Grant 0655807T (to C. Cabo) as well as American Heart Association Grant 0535084 and National Heart, Lung, and Blood Institute Grant NIH-RO1-HL083205 (to H. S. Duffy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.B., C.C., and H.S.D. conception and design of research; J.R.B., B.L., C.C., and H.S.D. performed experiments; J.R.B., B.L., C.C., and H.S.D. analyzed data; J.R.B., B.L., C.C., and H.S.D. interpreted results of experiments; J.R.B., B.L., C.C., and H.S.D. prepared figures; J.R.B., C.C., and H.S.D. drafted manuscript; J.R.B., C.C., and H.S.D. edited and revised manuscript; J.R.B., C.C., and H.S.D. approved final version of manuscript.
REFERENCES
- 1. Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol 293: H1223–H1230, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res 80: 88–94, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Baba S, Dun W, Cabo C, Boyden PA. Remodeling in cells from different regions of the reentrant circuit during ventricular tachycardia. Circulation 112: 2386–2396, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cabo C, Boyden PA. Electrical remodeling of the epicardial border zone in the canine infarcted heart: a computational analysis. Am J Physiol Heart Circ Physiol 284: H372–H384, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Cabo C, Yao J, Boyden PA, Chen S, Hussain W, Duffy HS, Ciaccio EJ, Peters NS, Wit AL. Heterogeneous gap junction remodeling in reentrant circuits in the epicardial border zone of the healing canine infarct. Cardiovasc Res 72: 241–249, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res 94: 828–835, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Chilton L, Giles WR, Smith GL. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J Physiol 583: 225–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res 95: 1035–1041, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duffy HS, Ashton AW, O′Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. Regulation of connexin43 protein complexes by intracellular acidification. Circ Res 94: 215–222, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Duffy HS, John GR, Lee SC, Brosnan CF, Spray DC. Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1beta in primary human fetal astrocytes. J Neurosci 20: RC114, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Froes MM, Correia AH, Garcia-Abreu J, Spray DC, Campos de Carvalho AC, Neto MV. Gap-junctional coupling between neurons and astrocytes in primary central nervous system cultures. Proc Natl Acad Sci U S A 96: 7541–7546, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res 88: 333–339, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iravanian S, Sovari AA, Lardin HA, Liu H, Xiao HD, Dolmatova E, Jiao Z, Harris BS, Witham EA, Gourdie RG, Duffy HS, Bernstein KE, Dudley SC., Jr Inhibition of renin-angiotensin system (RAS) reduces ventricular tachycardia risk by altering connexin43. J Mol Med (Berl) 89: 677–687, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. John GR, Scemes E, Suadicani SO, Liu JS, Charles PC, Lee SC, Spray DC, Brosnan CF. IL-1beta differentially regulates calcium wave propagation between primary human fetal astrocytes via pathways involving P2 receptors and gap junction channels. Proc Natl Acad Sci U S A 96: 11613–11618, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, Hirst-Jensen BJ, Duffy HS, Sorgen PL. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res 104: 1103–1112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84: 431–488, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Kohl P, Kamkin AG, Kiseleva IS, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol 79: 943–956, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Long CS, Brown RD. The cardiac fibroblast, another therapeutic target for mending the broken heart? J Mol Cell Cardiol 34: 1273–1278, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Macia E, Dolmatova E, Cabo C, Sosinsky AZ, Dun W, Coromilas J, Ciaccio EJ, Boyden PA, Wit AL, Duffy HS. Characterization of gap junction remodeling in epicardial border zone of healing canine infarcts and electrophysiological effects of partial reversal by rotigaptide. Circ Arrhythm Electrophysiol 4: 344–351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res 98: 801–810, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res 39: 280–300, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol 287: H1762–H1770, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol Cell Physiol 277: C1–C9, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Prestia KA, Sosunov EA, Anyukhovsky EP, Dolmatova E, Kelly CW, Brink PR, Robinson RB, Rosen MR, Duffy HS. Increased cell-cell coupling increases infarct size and does not decrease incidence of ventricular tachycardia in mice. Front Physiol 2: 1, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rash JE, Duffy HS, Dudek FE, Bilhartz BL, Whalen LR, Yasumura T. Grid-mapped freeze-fracture analysis of gap junctions in gray and white matter of adult rat central nervous system, with evidence for a “panglial syncytium” that is not coupled to neurons. J Comp Neurol 388: 265–292, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Rohr S. Myofibroblasts in diseased hearts: new players in cardiac arrhythmias? Heart Rhythm 6: 848–856, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Rozental R, Andrade-Rozental AF, Zheng X, Urban M, Spray DC, Chiu FC. Gap junction-mediated bidirectional signaling between human fetal hippocampal neurons and astrocytes. Dev Neurosci 23: 420–431, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Sohlenius AK, Reinfeldt M, Backstrom K, Bergstrand A, DePierre JW. Hepatic peroxisome proliferation in vitamin A-deficient mice without a simultaneous increase in peroxisomal acyl-CoA oxidase activity. Biochem Pharmacol 51: 821–827, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Spach MS, Heidlage JF. The stochastic nature of cardiac propagation at a microscopic level. Electrical description of myocardial architecture and its application to conduction. Circ Res 76: 366–380, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Ursell PC, Gardner PI, Albala A, Fenoglio JJ, Jr, Wit AL. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res 56: 436–451, 1985 [DOI] [PubMed] [Google Scholar]
- 31. Yankelson L, Feld Y, Bressler-Stramer T, Itzhaki I, Huber I, Gepstein A, Aronson D, Marom S, Gepstein L. Cell therapy for modification of the myocardial electrophysiological substrate. Circulation 117: 720–731, 2008 [DOI] [PubMed] [Google Scholar]