Abstract
Cardiovascular disease (CVD) is the number-one killer of women. Women with primary ovarian insufficiency (POI) may be more burdened by cardiovascular disease, such as myocardial infarction and stroke, as compared with women with normal menopause. The increased burden may be mediated by a worsening of cardiovascular risk factors, such as lipids, corresponding with the loss of ovarian function. In contrast, the increased burden may be caused by factors that precede and potentially contribute to both CVD events and ovarian decline, such as X-chromosome abnormalities and smoking. Regardless of the cause, women with POI may serve as an important population to target for CVD screening and prevention strategies. These strategies should include the use of CVD risk stratification tools to identify women that may benefit from lifestyle modification and pharmacological therapy to prevent CVD. Sex steroid therapy for the sole purpose of CVD prevention in women with POI cannot be recommended, based on a lack of evidence.
Keywords: POI, MI, stroke, heart disease, cardiovascular disease
PRIMARY OVARIAN INSUFFICIENCY AND CARDIOVASCULAR DISEASE
Overview
Heart disease is the number-one killer of women, and stroke is the third leading cause of death.1 Epidemio-logical studies suggest that women with loss of ovarian function at early ages may be especially burdened by cardiovascular disease (CVD). However, many of the studies showing a strong relationship between early age at menopause and CVD have a preponderance of surgically menopausal women.2,3 Less strong is the association between early age at menopause and CVD in women with natural menopause.4
All women who live long enough will experience menopause. In epidemiological studies, the distribution of age at menopause is skewed with a long left tail.5 Some authors have suggested that the distribution of age at menopause is more likely bimodal, with women with ovarian insufficiency <40 years of age, that is primary ovarian insufficiency (POI), forming a distinct population.6 Currently, underlying genetics, rather than environmental exposures, is thought to be the major contributor to age at menopause in the general population.7 However, more research is needed, especially in women with menopause at the youngest ages.
Women with spontaneous POI have a different distribution of causes of their ovarian failure, as compared with women with normal menopause. In these women, X-chromosome abnormalities and autoimmunity are more prevalent than in women with later menopause. However, the etiology of >90% of cases of spontaneous 46,XX POI is unknown.8 It is likely that in women with POI, especially those with spontaneous POI in their late 30s, determining the genetic and environmental contributors to the timing of normal menopause will have relevance.
Full absence of the X chromosome (45,X) and X-chromosome mosaicism are estimated to occur in ~50 per 100,000 live-born girls.9 Many of these girls experience primary amenorrhea rather than secondary amenorrhea. Women with X chromosome–related POI (i.e., Turner’s syndrome) are a distinct group6 with unique medical needs.10
Data on spontaneous 46,XX POI and its relationship with CVD are sparse. Studies of women with spontaneous POI and CVD frequently include women with X chromosome–related ovarian failure or insufficiency. Thus X chromosome–related ovarian failure is discussed in this article. Because more data exist regarding early natural menopause (menopause at ages near or < 45 years) and CVD than for POI (menopause <40 years of age), much of this article discusses early natural menopause.
AGE AT MENOPAUSE AND CARDIOVASCULAR DISEASE MORTALITY
Cardiovascular Disease Mortality in General Populations
Researchers have investigated the association between early age at menopause and longevity in multiple large-scale epidemiological studies. These studies have primarily been in European and white cohorts. In the late 1980s, Snowden et al reported a modest increase in all-cause mortality in Seventh-Day Adventist women with natural menopause at ages <40 years, as compared with women with natural menopause at ages 50 to 54 (age-adjusted odds ratio: 1.95; 95% confidence interval [CI]: 1.24 to 3.07).11 In a separate publication using the Seventh-Day Adventist data, researchers estimated that each 1-year decrease in age at natural menopause before age 47 was associated with a 0.53-year increase in postmenopausal life (p =0.04) and a 0.47-year earlier age at death (p =0 0.04).12
In the 1990s, additional research groups compared mortality rates in women with early menopause (~40 years) versus women with more average ages at menopause (~50 years) and found increased mortality rates in the early menopause group.. However, only rarely did this increase meet statistical significance. For example, in the National health and Nutrition Examination Survey, researchers found an increased mortality rate ratio that was statistically insignificant for their group of women with menopause at age <40 years (mortality rate ratio [MRR]: 1.50; 95% CI, 0.97 to 2.34).13 In a sample of college-educated Minnesota women, researchers found only slightly higher mortality in women with menopause at age ≤ 45 (adjusted rate ratio [RR]: 1.39; 95% CI, 0.63 to 3.04).14 In a Norwegian cohort, researchers found increased mortality in women with menopause at an age <40 years that also did not meet statistical significance (MRR: 1.06; 95% CI, 0.99 to 1.44). However, they found a small but statistically significant relationship between age at menopause and all-cause mortality in the overall cohort, with a 1.6% decrease in mortality for every 3-year increase in age at menopause.15
Several studies from the late 1990s in European cohorts focused on age at menopause and ischemic heart disease mortality16,17 rather than general mortality. In a Norwegian cohort, Jacobsen et al reported a weak inverse relationship between age at menopause and cardiovascular disease mortality.16 In a cohort from the Netherlands, Van der Schouw et al reported a quantifiable 2% decrease in cardiovascular mortality risk for each year that menopause was delayed.17 In an American Seventh-Day Adventist cohort, Jacobsen et al also found increased mortality due to ischemic heart disease in Seventh-Day Adventist women with natural menopause at ages <40.18
MORTALITY IN WOMEN WITH X-CHROMOSOME ABNORMALITIES
Women with Turner syndrome (TS) appear to have worse mortality than women from the general population. British women with TS have a threefold worse mortality as compared with other women, and mortality was higher for almost all major causes of death.19 The greatest differences in mortality between women with TS and other women were for vascular diseases that appeared congenital in origin (cardiovascular congenital anomalies, aortic aneurysm, and aortic valve disease); in fact, reported standardized mortality ratios (SMR)s were >10 for all of these causes of death. However, death from diabetes and hypertension was also increased (SMR: 11.3, 95% CI, 5.8 to 19.7; SMR: 6.0, 95% CI, 1.2 to 17.5). These data exemplify the difficulty in classifying vascular disease as congenital or atherosclerotic in women with TS. Experts have concluded that women with TS are likely burdened by atherosclerotic disease in additional to congenital vascular disease.10,20 However, the underlying congenital disease and the absence of a second normal X chromosome may make these women very different from those POI women who have two normal X chromosomes.
AGE AT MENOPAUSE AND RISK OF CARDIOVASCULAR DISEASE EVENTS
Heart Disease
In studies from the 1970s and 1980s, menopause and heart disease appeared to be tightly linked. In a case series of 145 young women with ischemic heart disease, 20% of the women had an early menopause (age <40 years), although only 11 of 18 of this early menopause subgroup could be classified as POI, with the rest a result of surgical menopause or chemotherapy.21 In early studies of general populations, a link between bilateral oophorectomy and coronary heart disease (CHD) was often reported, but a link between natural menopause and heart disease was not consistently found.22–24
In studies from the 1990s, associations between early age at menopause and CVD were seen more consistently, likely because of the larger sample size of these studies. In a cross-sectional survey of 14,620 women for the Study of Women Across the Nation (SWAN), women who self-reported a history of heart disease gave an age at natural menopause that occurred 1.4 years earlier on average than women who did not report a history of heart disease.25 In analyses of the Nurses Health Study, Hu et al found a significant association between POI (age at menopause <40 versus reference age 50 to 54) and myocardial infarction (age-adjusted RR: 1.95; 95% CI, 1.21 to 3.13).26 The association was attenuated with additional adjustment for smoking (RR: 1.66; 95% CI, 1.03 to 2.68). The statistically significant association disappeared when only nonsmokers were included in the analyses (age-adjusted RR: 1.07; 95% CI, 0.26 to 4.34). The researchers concluded that confounding by smoking might therefore explain the relationship between early menopause and CHD in their study as well as in others.
More recently, Løkkegaard et al examined the relationship between early menopause and incident CHD in a Danish cohort. In age-corrected hazard models that were also adjusted for smoking and multiple other potential confounders, women with natural menopause at an age <40 had a twofold increased risk of CHD (hazard ratio [HR]: 2.2; 95% CI, 1.0 to 4.9). However, although they did adjust for smoking in multivariable analyses, they did not report analyses stratified by smoking status,27 as Hu et al did.
Stroke
Most studies do not find a link between age at menopause and stroke. In the Nurses Health Study, Hu et al did not find a relationship between age at natural menopause and ischemic stroke (RR: 1.01; 95% CI, 0.97 to 1.04) or hemorrhagic stroke (RR: 1.03; 95% CI, 0.97 to 1.10).26 In a Norwegian cohort, Jacobsen et al also noted that age at menopause and stroke appeared unrelated.28 In a Japanese study, Baba et al29 found an association between menopause at age <40 and stroke (HR: 2.18; 95% CI, 1.20 to 5.49), but this finding was driven by their sample, with an early age at surgical menopause, and not by the sample with an early age at natural menopause (HR: 2.18; CI, 0.44 to 10.83 versus HR: 0.94; 95% CI, 0.12 to 7.07, respectively). In a 2009 study of the Framingham cohort, Lisabeth et al found that women with natural menopause before age 42 had twice the stroke risk compared with women without early menopause, after adjustment for multiple confounders including smoking (HR: 2.03; 95% CI, 1.16 to 3.56).30 This finding persisted when the sample was restricted to women who never smoked. Their finding that the effect was independent of smoking is compelling, but validation in other cohorts is needed.
Hypertension
BLOOD PRESSURE DURING THE MENOPAUSE TRANSITION
Few studies have followed young premenopausal women (i.e., women at risk of spontaneous POI) through the menopause transition. In the Framingham cohort, Kok et al31 studied 695 premenopausal women who were ages 34 to 55 at study entry and subsequently underwent a natural menopause at ages 38 to 58. This study thus included some women with late POI (ages 38 to 39 at menopause) but no women with earlier POI.
The researchers did not find a significant relationship between baseline (premenopausal) blood pressure and subsequent age at menopause. However, they did find an association between increasing blood pressure during the premenopausal time period and an earlier age at menopause. They found that after controlling for smoking, for each 10-mm increase in systolic blood pressure over the premenopausal time period, age at menopause occurred 3.45 years earlier on average. The study authors concluded that blood pressure (and other traditional CVD risk factors) may contribute to early menopause. However, it is not possible to determine a causal pathway from these data. It is still plausible, given these data, that increasing blood pressure and ovarian failure occur concomitantly, rather than one event preceding the other.32
Several other studies have investigated whether the transition through menopause is linked to increasing blood pressure. In studies of women going through menopause at normal ages, this link was not seen.33 However, as stated previously, few studies have followed young women. In a Japanese cohort, Akahoshi et al investigated whether blood pressure increased differentially during the menopause transition in young women with an early age at menopause versus older women with more average ages of menopause. Before menopause, younger women had lower blood pressures than older women. Young women with early menopause (<44 years of age) also had lower blood pressures during the menopause transition as compared with older women who transitioned through menopause at older ages.34 From these data, the authors concluded that the menopause transition does not have an effect on blood pressure. However, this study did not contain a young control group who remained premenopausal for blood pressure comparison, so it is unclear whether ovarian failure during young life may have an independent effect on blood pressure.
Blood Pressure and X-Chromosome Abnormalities
Women with TS are burdened by vascular disease. They are known to have more congenital vascular disease, such as aortic coarctation and renovascular anomalies. In addition, they have more hypertension than similar age women from the general population.20,35 However, the exact cause of the increased prevalence of hypertension is difficult to elucidate and may reflect secondary hypertension from occult congenital vascular disease rather than primary or essential hypertension. The vascular similarities and differences in these women versus women with spontaneous 46,XX POI are unknown because direct comparisons of these two groups have not been reported.
AGE AT MENOPAUSE AND TRADITIONAL CARDIOVASCULAR DISEASE RISK FACTORS
Smoking
Smoking and an earlier age at menopause are related in many studies.25,36–39 Women who smoke have a menopause that occurs 1 to 2 years earlier than nonsmokers, although some studies suggest it is only active smoking that affects the timing of menopause.40,41 Multiple researchers have concluded that the relationship between age at menopause and cardiovascular mortality and disease events is highly confounded by smoking.26,38
Lipids
LIPIDS AND NATURAL MENOPAUSE
A preponderance of evidence links the menopausal transition to changes in lipids, most notably an increase in low-density lipoprotein (LDL) and total cholesterol.42–45 In contrast, high-density lipoprotein (HDL) cholesterol does not appear to change with menopause.45–48 A well-designed study by Matthews et al assessed women from the SWAN cohort who transitioned through natural menopause at normal ages. They found that total cholesterol, LDL cholesterol, and apolipoprotein B all increased substantially during the interval from 1 year before to 1 year after the final menstrual period. Using sophisticated modeling techniques, the researchers were able to conclude that the pattern of these increases was consistent with an effect of menopause and was not likely due to aging alone.33 This study may have relevance to women with early menopause and possibly POI, although a limitation is that the SWAN cohort included only women ≥42 years and those women had to still have at least intermittent menses at the time of recruitment.
Chu et al found that lipids change very early in the menopause transition, even before the beginning of irregular menses that typically define the menopause transition. They found that in regularly cycling women, total and LDL cholesterol increase in proportion to a common surrogate marker of ovarian function, the follicle-stimulating hormone (FSH) level on day 3 of the follicular phase of the menstrual cycle.49 They further reported that this increase in cholesterol with increasing FSH level was independent of estradiol level.50 This study suggests a link between hormones of the hypothalamic-pituitary-ovarian axis and total and LDL cholesterol levels that is not explained solely by the waning of estrogen.
LIPIDS AND EARLY MENOPAUSE
In women transitioning through an early menopause, cholesterol appears to change similarly to women with menopause at normal age, with a few differences. Akahoshi et al observed that total cholesterol increased in women of all ages as they transitioned through menopause. However, they found that women with early menopause (<44 years) had a higher magnitude of increase in cholesterol as compared with women with menopause at later ages. In the postmenopausal time period, the women with early menopause had cholesterol levels that were similar to women who had gone through menopause at later ages, even though the women with early menopause were younger. Their findings suggested that women with early menopause may be burdened by an increased duration of lipid derangement, as well as a more abrupt increase in magnitude of lipid derangement attributable to early menopause.34
LIPIDS AND PRIMARY OVARIAN INSUFFICIENCY
Very little is known about lipid profiles in women with ovarian failure at <40 years of age. Knauff et al compared lipid profiles in women with POI to women without POI.51 All women were off hormone replacement therapy or oral contraceptive pills at the time of the study. In addition, women with POI and women without POI were very similar in terms of body mass index (BMI), waist circumference, and smoking status. Surprisingly, no difference in total cholesterol or LDL cholesterol was observed between the two groups. However, a significantly higher triglyceride level and borderline lower HDL cholesterol level was observed in the POI group. The authors concluded that the subtle lipid abnormalities that they observed in triglycerides were likely causally related to lack of endogenous ovarian function. Yet why these women would manifest lipid changes (i.e., higher triglyceride levels and potentially lower HDL levels) that are common in the setting of insulin resistance but unique from the lipid changes typically seen in women with menopause at later ages is unclear.
LIPIDS AND X-CHROMOSOME ABNORMALITIES
Cooley et al compared lipid profiles of women with Turner’s syndrome to age-matched 46,XX women with spontaneous POI. All subjects were off sex hormone therapy at the time of study. They found that women with TS had a higher total cholesterol, LDL cholesterol, and triglycerides, as compared with the 46,XX women with spontaneous POI. They also noted a potentially more atherogenic profile in women with Turner’s syndrome, with more having a high proportion of small dense LDL.52 They concluded that the lipid abnormalities were related to an as yet to be identified abnormal X-linked gene.53
LIPIDS SUMMARY
High LDL and total cholesterol levels are linked to a high risk of future heart disease events, although this relationship may not be as strong in women as it is in men.45 Non-HDL cholesterol is also an important marker of cardiovascular risk in women. A recent study of >300,000 subjects from multiple prospective studies of CVD drugs found that non-HDL cholesterol (total cholesterol minus HDL cholesterol) predicted future CVD as well as LDL cholesterol in both men and women.54 Smaller studies have shown superiority of non-HDL cholesterol over LDL cholesterol in predicting CVD events, especially in women.55,56 Thus, given the lipid abnormalities and increased CVD risk seen in observational studies of women with POI and Turner’s syndrome, the monitoring of lipid profiles, especially non-HDL cholesterol, may have some important clinical usefulness in predicting future CVD in women with POI.
It is difficult to discuss lipid profiles in women with ovarian insufficiency without commenting on the lipid changes associated with hormone therapy. In the 1990s, the LDL and total cholesterol changes that accompany natural menopause were implicated as the main culprit in the association between early menopause and CVD. Observational studies of hormone therapy at the time of menopause had found that hormone therapy prevented the increase in total cholesterol typically seen with menopause47 and also appeared to prevent CVD events.57 This finding in part led to the placebo-controlled randomized trials of conjugated equine estrogen (CEE) and CEE + progestin (CEE + P) performed by the Women’s Health Initiative (WHI).
In both of the WHI trials, hormone therapy given to postmenopausal women reversed the lipid changes typically associated with menopause; it decreased total cholesterol and LDL cholesterol. In addition, it increased HDL cholesterol. However, it also increased triglyceride levels and C-reactive protein (CRP) significantly.58,59 In these trials, neither CEE nor CEE + P conferred protection from CHD, and both therapies caused stroke.60–62
The results of the WHI randomized trials have sparked intense debate regarding the underlying reason for these findings. The type of hormone therapy, the hormone therapy delivery system, and the timing of therapy initiation have all been proposed as possible contributors. Secondary analyses of the WHI trial data to address some of the potential contributors are ongoing.
Both WHI randomized trials included some women with early menopause, either natural or surgical. One recent WHI publication revealed a positive interaction between baseline LDL cholesterol levels and CEE + P for CHD that was independent of age and multiple other confounders.59 This finding suggests that a woman with POI and a high baseline LDL cholesterol level may have a higher risk of having a CHD event if treated with CEE + P. More studies of the sample of WHI subjects with POI are needed, as are studies investigating whether lowering LDL cholesterol before sex steroid therapy can reduce the risk of CHD events.
Body Mass, Glucose, and Insulin
BODY MASS INDEX, NATURAL MENOPAUSE, AND PRIMARY OVARIAN INSUFFICIENCY
BMI is associated with age at menopause, with several studies showing that heavier women experience a later menopause. However this relationship is highly confounded by smoking; that is, current smokers tend to weigh less than nonsmokers37 and smokers are known to have earlier menopause. When comparing women transitioning through menopause at an early age (<44 years) versus a later age, women with early menopause gain weight at the same rate as women with later menopause.34 Whether young women <40 years of age who undergo 46,XX spontaneous primary ovarian insufficiency gain more weight over time, as compared with women who retain normal ovarian function, is unknown. Women with Turner’s syndrome appear to weigh more than women with 46,XX primary ovarian insufficiency; however, this difference may be related to decreased physical activity levels35 seen in women with Turner’s syndrome.
THE ASSOCIATION BETWEEN GLUCOSE METABOLISM AND NATURAL MENOPAUSE
Parallel to the fact that heavier women appear to have later menopause, women with insulin resistance may also have later menopause. In a small case-control study of ~400 women, those with type 2 diabetes had a slightly later age at menopause as compared with women without diabetes, although this difference was very small and not statistically significant.63 A group of women with unique reproductive issues who are frequently burdened by insulin resistance are women with polycystic ovary syndrome (PCOS).
PCOS is commonly defined as the presence of menstrual irregularity due to oligo- or amenorrhea and signs or symptoms of androgen excess.64 The definition may also include the presence of multiple (≥12) small follicles on one or both ovaries.65 Because of associated amenorrhea, these women may appear clinically similar to women with spontaneous POI, especially in women with onset of these disorders in their late 30s. One obvious distinguishing feature is hypergonadotropism,66 with POI women having low estrogen/high FSH and PCOS women having normal or low FSH and often a high luteinizing hormone to FSH ratio.67
Some evidence suggests that women with PCOS may have a higher ovarian reserve than women without PCOS,68 although no long-term studies that follow women with PCOS through the menopause transition exist. This has relevance to the study of age at menopause and its association with CVD risk because the relationship between the two may be U-shaped. For example, women with both early and late menopause may have increased CVD risk factors, such as insulin resistance. A U-shaped association will not be detected by statistical analyses that assume a linear relationship, and more appropriate techniques must be used.
CHANGES IN GLUCOSE METABOLISM WITH NORMAL MENOPAUSE
Glucose metabolism may change with normal menopause, with worsening insulin resistance frequently reported. However, it is very difficult to tease out metabolic changes caused by aging (such as insulin resistance caused by aging-related fat gain) versus changes that are directly attributable to the menopause. Carr’s article, “The Emergence of the Metabolic Syndrome with Menopause,” is a good overview of the complexity of this topic.69 In addition, a recent publication by Matthews et al from the SWAN cohort provides information on glucose and insulin in women transitioning through a natural menopause at normal ages. They observed a decrease in glucose and an increase in insulin over time that appeared related to aging rather than to a direct effect of menopause.33 However, it is unclear from their article whether women became more or less insulin resistant with aging. Analyses of the SWAN data that includes changes in weight and BMI in relation to changes in glucose metabolism would provide more insight.
GLUCOSE METABOLISM AND PRIMARY OVARIAN INSUFFICIENCY
Glucose metabolism may be abnormal in women with POI, and autoimmunity may contribute to a small degree. Autoimmunity is a cause of both type 1 diabetes and POI, and these two disorders can coexist in persons with the most common polyglandular autoimmune syndrome, polyglandular autoimmune syndrome type 2 (PAS 2). PAS 2 is typically defined as the presence of autoimmune adrenal failure, plus a second immunoendocrinopathy (most often autoimmune thyroid disease or type 1 diabetes).
The effect of autoimmunity on the relationship between glucose metabolism and POI appears to be minor. Even when autoimmunity is highly suspected in a group of women with POI, diabetes is not frequently diagnosed. A 1997 study of 119 women referred to a tertiary academic center where patients with spontaneous 46,XX POI revealed a high prevalence of coexisting endocrine disease; however 27% of these women (32 of 119) had thyroid disease, whereas only 2.5% had adrenal disease (3 of 119) and only 2.5% had glucose abnormalities (3 of 119). The researchers attributed these glucose abnormalities to autoimmune mediated β-cell dysfunction, but antibody testing was not performed.70 Based on this one study and an estimated prevalence of diabetes in women ages 20 to 39 years of 1.7% during this same time period,71 it appears that women with spontaneous 46,XX POI are slightly more burdened by glucose abnormalities than the general population.
GLUCOSE METABOLISM AND X-CHROMOSOME ABNORMALITIES
Women with TS appear to have more abnormalities of glucose metabolism and more overt diabetes, as compared with women in the general population.20 A recent study of 224 self-referred individuals with Turner’s syndrome found a 25% prevalence of type 2 diabetes and a 0.5% prevalence of type 1 diabetes in these women. This compared with an expected 6.7% prevalence of diabetes for the general population. Researchers found that haploinsufficiency for Xp especially increased the risk of type II diabetes for women with Turner’s syndrome.72 Given previous studies showing lower glucose-dependent insulin secretion in women with Turner’s syndrome, as compared with women with 46,XX POI,71 authors hypothesized that X-chromosome gene dosing contributes to β-cell dysfunction in women with Turner’s syndrome.72
CARDIOVASCULAR DISEASE PREVENTION STRATEGIES IN WOMEN WITH PRIMARY OVARIAN INSUFFICIENCY
Based on the available evidence, it appears that women with POI have a higher risk of CVD and CVD mortality as compared with the general population, although this increased risk appears small. A decade ago, multiple reports concluded that young women with heart disease events had especially high mortality.73–76 On a positive note, heart disease mortality in young women has significantly improved since the 1990s, possibly because of better recognition and management of heart disease and its risk factors.77 Continued early identification of women at high risk for CVD is needed to maintain these gains, and women with POI may serve as an important population to target for CVD screening and preventive strategies.
CARDIOVASCULAR DISEASE RISK SCREENING AND RISK STRATIFICATION TOOLS
Adult Treatment Panel III
The Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) was published in 200178 and updated in 2004.79 The ATP IV report is due out in the fall of 2011. The 1994 ATP II guidelines included “premature menopause without estrogen replacement therapy” as a major risk factor for CVD,80 but premature menopause without estrogen replacement therapy is no longer listed as a risk factor in the ATP III reports. The ATP III panel now deems only cigarette smoking, family history of premature CHD, low HDL cholesterol (<40 mg/dL), and hypertension (blood pressure ≥140/90 mm Hg) as major risk factors for CVD. It also deems diabetes as a CVD equivalent for risk stratification purposes.
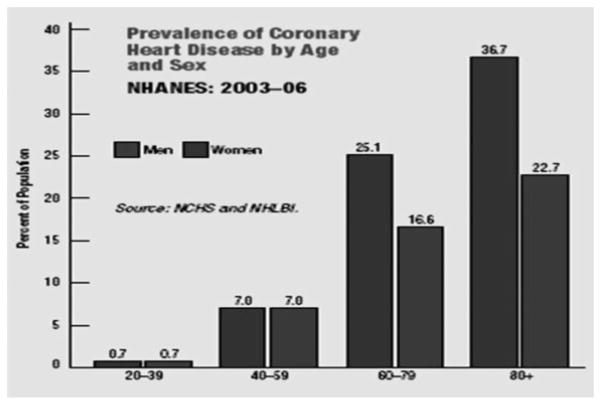
The panel recommends the use of the Framingham risk assessment tool for evaluating 10-year risk of developing myocardial infarction and CHD death (Fig. 1). The Framingham risk assessment tool incorporates age, gender, total cholesterol, HDL cholesterol, smoking status, blood pressure, and blood pressure treatment into the CVD risk calculation. This risk calculator is intended for adults ≥20 years of age who do not have known heart disease or diabetes. The Framingham risk assessment tool was developed based on a population that included young women; however, data indicate that women <40 years comprise a very low-risk group (Fig. 2).
Figure 1.
Risk Assessment Tool for Estimating Your 10-Year Risk of Having a Heart Attack.110 CHD, coronary heart disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Figure 2.
National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention and National Heart Lung and Blood Institute (NHLBI), National Institutes of Health. U.S. Department of Health and Human Services. Prevalence of Coronary Heart Disease by Age and Sex. In: Heart Disease and Stroke Statistics—2010 Update. American Heart Association. Available at: http://www.americanheart.org/downloadable/heart/1265665152970DS-3241%20HeartStrokeUpdate_2010.pdf. Accessed November 9, 2010. NHANES, National Health and Nutrition Examination Survey.
Long-term prospective studies reveal that elevated serum cholesterol detected in young adulthood predicts a higher rate of premature CHD in middle age. The ATP III guidelines emphasize that risk factor identification in young adults is important for long-term prevention strategies. Thus women with POI may serve as an important group to target for risk factor identification.
Reynolds Risk Score
The Reynolds risk score is a risk stratification tool that some have argued is superior for CVD risk prediction (heart attack, stroke, or other major heart disease) in women. The tool includes age, smoking status, systolic blood pressure, total cholesterol, HDL cholesterol, high-sensitivity CRP, and family history of heart disease. This tool was developed in postmenopausal women who were at least 45 years old at study entry. This risk calculator is intended for adults who do not have known heart disease or diabetes. Studies to date using the Reynolds risk score for risk stratification in women have focused on postmenopausal women,81 and it may not have as much relevance to young women with POI.
CARDIOVASCULAR DISEASE RISK REDUCTION
Lifestyle Modification
The first line of treatment for reducing cardiovascular disease risk is lifestyle modification.45 Of special importance to women with POI is smoking, which has been linked with earlier menopause in multiple epidemiological studies of the timing of menopause. Because a preponderance of evidence shows that limiting exposure to smoking reduces cardiovascular risk, all women who smoke, regardless of ovarian functional status, should be counseled and provided assistance in quitting.
In addition to smoking cessation, weight management, adherence to a healthy diet, and regular physical activity have beneficial effects in reducing CVD risk in women. Lifestyle modification should thus be recommended to all women with increased CVD risk, as outlined in current prevention guidelines.82
Pharmacological Therapy
LIPID-LOWERING THERAPY
In most clinical trials of lipid-lowering therapies, women are in the minority, and the power to detect clinical efficacy in this group is minimal. However, the Cholesterol Treatment Trialists’ collaboration has performed meta-analysis of lipid-lowering trials and concluded that, in persons with comparable CVD risk, statins appear to have equal efficacy in men and women.83,84 Although lipid-lowering therapy appears beneficial in meta-analyses of combined primary and secondary prevention trials,85 some argue that this benefit is not robust enough to recommend statins solely for primary prevention in women.86,87 No studies of lipid-lowering therapies in women with POI exist.
The ATP III’s general approach to the primary prevention of cardiovascular disease is the same for women and men. It is reasonable to use the ATP III recommendations to guide the management of CVD risk in women with POI.78 ATP III recommends taking into account the later onset of CHD for women in clinical decisions about using cholesterol-lowering drugs. However, given that women with early menopause appear to experience more and earlier CHD events, this recommendation may not apply to them. The ATP panel notes that particular attention should be given to young men who smoke and have a high LDL cholesterol (160 to 189 mg/dL) because they may be appropriate candidates for LDL-lowering drugs. It may also be reasonable to give particular attention to young women with POI who smoke and have high LDL. In addition, recommendations regarding non-HDL targets may be particularly relevant to women with POI, given the lipid abnormalities reported in POI.
Outcomes data in women treated with lipid-lowering medications other than statins are limited88 and do not exist in women with POI. However, the most commonly used drugs for lipid lowering in women in general are statins. Given that 5 to 10% of women with POI achieve spontaneous pregnancies, it is relevant that statins are pregnancy category X and should not be used in women with POI who may develop a spontaneous pregnancy.45,89,90
Sex Steroid Therapy
Cardiovascular risk should be taken into account when and if prescribing sex steroids in women with POI. A summary of the various sex steroid preparations available for the treatment of POI is beyond the scope of this review. Thus estrogen-progestogen therapy and testosterone therapy are discussed only broadly here.
ESTROGEN-PROGESTOGEN THERAPY
The North American Menopause Society’s 2010 position statement on hormone therapy (HT), which encompasses both estrogen only and estrogen-progestogen therapy, notes that HT is not recommended for heart disease protection in women of any age. The authors distinguish between menopause at usual ages and menopause <40 years of age and note that there are inadequate data regarding the use of HT in women with menopause <40 years. They feel the existing data regarding the cardiovascular risk of HT in women experiencing menopause at the median age should not be extrapolated to women experiencing “premature menopause.” They acknowledge that no comparative data exist on the risks and benefits of HT initiation in women with menopause <40 years and those with menopause at more average ages. However, even though evidence is lacking, their tone is favorable toward the use of HT in women with POI, stating that “the risks attributable to HT use by these young women receiving HT may be smaller and the benefits potentially greater than those in older women who commence HT at or beyond the median age of menopause.”91
The Endocrine Society’s scientific statement on postmenopausal hormone therapy was published in 2010. Their statement acknowledges that a paucity of large randomized controlled trials (RCTs) is available to guide clinical decision making with regard to the use of HT for “premature menopause” and that evidence from studies of older women may not apply. They state that “in women with ovarian failure or surgical menopause before the age of 40 years, risk-vs.-benefit data must rely on observational studies and small RCTs.”92
Potential estrogen-progestogen therapies for the treatment of ovarian failure are numerous, but studies of these therapies are rare. The Women’s Health Initiative Trials included one type of estrogen (conjugated equine estrogen [CEE]) and contained a small proportion of women with POI. However, as discussed previously, information on the use of CEE + medroxyprogesterone medroxyprogesterone in POI may be derived from WHI publications.
A few additional hormone preparations have been studied in women with POI. A recent small crossover study of 34 women with POI (including women with TS) randomized women to receive either transdermal estradiol ~100 μg plus vaginal progesterone or oral ethinyl estradiol 30 μg plus norethisterone. Only 19 women completed the protocol. In this sample, the women randomized to transdermal estradiol achieved significantly lower blood pressures during therapy than the oral estradiol group.93 Whether lower blood pressures would have occurred in the oral ethinyl estradiol group if a different progestogen such as drospirenone that has antimineralocorticoid effects94 had been used is unknown, as is the effect of this therapy on hard CVD outcomes like myocardial infarction and stroke.
TESTOSTERONE THERAPY
Women with POI may have low androgen levels, although the data are not consistent,95 and there is considerable overlap in androgen levels when comparing women with POI to normally cycling women.96 Based on limited human data that show that neither testosterone deficiency nor testosterone therapy appear to affect length of life in either gender, it is unlikely that testosterone deficiency is the mediator of the association between POI97 and excess CVD risk. Oral testosterone therapy in oophorectomized and naturally postmenopausal women lowers HDL cholesterol.98,99 In addition, oral testosterone therapy appears to increase insulin resistance in postmenopausal women,98,99 as well as in normal cycling women.100
Testosterone therapy is not currently recommended for treatment of women with POI. However, a placebo-controlled trial of transdermal testosterone therapy for bone health in women with POI is currently ongoing.101 Although CVD is not a primary outcome of this study, it may still provide valuable information on the CVD effects of this treatment in women with POI.
Antihypertensive Therapy
There is no clear evidence that women with spontaneous 46,XX POI are predisposed to blood pressure abnormalities. However, it is appropriate to treat women with POI who develop hypertension based on current guidelines.102 Of particular relevance to women with POI is that some antihypertensive medications may be contraindicated in pregnancy and may not be appropriate for women with POI who may develop a spontaneous pregnancy. For example, angiotensin-converting enzyme inhibitors and thiazide diuretics are both category D.
Women with X-chromosome abnormalities, such as TS, do appear predisposed to blood pressure abnormalities. Researchers have suggested that an unidentified X-linked gene or genes are likely the culprits. Special focus should be paid to the CVD management of adult women with Turner’s syndrome because a study showed that these women are significantly less likely to have undergone recommended cardiac screening as compared with pediatric patients.103 Cardiac and metabolic issues in TS are discussed more thoroughly in Gravholt’s “Medical Problems of Adult Turner’s Syndrome”10 and the 2007 Guideline on the Care and of Girls and Women with Turner Syndrome.104 As our ability to assess for microdeletions and other abnormalities of the X chromosome increases and as the cost of these technologies decreases, identification of new X-linked genes linked to vascular disease may emerge and may have relevance to women with spontaneous POI with currently unidentified X-chromosome abnormalities.
Assisted Reproductive Therapy
Many women with POI undergo treatment with assisted reproductive technologies, especially the use of donor oocytes. Several studies suggest that women carrying donor oocyte pregnancies have more pregnancy-induced hypertension as compared with nondonor in vitro fertilization pregnancies.105,106 Thus women with POI may require more high-risk obstetric care.107 Of note, pregnancies in women with Turner’s syndrome are associated with an increased risk of cardiac-related death, and, in general, the Practice Committee of the American Society of Reproductive Medicine considers Turner’s syndrome to be a relative contraindication to pregnancy.108 Careful cardiovascular screening before attempting pregnancy and monitoring of women with Turner’s syndrome during pregnancy is recommended in multiple clinical guidelines.108,109
CONCLUSIONS
The relationship between early age at natural menopause and cardiovascular disease is complex. Although a small increased risk of CVD is associated with early natural menopause, the causal pathway has not been determined.
Cardiovascular risk screening should be performed early and at appropriate intervals to identify women that may benefit from lifestyle modification and pharmacological therapy to prevent CVD.
Smoking is strongly associated with early age at menopause. Smoking cessation should be encouraged in all women—both with and without POI.
Dyslipidemia is seen in POI and early menopause and should be managed based on current (2004) and upcoming (2010) ATP guidelines.
Sex steroid therapy for the sole purpose of CVD prevention cannot be recommended based on a lack of evidence in women with POI.
Decisions regarding sex steroid therapy must be based on a weighing of all known and potential risks and benefits of HT, of which CVD is only a part.
References
- 1.Leading causes of death in females, United States. Atlanta, GA: Centers for Disease Control and Prevention; 2006. [Accessed July 27, 2010]. Available at: http://www.cdc.gov/women/lcod/ [Google Scholar]
- 2.Amagai Y, Ishikawa S, Gotoh T, Kayaba K, Nakamura Y, Kajii E. Age at menopause and mortality in Japan: the Jichi Medical School Cohort Study. J Epidemiol. 2006;16(4):161–166. doi: 10.2188/jea.16.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framing-ham study. Ann Intern Med. 1976;85(4):447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 4.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 5.Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. 2003;18(1):199–206. doi: 10.1093/humrep/deg005. [DOI] [PubMed] [Google Scholar]
- 6.Rebar RW, Connolly HV. Clinical features of young women with hypergonadotropic amenorrhea. Fertil Steril. 1990;53(5):804–810. [PubMed] [Google Scholar]
- 7.Murabito JM, Yang Q, Fox C, Wilson PW, Cupples LA. Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90(6):3427–3430. doi: 10.1210/jc.2005-0181. [DOI] [PubMed] [Google Scholar]
- 8.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gravholt CH. Epidemiological, endocrine and metabolic features in Turner syndrome. Eur J Endocrinol. 2004;151(6):657–687. doi: 10.1530/eje.0.1510657. [DOI] [PubMed] [Google Scholar]
- 10.Bondy CA Turner Syndrome Study Group. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92(1):10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 11.Snowdon DA, Kane RL, Beeson WL, et al. Is early natural menopause a biologic marker of health and aging? Am J Public Health. 1989;79(6):709–714. doi: 10.2105/ajph.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snowdon DA. Early natural menopause and the duration of postmenopausal life. Findings from a mathematical model of life expectancy. J Am Geriatr Soc. 1990;38(4):402–408. doi: 10.1111/j.1532-5415.1990.tb03537.x. [DOI] [PubMed] [Google Scholar]
- 13.Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol. 1998;8(4):229–235. doi: 10.1016/s1047-2797(97)00207-x. [DOI] [PubMed] [Google Scholar]
- 14.Cooper GS, Baird DD, Weinberg CR, Ephross SA, Sandler DP. Age at menopause and childbearing patterns in relation to mortality. Am J Epidemiol. 2000;151(6):620–623. doi: 10.1093/oxfordjournals.aje.a010250. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen BK, Heuch I, Kvåle G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol. 2003;157(10):923–929. doi: 10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen BK, Nilssen S, Heuch I, Kvåle G. Does age at natural menopause affect mortality from ischemic heart disease? J Clin Epidemiol. 1997;50(4):475–479. doi: 10.1016/s0895-4356(96)00425-8. [DOI] [PubMed] [Google Scholar]
- 17.van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347(9003):714–718. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol. 1999;52(4):303–307. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 19.Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, Jacobs PA United Kingdom Clinical Cytogenetics Group. Mortality in women with Turner syndrome in Great Britain: a national cohort study. J Clin Endocrinol Metab. 2008;93(12):4735–4742. doi: 10.1210/jc.2008-1049. [DOI] [PubMed] [Google Scholar]
- 20.Gravholt CH, Juul S, Naeraa RW, Hansen J. Morbidity in Turner syndrome. J Clin Epidemiol. 1998;51(2):147–158. doi: 10.1016/s0895-4356(97)00237-0. [DOI] [PubMed] [Google Scholar]
- 21.Oliver MF. Ischaemic heart disease in young women. BMJ. 1974;4(5939):253–259. doi: 10.1136/bmj.4.5939.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157–161. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg L, Hennekens CH, Rosner B, Belanger C, Rothman KJ, Speizer FE. Early menopause and the risk of myocardial infarction. Am J Obstet Gynecol. 1981;139(1):47–51. doi: 10.1016/0002-9378(81)90410-5. [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB. Metabolic risk factors for coronary heart disease in women: perspective from the Framingham Study. Am Heart J. 1987;114(2):413–419. doi: 10.1016/0002-8703(87)90511-4. [DOI] [PubMed] [Google Scholar]
- 25.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 26.Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159(10):1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 27.Løkkegaard E, Jovanovic Z, Heitmann BL, Keiding N, Ottesen B, Pedersen AT. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53(2):226–233. doi: 10.1016/j.maturitas.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen BK, Heuch I, Kvåle G. Age at natural menopause and stroke mortality: cohort study with 3561 stroke deaths during 37-year follow-up. Stroke. 2004;35(7):1548–1551. doi: 10.1161/01.STR.0000131746.49082.5c. [DOI] [PubMed] [Google Scholar]
- 29.Baba Y, Ishikawa S, Amagi Y, et al. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause. 2010;17:506–510. doi: 10.1097/gme.0b013e3181c7dd41. [DOI] [PubMed] [Google Scholar]
- 30.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009;40(4):1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kok HS, van Asselt KM, van der Schouw YT, et al. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47(10):1976–1983. doi: 10.1016/j.jacc.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 32.Bittner V. Menopause and cardiovascular risk cause or consequence? J Am Coll Cardiol. 2006;47(10):1984–1986. doi: 10.1016/j.jacc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 33.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akahoshi M, Soda M, Nakashima E, et al. Effects of age at menopause on serum cholesterol, body mass index, and blood pressure. Atherosclerosis. 2001;156(1):157–163. doi: 10.1016/s0021-9150(00)00609-2. [DOI] [PubMed] [Google Scholar]
- 35.Landin-Wilhelmsen K, Bryman I, Wilhelmsen L. Cardiac malformations and hypertension, but not metabolic risk factors, are common in Turner syndrome. J Clin Endocrinol Metab. 2001;86(9):4166–4170. doi: 10.1210/jcem.86.9.7818. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman DW, Slone D, Rosenberg L, Miettinen OS, Shapiro S. Cigarette smoking and age at natural menopause. Am J Public Health. 1980;70(4):420–422. doi: 10.2105/ajph.70.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117(6):651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 38.Brambilla DJ, McKinlay SM. A prospective study of factors affecting age at menopause. J Clin Epidemiol. 1989;42(11):1031–1039. doi: 10.1016/0895-4356(89)90044-9. [DOI] [PubMed] [Google Scholar]
- 39.van Noord PA, Dubas JS, Dorland M, Boersma H, te Velde E. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril. 1997;68(1):95–102. doi: 10.1016/s0015-0282(97)81482-3. [DOI] [PubMed] [Google Scholar]
- 40.Cooper GS, Sandler DP, Bohlig M. Active and passive smoking and the occurrence of natural menopause. Epidemiology. 1999;10(6):771–773. [PubMed] [Google Scholar]
- 41.McKinlay SM, Bifano NL, McKinlay JB. Smoking and age at menopause in women. Ann Intern Med. 1985;103(3):350–356. doi: 10.7326/0003-4819-103-3-350. [DOI] [PubMed] [Google Scholar]
- 42.Akahoshi M, Soda M, Nakashima E, Shimaoka K, Seto S, Yano K. Effects of menopause on trends of serum cholesterol, blood pressure, and body mass index. Circulation. 1996;94(1):61–66. doi: 10.1161/01.cir.94.1.61. [DOI] [PubMed] [Google Scholar]
- 43.Hjortland MC, McNamara PM, Kannel WB. Some atherogenic concomitants of menopause: The Framingham Study. Am J Epidemiol. 1976;103(3):304–311. doi: 10.1093/oxfordjournals.aje.a112228. [DOI] [PubMed] [Google Scholar]
- 44.van Beresteijn EC, Korevaar JC, Huijbregts PC, Schouten EG, Burema J, Kok FJ. Perimenopausal increase in serum cholesterol: a 10-year longitudinal study. Am J Epidemiol. 1993;137(4):383–392. doi: 10.1093/oxfordjournals.aje.a116686. [DOI] [PubMed] [Google Scholar]
- 45.Bittner V. Perspectives on dyslipidemia and coronary heart disease in women. J Am Coll Cardiol. 2005;46(9):1628–1635. doi: 10.1016/j.jacc.2005.05.089. [DOI] [PubMed] [Google Scholar]
- 46.Do KA, Green A, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Longitudinal study of risk factors for coronary heart disease across the menopausal transition. Am J Epidemiol. 2000;151(6):584–593. doi: 10.1093/oxfordjournals.aje.a010246. [DOI] [PubMed] [Google Scholar]
- 47.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 48.Matthews KA, Wing RR, Kuller LH, Meilahn EN, Plantinga P. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch Intern Med. 1994;154(20):2349–2355. [PubMed] [Google Scholar]
- 49.Chu MC, Rath KM, Huie J, Taylor HS. Elevated basal FSH in normal cycling women is associated with unfavourable lipid levels and increased cardiovascular risk. Hum Reprod. 2003;18(8):1570–1573. doi: 10.1093/humrep/deg330. [DOI] [PubMed] [Google Scholar]
- 50.Taylor HS. Genetic vs hormonal factors in lipid metabolism in women. JAMA. 2004;291(4):424–425. doi: 10.1001/jama.291.4.424-c. author reply 425. [DOI] [PubMed] [Google Scholar]
- 51.Knauff EA, Westerveld HE, Goverde AJ, et al. Lipid profile of women with premature ovarian failure. Menopause. 2008;15(5):919–923. doi: 10.1097/gme.0b013e31816b4509. [DOI] [PubMed] [Google Scholar]
- 52.Van PL, Bakalov VK, Bondy CA. Monosomy for the X-chromosome is associated with an atherogenic lipid profile. J Clin Endocrinol Metab. 2006;91(8):2867–2870. doi: 10.1210/jc.2006-0503. [DOI] [PubMed] [Google Scholar]
- 53.Cooley M, Bakalov V, Bondy CA. Lipid profiles in women with 45,X vs 46,XX primary ovarian failure. JAMA. 2003;290(16):2127–2128. doi: 10.1001/jama.290.16.2127. [DOI] [PubMed] [Google Scholar]
- 54.Di Angelantonio E, Sarwar N, Perry P, et al. Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui Y, Blumenthal RS, Flaws JA, et al. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001;161(11):1413–1419. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 56.Bass KM, Newschaffer CJ, Klag MJ, Bush TL. Plasma lipoprotein levels as predictors of cardiovascular death in women. Arch Intern Med. 1993;153(19):2209–2216. [PubMed] [Google Scholar]
- 57.Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325(11):756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 58.Hsia J, Criqui MH, Herrington DM, et al. Women’s Health Initiative Research Group. Conjugated equine estrogens and peripheral arterial disease risk: the Women’s Health Initiative. Am Heart J. 2006;152(1):170–176. doi: 10.1016/j.ahj.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Rossouw JE, Cushman M, Greenland P, et al. Inflammatory, lipid, thrombotic, and genetic markers of coronary heart disease risk in the women’s health initiative trials of hormone therapy. Arch Intern Med. 2008;168(20):2245–2253. doi: 10.1001/archinte.168.20.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 61.Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 62.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. WHI Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289(20):2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 63.López-López R, Huerta R, Malacara JM. Age at menopause in women with type 2 diabetes mellitus. Menopause. 1999;6(2):174–178. [PubMed] [Google Scholar]
- 64.Zawadzki J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens J, Haseltine F, Merriam G, editors. Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 65.Rotterdam ESHRE/ASRM -Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Legro RS. A 27-year-old woman with a diagnosis of polycystic ovary syndrome. JAMA. 2007;297(5):509–519. doi: 10.1001/jama.297.5.509. [DOI] [PubMed] [Google Scholar]
- 67.Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. 1976;57(5):1320–1329. doi: 10.1172/JCI108400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hudecova M, Holte J, Olovsson M, Sundström Poromaa I. Long-term follow-up of patients with polycystic ovary syndrome: reproductive outcome and ovarian reserve. Hum Reprod. 2009;24(5):1176–1183. doi: 10.1093/humrep/den482. [DOI] [PubMed] [Google Scholar]
- 69.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 70.Kim TJ, Anasti JN, Flack MR, Kimzey LM, Defensor RA, Nelson LM. Routine endocrine screening for patients with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol. 1997;89(5 Pt 1):777–779. doi: 10.1016/s0029-7844(97)00077-x. [DOI] [PubMed] [Google Scholar]
- 71.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 72.Bakalov VK, Cheng C, Zhou J, Bondy CA. X-chromosome gene dosage and the risk of diabetes in Turner syndrome. J Clin Endocrinol Metab. 2009;94(9):3289–3296. doi: 10.1210/jc.2009-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaccarino V, Horwitz RI, Meehan TP, Petrillo MK, Radford MJ, Krumholz HM. Sex differences in mortality after myocardial infarction: evidence for a sex-age interaction. Arch Intern Med. 1998;158(18):2054–2062. doi: 10.1001/archinte.158.18.2054. [DOI] [PubMed] [Google Scholar]
- 74.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341(4):217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 75.Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med. 2001;134(3):173–181. doi: 10.7326/0003-4819-134-3-200102060-00007. [DOI] [PubMed] [Google Scholar]
- 76.MacIntyre K, Stewart S, Capewell S, et al. Gender and survival: a population-based study of 201,114 men and women following a first acute myocardial infarction. J Am Coll Cardiol. 2001;38(3):729–735. doi: 10.1016/s0735-1097(01)01465-6. [DOI] [PubMed] [Google Scholar]
- 77.Vaccarino V, Parsons L, Peterson ED, Rogers WJ, Kiefe CI, Canto J. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch Intern Med. 2009;169(19):1767–1774. doi: 10.1001/archinternmed.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 79.Grundy SM, Cleeman JI, Merz CN, et al. Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44(3):720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 80.Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) JAMA. 1993;269(23):3015–3023. [PubMed] [Google Scholar]
- 81.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 82.Mosca L. Guidelines for prevention of cardiovascular disease in women: a summary of recommendations. Prev Cardiol. 2007;10(Suppl 4):19–25. doi: 10.1111/j.1520-037x.2007.07255.x. [DOI] [PubMed] [Google Scholar]
- 83.Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 84.Kearney PM, Blackwell L, Collins R, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 85.Grundy SM. Should women be offered cholesterol lowering drugs to prevent cardiovascular disease? Yes BMJ. 2007;3 34(7601):982. doi: 10.1136/bmj.39202.399942.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kendrick M. Should women be offered cholesterol lowering drugs to prevent cardiovascular disease? No BMJ. 2007;334(7601):983. doi: 10.1136/bmj.39202.397488.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abramson J, Wright JM. Are lipid-lowering guidelines evidence-based? Lancet. 2007;369(9557):168–169. doi: 10.1016/S0140-6736(07)60084-1. [DOI] [PubMed] [Google Scholar]
- 88.Walsh JM, Pignone M. Drug treatment of hyperlipidemia in women. JAMA. 2004;291(18):2243–2252. doi: 10.1001/jama.291.18.2243. [DOI] [PubMed] [Google Scholar]
- 89.Kenis I, Tartakover-Matalon S, Cherepnin N, et al. Simvastatin has deleterious effects on human first trimester placental explants. Hum Reprod. 2005;20(10):2866–2872. doi: 10.1093/humrep/dei120. [DOI] [PubMed] [Google Scholar]
- 90.Edison RJ, Muenke M. Central nervous system and limb anomalies in case reports of first-trimester statin exposure. N Engl J Med. 2004;350(15):1579–1582. doi: 10.1056/NEJM200404083501524. [DOI] [PubMed] [Google Scholar]
- 91.North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause. 2010;17(2):242–255. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 92.Santen RJ, Allred DC, Ardoin SP, et al. Endocrine Society. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95(7, Suppl 1):s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Langrish JP, Mills NL, Bath LE, et al. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension. 2009;53(5):805–811. doi: 10.1161/HYPERTENSIONAHA.108.126516. [DOI] [PubMed] [Google Scholar]
- 94.Krattenmacher R. Drospirenone: pharmacology and pharmacokinetics of a unique progestogen. Contraception. 2000;62(1):29–38. doi: 10.1016/s0010-7824(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 95.Benetti-Pinto CL, Bedone AJ, Magna LA. Evaluation of serum androgen levels in women with premature ovarian failure. Fertil Steril. 2005;83(2):508–510. doi: 10.1016/j.fertnstert.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 96.Kalantaridou SN, Calis KA, Vanderhoof VH, et al. Testosterone deficiency in young women with 46,XX spontaneous premature ovarian failure. Fertil Steril. 2006;86(5):1475–1482. doi: 10.1016/j.fertnstert.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 97.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24(3):313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 98.Flöter A, Nathorst-Böös J, Carlström K, von Schoultz B. Serum lipids in oophorectomized women during estrogen and testosterone replacement therapy. Maturitas. 2004;47(2):123–129. doi: 10.1016/s0378-5122(03)00246-9. [DOI] [PubMed] [Google Scholar]
- 99.Zang H, Carlström K, Arner P, Hirschberg AL. Effects of treatment with testosterone alone or in combination with estrogen on insulin sensitivity in postmenopausal women. Fertil Steril. 2006;86(1):136–144. doi: 10.1016/j.fertnstert.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 100.Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab. 1998;83(12):4420–4425. doi: 10.1210/jcem.83.12.5333. [DOI] [PubMed] [Google Scholar]
- 101.Kalantaridou SN, Calis KA, Mazer NA, Godoy H, Nelson LM. A pilot study of an investigational testosterone transdermal patch system in young women with spontaneous premature ovarian failure. J Clin Endocrinol Metab. 2005;90(12):6549–6552. doi: 10.1210/jc.2005-0692. [DOI] [PubMed] [Google Scholar]
- 102.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 103.Bondy C, Bakalov VK, Lange ED, Ceniceros I. Deficient medical care for adults with the Turner syndrome. Ann Intern Med. 2006;145(11):866–867. doi: 10.7326/0003-4819-145-11-200612050-00020. [DOI] [PubMed] [Google Scholar]
- 104.Gravholt CH. Medical problems of adult Turner’s syndrome. Horm Res. 2001;56(Suppl 1):44–50. doi: 10.1159/000048134. [DOI] [PubMed] [Google Scholar]
- 105.Wiggins DA, Main E. Outcomes of pregnancies achieved by donor egg in vitro fertilization—a comparison with standard in vitro fertilization pregnancies. Am J Obstet Gynecol. 2005;192(6):2002–2006. doi: 10.1016/j.ajog.2005.02.059. discussion 2006–2008. [DOI] [PubMed] [Google Scholar]
- 106.Söderström-Anttila V, Tiitinen A, Foudila T, Hovatta O. Obstetric and perinatal outcome after oocyte donation: comparison with in-vitro fertilization pregnancies. Hum Reprod. 1998;13(2):483–490. doi: 10.1093/humrep/13.2.483. [DOI] [PubMed] [Google Scholar]
- 107.Pados G, Camus M, Van Steirteghem A, Bonduelle M, Devroey P. The evolution and outcome of pregnancies from oocyte donation. Hum Reprod. 1994;9(3):538–542. doi: 10.1093/oxfordjournals.humrep.a138541. [DOI] [PubMed] [Google Scholar]
- 108.Practice Committee of American Society for Reproductive Medicine. Increased maternal cardiovascular mortality associated with pregnancy in women with Turner syndrome. Fertil Steril. 2008;90(5, Suppl):S185–S186. doi: 10.1016/j.fertnstert.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 109.Hiratzka LF, Bakris GL, Beckman JA, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55(14):e27–e129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 110.National Heart, Lung and Blood Institute. [Accessed November 3, 2010];National Institutes of Health. Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Risk Assessment Tool for Estimating Your 10-year Risk of Having a Heart Attack. Available at: http://hp2010.nhlbihin.net/atpiii/calculator.asp.