Abstract
Aims
Vascular cartilaginous metaplasia and calcification are common in patients with atherosclerosis. However, sources of cells contributing to the development of this complication are currently unknown. In this study, we ascertained the origin of cells that give rise to cartilaginous and bony elements in atherosclerotic vessels.
Methods and results
We utilized genetic fate mapping strategies to trace cells of smooth muscle (SM) origin via SM22α-Cre recombinase and Rosa26-LacZ Cre reporter alleles. In animals expressing both transgenes, co-existence within a single cell of β-galactosidase [marking cells originally derived from SM cells (SMCs)] with osteochondrogenic (Runx2/Cbfa1) or chondrocytic (Sox9, type II collagen) markers, along with simultaneous loss of SM lineage proteins, provides a strong evidence supporting reprogramming of SMCs towards osteochondrogenic or chondrocytic differentiation. Using this technique, we found that vascular SMCs accounted for ∼80% of Runx2/Cbfa1-positive cells and almost all of type II collagen-positive cells (∼98%) in atherosclerotic vessels of LDLr−/− and ApoE−/− mice. We also assessed contribution from bone marrow (BM)-derived cells via analysing vessels dissected from chimerical ApoE−/− mice transplanted with green fluorescence protein-expressing BM. Marrow-derived cells were found to account for ∼20% of Runx2/Cbfa1-positive cells in calcified atherosclerotic vessels of ApoE−/− mice.
Conclusion
Our results are the first to definitively identify cell sources attributable to atherosclerotic intimal calcification. SMCs were found to be a major contributor that reprogrammed its lineage towards osteochondrogenesis. Marrow-derived cells from the circulation also contributed significantly to the early osteochondrogenic differentiation in atherosclerotic vessels.
Keywords: Atherosclerosis, Circulating progenitors, Osteochondrogenic differentiation, Smooth muscle cells, Vascular calcification
1. Introduction
Vascular calcification (VC), a complication arising from calcium-phosphate salt deposition in the form of hydroxyapatite in vasculature, is commonly associated with ageing and is highly prevalent in patients with atherosclerosis, type II diabetes mellitus (T2D), and end-stage renal disease (ESRD).1–3 Clinical consequences of VC are largely dependent upon its location, extent, and organs affected. In atherosclerosis, calcification is found mainly in the intima of blood vessels, in the form of dispersed, punctate nanocrystals. As lesion development proceeds, these calcium-phosphate crystals aggregate to produce larger crystals associated with the necrotic core of atheromas. The presence and extent of intimal calcification has been reported to be highly correlated with atherosclerotic plaque burden in addition to the risk of myocardial infarction, plaque instability, and stroke.1 The notion that VC contributes to the susceptibility of plaque rupture was recently confirmed in an elegant study by Ehara et al.4 that spotty calcification typified the culprit plaques in patients with acute myocardial infarction. VC also occurs in the media of blood vessels with or without association to atherosclerosis.3,5 Calcium-phosphate salt typically deposits circumferentially along the elastic lamina of arterial media,5 leading to increased arterial stiffness and pulse wave velocity and pressure that impairs cardiovascular function.6 In T2D and ESRD patients, arterial medial calcification is highly prevalent and considered a major contributor to the heightened risk of cardiovascular mortality, stroke, and lower-limb amputation observed in this population.2,3,6 Despite the associated risk factors for cardiovascular disease, there are no drug therapies currently available to treat or prevent VC. To advance the development of targeted therapeutics, it is important to understand mechanisms underlying this pathology, especially what cell types give rise to osteochondrogenic precursor-, osteoblast-, and chondrocyte-like cells in calcifying vasculature and whether this is a common phenomenon among various disease conditions.
A growing number of mechanistic studies have highlighted VC as an actively regulated process, potentially involving osteochondrogenic differentiation of vascular wall cells in response to disease-specific environmental cues.5,7–10 Molecules that initiate and regulate osteoblastic and chondrocytic differentiation, e.g. Runx2/Cbfa1, BMP2, Msx2, osterix, and Sox9, were frequently observed in the early stages of VC.7,9 Cells with osteoblastic and/or chondrocytic properties, as indicated by expression of bone and/or cartilage marker proteins, e.g. alkaline phosphatase, bone Gla protein, type II collagen (Col II), and osteopontin,5,9 were often co-localized with calcium-phosphate deposits within the vessel wall. In fact, outright ossification was observed in ∼10–25% of calcified atherosclerotic arteries, as evidenced by bone marrow (BM), cartilage, and mature lamellar bone.5,11 More importantly, osteochondrogenic differentiation and matrix calcification were reproduced in vitro using cells isolated from normal vasculature, such as calcifying vascular cells cloned from aortic media8 and uncloned heterogeneous vascular smooth muscle cells (SMCs).10
The most direct evidence supporting a critical role of mural cells in VC is from the study of matrix Gla protein knockout (MGP−/−) mice. These mice develop VC arterial medial calcification early in life with features similar to Monckeberg's medial sclerosis observed in T2D and ESRD patients.5,10 Using a Cre-loxP site-specific recombination technique that allows genetic fate mapping of specific cell types in vivo,12,13 vascular SMCs were found to contribute to essentially all of the chondrocyte-like cells (∼97%) in calcifying MGP−/− vessels, as evidenced by the loss of SMC lineage markers (SM-MHC, SM22α, and SMα-actin) coupled with the gain of osteochondrogenic markers (Runx2/Cbfa1, osteopontin, and Col II) within cells of β-galactosidase activity (cells that had once exhibited SMC property). In these mice, circulating progenitors did not contribute to osteochondrogenic precursor- or chondrocyte-like cells.
The MGP−/− genetic fate mapping study provided significant insight into the role of SMCs in a medial calcification model resembling human autosomal recessive genetic disorder, Keutel syndrome,14 and long-term warfarin treatment.15 Numerous studies suggest that intimal calcification in atherosclerotic vessels differs from medial calcification of Monckeberg's medial sclerosis by its prevalent diseases, pathogenesis, histoanatomic locations, and possibly histological appearance.2–5,7,16 Additionally, potential cell sources for osteogenic lineages in atherosclerotic lesions include not only vascular SMCs, but also local and circulating multipotent progenitor cells,7,8,17 although there has been no genetic fate mapping study that directly tested these possibilities. In the present study, we applied the Cre-loxP site-specific recombination system to genetically label cells of SM origin in low-density lipoprotein receptor mutant (LDLr−/−) mice and apolipoprotein mutant (ApoE−/−) mice, providing a direct test of whether SMCs undergo lineage reprogramming and contribute to the development of cartilaginous metaplasia and calcification in atherosclerotic vessels. We also employed a BM transplantation (BMT) technique to trace cells derived from circulation in ApoE−/− mice,18 allowing us to determine the contribution of BM-derived cells. Our studies are the first to provide definitive evidence of cell sources that play an important role in the osteochondrogenic processes observed in atherosclerotic vessels.
2. Methods
2.1. Genetic fate mapping of LDLr mutant and ApoE mutant mice
Cells of SM origin were marked by SM22α-Cre and R26R transgenes. Briefly, LDLr−/− mice (002207) and ApoE−/− mice (002052) were purchased from the Jackson Laboratory and bred to SM22α-Cre (gift from Dr Herz, UT) and R26R (gift from Dr Soriano, FHCRC) transgenic alleles to produce SM22α-Cre+/0:R26R+/0:LDLr−/− and SM22α-Cre+/0:R26R+/0:ApoE−/− mice, respectively, for the study. Ten-week-old SM22α-Cre+/0:R26R+/0:LDLr−/− mice were fed with a high-fat, high-cholesterol diet (HFD; Research Diets Inc., 1.25% cholesterol, 39.9% kcal fat, 40% kcal carbohydrate) to induce atherosclerosis and VC. Normal chow was used as a diet control. SM22α-Cre0/0:R26R+/0:LDLr−/− mice were used as controls to determine specificity and efficiency of Cre recombination. SM22α-Cre+/0:R26R+/0:LDLr−/− mice were anaesthetized with 50–180 mg/kg pentobarbital intraperitoneally followed by exsanguination via cardiac puncture for blood collection at 18–30 weeks on diet. SM22α-Cre+/0:R26R+/0:ApoE−/− mice were fed with normal chow and sacrificed by pentobarbital injection (300 mg/kg) at 45–60 weeks of age. Fasted sera were collected for blood chemical analyses; aortic arches and innominate arteries were collected for histology. A total of 64 LDLr−/− mice and 12 ApoE−/− mice were examined.
2.2. Marrow transplantation to generate ApoE−/−:GFP+/0 chimeric mice
Male ApoE−/−:GFP+/0 mice of 8–9 weeks old were sacrificed by Nembutal injection on the day of transplantation and marrow cells were harvested from femurs. The cells were pelleted and re-suspended with sterile phosphate-buffered saline (PBS) to a final concentration of 4 × 107 cells/mL. Recipient ApoE−/− mice received neomycin water (2 mg/mL) 1 week prior to BMT and 2 weeks thereafter and were lethally irradiated by 9.5 Gy (Cesium-137 γ-ray source) 24 h prior to BMT. On the day of BMT, each recipient mice was anaesthetized with isoflurane inhalation (3–5% for induction and 1–3% for maintenance) and transplanted with ∼1.2 × 107 ApoE−/−:GFP+/0 marrow cells via retro-orbital sinus injection. ApoE−/−:GFP+/0 chimeric mice were maintained in a specific pathogen-free environment, fed with normal chow, and euthanized for study by pentobarbital injection (300 mg/kg) 10 weeks after BMT. Engraftment rate was assessed by flow cytometric analysis of peripheral blood collected from the chimeric ApoE−/− mice. Briefly, whole blood was lysed with erythrocyte lysis buffer (15.5 mM NH4Cl, 1.0 mM KHCO3, 0.1 mM EDTA). Nucleated cells were collected and re-suspended in ice-cold PBS containing 2% foetal bovine serum and 5 U/mL heparin. The cells were stained with propidium iodide and analysed by flow cytometry for GFP-positive cells. More than 83 ± 5% of peripheral blood cells in the ApoE−/−:GFP+/0 chimeric mice were of donor origin.18
Animals were maintained in a specific pathogen-free environment and genotypes were determined as described.19 All protocols are in compliance with the NIH Guideline for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee, University of Washington.
2.3. X-gal staining
Tissues dissected from SM22α-Cre+/0:R26R-LacZ+/0:LDLr−/−, SM22α-Cre0/0:R26R-LacZ+/0:LDLr−/−, SM22α-Cre+/0:R26R-LacZ+/0:ApoE−/−, and SM22-Cre0/0:R26R+/0:ApoE−/− mice were stained using a β-galactosidase stain kit (Special Media) as described previously.19 Specifically, tissues were fixed on ice with tissue fixative (catalog # BG-5-C) for 1.5 h, followed by a 30-min wash in tissue rinse solution A (catalog # BG-6-B) at room temperature and a 5 min wash in tissue rinse solution B (catalog # BG-7-B). The tissues were then stained with freshly prepared β-gal tissue stain solution (catalog # BG-8-C and BG-3-G) at 37°C for 2–4 h depending on the tissue type and size.
2.4. Tissue preparation and histochemical and immunohistochemical staining
X-gal-stained tissues were post-fixed with Methyl Carnoy's fixative prior to processing and embedding in paraffin. Tissues dissected from ApoE−/−:GFP+/0 chimeras were fixed with 10% buffered formalin and embedded in paraffin. Five-micrometre sections were used for histochemical and immunohistochemical analyses. Movat Pentachrome stain was used to visualize morphology, von Kossa stain was used to detect calcium-phosphate minerals, antibodies recognizing SM22α (ab10135, Abcam; 0.25 μg/mL), MOMA-2 (YSRTMCA519G, Accurate Chemical & Scientific Corporation; 10 μg/mL), Runx2/Cbfa1 (MAB2006, R&D Systems; 5.0 μg/mL), Sox-9 (sc-20095, Santa Cruz; 3.0 μg/mL), type II collagen (AB761, Millipore; 50 μg/mL), and green fluorescence protein (A11122, Invitrogen; 3.3 μg/mL) were used to detect SMCs, macrophages, osteochondrogenic precursors, chondrocytes, and cells carrying GFP transgenes. All immunohistochemical staining were validated with non-specific IgG controls as well as negative and positive control tissues, e.g. aortic sections from LDLr−/− mice fed with normal chow as negative controls and femur sections as positive controls for osteochondrogenic and chondrocytic marker proteins. Sections were counterstained with either methyl green or nuclear fast red (Vector) as indicated in the figure legends.
To quantify osteochondrogenic precursors that were derived from vascular SMCs or BM cells, triple-stained aortic sections (β-galactosidase or GFP, Runx2/Cbfa1, and nuclear fast red) were used. In each group, slides from seven randomly selected animals were imaged. Total cell numbers, cells positive for Runx2/Cbfa1, and cells positive for both Runx2/Cbfa1- and β-galactosidase (or GFP) in the entire cartilaginous and calcifying intimal lesions were counted. Cell numbers were normalized to the lesion areas. The percentage of Runx2/Cbfa1-positive cells in cartilaginous metaplasia and calcification lesions was used to evaluate osteochondrogenic differentiation. The percentage of Runx2/Cbfa1-positive cells with β-galactosidase activity (stained blue by X-gal) is noted as SM-derived osteochondrogenic cells. The percentage of Runx2/Cbfa1-positive cells that were also positive for GFP is noted as BM-derived osteochondrogenic cells. A similar approach was employed to quantify chondrocyte-like cells that were derived from vascular SMCs using triple-stained sections for X-gal, type II collagen, and nuclear fast red.
2.5. Statistical analysis
Data, shown as means ± SD, were analysed with Student's t-test or ANOVA to determine the significance of differences. Data were considered to be statistically significant at a P–value of <0.05.
3. Results
3.1. LDLr−/− mice fed with HFD developed cartilaginous metaplasia and calcification in atherosclerotic intima and arterial media
To determine what cell types contribute to the development of VC associated with atherosclerosis, we genetically marked cells of SM origin in LDLr−/− mice (see Supplementary material online, Figure S1A–D).19 The mice were fed with HFD to induce atherosclerosis and VC and were terminated at 18–30 weeks on diet. As seen in Table 1, mice fed with HFD showed an increase in serum cholesterol and triglyceride levels at all time points (P ≤ 0.001–0.05) compared with the normal chow counterparts. The HFD group gained more body weight, statistically significant only at 18-week diet-fed (32 ± 5 vs. 22 ± 3 g), while their fasted glucose levels were similar to mice fed with normal chow, suggesting that these mice were not diabetic. In addition, no renal failure and related hyperparathyroidism were found in these animals as determined by their serum blood urea nitrogen, phosphorus, and alkaline phosphatase levels.
Table 1.
Body weight and blood chemistry of SM22α-Cre+/0:R26R-LacZ+/0: LDLr−/− mice fed with D12108 diet or normal chow
| 18 weeks on diet |
24 weeks on diet |
28 weeks on diet |
||||
|---|---|---|---|---|---|---|
| Normal chow (n= 8) | D12108 (n= 13) | Normal chow (n= 11) | D12108 (n= 12) | Normal chow (n= 6) | D12108 (n= 11) | |
| Total cholesterol (mg/dL) | 274 ± 46† | 1433 ± 252† | 282 ± 29† | 1570 ± 362† | 270 ± 25† | 1539 ± 302† |
| Triglyceride (mg/dL) | 72 ± 27‡ | 146 ± 63‡ | 80 ± 16‡ | 172 ± 103‡ | 76 ± 16‡ | 225 ± 145‡ |
| Body weight (g) | 22 ± 3* | 32 ± 5* | 28 ± 4 | 35 ± 4 | 28.6 ± 7.0 | 32.2 ± 6.3 |
| Glucose (mg/dL) | 173 ± 55 | 162 ± 55 | 191 ± 60 | 182 ± 52 | 242 ± 42 | 211 ± 63 |
| BUN (mg/dL) | 30.3 ± 8.4 | 26.0 ± 4.8 | 30 ± 9 | 24 ± 5 | 27.50 ± 3.5 | 25.6 ± 4.1 |
| Phosphorus (mg/dL) | 7 ± 0.8 | 8 ± 1 | 8 ± 2 | 8 ± 0.9 | 9 ± 1.5 | 9 ± 1.8 |
| Alkaline phosphatase (U/L) | 83 ± 19 | 78 ± 34 | 65 ± 13 | 81 ± 25 | 85.5 ± 41 | 82 ± 29 |
†P = 0.0001.
‡P ≤ 0.05.
*P = 0.005.
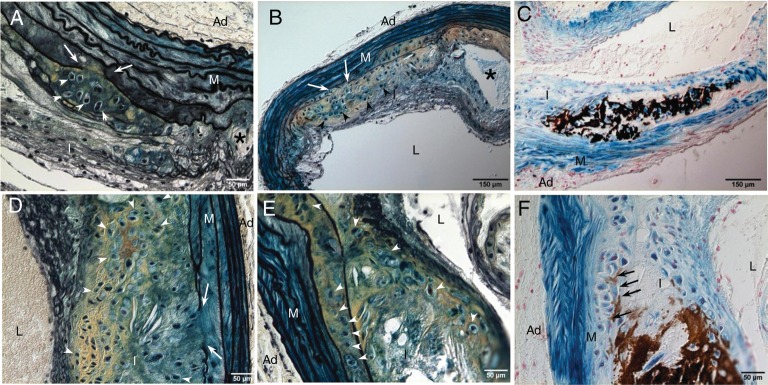
In arteries of LDLr−/− mice fed with HFD, cartilaginous matrices consisting of a collagen- (yellow) and proteoglycan- (blue) rich extracellular matrix embedded with chondrocyte-like cells characterized by relatively large amount of clear cytoplasm surrounded by a lacunar rim (arrowheads) were first found in deep intima and inner medial layers of the vessels (Figure 1A and B). Interestingly, cartilaginous metaplasia tended to occur adjacent to areas of elastic lamina breakage (arrows), outside the lipid core, and independent from areas containing macrophage foam cells. Cartilaginous metaplasia could be found in the absence of outright calcification and was observed as early as 18–20 weeks. Around 33% of LDLr−/− vessels displayed chondrocyte-like cells (Table 2), with only one out of 18 (5%) calcified at this stage (Figure 1C, dark brown). By 24 weeks, the animals showed rapid progression of vascular osteochondrogenesis, with large areas filled with chondrocyte-like cells (Figure 1D and E, arrowheads) in all vessels examined (13/13). Approximately 46% were calcified, mostly in the deep intima and inner medial layers adjacent to the lesions (Figure 1F, brown and arrows, and Table 2). By 30 weeks, all the animals (13/13) had lesions containing both cartilaginous metaplasia and calcification (Table 2). These results suggest that the development of cartilaginous metaplasia may have preceded calcification in LDLr−/− vessels, similar to the process of endochondral ossification in hard tissue. Finally, mice fed with normal chow did not develop atherosclerosis, cartilaginous metaplasia, or calcification in their vasculature (Table 2) and were negative for markers of osteochondrogenesis (Runx2/Cbfa1) and chondrocytes (Sox9 and Col II) (data not shown).
Figure 1.
Development of cartilaginous metaplasia and calcification in atherosclerotic vessels of LDLr−/− mice. Aortic arches were dissected from SM22α-Cre+/0:R26R+/0:LDLr−/− mice fed with HFD for 18–20 weeks (A–C) and 24 weeks (D–F). Cells of SM origin were stained by X-gal before embedding. Calcification was stained by the von Kossa method (C and F). Cells of chondrocyte morphology were visualized by Movat pentachrome staining (A, B, D, and E). White arrows designate elastic lamina breakage. White arrowheads designate chondrocyte-like cells. Black arrows designate medial calcification. Asterisk designates necrotic core. L, lumen; I, intima; M, media; Ad, adventitia.
Table 2.
Incidence of cartilaginous metaplasia and calcification in SM22α-Cre+/0:R26R-LacZ+/0:LDLr−/− vessels
| Cartilaginous metaplasia |
Calcification |
|||||
|---|---|---|---|---|---|---|
| 18–20 weeks | 24 weeks | 28–30 weeks | 18–20 weeks | 24 weeks | 28–30 weeks | |
| Normal chow | 0/5 (0%) | 0/3 (0%) | 0/7 (0%) | 0/5 (0%) | 0/3 (0%) | 0/7 (0%) |
| D12108 | 6/18 (33%) | 13/13 (100%) | 13/13 (100%) | 1/18 (5%) | 6/13 (46%) | 13/13 (100%) |
3.2. Cells of SM origin are the major source of osteochondrogenic precursors and chondrocytes seen in LDLr−/− vessels
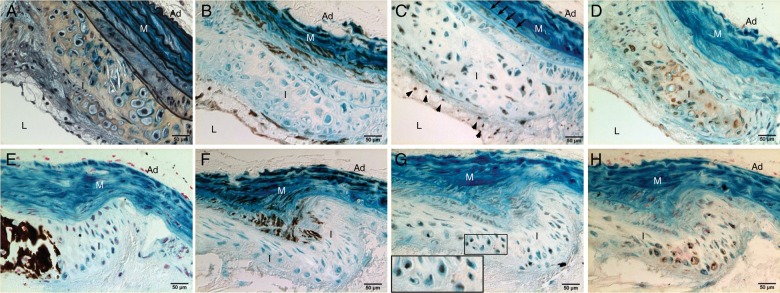
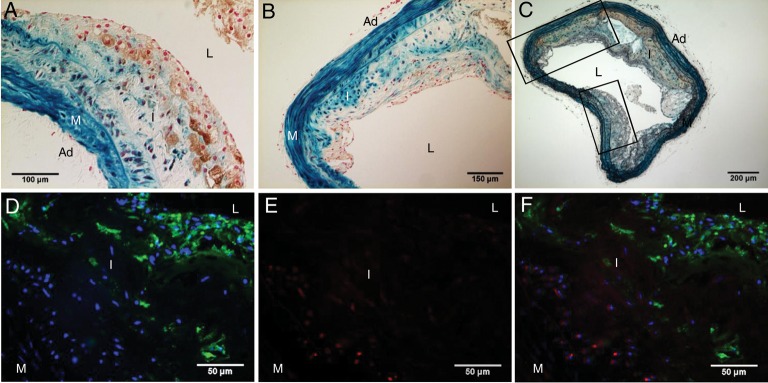
To determine whether SMCs contribute to vascular osteochondrogenic differentiation, SM22α-Cre+/0:R26R+/0:LDLr−/− vessels were stained with X-gal to identify cells of SM origin. As shown in Figures 1C and F, and 2, cells in cartilaginous metaplasia and calcified regions were primarily stained blue by X-gal, indicating that they had once acquired SMC characteristics. However, these cells no longer expressed SMC lineage proteins, e.g. SM22α (Figure 2B and F, lack of brown stain). Instead, they developed osteochondrogenic and chondrocytic properties as identified by transcriptional factors Runx2/Cbfa1 (Figure 2C) and Sox9 (Figure 2G), and chondrocyte marker protein, Col II (Figure 2D and H). Because these cells were also stained blue by X-gal, they were likely to have differentiated from vascular SMCs, either migrating from arterial media or from circulating multipotent cells that had expressed SM22α at an earlier time point. Since LDLr−/− mice fed with HFD also developed lipid-filled lesions, we stained these sections for macrophages with MOMA-2 antibody. As shown in Figure 3, MOMA-2-positive cells were mostly found in the lipid-laden areas (Figure 3A, brown; Figure 3D and F, green fluorescence) and were neither stained by X-gal (Figure 3A, lack of blue in brown area) nor co-localized with chondrocyte marker, Sox9 (Figure 3E and F, red fluorescence).
Figure 2.
SMCs gave rise to osteochondrogenic precursor- and chondrocyte-like cells in atherosclerotic LDLr−/− vessels. Aortic arches were dissected from SM22α-Cre+/0:R26R+/0:LDLr−/− mice fed with HFD for 20 weeks (A–D) and 28 weeks (E–H). Cells of SMC origin were stained by X-gal before embedding. Adjacent sections were stained by Movat pentachrome (A), von Kossa (E), and immunohistochemistry for SM22α (B and F), Runx2/Cbfa1 (C), Sox9 (G), and Col II (D and H). Insert in G. Higher-powered magnification of the boxed region shows colocalization of β-galactosidase (blue) and chondrocytic transcription factor, Sox9 (brown). L, lumen; I, intima; M, media; Ad, adventitia.
Figure 3.
Determination of macrophages in atherosclerotic vessels of LDLr−/− mice. Aortic arches were dissected from SM22α-Cre+/0:R26R+/0:LDLr−/− mice fed with HFD diet for 20 weeks. Cells of SM origin were stained by X-gal before embedding. MOMA-2 antibody was used to identify macrophages (A and B, brown; D and F, green fluorescence). Cells of chondrocyte morphology were visualized by Movat pentachrome staining (C, yellow) and immunohistochemistry for Sox9 (E and F, red fluorescence). Note that MOMA-2-positive cells were not stained blue by X-gal (A and B) and were negative for chondrocyte marker, Sox9 (F). A and B. Images taken from MOMA-2-stained adjacent sections in the boxed regions of C. L, lumen; I, intima; M, media; Ad, adventitia.
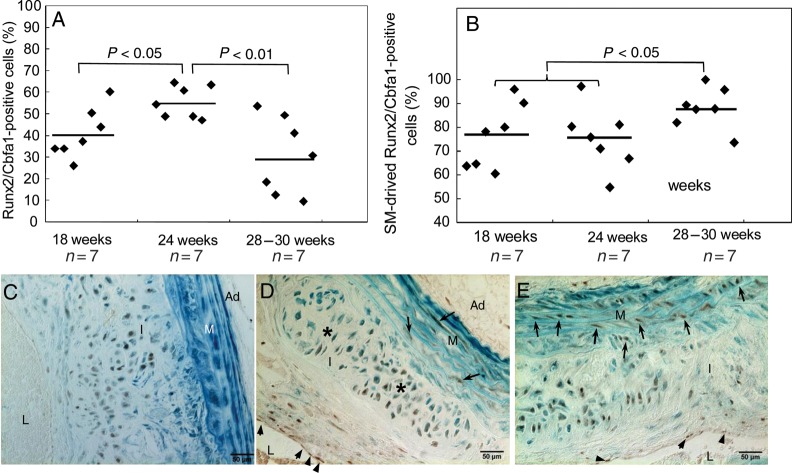
Runx2/Cbfa1 is a critical transcription factor that governs early osteochondrogenic differentiation and chondrocyte maturation in skeletal tissue.20 To better understand osteochondrogenic differentiation of cells in atherosclerotic vessels, we analysed the expression patterns of Runx2/Cbfa1 at multiple stages of vascular cartilaginous metaplasia and calcification. Interestingly, Runx2/Cbfa1 appeared in LDLr−/− vessels as early as 18 weeks, accounting for 40.9% of cells inside the intimal lesions (Figure 4A). Most of the Runx2/Cbfa1-positive cells were found in the intimal areas of cartilaginous metaplasia, notably areas containing early chondrocytic cells and cartilaginous matrix (Figure 4C, brown, vs. Figure 1D, arrowheads and yellow), as well as in areas in which cells were organized in a pattern similar to that found in proliferating zones of growth plates (Figure 4D, asterisks). Some Runx2/Cbfa1-positive cells were found in media adjacent to cartilaginous lesions (arrows) and in intima towards the lumen (arrowheads). Few were also seen in the adventitia (Figures 2C and 4D and E, brown nuclear stain). The expression of Runx2/Cbfa1 peaked at 24 weeks and decreased thereafter (Figure 3A). At later time points, Runx2/Cbfa1 was found in cells with morphologies resembling hypertrophic chondrocytes, with and without calcification (Figures 2C and 4D). Most of the Runx2/Cbfa1-positive cells were stained blue by X-gal, consistent with the theory of SMCs undergoing lineage reprogramming towards osteochondrogenesis. In addition, cells residing in the intima adjacent to the lumen were not stained by X-gal, identifying their non-SMC source (Figures 2C and 4D and E; arrowheads). Contribution of SMCs to osteochondrogenic precursors was further quantified using X-gal, Runx2/Cbfa1 antibody, and nuclear fast red triple-stained sections. Of ∼1000–2000 Runx2/Cbfa1-positive cells from lesion areas of seven randomized animals examined at each time point, 75–88% was stained blue by X-gal (Figure 4B), supporting the notion that vascular SMCs are the major source of osteochondrogenic precursors observed in cartilaginous metaplasia and calcification lesions of LDLr−/− vessels. Finally, we quantified the proportion of chondrocyte-like cells that were derived from SMCs using X-gal, Col II antibody, and nuclear fast red triple-stained sections. Of 2720 Col II-positive cells from sections of 14 randomly chosen animals at 24- to 30-week diet-fed, 2664 cells were stained blue by X-gal (98%; see Supplementary material online, Figure S2A), suggesting that chondrocytic cells found in LDLr−/− vessels were either originally derived from SMCs or gained partial SMC feature, such as SM22α expression, on their way to differentiating into chondrocytes.
Figure 4.
Quantitative analysis of osteochondrogenic precursor-like cells in atherosclerotic vessels of LDLr−/− mice. (A). The percentage of Runx2/Cbfa1-positive cells in calcified atherosclerotic vessels of LDLr−/− mice. (B). The percentage of Runx2/Cbfa1-positive cells with β-galactosidase activity. (C–E) Aortic arches of SM22α-Cre+/0:R26R+/0:LDLr−/− mice fed with HFD were stained by X-gal before embedding. Osteochondrogenic precursor cells were stained by Runx2/Cbfa1 antibody. L, lumen; I, intima; M, media; Ad, adventitia.
3.3. SMCs also give rise to osteochondrogenic precursors and chondrocytes in atherosclerotic ApoE−/− vessels
To confirm our findings in atherosclerotic LDLr−/− mice, we employed the same genetic fate mapping strategy to ApoE−/− mice, a different atherosclerotic mouse model, to determine whether SMCs play a similar role in vascular cartilaginous metaplasia and calcification. As shown in Figure 5, ApoE−/− mice developed not only atherosclerosis but also cartilaginous metaplasia and calcification at 45–60 weeks of age. Similar to atherosclerotic LDLr−/− vessels, cells once exhibited SMC features (stained blue by X-gal) in cartilaginous metaplasia and calcification areas ceased to express SMC lineage proteins (Figure 5B and F), and instead, gained osteochondrogenic (Figure 5C) and chondrocytic (Figure 5G and H) properties. Approximately 76–83% Runx2/Cbfa1-positive cells had β-galactosidase activity in atherosclerotic lesions of 44- to 60-week-old ApoE−/− mice (Figure 5D), identifying that SMCs were the major contributors to osteochondrogenic differentiation of ApoE−/− vessels. Finally, the contribution of SMCs to chondrocyte-like cells of ApoE−/− vessels was quantified with a similar approach used for LDLr−/− vessels, and the majority of the Col II-positive cells (95.5%) had once possessed SMC features (see Supplementary material online, Figure S2B).
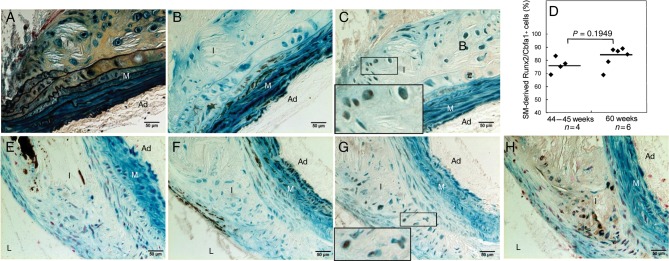
Figure 5.
SMCs gave rise to osteochondrogenic precursor- and chondrocyte-like cells in atherosclerotic ApoE−/− vessels. Aortic arches were dissected from 45-week-old (A–C) and 60-week-old (E–H) SM22α-Cre+/0:R26R+/0:ApoE−/− mice. Cells of SMC origin were stained by X-gal before embedding. Adjacent sections were stained by Movat pentachrome (A), von Kossa (E), and immunohistochemistry for SM22α (B and F), Runx2/Cbfa1 (C), Sox9 (G), and Col II (H). Insert in C and G. Higher-powered magnification of the boxed region shows colocalization of β-galactosidase (blue) with osteochondrogenic marker Runx2/Cbfa1 (C, brown) or with chondrocytic transcription factor, Sox9 (G, brown). (D). The percentage of Runx2/Cbfa1-positive cells in calcified atherosclerotic vessels of LDLr−/− mice. L, lumen; I, intima; M, media; Ad, adventitia.
3.4. Contribution of BM-derived cells to vascular cartilaginous metaplasia and calcification
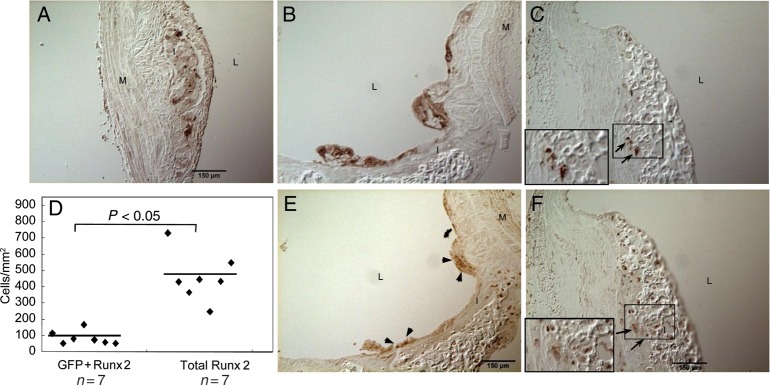
To determine whether BM-derived cells contribute to the development of cartilaginous metaplasia and calcification in atherosclerotic blood vessels, we assessed aortic arch specimens available from the control group of a previous study.18 In this study, GFP-expressing BM cells were engrafted into 35-week-old ApoE−/− mice that were studied 10-week post-transplantation. ApoE−/− mice of 35 weeks old had only a few foam cells in their aortic arches (negative for Runx2/Cbfa1), suggesting that BM-derived cells that showed osteochondrogenic properties in aortic arches at later time points had entered the vessel wall primarily after 35 weeks of age. By 45 weeks of age, these mice developed cartilaginous metaplasia and calcification similar to LDLr−/− mice fed with HFD for 24–28 weeks. As shown in Supplementary material online, Figure S3, 45-week-old ApoE−/− mice developed not only atherosclerosis (see Supplementary material online, Figure S3A and D) but also cartilaginous metaplasia and calcification (see Supplementary material online, Figure S3B, C, E, and F) in aortic arches. In smaller lesions, foam cells and lipid-filled areas were distinct from areas of cartilaginous metaplasia and calcification. BM-derived GFP-positive cells were mostly found in lesion shoulders (data not shown), lipid cores, and areas filled with foam cells (Figure 6A and B, brown, vs. Supplementary material online, Figures S3D and E), accounting for 87% of GFP-positive cells observed in the vessel wall. Significantly, ∼13% of GFP-positive cells were also positive for Runx2/Cbfa1, residing mostly on the surface of lesions (Figure 6E vs. B; see Supplementary material online, Figure S4, arrowheads) with only a few at the sites of cartilaginous metaplasia and calcification (Figure 6F vs. C, arrows). These GFP-positive osteochondrogenic cells accounted for 20.1% of total Runx2/Cbfa1-positive cells found in ApoE−/− vessels (Figure 6D), suggesting that BM-derived cells also make a significant contribution to the osteochondrogenic differentiation of atherosclerotic vessels.
Figure 6.
Transplantation of GFP-positive marrow cells to ApoE−/− mice. GFP marrow cells were transplanted to 35-week-old ApoE−/− mice. Aortic arches were collected for the study 10 weeks after transplantation. Adjacent sections were stained immunohistochemically for GFP (A–C, brown) and Runx2/Cbfa1 (E and F, brown). (D). Quantitative analysis of GFP-positive osteochondrogenic precursor-like cells in atherosclerotic ApoE−/− vessels. Inset in C and F. Higher-powered magnification of the boxed region shows colocalization of GFP (brown, arrows) with osteochondrogenic marker Runx2/Cbfa1 (brown, arrows). L, lumen; I, intima; M, media; Ad, adventitia.
4. Discussion
In the present study, we used a genetic fate mapping strategy to identify sources of cells that give rise to osteochondrogenic precursor- and chondrocyte-like cells in atherosclerotic vessels. We introduced to atherosclerotic LDLr−/− mice a −2.8 kb SM22α promoter-driven Cre recombinase transgenic allele and its reporter R26R transgene that expresses β-galactosidase activity only following Cre-mediated excision. Because the −2.8 kb SM22α promoter contains regulatory sequences that direct arterial SMC-restricted expression21 and the Cre-dependent β-galactosidase activity in mice carrying SM22α-Cre and R26R transgenes was determined to be confined to SMCs and cardiomyocytes,12 the −2.8 kb SM22α promoter-driven Cre recombinase has long been used to perform loss-of-function studies of vascular SMCs in vivo.13 Therefore, application of SM22α-Cre and R26R transgenes to LDLr−/− mice leads to a permanent mark of cells with SMC properties prior to the development of atherosclerosis, cartilaginous metaplasia, and calcification. Co-existence within a single vascular cell of β-galactosidase activity with osteochondrogenic (Runx2/Cbfa1) or chondrocytic (Sox9 and Col II) marker proteins, along with simultaneous loss of SM lineage proteins, provides strong evidence supporting lineage reprogramming of SMCs towards osteochondrogenic differentiation. According to this reasoning, our experiments revealed that the majority of the osteochondrogenic precursor-like cells (∼75–88%) and almost all of the chondrocyte-like cells (∼98%) observed in atherosclerotic LDLr−/− vessels were derived from SMCs. In early stages of lesion development, these cells were mostly clustered in the deep intimal and inner medial layers, adjacent to elastic lamina breakage, and distinct from areas containing lipid and macrophage foam cells. Despite wide acceptance of BM-derived cells as sources of cells that contribute to lipid-laden areas of atherosclerotic lesion,22 our studies are consistent with an electron microscopic study showing cells with hybrid SMC and chondrocyte properties, termed ‘myochondrocytes’, in human atherosclerotic lesions.23 Cells of SM origin, possibly migrating from local vascular media or, to a lesser extent, differentiating from BM-derived cells, were likely the major sources of cells that contribute to the development of cartilaginous and calcifying lesions of atherosclerotic vessels. These results were also reproduced in another atherosclerotic mouse model, the ApoE−/− mice, using an identical genetic fate mapping strategy. These findings are the first to definitively demonstrate a role of vascular SMCs in cartilaginous metaplasia and calcification of atherosclerotic vessels.
BM-derived vascular progenitors are generally accepted as cells that have the ability to differentiate into vascular SMCs, consequently participating in pathogenesis of atherosclerosis and blood vessel repair.22 However, this concept has been challenged in recent studies from different laboratories using various lineage tracing strategies.24–26 In a model of post-angioplasty restenosis, a time-course analysis of BM-derived progenitors that differentiate into SMCs during neointima formation was performed. GFP-positive BM cells were found to only account for ∼2% of neointimal SMCs. Most of the GFP-positive BM cells were monocytes/macrophages occurring in the acute inflammatory response, accounting for ∼69% of the neointimal cells.25 In a transplant atherosclerosis model, engraftment of SM-LacZ β-galactosidase-expressing marrow cells into aortic allograft recipients revealed that neointimal and atherosclerotic lesions were negative for β-galactosidase staining.26 Finally, in a hyperlipidaemia-associated atherosclerotic model, sex-mismatched eGFP+ApoE−/− marrow cells were engrafted into ApoE−/− recipients. eGFP-positive cells were mostly seen in lipid-laden areas, co-localizing with macrophage foam cells, identical to our observations. In that study, no eGFP expression was found in ∼10 000 SMα-actin-positive cells, counted in 154 sections from multiple sites in arteries of 23 BM-transplanted mice.24 These authors also transplanted ApoE−/− arterial segments into carotids of eGFP+ApoE−/− mice and showed no significant eGFP-positive SMCs in lesions of the engrafted vessels.24
In contrast to the above strategies, we tracked cells of SM origin to determine whether vascular SMCs contribute to cartilaginous and bony elements seen in atherosclerotic vessels. We found that SMCs were mostly located in the fibrous cap of atheromas and areas of cartilaginous metaplasia and calcification. These cells have lost SMC marker protein expression (e.g. SM22α), with the rare exception of some cells on the lumen side of the fibrous cap. In both LDLr−/− and ApoE−/− vessels, cells exhibiting chondrocytic properties were largely derived from SMCs as identified by transgene β-galactosidase activity, localizing in the deep intima and inner medial layers near areas of elastic lamina breakage that signifies their likelihood of being derived from vascular medial SMCs. These findings are consistent with our previous observations in MGP−/− vessels, a model of solely arterial medial calcification, where all the chondrocytes were found to be derived from vascular SMCs.19 However, the sources of osteochondrogenic precursors that apparently give rise to chondrocytes in atherosclerotic lesions appear to be distinct from those observed in arterial medial calcification of MGP−/− mice. In atherosclerotic vessels, circulating BM-derived cells were shown to contribute to ∼20% of the Runx2/Cbfa1-positive osteochondrogenic precursors, whereas no BM-derived cells were found to participate in medial calcification of MGP−/− vessels.19 Our finding indicating BM cells as additional sources of osteochondrogenesis seen in atherosclerotic vessels is complemented by a recent study that used SM MHC-Cre recombinase and Cre reporter transgenes to trace BM-derived cells that had once developed SMC property,27 even though the study is limited by the restricted expression of SM MHC and a fairly low recombination efficiency of SM MHC-Cre.13 BM-derived cells may have gained SMC properties22 while differentiating towards osteochondrogenic precursors and chondrocytes after recruitment into the diseased vasculature. Although we did not see a co-localization of macrophages (MOMA-2-positive) with chondrocyte-like cells (Sox9-positive) in atherosclerotic vessels of the animals examined, our studies do not exclude the contribution of monocytes/macrophages to chondrocyte-like cells. It is possible that these cells have lost their phenotype in the diseased blood vessels and are therefore undetectable by lineage-specific antibodies.
BM-derived myeloid CD34+ CD13+ cells were recently reported to be the major sources of atherosclerotic intimal chondrocyte-like cells by Doehring et al.28 In that study, β-galactosidase-positive BM cells were transplanted into LDLr−/− mice fed with a high-fat diet containing 1.25% cholesterol and 0.5% sodium cholate. BM-derived β-galactosidase-positive cells were found to constitute ∼7–14% of the total plaque cellularity. Unlike our lineage studies of LDLr−/− and ApoE−/− mice, these vessels developed larger necrotic cores, and chondrocyte-like cells (recognized by Col II antibody) were mostly found in the fibrous cap where SMCs are typically found. In addition, ∼89% of the chondrocytes were found to be derived from BM, as marked by myeloid markers, CD34 and CD13.28 It is unclear whether these cells had once developed SMC properties due to the phenotypic plasticity of vascular SMCs in calcified lesions. Although LDLr−/− mice and high-fat diet were used in both studies, sodium cholate was only added to the diet used in Doehring's study. HFDs containing cholate are known to induce hepatic fibrosis resulting from enhanced macrophage infiltration to an inflammatory response,29 a likely cause of enhanced recruitment of BM myeloid cells and acceleration of lipid-laden and necrotic core formation in Doehring's study. Indeed, in our studies utilizing a GFP transgene to trace cells derived from BM in ApoE−/− mice, GFP-positive marrow cells were mostly seen in macrophages and foam cells, identifying their major role in inflammation and lipid laden of atherosclerotic lesions.22,24,25 Interestingly, these cells also contributed significantly (∼20%) to osteochondrogenic precursor-like cells found in cartilaginous and calcifying areas of atherosclerotic vessels. Transplantation of GFP marrow cells to ApoE−/− recipients in our study did not reveal any contribution of BM-derived cells prior to 35 weeks of age. Although only a few foam cells were visualized by this time, it is true that our finding showing ∼20% contribution of BM-derived cells represents only the marrow cells that have entered the blood vessels within 35–45 weeks of age. Considering the findings of our lineage studies of atherosclerotic LDLr−/− and ApoE−/− mice that essentially all chondrocyte-like cells (marked by Sox9 and Col II) were labelled with SMC-specific transgene β-galactosidase, the BM-derived osteochondrogenic cells were likely to have had gained partial SMC characteristics (such as SM22α expression) while differentiating into chondrocytes. Understanding this pathway holds promise for the development of novel therapeutic strategies that control the recruitment and accumulation of BM-derived cells in cartilaginous and bony elements of atherosclerotic vessels.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by NIH grants R01 HL081785 (C.M.G.), R01 HL62329 (C.M.G.), R01 HL080597 (D.A.D.), K01 DK075665 (M.Y.S.), and the New Investigator Award (M.Y.S.) administered by NIH P30 DK017047 grant.
Supplementary Material
Acknowledgements
We thank Dr David A. Dicheck (D.A.D.) for BM-transplanted ApoE−/− specimens and valuable discussion of the project.
Conflict of interest: none declared.
References
- 1.Taylor AJ, Burke AP, O'Malley PG, Farb A, Malcom GT, Smialek J, et al. A comparison of the Framingham risk index, coronary artery calcification, and culprit plaque morphology in sudden cardiac death. Circulation. 2000;101:1243–1248. doi: 10.1161/01.cir.101.11.1243. [DOI] [PubMed] [Google Scholar]
- 2.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 3.Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453–1464. doi: 10.1681/ASN.2008070692. [DOI] [PubMed] [Google Scholar]
- 4.Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–3429. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 5.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg's sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 6.Guerin AP, Pannier B, Metivier F, Marchais SJ, London GM. Assessment and significance of arterial stiffness in patients with chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:635–641. doi: 10.1097/mnh.0b013e32830dcd5c. [DOI] [PubMed] [Google Scholar]
- 7.Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278:45969–45977. doi: 10.1074/jbc.M306972200. [DOI] [PubMed] [Google Scholar]
- 8.Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 9.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 10.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, et al. Smooth muscle cell phenotypic transition associated with calcification—upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 11.Hunt JL, Fairman R, Mitchell ME, Carpenter JP, Golden M, Khalapyan T, et al. Bone formation in carotid plaques: a clinicopathological study. Stroke. 2002;33:1214–1219. doi: 10.1161/01.str.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 12.Lepore JJ, Cheng L, Min LM, Mericko PA, Morrisey EE, Parmacek MS. High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22alpha-Cre transgenic mice. Genesis. 2005;41:179–184. doi: 10.1002/gene.20112. [DOI] [PubMed] [Google Scholar]
- 13.Frutkin AD, Shi H, Otsuka G, Leveen P, Karlsson S, Dichek DA. A critical developmental role for tgfbr2 in myogenic cell lineages is revealed in mice expressing SM22-Cre, not SMMHC-Cre. J Mol Cell Cardiol. 2006;41:724–731. doi: 10.1016/j.yjmcc.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 14.Munroe PB, Olgunturk RO, Fryns J-P, Maldergem LV, Ziereisen F, Yuksel B, et al. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat Genet. 1999;21:142–144. doi: 10.1038/5102. [DOI] [PubMed] [Google Scholar]
- 15.Schori TR, Stungis GE. Long-term warfarin treatment may induce arterial calcification in humans: case report. Clin Invest Med. 2004;27:107–109. [PubMed] [Google Scholar]
- 16.Clarke MC, Littlewood TD, Figg N, Maguire JJ, Davenport AP, Goddard M, et al. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res. 2008;102:1529–1538. doi: 10.1161/CIRCRESAHA.108.175976. [DOI] [PubMed] [Google Scholar]
- 17.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 18.Hu JH, Du L, Chu T, Otsuka G, Dronadula N, Jaffe M, et al. Overexpression of urokinase by plaque macrophages causes histological features of plaque rupture and increases vascular matrix metalloproteinase activity in aged apolipoprotein e-null mice. Circulation. 2010;121:1637–1644. doi: 10.1161/CIRCULATIONAHA.109.914945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin W-L, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Miano JM, Mercer B, Olson EN. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol. 1996;132:849–859. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 23.Bobryshev YV. Transdifferentiation of smooth muscle cells into chondrocytes in atherosclerotic arteries in situ: implications for diffuse intimal calcification. J Pathol. 2005;205:641–650. doi: 10.1002/path.1743. [DOI] [PubMed] [Google Scholar]
- 24.Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2696–2702. doi: 10.1161/01.ATV.0000247243.48542.9d. [DOI] [PubMed] [Google Scholar]
- 25.Daniel JM, Bielenberg W, Stieger P, Weinert S, Tillmanns H, Sedding DG. Time-course analysis on the differentiation of BM-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol. 2010;30:1890–1896. doi: 10.1161/ATVBAHA.110.209692. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y, Davison F, Ludewig B, Erdel M, Mayr M, Url M, et al. Smooth muscle cells in transplant atherosclerotic lesions are originated from recipients, but not BM progenitor cells. Circulation. 2002;106:1834–1839. doi: 10.1161/01.cir.0000031333.86845.dd. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Stoneman V, Clarke M, Figg N, Xin HB, Kotlikoff M, et al. BM-derived smooth muscle-like cells are infrequent in advanced primary atherosclerotic plaques but promote atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1291–1299. doi: 10.1161/ATVBAHA.110.218578. [DOI] [PubMed] [Google Scholar]
- 28.Doehring LC, Heeger C, Aherrahrou Z, Kaczmarek PM, Erdmann J, Schunkert H, et al. Myeloid CD34+ CD13+ precursor cells transdifferentiate into chondrocyte-Like cells in atherosclerotic intimal calcification. Am J Pathol. 2010;177:473–480. doi: 10.2353/ajpath.2010.090758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong WI, Jeong DH, Do SH, Kim YK, Park HY, Kwon OD, et al. Mild hepatic fibrosis in cholesterol and sodium cholate diet-fed rats. J Vet Med Sci. 2005;67:235–242. doi: 10.1292/jvms.67.235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.