Abstract
Aims
Binge drinking often triggers compromised myocardial contractile function while activating AMP-activated protein kinase (AMPK). Given the role of AMPK in the initiation of autophagy through the mammalian target of rapamycin complex 1 (mTORC1) and Unc51-like kinase (ULK1), this study was designed to examine the impact of AMPK deficiency on cardiac function and the mechanism involved with a focus on autophagy following an acute ethanol challenge.
Methods and results
Wild-type (WT) and transgenic mice overexpressing a kinase-dead (KD) α2 isoform (K45R mutation) of AMPK were challenged with ethanol. Glucose tolerance, echocardiography, Langendorff heart and cardiomyocyte contractile function, autophagy, and autophagic signalling including AMPK, acetyl-CoA carboxylase (ACC), mTOR, the mTORC1-associated protein Raptor, and ULK1 were examined. Ethanol exposure triggered glucose intolerance and compromised cardiac contraction accompanied by increased phosphorylation of AMPK and ACC as well as autophagosome accumulation (increased LC3II and p62), the effects of which were attenuated or mitigated by AMPK deficiency or inhibition. Ethanol dampened and stimulated, respectively, the phosphorylation of mTOR and Raptor, the effects of which were abolished by AMPK deficiency. ULK1 phosphorylation at Ser757 and Ser777 was down-regulated and up-regulated, respectively, by ethanol, the effect of which was nullified by AMPK deficiency or inhibition. Moreover, the ethanol challenge enhanced LC3 puncta in H9c2 cells and promoted cardiac contractile dysfunction, and these effects were ablated by the inhibition of autophagy or AMPK. Lysosomal inhibition failed to accentuate ethanol-induced increases in LC3II and p62.
Conclusion
In summary, these data suggest that ethanol exposure may trigger myocardial dysfunction through a mechanism associated with AMPK-mTORC1-ULK1-mediated autophagy.
Keywords: Ethanol, AMPK deficiency, Autophagy, Cardiac function, ULK1
1. Introduction
Binge drinking often leads to unfavourable pathological sequelae of the heart including disruption of myofibrillary architecture and compromised myocardial contractile function.1,2 Although a number of scenarios have been postulated with regard to the onset and progression of alcohol (ethanol)-induced myopathic changes including toxicity of ethanol and its metabolites, reactive oxygen species, apoptosis, mitochondrial damage, accumulation of fatty acid ethyl esters, as well as modification of lipoprotein and apolipoprotein particles,3–6 the precise mechanism(s) underlying alcohol-elicited cardiac anomalies remains elusive. Recent evidence from our group has demonstrated that an acute ethanol challenge significantly enhanced the AMP-to-ATP ratio and LKB1 levels in the heart, en route to hyperactivation of AMP-activated protein kinase (AMPK) and cardiac contractile dysfunction.7 This finding depicted a potential role of the metabolic sensor AMPK in alcoholic cardiac damage. AMPK has long been known to serve as a potential target in heart failure development. In particular, AMPK senses the energy state and orchestrates a global metabolic response to energy deprivation in the heart, such as in failing hearts.8–11 However, the precise mechanism behind AMPK-mediated maintenance of cardiac energy homoeostasis and contractile function under alcoholism remains unclear.
Autophagy, the highly orchestrated intracellular bulk degradation, refers to three types of processes, namely microautophagy, chaperon-mediated autophagy, and macroautophagy, which is the main machinery for cytoplasm-to-lysosome delivery. Autophagy plays a pivotal role in the maintenance of cardiac geometry and contractile function.12 Impaired autophagy has been found in a number of heart diseases, including ischaemia/reperfusion injury.12 To the contrary, excessive and uncontrolled autophagy leads to loss of functional protein, depletion of essential molecules, oxidative stress, loss of ATP, collapse of cellular catabolic machinery, and ultimately cell death in the heart.12,13 Recent evidence has revealed a likely role of autophagy in alcoholic liver diseases.14,15 Moreover, initiation of autophagy and suppression of lysosomal function have been suggested to facilitate tissue damage including viral infection and steatosis in alcoholics.15 More recent reports from our laboratory have depicted a role of autophagy in the onset and progression of alcoholic cardiomyopathy.16,17 Nonetheless, the mechanism behind autophagy and signalling cascades involved in alcoholism remains unknown. Given the close tie between alcoholism and AMPK,7,16 it is plausible to speculate a role of AMPK in autophagic regulation and subsequently changes in cardiac function following an alcohol challenge. AMPK is known to promote autophagy through activation of Ca2+/Calmodulin-dependent kinase kinase-β, an essential signalling molecule required for Ca2+-induced autophagy through the mammalian target of rapamycin complex 1 (mTORC1) regulation.18 In particular, AMPK promotes autophagy via inhibition of mTORC1 by way of phosphorylation of the mTORC1-associated protein Raptor19 and tuberous sclerosis complex 2.20 Two seminal reports have depicted that energy stress triggers autophagy through AMP activation, which phosphorylates the homologue of Atg1, namely Unc51-like kinase (ULK1), at different sites from its Ser/Thr-rich domain binding to the complex with Atg13 and FIP200.21,22 ULK1 may be phosphorylated and negatively regulated by mTORC1.23 High mTOR activity prevents ULK1 activation via ULK1 phosphorylation at Ser757 to disrupt the interaction between ULK1 and AMPK.22 To this end, we took advantage of a transgenic mouse model with overexpression of the dominant-negative AMPKα2 subunit to examine the impact of AMPK deficiency on acute ethanol exposure-induced cardiac anomalies and the underlying mechanisms with a focus on autophagy. Echocardiographic, Langendorff heart perfusion, cardiomyocyte contractile function, and autophagic markers including Beclin-1, LC3, and p62 were monitored. Expression and activation of AMPK and its downstream signalling molecules including acetyl-CoA carboxylase (ACC), mTOR, Raptor, and ULK1 were examined in hearts from wild-type (WT) and AMPK-deficient transgenic mice with or without acute ethanol challenge. An intraperitoneal glucose tolerance test (IPGTT) was performed for overall assessment of glucose-handling capacity. ULK1 signalling was detected in H9C2 myoblasts. In vitro H9C2 myoblast cell culture was also used for transfection of green fluorescent protein (GFP)-LC3 to assess the potential AMPK signalling mechanism involved in short-term ethanol exposure-induced autophagy. Given that ethanol may interrupt autophagic flux in cardiomyocytes,16 LC3II and the autophagosome cargo protein p62 were evaluated in myocytes subjected to ethanol exposure or AMPK inhibition in the absence (steady-state autophagosomes) or the presence (cumulative autophagosomes) of a mixture of lysosomal inhibitors.
2. Methods
For details, refer to the Supplemental material online.
2.1. Experimental animals and acute ethanol exposure
All animal procedures described here were in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and were approved by the University of Wyoming Animal Care and Use Committee. Adult male mice overexpressing the dominant-negative AMPKα2 subunit (kinase dead, KD, K45R mutation, KD1 line) and their WT littermates with expression of the α2 subunit of AMPK were used. All mice were housed in a temperature-controlled room (22.8 ± 2.0°C, 45–50% humidity) with a 12/12-light/dark cycle and allowed access to tap water ad libitum. For the acute ethanol challenge, mice (4 month old) were injected intraperitoneally with ethanol (3 g/kg/day, ∼100 μL injected volume per day) for three consecutive days5 and were used 24 h after the last injection. Mice not treated with ethanol received an equal volume of saline each day.
2.2. Assessment of ethanol level and intraperitoneal glucose tolerance test
Plasma ethanol levels were measured24 and the IPGTT was performed as described.25
2.3. Echocardiographic assessment
Cardiac geometry and function were evaluated in anaesthetized (ketamine 80 mg/kg and xylazine 12 mg/kg, ip) mice using a two-dimensional (2D) guided M-mode echocardiography (Phillips Sonos 5500) equipped with a 15–6 MHz linear transducer (Phillips Medical Systems, Andover, MD, USA). The adequate depth of anaesthesia was monitored using the toe reflex. The heart was imaged in the 2D mode in the parasternal long-axis view with a depth setting of 2 cm. The M-mode cursor was positioned perpendicularly to the interventricular septum and the posterior wall of the left ventricle (LV) at the level of papillary muscles from the 2D mode. The sweep speed was 100 mm/s for the M-mode. Diastolic wall thickness, end-diastolic dimension (EDD), and end-systolic dimension (ESD) were measured. All measurements were done from leading edge to a leading edge in accordance with the Guidelines of the American Society of Echocardiography.26 The percentage of LV fractional shortening was calculated as [(EDD − ESD)/EDD] × 100. The ejection fraction was calculated as [(EDD3 − ESD3)/EDD3] × 100. Heart rates were averaged over 10 cardiac cycles.27
2.4. Langendorff perfused heart function
Mice were sacrificed under anaesthesia (ketamine 80 mg/kg and xylazine 12 mg/kg, ip). The left ventricular developed pressure (LVDP) and the first derivative of the LVDP (±dP/dt) were recorded.5
2.5. Isolation of murine cardiomyocytes and cell mechanics
Mice were sacrificed under anaesthesia (ketamine 80 mg/kg and xylazine 12 mg/kg, ip). Hearts were digested using Liberase Blendzyme. To assess the role of AMPK in acute ethanol exposure-induced cardiomyocyte contractile response, cardiomyocytes from adult WT mice were treated with ethanol (240 mg/dL) at 37°C for 4 h in the absence or presence of the AMPK inhibitor compound C (5 μM) or the autophagy inhibitor 3-methyladenine (3-MA, 10 mM).28 To evaluate the autophagy flux in response to ethanol and AMPK deficiency, cardiomyocytes from WT and AMPK KD mice were incubated with a mixture of lysosomal inhibitors [bafilomycin A1 (50 nM), E64D (2.5 μg/mL), and pepstatin A methyl ester (5 μg/mL)]16 along with an ethanol challenge. Mechanical properties of cardiomyocytes were assessed using a SoftEdge MyoCam system (IonOptix Corporation, Milton, MA, USA).29 Cell shortening and re-lengthening were assessed using the following indices: peak shortening (PS), maximal velocities of cell shortening and re-lengthening (±dL/dt), time-to-PS (TPS), and time-to-90% re-lengthening (TR90).
2.6. LC3B-GFP-adenovirus production, infection, and quantification in H9C2 cells
Owing to the technical difficulty of viral transfection in murine cardiomyocytes, H9c2 cells were used to assess autophagy. H9c2 cells were infected with adenoviruses expressing GFP-LC3 fusion protein. H9c2 cells transfected with GFP-LC3 adenovirus were treated with or without ethanol (240 mg/dL) at 37°C for 4 h in the absence or presence of the AMPK inhibitor compound C (5 µM) or the autophagy inhibitor 3-MA (10 mM).30 Rapamycin (5 µM) was used as the positive control for autophagy induction.31 To evaluate the autophagic flux, GFP-LC3-positive cells were evaluated in H9c2 cells pre-incubated with a mixture of lysosomal inhibitors [bafilomycin A1 (50 nM), E64D (2.5 μg/mL), and pepstatin A methyl ester (5 μg/mL)] or compound C prior to ethanol treatment. Cells were visualized using a fluorescence microscope and the percentage of GFP-LC3-positive cells showing GFP-LC3 puncta (>10 dots/cell) were scored as described.32
2.7. Western blot analysis
Protein was prepared as described.29 Quantification of the gel density was determined using Quantity One software (Bio-Rad, version 4.4.0, ChemiDoc XRS) and reported in optical density per square millimetre. For details, refer to the Supplementary material online.
2.8. Data analysis
Data are mean ± SEM. The difference was calculated by repeated measures analysis of variance (ANOVA) followed by Tukey's post hoc analysis. A P-value < 0.05 was considered significant.
3. Results
3.1. General features and IPGTT of WT and AMPK KD mice with or without ethanol
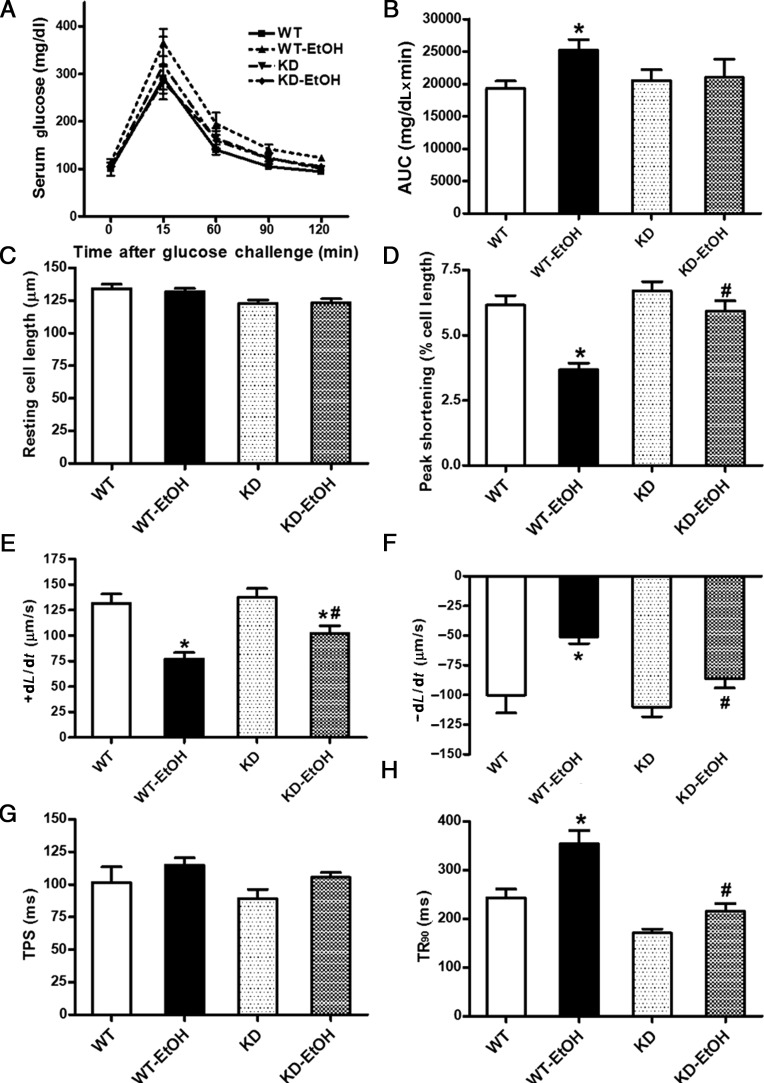
Neither ethanol treatment nor AMPK deficiency affected body and organ (heart, liver, and kidney) weights or size (organ-to-body weight ratio). As expected, the ethanol challenge elicited comparable rises in blood alcohol levels in both WT and AMPK KD mice. Systolic and diastolic blood pressures were similar between WT and AMPK KD mice in the absence of ethanol exposure. The acute ethanol challenge elicited a trend of decrease in both systolic and diastolic blood pressures (P > 0.05) in both WT and AMPK KD mice (Table 1). Following the ip glucose challenge, serum glucose levels in all four mouse groups began to drop after peaking at 15 min and returned towards near baseline values after 120 min. Ethanol-treated WT (WT-EtOH) mice displayed a slightly higher serum glucose levels between 15 and 120 min after the glucose challenge compared with WT mice without the ethanol challenge (although it failed to reach statistical significance). This is supported by the significantly greater area underneath the IPGTT curve (AUC) in ethanol-treated WT mice, indicating glucose intolerance in ethanol-treated WT mice. Although AMPK deficiency did not affect the IPGTT curve and the AUC in the absence of the ethanol challenge, it significantly attenuated ethanol-induced increase in glucose intolerance (as evidenced by the AUC, Figure 1A and B).
Table 1.
Biometric and echocardiographic parameters of WT and AMPK KD mice challenged with ethanol (3 g/kg, ip for 3 days)
| Parameter | WT | WT-EtOH | KD | KD-EtOH |
|---|---|---|---|---|
| Body weight (g) | 24.2 ± 0.6 | 22.0 ± 1.0 | 24.2 ± 0.8 | 22.2 ± 0.9 |
| Heart weight (mg) | 126 ± 8 | 116 ± 4 | 116 ± 4 | 115 ± 4 |
| Heart/body weight (mg/g) | 5.18 ± 0.26 | 5.44 ± 0.16 | 4.83 ± 0.16 | 5.23 ± 0.15 |
| Liver weight (g) | 1.20 ± 0.06 | 1.06 ± 0.03 | 1.24 ± 0.06 | 1.18 ± 0.06 |
| Liver/body weight (mg/g) | 49.2 ± 1.8 | 48.3 ± 1.5 | 51.5 ± 0.8 | 53.1 ± 2.3 |
| Kidney weight (g) | 0.28 ± 0.01 | 0.27 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.01 |
| Kidney/body weight (mg/g) | 11.6 ± 0.4 | 12.7 ± 0.2 | 12.6 ± 0.3 | 13.5 ± 0.4 |
| Blood alcohol (mg/dL) | Undetectable | 70.1 ± 7.1* | Undetectable | 73.6 ± 8.1* |
| Diastolic blood pressure (mmHg) | 79.0 ± 1.4 | 74.4 ± 0.8 | 79.3 ± 1.5 | 74.5 ± 0.8 |
| Systolic blood pressure (mmHg) | 106.9 ± 2.4 | 101.9 ± 2.3 | 105.5 ± 2.6 | 99.4 ± 1.2 |
| Heart rate (b.p.m.) | 493 ± 13 | 505 ± 24 | 497 ± 13 | 500 ± 24 |
| Diastolic wall thickness (mm) | 0.96 ± 0.03 | 0.94 ± 0.03 | 0.97 ± 0.03 | 0.89 ± 0.08 |
| LV ESD (mm) | 1.32 ± 0.07 | 1.42 ± 0.11 | 1.25 ± 0.06 | 1.07 ± 0.06# |
| LV EDD (mm) | 2.65 ± 0.13 | 2.28 ± 0.13* | 2.58 ± 0.12 | 2.18 ± 0.09* |
| Fractional shortening (%) | 50.3 ± 1.1 | 42.3 ± 1.8* | 51.4 ± 0.8 | 50.9 ± 1.8# |
| Ejection fraction (%) | 87.5 ± 0.9 | 80.0 ± 1.9* | 88.5 ± 0.7 | 87.7 ± 1.5# |
LV ESD, left ventricular end-systolic diameter; LV EDD, left ventricular end-diastolic diameter; Mean ± SEM, n = 10–14 mice/group.
*P < 0.05 vs. WT group.
#P < 0.05 vs. WT-EtOH group.
Figure 1.
Intraperitoneal glucose tolerance test (IPGTT, 2 g/kg) and cardiomyocyte contractile properties in adult WT and KD mice with or without acute ethanol (EtOH) challenge (3 g/kg, ip for 3 days). (A) Serum glucose levels following acute glucose challenge; (B) area underneath the IPGTT curve (AUC); (C) resting cell length; (D) peak shortening (normalized to cell length); (E) maximal velocity of shortening (+dL/dt); (F) maximal velocity of re-lengthening (−dL/dt); (G) Time-to-PS (TPS); and (H) time-to-90% re-lengthening (TR90). Mean ± SEM, n = 10–14 mice or 62–83 cells from 3 to 4 mice per group, *P < 0.05 vs. WT group; #P < 0.05 vs. WT-EtOH group.
3.2. Echocardiographic characteristics and cardiomyocyte contractile properties
The heart rate was comparable among WT and AMPK KD mice regardless of acute ethanol treatment. The acute ethanol challenge significantly reduced the LVEDD, fractional shortening, and ejection fraction without affecting the LVESD and diastolic wall thickness in WT mice. Although AMPK deficiency failed to affect any of the echocardiographic parameters measured in the absence of ethanol exposure, it mitigated the ethanol-induced decrease in fractional shortening and the ejection fraction as well as unmasked an ethanol-induced decrease in the LVESD without affecting the ethanol-induced drop in the LVEDD (Table 1). Neither the ethanol challenge nor AMPK deficiency affected the resting cell length. However, cardiomyocytes from ethanol-treated WT mice displayed significantly reduced PS and ±dL/dt associated with prolonged TR90 and an unchanged TPS. AMPK deficiency significantly attenuated or ablated acute ethanol challenge-induced cardiomyocyte mechanical dysfunctions without eliciting any obvious effect itself (Figure 1C–H).
3.3. Effect of ethanol exposure and AMPK deficiency on Langendorff perfused heart function
To further assess the impact of acute ethanol exposure and AMPK deficiency on cardiac contractile function in the whole heart setting, the Langendroff perfused heart function was evaluated in WT and AMPK KD mice with or without the ethanol challenge. Our data revealed that the acute ethanol challenge resulted in a significant decline in the LVDP and ± dP/dt in WT mice, the effects of which were significantly alleviated by AMPK deficiency. AMPK deficiency alone did not affect whole heart contractile function manifested by an unchanged LVDP and ± dP/dt (Supplementary material online, Figure S1).
3.4. Effect of acute ethanol challenge and AMPK deficiency on AMPK and ACC
To explore the potential role of AMPK in ethanol-induced cardiac mechanical responses, pan protein expression and phosphorylation of AMPK and its downstream signalling target ACC were examined. Acute ethanol exposure down-regulated pan protein expression of AMPKα in both WT and AMPK KD mice. AMPK-deficient KD mice displayed elevated protein expression of AMPKα (reflecting overexpression of the dominant-negative AMPKα2 subunit). Moreover, the acute ethanol challenge enhanced the phosphorylation of AMPK (either in absolute or normalized value), the effect of which was obliterated by AMPK deficiency. AMPK deficiency itself did not affect the phosphorylation of AMPKα, possibly due to the compensatory effect of the α1 subunit as reported previously.33 Moreover, neither acute ethanol treatment nor AMPK deficiency (or both) affected the pan protein expression or phosphorylation of ACC. However, the normalized ACC phosphorylation (pACC-to-ACC ratio) was significantly increased following the acute ethanol challenge in WT mice. AMPK deficiency mitigated the ethanol-induced increase in the pACC-to-ACC ratio without eliciting any effect by itself (Supplementary material online, Figure S2).
3.5. Effect of acute ethanol challenge and AMPK deficiency on autophagic markers and autophagic flux
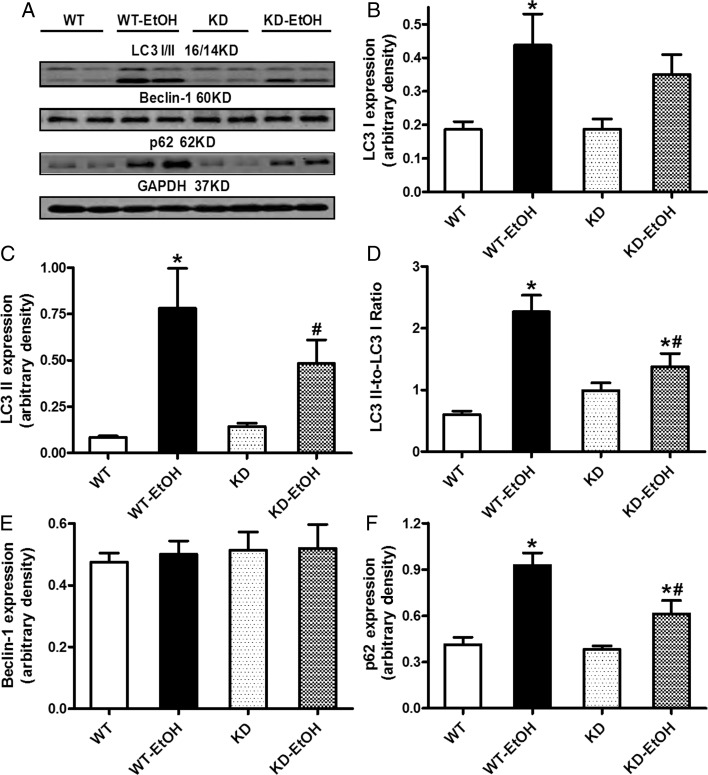
To examine the potential role of autophagy in alcoholic heart damage, expression of the autophagic markers Beclin-1, LC3I/II, and the cargo receptor p62 was evaluated in the myocardium from WT and KD mice following acute ethanol treatment. Immunoblotting results shown in Figure 2 reveal overtly elevated levels of LC3I and LC3II (and the LC3II-to-LC3I ratio) and p62 in the myocardium from WT mice following an ethanol challenge. Although AMPK deficiency itself did not affect the expression of these autophagic markers, it significantly attenuated or obliterated the ethanol-induced rise in autophagy. Last but not least, neither ethanol treatment nor AMPK deficiency significantly affected Beclin-1 levels. To assess the effect of the acute ethanol challenge on the autophagic flux, cardiomyocytes from WT and KD mice were treated with ethanol (240 mg/dL) for 4 h in the absence or presence of mixed lysosomal inhibitors [bafilomycin A1 (50 nM), E64D (2.5 μg/mL), and pepstatin A methyl ester (5 μg/mL)]16 prior to assessment of the autophagic markers LC3II and p62. Our data indicated that lysosomal inhibitors affect neither a basal nor ethanol challenge-induced rise of LC3II and p62 in cardiomyocytes from WT mice. Similarly, mixed lysosomal inhibitors failed to affect either basal or ethanol challenge-induced rise of LC3II and p62 levels AMPK KD group (Supplementary material online, Figure S3), not favouring a major role of the autophagic flux in AMPK-deficiency-induced regulation of autophagy.
Figure 2.
Expression of autophagic markers in hearts from WT and KD mice with or without acute ethanol challenge (3 g/kg, ip for 3 days). (A) Representative gel blots depicting LC3 I, LC3 II, p62, Beclin-2, and GAPDH (loading control) using specific antibodies; (B) LC3 I expression; (C) LC3 II expression; (D) LC3 II-to-LC3 I ratio; (E) Beclin-1 expression; and (F) p62 expression. Mean ± SEM, n = 7–10 mice per group, *P < 0.05 vs. WT group, #P < 0.05 vs. WT-EtOH group.
3.6. Effect of acute ethanol challenge and AMPK deficiency on AMPK-mTOR-ULK1 signalling
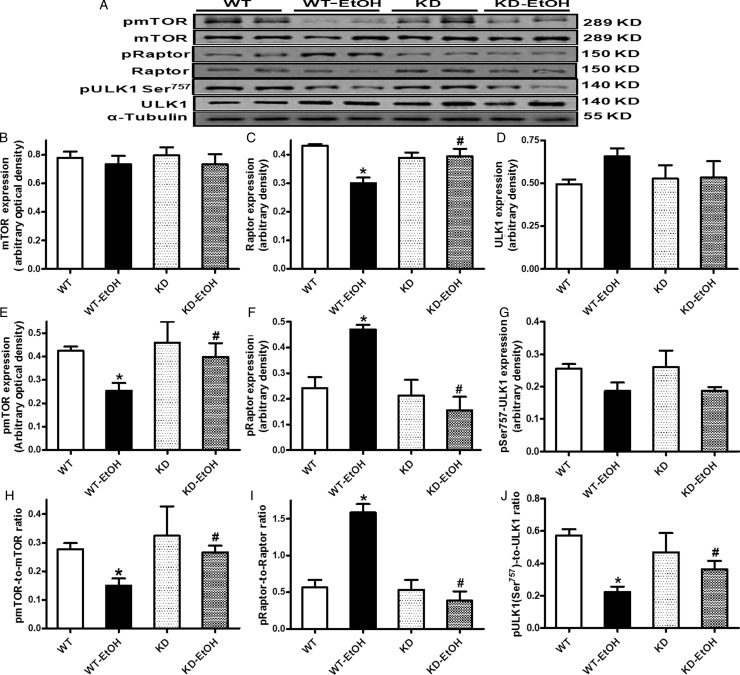
To better understand the cell signalling mechanism(s) involved in ethanol- and/or AMPK-deficiency-induced autophagic responses, the AMPK-related autophagic signalling molecules including mTOR, Raptor, and ULK1 were examined. Our data shown in Figure 3 reveal that the acute ethanol challenge significantly dampened mTOR phosphorylation (absolute or normalized value), pan protein expression of Raptor, and ULK1 phosphorylation at Ser757 (normalized value). Although AMPK deficiency itself failed to alter pan or phosphorylated levels of these signalling molecules, it effectively mitigated acute ethanol exposure-induced changes in these signalling molecules. Neither ethanol treatment nor AMPK deficiency (or both) elicited any notable effect on the pan protein expression of mTOR and ULK1 or the absolute phosphorylation of ULK1 (Ser757).
Figure 3.
Expression of mTORC1-related autophagic signalling proteins in WT and KD mice with or without acute ethanol challenge (3 g/kg, ip for 3 days). (A) Representative gel blots depicting mTOR, phospho-mTOR (pmTOR, Ser2448), Raptor, phospho-Raptor (pRaptor, Ser792), ULK1, phosphoULK1 (pULK1, Ser757), and α-tubulin (loading control) using specific antibodies; (B) pan mTOR; (C) pan Raptor; (D) pan ULK1; (E) pmTOR; (F) pRaptor; (G) pULK1; (H) pmTOR-to-mTOR ratio; (I) pRaptor-to-Raptor ratio; and (J) pULK1-to-ULK1 ratio. Mean ± SEM, n = 5–6 mice per group, *P < 0.05 vs. WT group, #P < 0.05 vs. WT-EtOH group.
3.7. Effect of compound C on ethanol-induced responses of AMPK-mTORC1-ULK1 signalling
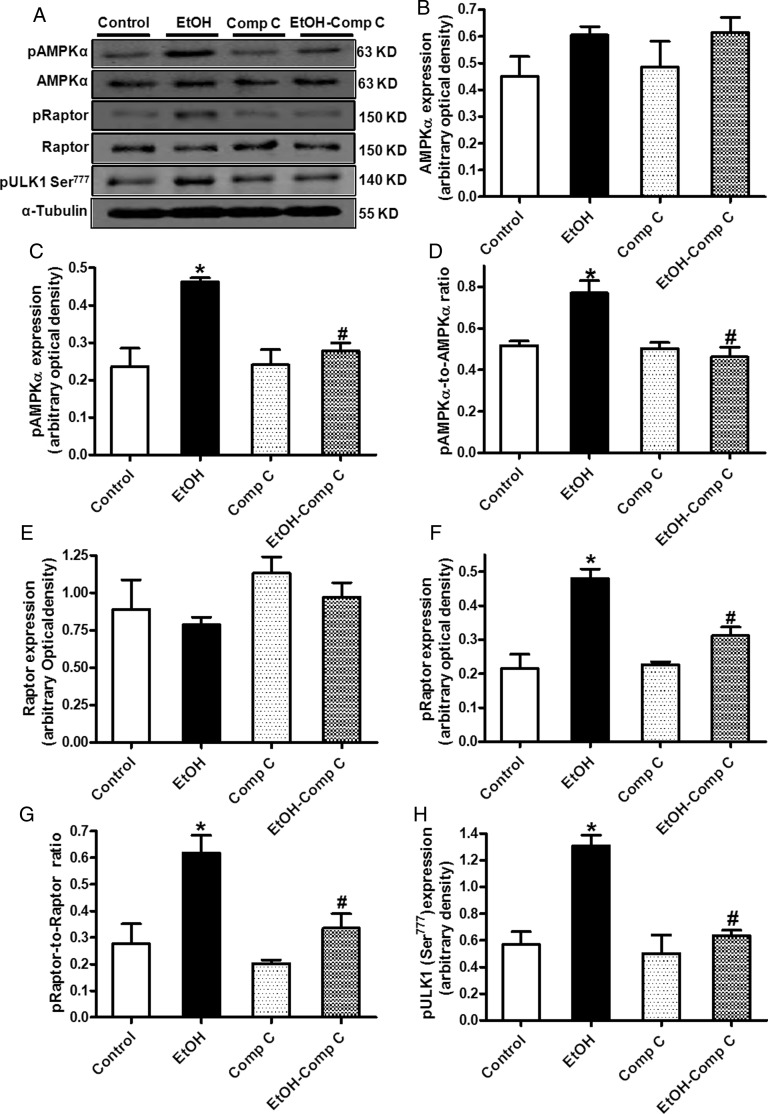
To further assess the role of AMPK in the ethanol-induced autophagic response, the effect of ethanol on AMPK-related autophagic signalling including Raptor and ULK1 was examined in the absence or presence of the AMPK inhibitor compound C. Short-term ethanol (240 mg/dL) exposure significantly increased the phosphorylation of AMPK and Raptor (both absolute and normalized values), as well as ULK1 (Ser777) without affecting the pan protein expression of AMPK and Raptor. Although compound C itself failed to affect the pan and phosphorylated levels of these proteins, it abrogated ethanol-induced changes in these autophagic signalling molecules (Figure 4).
Figure 4.
The effect of compound C (5 µM) on AMPK-mTORC1-ULK1 signalling in the absence or presence of ethanol exposure (240 mg/dL, 4 h) in H9c2 cells. (A) Representative gel blots depicting pan and phosphorylated AMPK, Raptor, ULK1, and α-tubulin (loading control) using specific antibodies; (B) AMPKα; (C) phospho-AMPKα (pAMPK, Thr172); (D) pAMPKα-to-AMPKα ratio; (E) pan Raptor; (F) phospho-Raptor (pRaptor, Ser792); (G) pRaptor-to-Raptor ratio; and (H) pULK1 (Ser777). Mean ± SEM, n = 5–6 mice per group, *P < 0.05 vs. WT group, #P < 0.05 vs. WT-EtOH group.
3.8. Effect of 3-MA and compound C on ethanol-induced cardiomyocyte contractile dysfunction
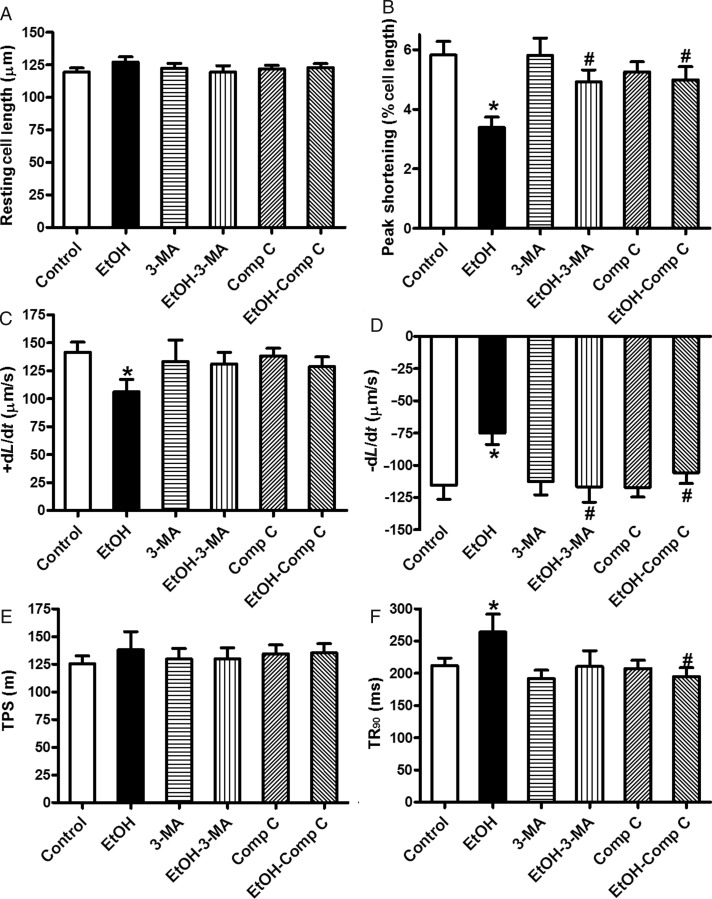
To further examine the role of AMPK and autophagy in ethanol-induced cardiac contractile response, freshly isolated cardiomyocytes from WT mice were exposed to ethanol in the absence or presence of the autophagy inhibitor 3-MA and the AMPK inhibitor compound C. Our data shown in Figure 5 reveal that ethanol significantly depressed PS, and maximal velocity of shortening/re-lengthening, and prolonged the duration of re-lengthening without affecting the resting cell length and duration of shortening. Although 3-MA and compound C themselves did not affect the mechanical properties of cardiomyocytes in the absence of ethanol exposure, it ablated or significantly attenuated ethanol-induced cardiomyocyte mechanical anomalies. These data consolidated the role of AMPK and autophagy in ethanol-induced cardiac contractile dysfunction.
Figure 5.
The effect of 3-MA and compound C on ethanol-induced cardiomyocyte contractile defects. Freshly isolated cardiomyocytes from WT mice were incubated with ethanol (240 mg/dL) in the presence or absence of 3-MA (10 mM) or compound C (5 µM) prior to mechanical assessment. (A) Resting cell length; (B) peak shortening (normalized to the resting cell length); (C) maximal velocity of shortening (+dL/dt); (D) maximal velocity of re-lengthening (−dL/dt); (E) time-to-peak shortening (TPS); and (F) time-to-90% re-lengthening (TR90). Mean ± SEM, n = 55–70 cells from three mice per group, *P < 0.05 vs. control group, #P < 0.05 vs. ethanol (EtOH) group.
3.9. Effect of 3-MA, compound C, and the mixed lysosomal inhibitors on ethanol-induced autophagosome accumulation in H9c2 cells
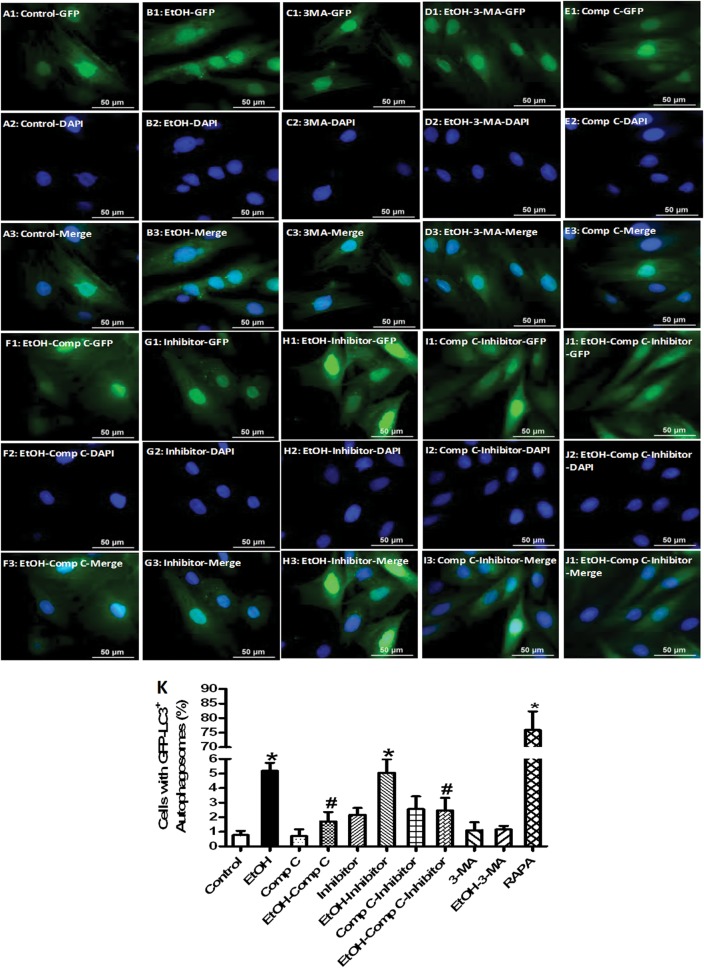
To confirm the role of AMPK and autophagy in ethanol-induced autophagy induction, H9c2 myoblasts were transfected with an adenovirus expressing GFP-LC3 fusion protein for 24 h prior to exposure to ethanol (240 mg/dL, 4 h) in the absence or presence of the autophagy inhibitor 3-MA or the AMPK inhibitor compound C. Evaluation of autophagosome formation using GFP-LC3 puncta revealed that both 3-MA and compound C blocked the ethanol-induced rise in GFP-LC3 puncta. Neither 3-MA nor compound C affected GFP-LC3 puncta in the absence of ethanol exposure. The autophagy inducer rapamycin was employed as a positive control for GFP-LC3 puncta formation. These findings supported a role of AMPK activation in ethanol-induced autophagosome accumulation. To evaluate the autophagic flux, H9c2 cells transfected with an adenovirus expressing GFP-LC3 fusion protein were incubated with ethanol in the absence or presence of a mixture of lysosomal inhibitors [bafilomycin A1 (50 nM), E64D (2.5 μg/mL) and pepstatin A methyl ester (5 μg/mL)].16 Our data revealed that the lysosomal inhibitors failed to affect the number of GFP-LC3 puncta in the absence of ethanol not did these agents further promote an ethanol-induced rise in GFP-LC3 puncta. Moreover, lysosomal inhibition did not significantly alter the beneficial effect of compound C against ethanol-induced GFP-LC3 puncta formation, suggesting a lesser role of autophagic flux here. Compound C alone did not significantly affect the number of GFP-LC3 puncta, the effect of which was unaffected by lysosomal inhibition (Figure 6).
Figure 6.
The effect of 3-MA, compound C and mixed lysosomal inhibitor on ethanol exposure-induced autophagosome formation and autophagic flux in H9c2 myoblasts. H9c2 cells were transfected with adenovirus for 24 h to express the GFP-LC3 fusion protein. Cells were then exposed to ethanol (240 mg/dL) for 4 h in the absence or presence of the autophagosome inhibitor 3-MA (10 mM), the AMPK inhibitor compound C (5 μM), or lysosomal inhibitors. Rapamycin (5 μM) served as the positive control. DAPI staining was used for identification of the nucleus. Representative images of GFP (A1–J1), DAPI (A2–J2), and the merged images (A3–J3) depicting GFP-LC3 puncta in H9c2 cells; and (K) percentage of cells with autophagosomes. Cells with 10 or more punctate spots were scored as positive for autophagosomes. Mean ± SEM, n = 300–400 cells per group, *P < 0.05 vs. control group, #P < 0.05 vs. ethanol (EtOH) group.
4. Discussion
The salient findings from this study indicated that AMPK deficiency protected against acute ethanol challenge-induced cardiac contractile dysfunction and glucose intolerance, in line with our earlier observation of overt cardiac functional anomalies accompanied with enhanced AMPK activation in the same experimental setting.7 Furthermore, data from our current study revealed the initiation of autophagy in response to ethanol exposure, the effect of which was reversed by AMPK deficiency. More importantly, our findings provided evidence for the first time of a likely role for AMPK-mTORC1-ULK1 signalling in ethanol-elicited autophagic response, which may underscore ethanol-induced cardiac contractile dysfunction. Assessment of the autophagic flux using the mixed lysosomal inhibitors revealed that ethanol exposure-induced autophagy may be due to both inhibition of the late-stage autophagic flux and elevation of the early-stage autophagosome formation. This is supported by enhanced levels of LC3II and autophagosome cargo protein p62 following the ethanol challenge. Our observation using lysosomal inhibitors depicted a minor role of autophagic flux in AMPK deficiency or inhibition-induced preservation of mechanical and autophagic homoeostasis following the ethanol challenge. These observations are in favour of the notion that acute ethanol toxicity induces myocardial dysfunction and glucose intolerance through induction of autophagy (autophagosome accumulation) by way of over-stimulation of the cellular fuel AMPK, inhibition of mTORC1, and phosphorylation of ULK1.
Cardiac damage may be seen following binge ethanol ingestion at 90–100 g/day (∼1.5 g/kg) in human subjects.34 In this study, an ethanol dosage at 3 g/kg was used to closely resemble the state of heavy ethanol binge intake (given the doubled ED50 in rodents). Blood alcohol levels measured following acute ethanol challenge (∼70 mg/dL) match those detected in humans after binge drinking.35 Data from our current study revealed that AMPK deficiency rescued against acute ethanol exposure-induced cardiac dysfunction (reduced LV fractional shortening, ejection fraction, depressed LVDP, ±dP/dt, cardiomyocyte PS, and ±dL/dt as well as prolonged TR90). These findings are in agreement with our earlier observations that acute ethanol exposure elicits myocardial dysfunction accompanied with overtly elevated AMPK phosphorylation in the heart.7 In our study, neither ethanol exposure nor AMPK deficiency affected the heart rate or blood pressure (despite a trend of reduced blood pressure following ethanol exposure). AMPK deficiency failed to affect ethanol-induced decrease in the LVEDD (despite preserved LV fractional shortening and ejection fraction). The decrease in the LVEDD in conjunction with an unchanged LVESD following acute ethanol exposure is somewhat surprising as alcohol abuse may elicit cardiomyopathy indistinguishable from other types of dilated non-ischaemic cardiomyopathy,4 similar to our recent finding (greater LVEDD) using a similar acute ethanol challenge protocol albeit in a different mouse strain (FVB).16 Although such a discrepancy in the LVEDD following the ethanol challenge is not entirely clear, it may be speculated that certain haemodynamic factors (such as the non-significant decreases in systolic and diastolic blood pressure seen in our present study) may contribute to ethanol-induced geometric changes in the left ventricle. However, given that AMPK deficiency significantly reconciled ethanol challenge-induced decrease in Langendorff heart contractility but not the ethanol-induced responses in blood pressure and the LVEDD, the AMPK-deficiency-induced beneficial cardiac effect likely originates from the heart as opposed to from systemic circulatory factors. The observation that AMPK deficiency itself did not affect general biometric and cardiac mechanical properties is in line with our previous report,33 indicating that AMPK deficiency may not be innately harmful to cardiac contractile function,36,37 Furthermore, AMPK deficiency significantly ameliorated acute ethanol exposure-induced glucose intolerance, in line with our earlier finding of dampened cardiac insulin signalling following an acute ethanol challenge associated with the over-phosphorylation of AMPK.7 AMPK is deemed a key signalling molecule in the metabolic actions of insulin.8,9 However, AMPK activation may also serve as a double-edged sword in glucose homoeostasis as AMPK activation, if excessive, triggers up-regulation of autophagy to promote gluconeogenesis.38
Our present data revealed a remarkable rise in AMPKα phosphorylation accompanied with decreased pan protein expression of AMPKα following ethanol treatment, the effect of which was reversed by AMPK deficiency. AMPK KD mice displayed a slightly lowered (although not significantly) pAMPKα-to-AMPKα ratio possibly due to the elevation of pan AMPKα expression (from overexpression of the dominant-negative α2 subunit). Interestingly, AMPK deficiency ablated the acute ethanol exposure-induced increase in AMPK phosphorylation. This is in concert with the changes in phosphorylation of ACC (pACC-to-ACC ratio), a downstream target of AMPK, in WT and AMPK KD mice following the ethanol challenge. The down-regulated AMPKα levels in response to the acute ethanol challenge may be due to the loss of the endogenous AMPK as WT mice displayed a ∼26% loss in AMPK expression following the ethanol challenge. Given that a much higher degree (43%) of loss in AMPK protein was observed in AMPK KD mice following ethanol exposure, a possible contribution from exogenous AMPKα cannot be excluded. In fact, it is rather difficult to precisely discern the origin of ethanol-induced loss of AMPK protein as overexpression of the KD α2 isoform (K45R mutation) used in this study leads to a conformational change in endogenous AMPK structural domains in its subunits.
Perhaps the most significant finding from our study is that AMPK deficiency or inhibition attenuated ethanol-induced cardiac autophagosome accumulation, manifested as increased LC3II and the autophagosome cargo protein p62 along with contractile anomalies following the acute ethanol challenge, the effects of which were unaffected by lysosomal inhibition. These findings support a causal role of autophagy in ethanol-elicited cardiac damage, possibly initiated by the excess production of autophagosomes and impaired autophagic flux, which received convincing support from the in vitro finding that autophagy inhibition using 3-MA reversed, whereas lysosomal inhibition mimicked ethanol-induced cardiomyocyte dysfunction or autophagosome accumulation. Likewise, AMPK inhibition with compound C ablated ethanol-induced autophagosome formation in H9C2 cells (Figure 6), the effect of which was less affected by lysosomal inhibition. These findings favour a role of AMPK inhibition in alleviating autophagosome formation rather than interrupted autophagic flux in response to ethanol exposure. It has been suggested that impaired autophagosome removal and resultant autophagosome accumulation may be more detrimental than impaired autophagosome formation.39 Thus the relative roles of autophagosome formation and autophagic flux in alcoholic heart injury deserve further scrutiny. It is possible that the initial autophagosome formation may be extremely low under AMPK deficiency or inhibition, thus autophagosome accumulation cannot be further enhanced with interruption of the lysosomal outflow.
The energy sensor AMPK triggers autophagy through its ability to inactivate mTORC1.40 Autophagosome formation, one of the early steps in the autophagic process, may be initiated by several multimolecular complexes such as VPS34-Beclin-1 class III PI3-kinase complex, ULK1 protein-kinase complex, Atg9-Atg2-Atg18 complex, and the Atg5-Atg12-Atg16 and Atg8/LC3 conjugation systems.14 The first autophagic gene identified was Atg1, which encodes the catalytic subunit of a protein kinase complex to trigger autophagy.40,41 The mammalian homologue of Atg1 ULK1 was thought to play a pivotal role in the initial stages of autophagy although the precise mechanisms remain unclear.23 Recent evidence suggested that autophagy may be regulated through the direct phosphorylation of ULK1 by AMPK and mTORC1.22 Our study depicted decreased mTOR phosphorylation and pan protein expression of Raptor, as well as elevated Raptor phosphorylation associated with enhanced AMPK phosphorylation following the acute ethanol challenge, the effects of which were alleviated by AMPK deficiency. It was demonstrated that phosphorylation of Raptor at Ser792 by AMPK is essential for mTORC1 inhibition under energy stress,19 consistent with our observation that AMPK deficiency reversed ethanol-induced elevation of Raptor phosphorylation. Our results also revealed that AMPK deficiency significantly suppressed ethanol-induced decrease of ULK1 phosphorylation at Ser757, which is negatively regulated by mTOR.42 It is demonstrated that the active mTORC1 may phosphorylate ULK1 on Ser757 to prevent ULK1 from interacting with AMPK. Dephosphorylated ULK1 is in fact enzymatically active, and the kinase activity of ULK1 is thought to be important for the recruitment of other downstream Atg proteins and the subsequent autophagosome formation.43 Furthermore, our in vitro data reveal that ethanol-induced distinct ULK1 phosphorylation at Ser777 and Raptor phosphorylation in concert with elevated AMPK phosphorylation, the effects of which were reversed by AMPK inhibition using compound C. ULK1 is reported to be regulated by multiple phosphorylation and dephosphorylation processes, one of which is Ser777.23 AMPK is capable of promoting autophagy through the direct activation of ULK1 at Ser317 and Ser777 phosphorylation sites,22 although other phosphorylation sites such as Ser555 and Ser467 also play a role in AMPK-stimulated activation of ULK1.21 In addition, our finding indicated that ethanol compromised cardiomyocyte contractile properties which were ameliorated by 3-MA and compound C. These findings supported a crucial role of autophagy in ethanol-induced cardiac contractile dysfunction related with overload AMPK, suggesting that the ethanol-induced initiation of autophagy may primarily be a pro-death rather than a pro-survival mechanism. A schematic diagram is provided to layout acute ethanol exposure-initiated autophagic and contractile responses through AMPK, and subsequently the inhibition of mTORC1 and the phosphorylation of ULK1 (Supplementary material online, Figure S4).
In conclusion, the present study provided convincing evidence for the first time that AMPK deficiency is capable of ameliorating ethanol-induced myocardial contractile dysfunction, possibly via AMPK-mTORC1-ULK1-mediated regulation of autophagosome formation. This is supported by the finding that inhibition of AMPK or autophagy protected against ethanol-induced cardiac anomalies. Although the acute ethanol challenge interrupted the autophagic flux, our data did not favour a major role of the autophagic flux in AMPK deficiency or inhibition-elicited beneficial effect against the ethanol challenge. Although it is still premature to discern the precise mechanism underneath AMPK-mediated autophagy following alcohol intake, our study should shed some light towards better understanding of the role of autophagy in alcoholic heart damage. Further investigation is warranted to elucidate the therapeutic value of the AMPK and AMPK-related autophagic signalling molecule in the management of binge drinking-induced myopathic anomalies.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported in part by National Institute of Health (NIH) 1R01 AA013412.
Supplementary Material
Acknowledgments
The AMPK KD founder mice were kindly provided by Dr Morris Birnbaum from University of Pennsylvania, Philadelphia, PA, USA.
Conflict of interests: none declared.
References
- 1.O'Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50:1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 2.Stolle M, Sack PM, Thomasius R. Binge drinking in childhood and adolescence: epidemiology, consequences, and interventions. Dtsch Arztebl Int. 2009;106:323–328. doi: 10.3238/arztebl.2009.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel VB, Why HJ, Richardson PJ, Preedy VR. The effects of alcohol on the heart. Adverse Drug React Toxicol Rev. 1997;16:15–43. [PubMed] [Google Scholar]
- 4.Ren J, Wold LE. Mechanisms of alcoholic heart disease. Ther Adv Cardiovasc Dis. 2008;2:497–506. doi: 10.1177/1753944708095137. [DOI] [PubMed] [Google Scholar]
- 5.Guo R, Ren J. Alcohol dehydrogenase accentuates ethanol-induced myocardial dysfunction and mitochondrial damage in mice: role of mitochondrial death pathway. PLoS One. 2010;5:e8757. doi: 10.1371/journal.pone.0008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannuksela ML, Liisanantti MK, Savolainen MJ. Effect of alcohol on lipids and lipoproteins in relation to atherosclerosis. Crit Rev Clin Lab Sci. 2002;39:225–283. doi: 10.1080/10408360290795529. [DOI] [PubMed] [Google Scholar]
- 7.Guo R, Scott GI, Ren J. Involvement of AMPK in alcohol dehydrogenase accentuated myocardial dysfunction following acute ethanol challenge in mice. PLoS One. 2010;5:e11268. doi: 10.1371/journal.pone.0011268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beauloye C, Bertrand L, Horman S, Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res. 2011;90:224–233. doi: 10.1093/cvr/cvr034. [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Tian R. Targeting AMPK for cardiac protection: opportunities and challenges. J Mol Cell Cardiol. 2011;51:548–553. doi: 10.1016/j.yjmcc.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolwicz SC, Jr, Tian R. Glucose metabolism and cardiac hypertrophy. Cardiovasc Res. 2011;90:194–201. doi: 10.1093/cvr/cvr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paiva MA, Rutter-Locher Z, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, et al. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300:H2123–2134. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemchenko A, Chiong M, Turer A, Lavandero S, Hill JA. Autophagy as a therapeutic target in cardiovascular disease. J Mol Cell Cardiol. 2011;51:584–593. doi: 10.1016/j.yjmcc.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie M, Morales CR, Lavandero S, Hill JA. Tuning flux: autophagy as a target of heart disease therapy. Curr Opin Cardiol. 2011;26:216–222. doi: 10.1097/HCO.0b013e328345980a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding WX, Manley S, Ni HM. The emerging role of autophagy in alcoholic liver disease. Exp Biol Med (Maywood) 2011;236:546–556. doi: 10.1258/ebm.2011.010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osna NA, Thomes PG, Donohue TM., Jr Involvement of autophagy in alcoholic liver injury and hepatitis C pathogenesis. World J Gastroenterol. 2011;17:2507–2514. doi: 10.3748/wjg.v17.i20.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge W, Guo R, Ren J. AMP-dependent kinase and autophagic flux are involved in aldehyde dehydrogenase-2-induced protection against cardiac toxicity of ethanol. Free Radic Biol Med. 2011;51:1736–1748. doi: 10.1016/j.freeradbiomed.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge W, Ren J. mTOR-STAT3-notch signaling contributes to ALDH2-induced protection against cardiac contractile dysfunction and autophagy under alcoholism. J Cell Mol Med. 2011;16:615–625. doi: 10.1111/j.1582-4934.2011.01347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyer-Hansen M, Jaattela M. AMP-activated protein kinase: a universal regulator of autophagy? Autophagy. 2007;3:381–383. doi: 10.4161/auto.4240. [DOI] [PubMed] [Google Scholar]
- 19.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 21.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan J, McFadden GE, Borgerding AJ, Norby FL, Ren BH, Ye G, et al. Overexpression of alcohol dehydrogenase exacerbates ethanol-induced contractile defect in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2002;282:H1216–1222. doi: 10.1152/ajpheart.00780.2001. [DOI] [PubMed] [Google Scholar]
- 25.Li SY, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol. 2008;44:992–1001. doi: 10.1016/j.yjmcc.2008.02.276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Manning WJ, Wei JY, Katz SE, Litwin SE, Douglas PS. In vivo assessment of LV mass in mice using high-frequency cardiac ultrasound: necropsy validation. Am J Physiol. 1994;266:H1672–1675. doi: 10.1152/ajpheart.1994.266.4.H1672. [DOI] [PubMed] [Google Scholar]
- 27.Gardin JM, Siri FM, Kitsis RN, Edwards JG, Leinwand LA. Echocardiographic assessment of left ventricular mass and systolic function in mice. Circ Res. 1995;76:907–914. doi: 10.1161/01.res.76.5.907. [DOI] [PubMed] [Google Scholar]
- 28.Ryu JH, Cho YS, Chun YS, Park JW. Myocardial SSAT induction via AMPK signaling and its implication for ischemic injury. Biochem Biophys Res Commun. 2008;366:438–444. doi: 10.1016/j.bbrc.2007.11.134. [DOI] [PubMed] [Google Scholar]
- 29.Ceylan-Isik AF, Zhao P, Zhang B, Xiao X, Su G, Ren J. Cardiac overexpression of metallothionein rescues cardiac contractile dysfunction and endoplasmic reticulum stress but not autophagy in sepsis. J Mol Cell Cardiol. 2010;48:367–378. doi: 10.1016/j.yjmcc.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marino G, Salvador-Montoliu N, Fueyo A, Knecht E, Mizushima N, Lopez-Otin C. Tissue-specific autophagy alterations and increased tumorigenesis in mice deficient in Atg4C/autophagin-3. J Biol Chem. 2007;282:18573–18583. doi: 10.1074/jbc.M701194200. [DOI] [PubMed] [Google Scholar]
- 31.Yuan H, Perry CN, Huang C, Iwai-Kanai E, Carreira RS, Glembotski CC, et al. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol. 2009;296:H470–479. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edick MJ, Tesfay L, Lamb LE, Knudsen BS, Miranti CK. Inhibition of integrin-mediated crosstalk with epidermal growth factor receptor/Erk or Src signaling pathways in autophagic prostate epithelial cells induces caspase-independent death. Mol Biol Cell. 2007;18:2481–2490. doi: 10.1091/mbc.E06-04-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turdi S, Kandadi MR, Zhao J, Huff AF, Du M, Ren J. Deficiency in AMP-activated protein kinase exaggerates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J Mol Cell Cardiol. 2011;50:712–722. doi: 10.1016/j.yjmcc.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spies CD, Sander M, Stangl K, Fernandez-Sola J, Preedy VR, Rubin E, et al. Effects of alcohol on the heart. Curr Opin Crit Care. 2001;7:337–343. doi: 10.1097/00075198-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Donovan JE. Estimated blood alcohol concentrations for child and adolescent drinking and their implications for screening instruments. Pediatrics. 2009;123:e975–981. doi: 10.1542/peds.2008-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, et al. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 37.Musi N, Hirshman MF, Arad M, Xing Y, Fujii N, Pomerleau J, et al. Functional role of AMP-activated protein kinase in the heart during exercise. FEBS Lett. 2005;579:2045–2050. doi: 10.1016/j.febslet.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 38.Cowherd RB, Asmar MM, Alderman JM, Alderman EA, Garland AL, Busby WH, et al. Adiponectin lowers glucose production by increasing SOGA. Am J Pathol. 2010;177:1936–1945. doi: 10.2353/ajpath.2010.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardie DG. AMPK and autophagy get connected. EMBO J. 2011;30:634–635. doi: 10.1038/emboj.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergheim I, Guo L, Davis MA, Lambert JC, Beier JI, Duveau I, et al. Metformin prevents alcohol-induced liver injury in the mouse: critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130:2099–2112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bach M, Larance M, James DE, Ramm G. The serine/threonine kinase ULK1 is a target of multiple phosphorylation events. Biochem J. 2011;440:283–291. doi: 10.1042/BJ20101894. [DOI] [PubMed] [Google Scholar]
- 43.Chan EY, Longatti A, McKnight NC, Tooze SA. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol Cell Biol. 2009;29:157–171. doi: 10.1128/MCB.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.