Abstract
Aims
Cardiolipin (CL) is a tetra-acyl phospholipid that provides structural and functional support to several proteins in the inner mitochondrial membrane. The majority of CL in the healthy mammalian heart contains four linoleic acid acyl chains (L4CL). A selective loss of L4CL is associated with mitochondrial dysfunction and heart failure in humans and animal models. We examined whether supplementing the diet with linoleic acid would preserve cardiac L4CL and attenuate mitochondrial dysfunction and contractile failure in rats with hypertensive heart failure.
Methods and results
Male spontaneously hypertensive heart failure rats (21 months of age) were administered diets supplemented with high-linoleate safflower oil (HLSO) or lard (10% w/w; 28% kilocalorie fat) or without supplemental fat (control) for 4 weeks. HLSO preserved L4CL and total CL to 90% of non-failing levels (vs. 61–75% in control and lard groups), and attenuated 17–22% decreases in state 3 mitochondrial respiration observed in the control and lard groups (P < 0.05). Left ventricular fractional shortening was significantly higher in HLSO vs. control (33 ± 2 vs. 29 ± 2%, P < 0.05), while plasma insulin levels were lower (5.4 ± 1.1 vs. 9.1 ± 2.3 ng/mL; P < 0.05), with no significant effect of lard supplementation. HLSO also increased serum concentrations of several eicosanoid species compared with control and lard diets, but had no effect on plasma glucose or blood pressure.
Conclusion
Moderate consumption of HLSO preserves CL and mitochondrial function in the failing heart and may be a useful adjuvant therapy for this condition.
Keywords: Heart failure, Polyunsaturated fatty acids, Cardiolipin, Mitochondria, Hypertension
1. Introduction
Modification of dietary fat content and composition is widely advocated as an important adjuvant therapy in the prevention of cardiovascular disease. Limiting fat consumption to ≤30% of total kilocalorie intake and favouring polyunsaturated over saturated and trans fatty acids are consistently recommended for the prevention of coronary artery disease.1–4 However, optimal dietary fat recommendations in the setting of chronic heart failure are less clear.5,6 We recently demonstrated that administration of a high-fat diet rich in linoleic acid (18:2n-6) improved survival in aged spontaneously hypertensive heart failure (SHHF) rats compared with a standard high-carbohydrate diet, whereas a diet rich in saturated fat (lard) had the opposite effect.7 The mechanisms responsible for the opposing effects of the diets were not clear from this study, but survival was closely associated with changes in the content and fatty acyl composition of cardiolipin (CL), a mitochondrial phospholipid that plays a critical role in maintaining mitochondrial function and myocardial health.8
CL is localized almost exclusively in mitochondria where it anchors cytochrome c to the inner membrane and provides essential structural and functional support to proteins involved in oxidative phosphorylation.8 In the healthy mammalian heart, the majority of CL molecules contain four linoleoyl acyl moieties (L4CL), a configuration generated by an acyl-chain remodelling process that requires sufficient bioavailability of linoleic acid, an essential fatty acid.8,9 A selective loss of CL and/or the proportion of L4CL species has been reported in the failing human heart and several rodent models of cardiac pathology,8 and is believed to be the primary cause of severe cardiomyopathy in Barth syndrome.10 While mitochondrial dysfunction has been implicated in the pathogenesis of these forms of cardiomyopathy, it remains unclear whether treatments aimed at restoring the CL content and/or its linoleate-rich composition are an effective means of preserving mitochondrial function and attenuating disease progression.
Given previous evidence that dietary linoleate supplementation reverses L4CL deficiency,7 we administered diets supplemented with moderate quantities of high-linoleic safflower oil (HLSO) or lard (total fat <30% kilocalorie intake) to SHHF rats in the present study during their progressive decline to end-stage heart failure when L4CL deficiency had been previously established.11,12 The effects of these diets on total CL and L4CL levels, mitochondrial respiration, and apoptotic signalling were examined and compared with values obtained from age-matched rats fed a standard chow diet without supplemental fat. In addition, effects on cardiac function, blood pressure, myocardial triglyceride accumulation, and circulating levels of leptin, glucose, insulin, and several bioactive lipid species derived from n-6 fatty acids were also examined based on previous studies implicating the effect of dietary fat interventions on these parameters during the progression of hypertensive heart disease.6,13,14
2. Methods
2.1. Animal model and diets
Male lean SHHF rats (Mccfacp −/−) were obtained from a colony maintained by Dr Sylvia McCune at the University of Colorado at Boulder. The SHHF rat model was selected for our studies due to its close similarity to the temporal, structural, and biochemical features of human hypertensive heart disease and dilated cardiomyopathy.15 Animals develop hypertension by 5 months of age, marked cardiac hypertrophy by 15 months, and classic dilated heart failure by 22–24 months of age,11,15 and respond to common pharmacotherapies at each stage of disease progression in a manner similar to what is seen in humans.16 As in humans, the precise mechanisms involved in the pathogenesis of heart failure in the SHHF rat are complex and multifactorial; however, the loss of L4CL is a feature common to humans, SHHF rats, and other animal models of cardiomyopathy.8,11
Animals were maintained on a standard low-fat diet (Purina 5001) ad libitum prior to being assigned to one of the following experimental groups: a 5-month-old baseline control group (5 month, n = 6), 21-month-old animals receiving standard chow (control, n = 10), HLSO (HLSO, n = 8), or lard diets (lard, n = 8) for 4 weeks until sacrifice at 22 months of age. In compliance with current dietary recommendations for the prevention of cardiovascular disease,4 total fat content of the HLSO and lard diets was limited to 28% of the total kilocalorie intake. All procedures in this investigation were approved by the Colorado State University Care and Use Committee and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
2.2. Echocardiography and blood pressure
Transthoracic echocardiography was performed non-invasively in rats under inhaled isoflurane anaesthesia (5% initial, 2% maintenance) using a 12 MHz paediatric transducer connected to a Hewlett Packard Sonos 5500 Ultrasound as previously described. Tail-cuff blood pressure measurements were obtained using the Kent Coda 6 system (Kent Scientific, Torrinton, CT, USA) in lightly isoflurane-anaesthetized rats.
2.3. Mitochondrial isolation and respiratory function
A detailed description of procedures for isolation of mitochondria, and assessment of respiratory function and enzyme activities is provided in the Supplementary material online, Methods. Briefly, following confirmation of deep anaesthesia (absence of hindlimb pinch reflex) with sodium pentobarbital (50 mg/kg ip), animals were sacrificed by midline thoracotomy and excision of the heart. Mitochondria were freshly isolated from ∼400 mg of left ventricular (LV) tissue in ice-cold Chappell-Perry buffer consisting of (in millimolar) 100 KCL, 50 MOPS, 1 EGTA, 5 MgSO4 · 7H2O, and 1 ATP (pH 7.4) with KOH by standard differential centrifugation methods as previously described.17 State 3 (ADP-stimulated) and state 4 (ADP-limited) mitochondrial respiratory function were measured in isolated mitochondria (0.25 mg protein) using a Clark-type electrode system (Strathkelvin) at 30°C with (in millimolar) pyruvate (5) + malate (5) or malate (5) + palmitoylcarnitine (PC) (0.04) as substrates to examine effects on lipid vs. carbohydrate-supported oxidation pathways.18 Cytochrome oxidase (COx) and citrate synthase activity were determined in frozen-thawed mitochondrial isolates using standard spectrophotometric methods on a Spectramax M2e spectrofluorometer (Molecular Devices).11,19 The relative protein abundance of OXPHOS complexes I–V (normalized to coomassie) was determined in mitochondrial isolates by standard immunoblotting methods using a commerically available antibody cocktail (Mitosciences).
2.4. Myocardial cardiolipin and global phospholipid fatty acid profiles
CL molecular species were determined in lipid extracts from 0.25 mg of mitochondrial protein by our published electrospray ionization mass spectrometry method.12 ‘Total’ CL represents the m/z sum of the 10 most prevalent CL species detected. To determine the fatty acid profile of total myocardial phospholipids, lipids were extracted from a 20–40 mg sample of LV tissue followed by extraction of the phospholipid fraction by thin layer chromatography and gas chromotography to determine the relative proportions of individual fatty acids. Detailed methods are available in the Supplementary material online.
2.5. Quantitative RT–PCR of CL metabolism genes and CL synthase activity
To determine the effect of the HLSO and lard diets on the mRNA expression of enzymes involved in CL metabolism, qRT–PCR was performed on mRNA extracted from LV tissue using methods and primers recently described in detail.20 Expression of the following genes were determined: Cytidinediphosphate-diacylglycerol (CDP-DAG) synthetase-2 (cds-2), a rate-limiting enzyme in polyglycerophospholipid biosynthesis essential for de novo synthesis of CL.21 CL synthase (crd-1), which catalyzes the formation of cardiolpin from phosphotidylglycerol and CDP-DAG. Tafazzin (taz), a CL transacylase involved in the fatty acyl remodelling of nacent CL into its predominant tetralinoleoyl (L4CL) configuration; and Acyl-CoA:lysocardiolipin acyltransferase-1 (alcat1) a polyglycerophospholipid acyltransferase capable of catalysing the acylation of lysocardiolipin putatively involved in the acyl-chain remodelling of CL. CL synthase enzyme activity was determined in isolated mitochondrial homogenates using methods previously described by Hatch and McClarty.22
2.6. Myocardial caspase activities and TUNEL staining
To provide an index of myocardial apoptotic signalling, Caspase-3/7, 8, and 9 activities were determined in LV homogenates (150 µg protein) by relative luminescence using the Caspase-Glo assays (Promega) according to the manufacturer's instructions on a luminescence spectrometer (Synergy 2; BioTek; Winooski, VT, USA). To confirm the presence of apoptosis in the heart, frozen myocardial tissue samples were obtained for TUNEL staining of apoptotic nuclei using a commercially available assay kit designed for cyropreserved tissue sections (GenScript, Piscataway, NJ, USA) according to the manufacturer's instructions (detailed methods available in the Supplementary material online).
2.7. Tissue triglycerides and plasma analyses
LV triglyceride content was determined using a spectrophotometric assay kit (BioVision). Non-fasted plasma was collected at the time of sacrifice and analysed using commercially available assays of plasma glucose (BioVision), insulin, and leptin (Crystal Chem) according to the manufacturers’ instructions.
2.8. Serum eicosanoid profile
Serum contents of arachidonic acid (20:4n6), docosahexaenoic acid (22:6n3), 9- and 13-hydroxyoctadecadienoic acids (9- and 13-HODE), and several eicosanoid species derived from 20:4n6 were quantified in lipid extracts obtained from 200 μL of serum by an LC/MS/MS in Dr Murphy's laboratory using deuterated standards (Cayman) as previously described in detail.23
2.9. Statistical analyses
All data are presented as group means ± SE. Group differences compared with baseline (5 month) values and among the 22-month groups examined by one-way ANOVA with Tukey tests post hoc. Within-group differences in echocardiography data from pre- to post-treatment were compared by paired t-tests. Statistical significance was established at P ≤ 0.05 for all analyses.
3. Results
3.1. Animal characteristics
Body weight increased in all animals by 21–22 months compared with 5-month baseline, but there was no significant effect of the HLSO or lard diet (Table 1). Heart weight increased from 5 to 22 months as expected in this model11,15 with a trend for greater heart weights in both HSLO and lard diets, but there were no independent effects of either diet on absolute or relative heart weight.
Table 1.
Animal morphology and blood pressure
| 5 Month | Control | HLSO | Lard | |
|---|---|---|---|---|
| BW-pre, g | NA | 434 ± 10 | 429 ± 18 | 419 ± 14 |
| BW-final, g | 239 ± 7 | 421 ± 14* | 426 ± 25* | 426 ± 8* |
| Heart, g | 1.24 ± 0.03 | 1.71 ± 0.07* | 1.80 ± 0.11* | 1.81 ± 0.07* |
| Brain, g | 1.88 ± 0.02 | 2.06 ± 0.38 | 2.10 ± 0.11 | 2.10 ± 0.09 |
| Heart/brain wt | 0.63 ± 0.02 | 0.79 ± 0.03* | 0.86 ± 0.05* | 0.86 ± 0.04* |
| Systolic BP, mmHg | 189 ± 5 | 194 ± 6 | 190 ± 5 | 194 ± 4 |
BP, blood pressure; BW, body weight.
Data are means ± SEM.
*P < 0.05 vs. 5 month.
3.2. Echocardiography and blood pressure
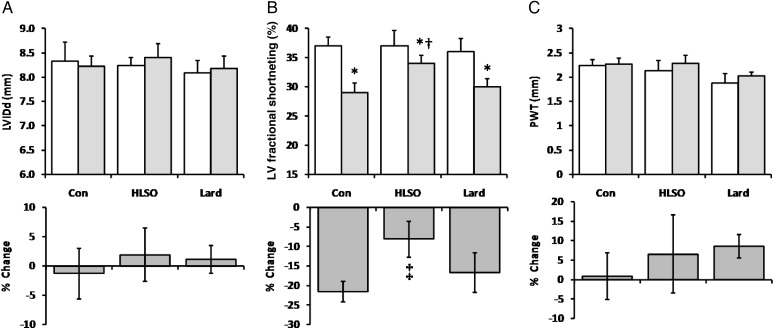
Animals were matched on all echocardiographic parameters at 21 months prior to beginning the experimental diets (Figure 1) and exhibited marked LV hypertrophy and systolic dysfunction compared with normal rats at this age, indicative of the early stages of decompensated heart failure well defined in this model.7,15 There were no changes in LV internal diameter in diastole (LVIDd) or posterior wall thickness (PWT) in any group over the 4-week-experimental period. LV fractional shortening significantly decreased in all groups, but this decline was attenuated by the HLSO diet. Blood pressure was significantly elevated in all groups compared with normal rats as previously reported,7,15 with no significant effect of either diet (Table 1).
Figure 1.
Echocardiography data from animals before (light bars; 21 months) and after (shaded bars; 22 months) the 4-week intervention period (n = 8/group). Data are means ± SEM for LV internal diameter in diastole (LVIDd; A), fractional shortening (FS; B), and LV posterior wall thickness (PWT; C). *P < 0.05 vs. pre-treatment levels; †P < 0.05 vs. Con and lard post-treatment. ‡P < 0.05 vs. Con and lard groups.
3.3. CL and phospholipid fatty acid profiles
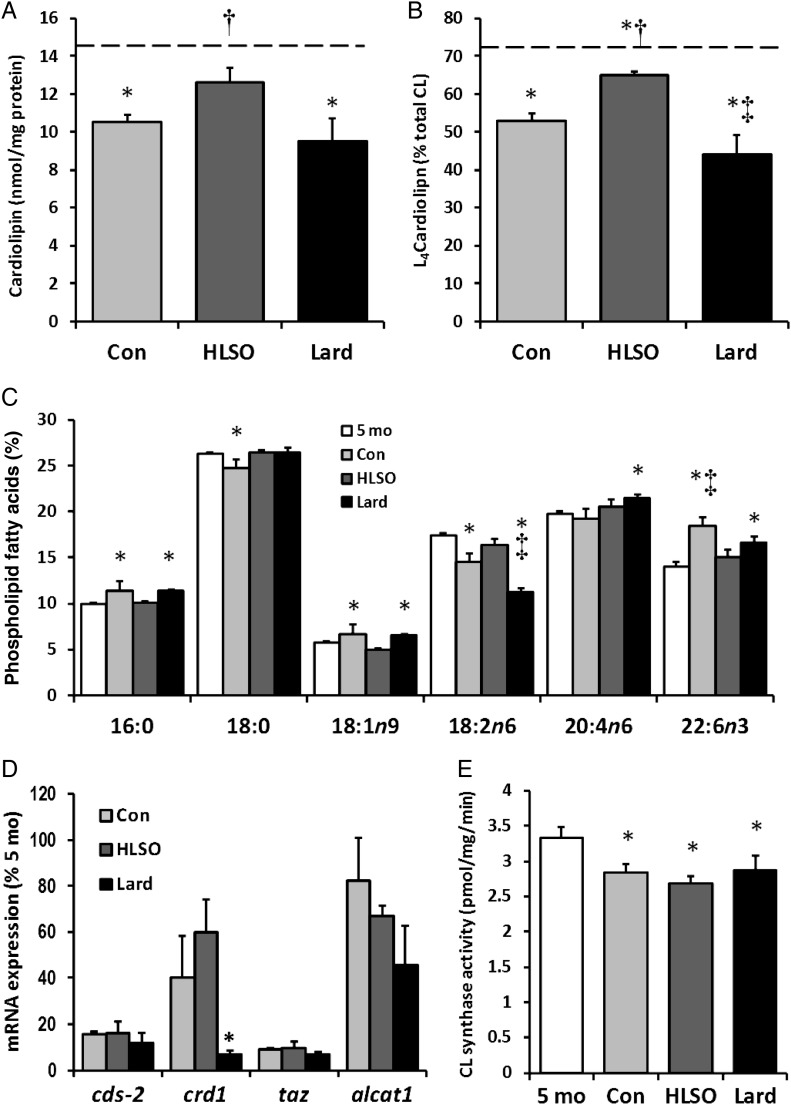
The total CL content of LV mitochondria decreased to 75 and 68% of 5 month levels in the control and lard groups by 22 months, respectively, but was maintained at 90% in the HLSO group (Figure 2). Similarly, the relative proportion of L4CL decreased to 74 and 61% of 5 month levels in the control and lard groups, respectively, but was restored to 90% of 5 month levels in the HLSO-fed animals (Figure 2B). This progressive loss of L4CL was associated with a 17 and 28% lower proportion of linoleate (18:2n-6) in the global myocardial phospholipid pool in the control and lard groups, respectively, which was restored to 94% of 5 month levels by HLSO feeding (Figure 2C). Relative proportions of palmitate (16:0), oleate (18:1n9), and DHA (22:6n3) were significantly greater in the 22-month control and lard groups compared with 5 month levels, but were maintained near 5 month levels by HLSO feeding. Both the HLSO and lard diets preserved levels of stearate (18:0) that declined in the 22-month vs. 5-month controls, and lard significantly increased arachidonate (20:4n6) levels compared with 5- and 22-month controls.
Figure 2.
Cardiolipin and membrane fatty acid profiles. Total cardiolipin content (A) and the relative proportion of L4CL (B) in LV mitochondria decreased in all 22-month groups vs. 5-month baseline, but this loss was attenuated by the HSLO diet. Several alterations in the myocardial phospholipid fatty acid profile resulted from heart failure and diets, (C; see text for details). Effect of the diets on mRNA expression of CL metabolism genes (D; see text for details). (E) CL synthase activity. Data are means ± SEM (n = 8/group). Dashed bars represent the mean values of 5-month baseline controls (n = 6). *P < 0.05 vs. 5-month control; †P < 0.05 vs. Con; ‡P < 0.05 vs. Con and HLSO.
3.4. CL metabolism genes and CL synthase activity
A recent study reported several significant age-related changes in the expression of enzymes involved in the biosynthesis and acyl-chain remodelling of CL in the SHHF rat model,20 therefore the effects of the HLSO and lard diets compared with 22-month controls were examined in the present study (Figure 2D). The HLSO diet tended to increase CL synthase (crd1) expression compared with control and lard groups (P = 0.10), but had no significant effects on the expression of other CL metabolism genes. The lard diet tended to decrease expression of all CL metabolism genes examined, and significantly suppressed expression of crd1 compared with control and HLSO groups (P < 0.05). Mitochondrial CL synthase activity was significantly lower in all 22-month groups compared with 5-month controls (P < 0.05), but was not significantly affected by the HLSO or lard diets.
3.5. Mitochondrial respiratory function
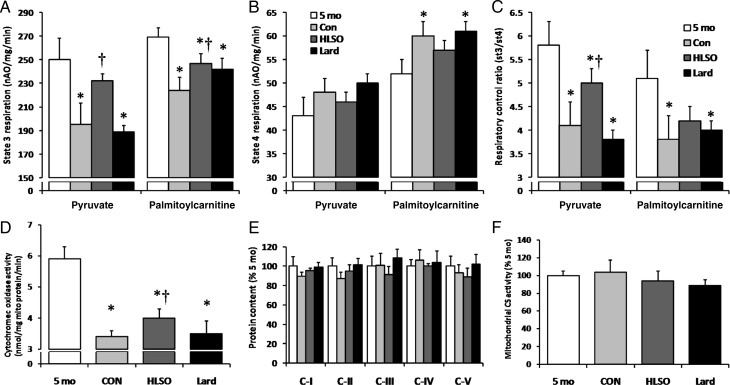
State 3 (ADP-dependent) respiration in isolated cardiac mitochondria declined to 78 and 83% of 5 month levels in the 22-month control animals using pyruvate and PC as substrates, respectively, indicating significant impairment of mitochondrial respiratory function with advancing heart failure in this model (Figure 3A). Administration of the HLSO diet maintained state 3 respiration levels to 95 and 91% of 5 month levels using pyruvate and PC, while the lard diet had no effect on pyruvate or PC respiration compared with 22-month controls. State 4 respiration tended to increase from 5 to 22 months in all groups using pyruvate, and increased to a statistically significant 16% above 5 month levels using PC in the control and lard, but not in the HLSO group (Figure 3B). These changes in respiratory states were reflected by a significant decrease in the respiratory control ratio (RCR; Figure 3C) in all 22-month groups that was attenuated by HLSO using pyruvate, but not PC as a substrate. Significant correlations were found between L4CL and state 3 (r = 0.59, 0.50), state 4 (r = −0.55, −0.54), and RCR (r = 0.88, 0.58) using pyruvate and PC as substrates, respectively, using paired data from individual animals in the control, HLSO, and lard groups (n = 5–6/group) (P < 0.05 for all analyses). The mitochondrial activity of COx, a CL-dependent respiratory enzyme, was significantly lower in all the 22-month groups compared with 5 month levels, but was significantly greater in the HLSO compared with lard and control groups (Figure 3D). There was no effect of diet or disease progression on the protein contents of OXPHOS complexes I–V or the activity of the matrix enzyme citrate synthase in LV mitochondria (Figure 3E and F).
Figure 3.
Left ventricular mitochondrial respiratory function. State 3 (ADP-dependent) respiration was lower in the control and lard groups using both pyruvate and palmitoylcarnitine as the substrate compared with 5-month baseline values, but was partially preserved in the HLSO-treated animals (A). State 4 (uncoupled) respiration tended to be elevated in the Con and lard groups vs. baseline, but this increase was attenuated in the HLSO group (B). The respiratory control ratio was lower in the control and lard groups using both pyruvate and palmitoylcarnitine as the substrate compared with 5-month baseline values, but was partially preserved in the HLSO-treated animals (C). Cytochrome oxidase activity in mitochondrial isolates was lower in all 22-month groups vs. 5-month baseline, but was significantly higher in the HLSO group compared with lard and control (D) groups. There were no significant effects of disease or diet on the relative protein contents of mitochondrial OXPHOS complexes (E) or citrate synthase activity (F). Data are means ± SEM (n = 8/group). *P < 0.05 vs. 5-month baseline; †P < 0.05 vs. Con; ‡P < 0.05 vs. all groups.
3.6. Myocardial caspase activities and TUNEL staining
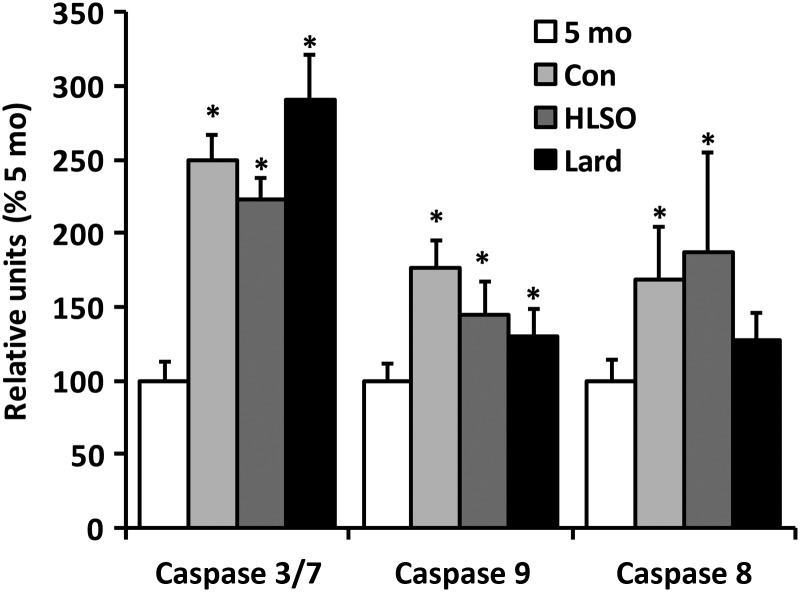
Myocardial caspase 3/7, 8, and 9 activities were significantly greater in the 22-month-old control group compared with 5 month levels (Figure 4), indicating significant induction of cardiac apoptotic signalling with disease progression in this model. The HSLO diet attenuated caspase-3/7 activity compared with control, whereas the lard diet increased activity above control and HLSO levels (P < 0.05). Both HLSO and lard tended to suppress caspase-9 activity, but no significant effects of diet were seen on caspase-9 or -8 activity. Despite significant effects on caspase-3/7, TUNEL staining of myocardial sections failed to confirm any effect of disease or diet on apoptosis in this model (Supplementary material online, Figure S2).
Figure 4.
Caspase activities from myocardial homogenates. Elevations in caspase activities were observed in all older animals vs. 5 month (P < 0.05) with the exception of caspase 8 in the lard group. Caspase 3/7 activity was significantly lower in the HLSO and higher in the lard group compared with control rats (P ≤ 0.05). Data are means ± SEM (n = 4–8/group). *P < 0.05 vs. 5-month baseline; †P < 0.05 vs. Con.
3.7. Tissue triglycerides and plasma analyses
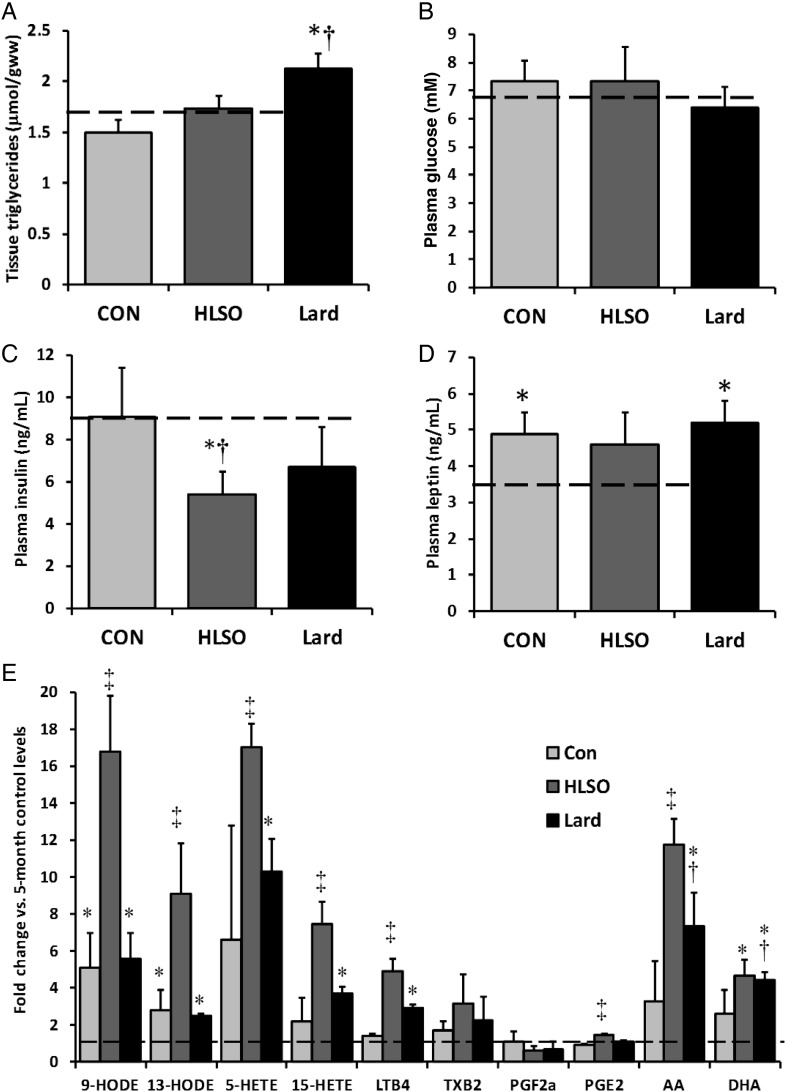
LV triglyceride content was not significantly altered by disease progression or the HLSO diet, but was increased by the lard diet (Figure 5A). There were no effects of disease or diet on fed plasma glucose or leptin levels (Figure 5B and D); however, plasma insulin tended to decrease with both fat supplemented diets, reaching statistical significance in the HLSO group only.
Figure 5.
Myocardial TAG content and blood analyses. Tissue TAG content remained similar to 5-month baseline control levels in the 22-month control and HLSO groups, but was elevated by the lard diet (P < 0.05) (A). Plasma glucose levels were unchanged by HF or diet (B), but plasma insulin was lower in the 22-month HLSO group vs. 5- and 22-month controls (C). Plasma leptin was slightly elevated in all 22-month groups vs. baseline, but was unaffected by diet (D). Serum arachidonic (AA), docosahexaenoic acid (DHA), and several products of the n-6 PUFA lipoxygenase pathways were elevated in 22-month groups vs. 5-month control, and were dramatically elevated by the HLSO diet (E; see text for details). Data are means ± SEM (n = 8/group). Dashed bars represent the mean values of 5-month baseline values (n = 6). *P < 0.05 vs. 5-month baseline; †P < 0.05 vs. Con; ‡P < 0.05 vs. all groups.
3.8. Serum eicosanoids
Given the possibility that dietary linoleate supplementation could augment production of bioactive lipid species by the cyclooxygenase and lipoxygenase pathways with potential relevance in inflammatory processes and cardiovascular health, the serum contents of several lipid species derived from linoleic (HODEs) and arachidonic acid metabolism (eicosanoids) were examined (Figure 5E). Plasma levels of HODEs were significantly elevated at 22 months compared with 5 month levels in rats fed the control and lard diets, along with a trend for higher levels of 5-, 12-, and 15-hydroxyeicosatetraenoic acid (HETE), thromboxane B2 (TXB2), 20:4n6 and 22:6n3, indicating up-regulation of polyunsaturated fatty acid desaturation, lipoxygenase, and cyclooxygenase pathways with disease progression in this model. HLSO significantly augmented production of HODEs, HETEs, leukotriene B4 (LTB4), and prostaglandin E2 compared with 22-month control and lard groups, but had no significant effect on TXB2 or PGF2-alpha. The lard diet increased serum LTB4, 20:4n6 and 22:6n3 compared with 22-month controls.
4. Discussion
The present study demonstrates that supplementing the diet with HLSO, limiting total fat consumption to <30% of kilocalorie intake, increases L4CL and total CL content in LV mitochondria, and attenuates the cardiac mitochondrial respiratory dysfunction associated with advanced hypertensive heart disease. HLSO also attenuated LV contractile dysfunction compared with the lard and control diets, but had no effect on blood pressure despite elevating serum levels of putatively vasoactive n-6 PUFA-derived eicosanoid species. These findings support accumulating evidence for the potential cardiovascular benefits of dietary linoleic acid7,24–26 and highlight a unique biological role of this essential fatty acid not routinely considered in nutritional studies that may have important implications for myocardial health and disease.
The present study corroborates previous evidence for a progressive loss of L4CL in SHHF rats11 that is attenuated by dietary linoleate and slightly worsened by lard supplementation.7 Diet-induced changes in L4CL paralleled changes in myocardial membrane 18:2n6 content, suggesting that HLSO and lard could have influenced L4CL levels by altering the bioavailability of phospholipid 18:2n6 for CL remodelling. Both the HLSO and lard diets elicited changes in crd-1 expression that reflected their effects on total CL levels; however, parallel effects on CL synthase enzymatic activity in mitochondrial homogenates were not observed. This finding is in agreement with a recent study by Lu et al.27 reporting that CL synthase mRNA levels do not necessarily correlate with enzyme activity or CL content, and suggests that diet-induced changes in total CL levels must have resulted from altered flux through the biosynthetic pathway upstream of CL synthase and/or CL degradation pathways. Mitochondrial CL levels are regulated by a complex interplay of hormonal, enzymatic, and non-enzymatic factors,28–31 any number of which might have been influenced by dietary fat content/composition and/or its effects on cardiac pathology in the present study. Additional studies investigating the interaction of dietary lipids on CL metabolism may yield important insights into how nutritional interventions may influence CL-dependent processes relevant to human health and disease.8,31
As previously reported,11 decreases in L4CL corresponded to reduced mitochondrial COx activity, which paralleled significant lower rates of state 3 respiration in the present study. COx is known to require L4CL for optimal activity,32 and so we hypothesized that preservation of L4CL by HLSO would at least partially restore COx activity and improve mitochondrial respiratory function. Indeed, mitochondria from the HLSO-fed animals exhibited greater COx activity and higher rates of state 3 respiration using both carbohydrate and fatty acid substrates compared with animals fed the control diet, whereas the lard diet had no effect. There were no effects of disease progression or diet on the protein contents of COx or the other OXPHOS complexes in LV mitochondria, indicating that HLSO-induced increases in L4CL and/or total CL may be, at least in part, responsible for the improvements in respiratory function. Decreases in the capacity and/or efficiency of mitochondrial oxidative phosphorlyation are widely hypothesized to contribute to cardiac dysfunction in heart failure,33,34 and therefore the benefits of HLSO on respiratory function, while relatively modest, could have contributed to its preservation of LV function in the current study.
CL is known to anchor cytochrome c to the inner mitochondrial membrane,35 and loss of its content or 18:2n6-rich configuration has been shown to initiate release of cytochrome c, triggering activation of caspase-9, and initiation of apoptosis in vitro.36 CL is also required for activation of caspase-8, implicating its role in mitochondria-dependent apoptosis in response to Fas stimulation as well.37 Given evidence supporting a role of mitochondria-mediated apoptosis in the development of cardiomyopathy,38 we examined whether changes in CL were associated with altered myocardial caspase activities and apoptosis in the present study. Significant increases in all three caspase activities were observed in the 22-month-old SHHF rats compared with young animals, providing novel evidence of increased apoptotic signalling during the development of cardiomyopathy in this model. However, we failed to detect any TUNEL positive nuclei in hearts from the control or diet groups, indicating that the actual level of myocardial apoptosis is either very low or non-existent. This finding is in agreement with previous studies reporting the occurrence of apoptosis as extremely low or undetectable in cardiomyopathic hearts from SHHF rats39 and humans.40,41 Nevertheless, very low rates of apoptotic myocyte loss could contribute significantly to cardiac dysfunction and failure in vivo,42 and therefore we cannot rule the potential effect of enhanced apoptotic signalling in the progression of cardiomyopathy in the present study. The respective effects of HLSO and lard on caspase-3/7 activity are consistent with previous evidence indicating pro- and anti-apoptotic effects of diets rich in saturated vs. unsaturated fatty acids, respectively.43 However, the involvement of CL alterations in these effects is questionable since both HLSO and lard tended to decrease caspase-9 activity, and neither significantly affected caspase-8. Taken together, these data suggest that induction of apoptotic signalling occurs during the early stages of heart failure in the SHHF rat model and may be influenced by dietary fat composition. However, the pathological significance and influence of CL alterations in this process requires more direct investigation.
In addition to its effects on mitochondria and CL, the HLSO diet might have improved LV function by other mechanisms. While there were no effects of disease progression or diet on plasma glucose levels, HLSO tended to suppress serum insulin to a greater extent than the lard diet. High levels of circulating insulin have been associated with insulin resistance and poor prognosis in heart failure.6,44 While insulin sensitivity was not assessed in the present study, the suppressive effect of HLSO on plasma insulin coupled with a greater capacity of mitochondria to oxidize pyruvate may reflect an improvement in myocardial glucose utilization, which could have improved LV function.45 It is also interesting to note that HLSO failed to elicit the significant increase in tissue triglycerides seen with the lard diet. Myocardial accumulation of triglycerides has been associated with cardiac dysfunction in humans and animal models;6,46 however, it is unlikely to explain the effects of lard and HLSO on cardiac function since the decline in fractional shortening in the lard group was similar to that of controls.
The HLSO diet also promoted an increase in the serum levels of several bioactive lipid metabolites derived from 18:2n6 and its desaturation-elongation product arachidonic acid (20:4n6) compared with the control and lard diet groups. The most dramatic increases were in HETEs and HODEs generated by the enzymatic lipoxygenation of 20:4n6 and 18:2n6, respectively. Effects on cyclooxygenase-derived TXB2, PGE2, and PGF2α were comparatively minor, suggesting a preferential increase in lipoxygenase activities in this model despite an abundance of exogenous substrate available to both enzyme systems. Up-regulation of 12/15 lipoxygenase has been linked to heart failure in mice,47 possibly through activation of inflammatory and/or other signalling pathways in cardiomyocytes.48 However, the present study and another7 support a beneficial effect of dietary HLSO supplementation in the SHHF rat model despite serum elevations in these molecules.
Interestingly, a very recent study by Galvao et al.49 reported improved survival in δ-sarcoglycan null cardiomyopathic hamsters fed a high-fat diet (45% kilocalorie fat) rich in saturated fat (from lard and cocoa butter) compared with a high-fat PUFA-rich diet (containing 18:2n6 and 18:3n3) or low-fat control diet, despite persistent mitochondrial defects and no improvement of cardiac function. This finding conflicts with the results of the current investigation and a previous study demonstrating improved survival in SHHF rats fed a high-fat HLSO diet compared with lard and control diets.7 Specific differences between the present study and that of Galvao et al. include the timing and duration of the dietary interventions, the specific fatty acid content/composition of the diets, and the species and disease phenotype of the animal model being investigated; all of which could contribute to the conflicting results. Accordingly, care should be taken when interpreting the findings of these and other animal studies in the context of human heart failure, which results from a variety of different aetiologies that might respond differently to dietary HLSO and lard supplementation.
In conclusion, the present study demonstrates that moderate HLSO supplementation preserves the content and linoleate enrichment of CL and attenuates the loss of mitochondrial and contractile function in the failing rat heart. These findings underscore potentially important biological effects of dietary linoleic acid and/or HLSO, and support the potential utility of this dietary intervention as an adjuvant therapy in the treatment of heart failure associated with hypertensive heart disease.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by grants from the American Heart Association (grant numbers 0620009Z and 0835545N to A.J.C., and 0265205Z to G.C.S.), a Colorado Agricultural Experimental Station (to A.J.C.), Heart and Stroke Foundation of Manitoba (to G.M.H.), and National Institutes of Health (grant numbers HL72770 to D.H.B., HL72790 to R.L.M., and U54 GM069338 to R.C.M.).
Supplementary Material
Acknowledgements
The authors thank Rachel Gioscia and David Bolden at the University of Colorado for their technical assistance and care of the animals. G.M.H. is a Canada Research Chair in Molecular Cardiolipin Metabolism.
Conflict of interest: none declared.
References
- 1.Hu FB. Diet and cardiovascular disease prevention the need for a paradigm shift. J Am Coll Cardiol. 2007;50:22–24. doi: 10.1016/j.jacc.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 3.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, et al. AHA dietary guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 5.Rennison JH, McElfresh TA, Chen X, Anand VR, Hoit BD, Hoppel CL, et al. Prolonged exposure to high dietary lipids is not associated with lipotoxicity in heart failure. J Mol Cell Cardiol. 2009;46:883–890. doi: 10.1016/j.yjmcc.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chess DJ, Stanley WC. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res. 2008;79:269–278. doi: 10.1093/cvr/cvn074. [DOI] [PubMed] [Google Scholar]
- 7.Chicco AJ, Sparagna GC, McCune SA, Johnson CA, Murphy RC, Bolden DA, et al. Linoleate-rich high-fat diet decreases mortality in hypertensive heart failure rats compared with lard and low-fat diets. Hypertension. 2008;52:549–555. doi: 10.1161/HYPERTENSIONAHA.108.114264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 9.Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 10.Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;580:5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, et al. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res. 2005;46:1196–1204. doi: 10.1194/jlr.M500031-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Nasjletti A, Arthur C. Corcoran memorial lecture. The role of eicosanoids in angiotensin-dependent hypertension. Hypertension. 1998;31:194–200. doi: 10.1161/01.hyp.31.1.194. [DOI] [PubMed] [Google Scholar]
- 14.Sweeney G. Cardiovascular effects of leptin. Nat Rev Cardiol. 2010;7:22–29. doi: 10.1038/nrcardio.2009.224. [DOI] [PubMed] [Google Scholar]
- 15.Heyen JR, Blasi ER, Nikula K, Rocha R, Daust HA, Frierdich G, et al. Structural, functional, and molecular characterization of the SHHF model of heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H1775–H1784. doi: 10.1152/ajpheart.00305.2002. [DOI] [PubMed] [Google Scholar]
- 16.Tamura T, Said S, Andersen SM, McCune SA, Mochizuki S, Gerdes AM. Temporal regression of myocyte hypertrophy in hypertensive, heart failure-prone rats treated with an AT1-receptor antagonist. J Card Fail. 2002;8:43–47. doi: 10.1054/jcaf.2002.32030. [DOI] [PubMed] [Google Scholar]
- 17.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 18.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955;217:383–393. [PubMed] [Google Scholar]
- 19.Srere P. Citrate snythase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- 20.Saini-Chohan HK, Holmes MG, Chicco AJ, Taylor WA, Moore RL, McCune SA, et al. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res. 2009;50:1600–1608. doi: 10.1194/jlr.M800561-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatch GM. Cardiolipin biosynthesis in the isolated heart. Biochem J. 1994;297(Pt 1):201–208. doi: 10.1042/bj2970201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatch GM, McClarty G. Regulation of cardiolipin biosynthesis in H9c2 cardiac myoblasts by cytidine 5′-triphosphate. J Biol Chem. 1996;271:25810–25816. doi: 10.1074/jbc.271.42.25810. [DOI] [PubMed] [Google Scholar]
- 23.Murphy RC, Barkley RM, Zemski Berry K, Hankin J, Harrison K, Johnson C, et al. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal Biochem. 2005;346:1–42. doi: 10.1016/j.ab.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC. The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. J Cardiovasc Med (Hagerstown) 2007;8(Suppl 1):S42–S45. doi: 10.2459/01.JCM.0000289275.72556.13. [DOI] [PubMed] [Google Scholar]
- 25.Laaksonen DE, Nyyssonen K, Niskanen L, Rissanen TH, Salonen JT. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med. 2005;165:193–199. doi: 10.1001/archinte.165.2.193. [DOI] [PubMed] [Google Scholar]
- 26.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 27.Lu B, Xu FY, Taylor WA, Feingold KR, Hatch GM. Cardiolipin synthase-1 mRNA expression does not correlate with endogenous cardiolipin synthase enzyme activity in vitro and in vivo in mammalian lipopolysaccharide models of inflammation. Inflammation. 2011;34:247–254. doi: 10.1007/s10753-010-9230-3. [DOI] [PubMed] [Google Scholar]
- 28.Schlame M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J Lipid Res. 2008;49:1607–1620. doi: 10.1194/jlr.R700018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatch GM. Cardiolipin: biosynthesis, remodeling and trafficking in the heart and mammalian cells (Review) Int J Mol Med. 1998;1:33–41. doi: 10.3892/ijmm.1.1.33. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Chen S, Thompson MN, Greenberg ML. New insights into the regulation of cardiolipin biosynthesis in yeast: implications for Barth syndrome. Biochim Biophys Acta. 2007;1771:432–441. doi: 10.1016/j.bbalip.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Claypool SM, Koehler CM. The complexity of cardiolipin in health and disease. Trends Biochem Sci. 2012;37:32–41. doi: 10.1016/j.tibs.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaoka-Koseki S, Urade R, Kito M. Cardiolipins from rats fed different dietary lipids affect bovine heart cytochrome c oxidase activity. J Nutr. 1991;121:956–958. doi: 10.1093/jn/121.7.956. [DOI] [PubMed] [Google Scholar]
- 33.Murray AJ, Cole MA, Lygate CA, Carr CA, Stuckey DJ, Little SE, et al. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol. 2008;44:694–700. doi: 10.1016/j.yjmcc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:2361–2367. doi: 10.1006/jmcc.2000.1266. [DOI] [PubMed] [Google Scholar]
- 35.Choi SY, Gonzalvez F, Jenkins GM, Slomianny C, Chretien D, Arnoult D, et al. Cardiolipin deficiency releases cytochrome c from the inner mitochondrial membrane and accelerates stimuli-elicited apoptosis. Cell Death Differ. 2007;14:597–606. doi: 10.1038/sj.cdd.4402020. [DOI] [PubMed] [Google Scholar]
- 36.Ostrander DB, Sparagna GC, Amoscato AA, McMillin JB, Dowhan W. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J Biol Chem. 2001;276:38061–38067. doi: 10.1074/jbc.M107067200. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ, et al. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, et al. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci U S A. 1999;96:8144–8149. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura T, Said S, Lu W, Harris J, Neufeld D, Burbach JA, et al. Is apoptosis present in progression to chronic hypertensive heart failure? J Card Fail. 2000;6:37–42. doi: 10.1016/s1071-9164(00)00010-5. [DOI] [PubMed] [Google Scholar]
- 40.Kanoh M, Takemura G, Misao J, Hayakawa Y, Aoyama T, Nishigaki K, et al. Significance of myocytes with positive DNA in situ nick end-labeling (TUNEL) in hearts with dilated cardiomyopathy: not apoptosis but DNA repair. Circulation. 1999;99:2757–2764. doi: 10.1161/01.cir.99.21.2757. [DOI] [PubMed] [Google Scholar]
- 41.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 42.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, et al. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–H44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 44.Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, et al. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30:527–532. doi: 10.1016/s0735-1097(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 45.Stanley WC, Chandler MP. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Fail Rev. 2002;7:115–130. doi: 10.1023/a:1015320423577. [DOI] [PubMed] [Google Scholar]
- 46.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 47.Kayama Y, Minamino T, Toko H, Sakamoto M, Shimizu I, Takahashi H, et al. Cardiac 12/15 lipoxygenase-induced inflammation is involved in heart failure. J Exp Med. 2009;206:1565–1574. doi: 10.1084/jem.20082596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jenkins CM, Cedars A, Gross RW. Eicosanoid signalling pathways in the heart. Cardiovasc Res. 2009;82:240–249. doi: 10.1093/cvr/cvn346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galvao TF, Brown BH, Hecker PA, O'Connell KA, O'Shea KM, Sabbah HN, et al. High intake of saturated fat, but not polyunsaturated fat, improves survival in heart failure despite persistent mitochondrial defects. Cardiovasc Res. 2012;93:24–32. doi: 10.1093/cvr/cvr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.