Abstract
Objectives
Electroencephalography (EEG) offers psychophysiologic tools to improve sensitivity for detecting objective effects in complementary and alternative medicine. This current investigation extended prior clinical research studies to evaluate effects of one of two different homeopathic remedies on resting EEG cordance after an olfactory activation protocol on healthy young adults with remedy-relevant, self-perceived characteristics.
Methods
Ninety-seven (7) young adults (N=97, mean age 19 years, 55% women) with good self-rated global health and screened for homeopathic constitutional types consistent with one of two remedies (either Sulphur or Pulsatilla) underwent three weekly laboratory sessions. At each visit, subjects had 5-minute resting, eyes-closed EEG recordings before and after a placebo-controlled olfactory activation task with their constitutionally relevant verum remedy. One remedy potency (6c, 12c, or 30c) used per week, was presented in a randomized order over the 3 sessions. Prefrontal resting EEG cordance values at Fp1 and Fp2 were computed from artifact-free 2-minute EEG samples from the presniffing and postsniffing rest periods. Cordance derives from an algorithm that incorporates absolute and relative EEG values.
Results
The data showed significant two-way oscillatory interactions of remedy by time for ß, α, θ, and δ cordance, controlling for gender and chemical sensitivity.
Conclusions
EEG cordance provided a minimally invasive technique for assessing objective nonlinear physiologic effects of two different homeopathic remedies salient to the individuals who received them. Time factors modulated the direction of effects. Given previous evidence of correlations between cordance and single-photon emission computed tomography, these findings encourage additional neuroimaging research on nonlinear psychophysiologic effects of specific homeopathic remedies.
Introduction
Psychophysiologic tools such as electroencephalography (EEG) can improve the objective assessment of a variety of modalities in the field of complementary and alternative medicine (CAM).1–5 The use of CAM therapies worldwide, involving both self-care and practitioner services, is extensive and growing.6–8 Historically, CAM clinicians and researchers often rely on patient self-reports rather than on laboratory measures for outcomes evaluation. However, an emerging challenge in CAM research is the need for developing sensitive biomarkers of early response to offset the small sample sizes, short study durations, relative insensitivity of self-report ratings, sometimes large placebo effects, and resultant low statistical power of many CAM study designs. At the same time, the ever-increasing use of CAM therapies by the public and the paucity of evidence-based CAM treatments highlights the need to identify objective measures for use in CAM research. One promising such biomarker is EEG cordance, an algorithm-based derivative of absolute and relative spectral EEG data.9 Empirically, EEG cordance offers a minimally invasive, radiation-free, more-accessible correlate of neuroimaging tests—such as single-photon emission computed tomography (SPECT), positron emission tomography (PET), or functional magnetic resonance imaging (fMRI)—that lends itself to repeated measurement over short periods of time.10–13
Leuchter, et al. at the University of California pioneered development of EEG cordance as a minimally invasive tool for distinguishing verum medication responders, placebo responders, and nonresponders to both verum and placebo in antidepressant drug trials.14 Their articles on nonclinical samples, neuropsychiatric patients, and persons with major depression suggest that the direction and magnitude of changes in EEG cordance can differentiate not only eventual responders from nonresponders but also verum responders from placebo responders early in treatment, even when subjective and/or observer-rated clinical outcomes appear to be indistinguishable.11
Within CAM research, Bell et al. adapted the EEG cordance approach successfully to a double-blinded placebo-controlled clinical trial of individualized homeopathy in persons with fibromyalgia.1 Homeopathy is a controversial 200-year-old whole system of CAM that originated in Germany.15 Homeopathy's reliance on medications (termed remedies) prepared from natural animal, mineral, or plant sources by a process of serial dilution and succussion (shaking) has engendered extensive debate. Skeptics claim that many homeopathic remedies are diluted past Avogadro's number of molecules and thus could act “only” as placebos.16 Proponents point to a body of basic-science, animal, preclinical, and clinical evidence that show empirically unique properties of remedies that differ from remedy-free controls (placebos).17,18 Recent studies suggest the possibilities of (1) measurable, persistent nanoparticles of source materials in remedies previously believed to contain no remaining source molecules19 and (2) nonlinear dose–response patterns to ultradilute doses20–24 (e.g., with hormetic, adaptive reversal in the direction of host responses, compared with higher dose toxicologic responses). Nanoparticles, which are very reactive, can be toxic at relatively higher doses25 but also elicit salutary hormetic responses at low doses.26
The homeopathy-fibromyalgia study1 found that the initial treatment EEG cordance response to sniffing verum homeopathic remedies distinguished patients who subsequently showed an excellent clinical response from patients who had limited clinical benefits from verum and patients who received placebo after 3 months of treatment. Olfactory administration is a less widely used, but nonetheless accepted, mode of treatment.27 The purpose of the present study was to extend the EEG cordance method to other aspects of homeopathic remedy research (i.e., the interaction of a phase I–like drug development study design in homeopathy—known as a proving or pathogenetic trial28,29—with individual differences in the neurophysiology of different constitutional types over time.
In pathogenetic trial research28,29 relatively healthy human subjects take test doses of a remedy under blinded conditions and record their observations of symptoms for a period of 1–30 days.30 The state dependency concept in homeopathy is that a substance that can cause symptoms in a healthy person can cure them in a sick person who has a similar presenting clinical pattern. However, not everyone responds to a given remedy; the “ideal” responder is the individual whose complete pattern of traits (constitutional type) and states match what the remedy could cause.22,31 Previous research in this laboratory showed complex interactions between trait and state variables of the individual exposed to a given remedy.32 Moreover, animal studies have shown that the direction of response to a given remedy can be biphasic, depending on the state of the recipient at the time of administration.33
The present study tested the hypotheses that: (1) resting EEG cordance changes in human subjects, after sniffing a specific homeopathic remedy,34 would fluctuate over sessions, modified by recent exposure history to the agent35–37; and (2) the EEG cordance patterns in two different groups of people, each receiving a constititutionally salient homeopathic remedy, would differ from each other, even after controlling for nonhomeopathic baseline subject characteristics.1,38
Materials and Methods
Subjects
Ninety-seven (97) college student volunteers, between ages 18 and 30, enrolled in the introductory psychology course at the University of Arizona and screened for inclusion/exclusion criteria, were deemed eligible to participate. Eligibility criteria included good global health (rating of 3 of 5 on a single-item screening question39) and either a score ≥25 for the remedy Sulphur on the Homeopathic Constitutional Type Questionnaire (CTQ),40 along with a score ≤21 on the CTQ for the remedy Pulsatilla or a score ≥24 for the remedy Pulsatilla together with a score ≤24 for the remedy Sulphur. The CTQ subscales for each remedy include 8 items that are self-rated on a 5-point Likert scale.41
In addition, subjects also completed baseline demographics and a validated trait questionnaire that covered individual differences on perceived sensitivity to low levels of environmental chemicals (Chemical Intolerance Index, CII).42 Previous research has shown that the CII identifies individuals who exhibit sensitization or oscillation of EEG responses to repeated exposures to the same agent over a several-week period.43–45 Textbooks in homeopathy specifically identify persons with chemical sensitivity as “universal provers” with heightened reactivity to homeopathic remedies in general30,46 and who are often clinically responsive to the class of remedies drawn from carbon-based sources, such as hydrocarbons or simple organic acids.47
Exclusion criteria were pregnancy or planning to become pregnant, major psychiatric or serious medical conditions, chronic use of medications other than contraceptives, history of anaphylactic shock, asthma, and/or migraine headaches. Study participants were paid $90 for completing the study. Both the screening process and the laboratory study were reviewed and approved by the institutional review board of The University of Arizona.
Study design
The design was a repeated-measures laboratory-based study in which each subject was tested in the laboratory at the same time of day once per week for 3 weeks. Participants chose the day and time of their visits according to their schedules. Any subject who missed a session was scheduled for follow-up immediately, and make-up sessions were scheduled at the same time of day. Study recruitment and implementation took place during the school year.
In this component of the study, each weekly visit involved only one homeopathic dilution (6c, 12c, or 30c, for which c potencies are diluted at a ratio of 1/100 parts over 6, 12, or 30 steps and then succussed (shaken vigorously after each dilution step). An additional aspect of the larger study involved evaluation of within-session transient EEG α-band effects from different numbers of succussions (stirred, 20, 40, or 100 succussions) performed on the verum and placebo sniff dilutions (detailed methods on succussion levels and sniff test results have been reported elsewhere).48 The contents were one of two well-documented, widely used homeopathic remedies, Sulphur (mineral) or Pulsatilla (plant), and associated control solvents (water or a water–ethanol mixture). The order of the dilutions used in a session was randomized, using computer-generated sequences over the 3 weeks for each subject.
The design was single-blinded for the subjects from the perspective of the identity of the remedy studied, but double-blinded from the perspective of the order of the dilutions from week to week and order of the presentation of verum and control solvent vials during the sniffing tasks for each week's session. As part of the olfactory activation component of the larger study, the constitutionally salient test remedy and two types of control solvents (distilled water or distilled water–alcohol) were each presented 32 times per session (at four different succussion levels each within each session), for a total of 96 sniffs between the resting EEG segments at the beginning and end of each weekly session. Results of the placebo-controlled olfactory activation component of the larger study that examined specific transient relative EEG α-effects from 2-second sniffs of remedy and placebos at different succussion levels were reported elsewhere in detail.48,* The data analysis and current article focus only on the pre- and postresting EEG segments as functions of time (week 1, 2, 3) and dilution (6c, 12c, 30c).
Laboratory procedure
Both research staff members and subjects were blinded to the contents of the vials during the laboratory procedures. The remedies and solvents were purchased from a U.S. Food and Drug Administration–regulated homeopathic pharmacy with experience in preparing materials for research purposes (Hahnemann Laboratories, San Rafael, CA). Although the pharmacy prepared the original remedy and water–ethanol solutions in accordance with the pharmacy's usual procedures, using 95% ethanol in a distilled water solvent, the test solutions that subjects sniffed were markedly diluted in distilled water in a final local preparation step (0.5 mL of test solution was placed in a cup with 150 mL of distilled water and stirred, followed by pouring 10 mL of this final water-diluted solution into the sniff vials). Subjects were instructed not to consume alcohol, caffeinated beverages, and/or tobacco for 6 hours prior to the recording. Subjects were also instructed not to wear strong perfumes or lotions the day of the recording.
Laboratory EEG recordings were performed using Compumedics E-Series equipment (El Paso, TX) and a 20 channel Quik-Cap. Electrodes on the Quik-Cap are situated according to the International 10-20 System. Recordings include 19 unipolar EEG channels referenced to contralateral mastoids, bilateral electro-oculograms (EOG), a two-lead electrocardiogram (bilateral subclavicle electrode placement), and a nasal pressure flow signal (Salter Labs Nasal EtCO2 Cannula). Electrode impedance levels were kept below 5 KΩ. Equipment settings for data acquisition included an EEG sampling rate of 512 Hz and a high pass filter set at 0.50 Hz. A 60-cycle notch filter was used to eliminate ambient electrical noise.
After hookup of the equipment and completion of state questionnaires, each laboratory visit included a resting 5-minute, eyes-closed EEG recording performed before and after the sniffing test periods during which the remedy and placebos were administered to the subject. In the sniffing activation task period, subjects were asked to take a series of two-second sniffs from the randomized and double-blinded vials of test liquids containing either remedy or placebos. An Austin Healthmate Junior air filter with 4-stage filtration (including prefilters for medium and large particles, an activated carbon/zeolite filter for volatile organic chemicals, and a medical grade high-energy particulate arresting filter) used on the low setting throughout each session to clear the air of trace residual odors. Only the pre- and postsession resting recordings were used for the EEG cordance analyses reported in the present article. As noted above, relative EEG α-frequency band findings from the sniffs per se are reported in other articles.48*
Cordance computation
A trained technician, who was blinded to group membership and outcomes, selected 2-minute artifact-free EEG segments (e.g., excluding epochs with eyeblink and muscle-movement artifacts defined as amplitudes>50 mV) from the 5-minute pre- and postresting eyes-closed segments of the laboratory procedure. Cordance was computed using a previously published algorithm with software available from a website of the University of California, Los Angeles (UCLA) Laboratory of Brain, Behavior, and Pharmacology, Los Angeles, CA (www.lbbp.org/lbbp).12,14,49 To prepare the data for cordance analysis, electrodes were first remontaged offline to bipolar channel pairs of nearest-neighbor electrodes that shared a common electrode. For example, Fp1 was referenced to Fp2, F3, and F7; and Fp2 was referenced to Fp1, F4, and F8, followed by averaging of the absolute power values for the bipolar pairs. Then, the EEG segments were subjected to fast Fourier transformation, setting the frequency range for EEG band frequencies as δ (0.5–4 Hz), θ (4–8 Hz), α (8–12 Hz), and ß (12–20 Hz).
Using the UCLA cordance software, EEG power values were computed for each band for absolute (amount of power in the band at a given electrode, in mV2) and relative power (percentage of power in the band relative to the total power in the overall frequency spectrum sampled by the cordance software (i.e., 0.5–20 Hz). Next, absolute and relative power values for the four frequency bands were normalized across electrode sites using a z-transformation statistic at each site. Finally, cordance was quantified by summing the z-scores for normalized absolute and relative power at each electrode site. The Fp1 and Fp2 sites were chosen for analysis in this article because of previous cordance study findings at these prefrontal sites in both antidepressant50–53 and homeopathy1 research.
Statistical analysis
Baseline comparisons
Demographic and baseline variables were compared by remedy group using chi-square analysis for categorical variables (gender and CII group) and t-tests for continuous variables (age, CTQ score, and baseline cordance value). To determine CII group in order to control for this trait, if necessary, participants were divided into high or low chemical sensitivity on the CII. High-sensitivity individuals were in the upper tercile (12 or higher of 25) and low-sensitivity individuals were the bottom two thirds of the sample. The cutoff was determined using this sample but was similar to cutoffs in previously published samples.42,44,45 The baseline cordance value was the first 5-minute cordance value obtained at visit 1 prior to performing the first sniff task or exposure to any remedy. Linear mixed models were also used to examine whether demographic characteristics (age, gender, CII group) and site (Fp1, Fp2) were associated with differences in baseline cordance values.
Cordance analysis
Cordance difference scores were created by subtracting the presession resting cordance value from the postsession resting cordance value. Negative change scores indicated a decrease in cordance following the session. A linear mixed-model regression analysis was performed, using SAS Proc Mixed, with person as the random effect. The regression equations used the subject's cordance change score at each visit as the dependent variable. Each model included gender, CII group, and baseline cordance value at the beginning of each weekly session.54 Main effects in the model were for the week of the study (visits 1, 2, and 3), the remedy received (Sulphur, Pulsatilla), the dilution received (6c, 12c, 30c), and the prefrontal electrode site (Fp1, Fp2). Interactions between week and remedy; and electrode site, week, and remedy were included. Stata 10.1 and SAS 9.2 were used for all data reduction and analyses. It was decided to report on the full set of comparisons performed for all bands, but not to apply the very conservative Bonferroni correction on probability levels for judging statistical significance. While protecting against a Type I error, a Bonferroni or multiple comparisons correction could lead to a Type II error at this exploratory stage of the research.55
Results
Fifty-two (52) constitutional-type Sulphur subjects received Sulphur, and 45 constitutional-type Pulsatilla subjects received Pulsatilla. Table 1 summarizes the baseline descriptive data for each treatment group. There were significantly more males in the Sulphur group than in the Pulsatilla group, and chemical intolerance scores were higher in the Pulsatilla group. These differences were anticipated. Homeopathic practice theory predicts the possibility of more male Sulphur types and more female Pulsatilla types.56,57 Moreover, many studies have shown that women score higher than men on scales for chemical sensitivity.58 There were no baseline differences in θ, δ, or ß–cordance bands, but the α-baseline cordance value was significantly higher in the Pulsatilla group than in the Sulphur group. Table 1 shows the values by frequency band.
Table 1.
Baseline Comparisons Between Remedy Groups: Means and Standard Deviations
| Variable | Sulphur group (n=52) | Pulsatilla group (n=45) |
|---|---|---|
| Age | 19.2±2.0 | 19.0±0.98 |
| Gender (% female) | 31% | 82%** |
| CII—Chemical intolerance/sensitivity | 9.5±0.5 | 11.0±0.6* |
| CTQ—Sulphur | 27.3±0.3 | 22.4±0.2** |
| CTQ—Pulsatilla | 18.7±0.3 | 25.8±0.3** |
| Baseline δ | 2.73±0.1 | 2.65±0.1 |
| Baseline θ | −2.27±0.2 | −1.94±0.2 |
| Baseline α | −2.87±0.01 | −2.65±0.1* |
| Baseline ß | −2.56±0.2 | −2.30±0.2 |
p<0.05; **p<0.001.
CII, Chemical Intolerance Index; CTQ=Constitutional Type Questionnaire.
In the models examining whether demographic and site variables account for differences in the baseline cordance values, only one model had a statistically significant difference. CII group was statistically significant in the baseline ß-cordance model (F(1, 93)=7.99; p=0.0058). Baseline cordance was higher for persons in the high-CII group (Mean±standard error [SE] –1.99±0.25) than the low-CII group (Mean±SE –2.86±0.17). To determine whether this difference varied by remedy group, an additional model including remedy and the interaction between remedy and CII group was conducted. In this model, CII group remained significant; however, remedy and the interaction were nonsignificant suggesting this difference is the result of CII group alone. Given the baseline differences for gender and CII, these variables are included in the cordance difference score models.
Cordance difference score analysis
Cordance changes were significantly different as a function of time (the week of the laboratory visit) and the remedy received for each of the four frequency bands (Table 2). There were no statistically significant differences by dilution level, CII, or significant lateralized differences between Fp1 and Fp2. Baseline cordance value was statistically significant in the models; however, its effect on cordance change score was controlled for, when interpreting the visit by remedy interaction. Only one band, θ, had a significant main effect for gender; males were more likely to increase (Mean±SE 0.17±0.11) and females to decrease (Mean±SE –0.20±0.10) θ band cordance. Cordance change scores by remedy over time and pairwise comparisons of means are presented in Table 3. Within the Pulsatilla group, δ-cordance increased following the olfactory activation task at visit 3 versus visit 2 (p<0.02) and, to a lesser extent, for visit 1 (p=0.07). In contrast, α-cordance decreased significantly for the Pulsatilla group at visit 3 versus both visits 1 and 2. In the Sulphur group, θ-cordance increased at visit 3 versus visit 2 and differed from Pulsatilla at visit 2.
Table 2.
Linear Mixed Models Results by Cordance Band: F-statistics and Significance for Main Effects and Interactions
| Independent Variables | df | δ | θ | α | ß |
|---|---|---|---|---|---|
| Visit | 2, 442 | 0.77 | 0.24 | 3.56* | 0.28 |
| Remedy | 1, 442 | 0.03 | 0.56 | 0.32 | 3.90* |
| Dilution | 2, 442 | 1.01 | 0.31 | 1.81 | 1.50 |
| Gender | 1, 442 | 0.46 | 4.77* | 2.01 | 0.57 |
| CII | 1, 442 | 1.96 | 0.55 | 0.22 | 0.66 |
| Electrode site | 1, 442 | 1.28 | 0.09 | 0.26 | 0.00 |
| Baseline | 1, 442 | 370.09*** | 230.09*** | 173.68*** | 244.1*** |
| Visit X remedy | 2, 442 | 3.13* | 3.84* | 4.98** | 6.77** |
| Visit X remedy X site | 5, 442 | 1.50 | 0.33 | 1.31 | 1.26 |
p<0.05; **p<0.01; ***p<0.001.
df, degrees of freedom; CII, Chemical Intolerance Index.
Table 3.
Means and Standard Error Values of Post–Pre Change in EEG Cordance for Each Frequency Band by Remedy Group
| |
|
Remedy group |
|
|---|---|---|---|
| Band | Visit | Sulphur | Pulsatilla |
| δ | 1 | 0.15 (0.10) | 0.15 (0.11)c |
| 2 | 0.23 (0.10) | 0.10 (0.10)b | |
| 3 | 0.13 (0.11) | 0.32 (0.11)a | |
| θ | 1 | −0.08 (0.15) | 0.01 (0.17) |
| 2 | −0.27 (0.16)a,c | 0.21 (0.17)d | |
| 3 | 0.08 (0.16)b | −0.02 (0.17) | |
| α | 1 | −0.15 (0.05) | −0.07 (0.05)b |
| 2 | −0.18 (0.05) | −0.07 (0.05)b | |
| 3 | −0.15 (0.05) | −0.23 (0.05)a | |
| ß | 1 | 0.39 (0.17)b,d | −0.37 (0.18)b,e |
| 2 | −0.04 (0.17)a | −0.06 (0.18)a | |
| 3 | 0.22 (0.18)d | −0.35 (0.18)c,e | |
δ: Pulsatilla a>bp<0.02; a>cp<0.07.
θ: Sulphur a>bp<0.02; Sulphur versus Pulsatilla c>dp<0.05.
α: Pulsatilla a<bp<0.0005.
ß: Sulphur a<bp<0.04; Pulsatilla a>bp<0.04; a>cp<0.06; Sulphur versus Pulsatilla d>ep<0.03.
EEG, electroencephalographic.
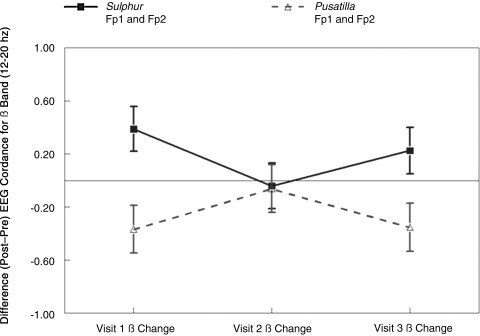
Although the interaction patterns of remedy by time were significant for all bands, the strongest evidence for oscillation was present in the ß-band (Fig. 1). In the ß-band, both remedies showed within-remedy oscillations over time, and the two remedies differed from one another at visit 1 and visit 3 on pairwise comparisons (Table 3). To summarize, within the Pulsatilla group, ß-cordance decreased significantly following the olfactory activation task at visits 1 and 3 versus no change in cordance at the second visit. This difference was statistically significant between visits 1 and 2, with a trend for significance between visits 2 and 3 (Table 3). The opposite pattern was observed in the Sulphur group, with significant increases in cordance following the session at visits 1 and 3 versus no pre–post-session change in cordance for the second visit. This difference was statistically significant between visits 1 and 2. In addition, between group differences in response to each remedy were observed at visits 1 and 3, with the Sulphur group increasing in cordance at these visits, while the Pulsatilla group decreased in cordance at these visits. Both groups showed no change in cordance at visit 2. The focus was on on change in resting ß-cordance because of Leuchter et al.'s earlier articles13, 59 (1) suggesting that ß-1 discordance was associated with lower regional brain perfusion on SPECT neuroimaging scans, and (2) their proposal that an activation task (in this case, repeated sniffing and olfactory stimulation) might have brought out differential blood flow in specific areas during a session that carried over into the postsniff task, end-of-session rest period.
FIG. 1.
Patterns of pre–post change in resting ß-electroencephalographic (EEG) cordance by visit.
Discussion
The data indicate that changes in resting EEG cordance from pre- to postsniffing (a central nervous system [CNS] activation task) differ from week to week, as a function of the constitutionally salient homeopathic remedy (Sulphur or Pulsatilla), even after controlling for baseline remedy group differences in gender and chemical sensitivity. The findings lend additional support to the growing literature demonstrating the ability to distinguish one homeopathic remedy from another, even at doses believed to be diluted beyond Avogadro's number, in both basic science18,19,60–62 and human studies.28,29,32
The remedy received was confounded intentionally by the subject-selection process, which was designed to mirror homeopathic practice theory,15 by preselecting and matching potentially reactive subjects to their respective remedies. Thus, the most conservative conclusion is that people who are Sulphur types and receive Sulphur show a different pattern of changes in their cordance-related prefrontal brain activity (e.g., perfusion) over time than do people who are Pulsatilla types and receive Pulsatilla. The findings persist even after controlling for gender and self-rated chemical sensitivity/intolerance. From a methodological perspective, an important finding of the present study is that cordance may be a useful biomarker of early response to homeopathic remedy administration in clinical testing.
The ß-cordance change pattern is nonlinear and oscillatory from session to session over time in opposite directions for the two remedies. A prior clinical trial study showed oscillatory effects of another homeopathically prepared remedy (dust mite 30c) on several different, non-EEG outcome measures assessed every 2 weeks, including both self-report ratings and lung function in patients with asthma.63,64 A recent review of basic and preclinical research on homeopathic remedies also concluded that homeopathic remedies produce nonlinear, bidirectional effects over time that appear to be dependent, in part, on subtle differences in the state of the recipient organisms at the time of administration.65 Time factors and prior history also play roles in the direction of effects with homeopathic remedies in animal and cellular models.22,33,37,66,67 Similarly, seasonal variants (e.g., pollen, temperature, light cycle, and barometric pressures), undocumented differences in menstrual cycles for female participants, or external stress levels for individual subjects over time (e.g., examination-related stress periods in students) could have could each also have confounded the current findings. However, none of those confounding factors was systematically built into the design of this exploratory study. About 4 subjects, with a maximum of 6 subjects, were run on a typical laboratory testing day, making it unlikely that specific environmental factors uniformly altered responses of all subjects in a given remedy type to generate an overall cohort effect. All sessions occurred at the same time of day for a given individual, reducing the likelihood that ultradian cycles could explain the data. Of course, to be more certain that one or more confounding factors do not explain these results, future replication studies can specifically examine the impact of each of these additional potential environmental confounding factors on EEG cordance changes. Nonetheless, the present data raise the possibility of adding psychophysiologic EEG measures to self-reported symptom patterns in distinguishing effects of one homeopathic remedy from another in future homeopathic pathogenetic trials.2,28,29,32
The phenomenon of time-dependent sensitization (TDS) may also be relevant to the current observations.34 TDS is the progressive amplification of host response by intermittent repeated exposures to an initially low-dose agent or stressor.68–70 In animal preparations pushed to their physiologic limits, the direction of the TDS response becomes nonlinear and oscillates up and down over successive weeks.35,36,71–73 Sensitization studies in animals and in cell cultures have shown that the prior exposure history of the individual to a given agent or a cross-sensitizing agent modulates the direction of effects during the next exposure.31,37,70,71,73–77 TDS adds the factor of time to explicate some variability of preclinical and clinical remedy effects, in addition to the nonlinear dose–response curves at low doses postulated for homeopathy on the basis of hormesis.24,78,79
Budgetary limitations in this exploratory study prevented a full between-group design in which each remedy type would have received both Sulphur and Pulsatilla or placebo controls. Thus, the current authors do not know if the findings are dependent specifically on the remedy received or on the basis of the remedy interacting with the “right” constitutional type person. The current research team examined this individual difference question in a separate exploratory study on sleep and mood effects of two other homeopathic remedies and personality types in relatively healthy young adults and found complex interactions between the type of person who received a specific remedy and the remedy given.32 Whether or not the current design would yield similar person-type by remedy-received interactions remains a testable but unexamined question.
Conclusions
In conclusion, the current findings suggest that repeated sessions involving short-term olfactory administration of homeopathic remedies can change the resting EEG cordance response across all frequency bands (δ, θ, α, and ß) with time. The ß-frequency band shows a particularly robust oscillatory pattern. Remedy-type effects interact with time to produce significant differences that imply a nonlinear variability in the changes over a macro time scale of several weeks. The data extend this research team's previous findings that early changes in EEG cordance were able to distinguish subsequent verum responders to homeopathy from verum nonresponders and from patients who received placebo.1
These data merit replication and extension in follow up studies of homeopathy using both EEG cordance analyses and eventually, neuroimaging techniques such as fMRI. fMRI has already been useful and sensitive for spatial resolution of CNS effects of other types of CAM modalities such as verum versus sham acupuncture.3,4,80 EEG cordance, as well as spectral EEG analyses per se, could provide a valuable objective tool for additional research on the temporal and dynamic nature of verum versus placebo responses to homeopathy81 and assist in more rigorous resolution of some of the long-standing disputes about the nature of remedy responses.
Footnotes
Bell IR, Brooks AJ, Howerter A, et al. Acute electroencephalographic effects from repeated olfactory administration of homeopathic remedies in individuals with self-reported chemical sensitivity. Submitted for publication 2011.
Acknowledgments
This study was supported by the National Institutes of Health/National Center for Complementary and Alternative Medicine (grant numbers R21 AT003212, K24 AT000057). The authors thank Dr. David Lee for supervising the preparation of the test vials and Kaya Belknap, Michael Biuso, Sarah David, Jeanette Garcia, Erica Morey, and Alivia Wieseler for their assistance in the collection and preparation of data.
Disclosure Statement
Drs. Bell and Brooks are consultants for Standard Homeopathic/Hyland's Inc., a homeopathic manufacturer whose products were not used in this study. Standard Homeopathic/Hyland's Inc. did not provide any funding for this investigation.
References
- 1.Bell IR. Lewis DAI. Schwartz GE, et al. Electroencephalographic cordance patterns distinguish exceptional clinical responders with fibromyalgia to individualized homeopathic medicines. J Altern Complement Med. 2004;10:285–299. doi: 10.1089/107555304323062275. [DOI] [PubMed] [Google Scholar]
- 2.Bell IR. Howerter A. Jackson N, et al. Effects of homeopathic medicines on polysomnographic sleep of young adults with histories of coffee-related insomnia. Sleep Med. 2011;12:505–511. doi: 10.1016/j.sleep.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napadow V. Dhond RP. Purdon P, et al. Correlating acupuncture FMRI in the human brainstem with heart rate variability. Conf Proc IEEE Eng Med Biol Soc; 2005. pp. 4496–4499. [DOI] [PubMed] [Google Scholar]
- 4.Napadow V. Dhond R. Park K, et al. Time-variant fMRI activity in the brainstem and higher structures in response to acupuncture. Neuroimage. 2009;47:289–301. doi: 10.1016/j.neuroimage.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra N. Muraleedharan KC. Paranjpe AS, et al. An exploratory study on scientific investigations in homeopathy using medical analyzer. J Altern Complement Med. 2011;17:705–710. doi: 10.1089/acm.2010.0334. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Arye E. Karkabi S. Shapira C, et al. Complementary medicine in the primary care setting: Results of a survey of gender and cultural patterns in Israel. Gend Med. 2009;6:384–397. doi: 10.1016/j.genm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Bucker B. Groenewold M. Schoefer Y. Schafer T. The use of complementary alternative medicine (CAM) in 1,001 German adults: Results of a population-based telephone survey. Gesundheitswesen. 2008;70:e29–e36. doi: 10.1055/s-2008-1081505. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PM. Bloom B. Nahin RL. National Health Statistics Reports. Vol. 12. Hyattsville, MD: U.S. Department of Health and Human Services; 2008. Complementary and Alternative Medicine Use Among Adults and Children: United States 2007. [PubMed] [Google Scholar]
- 9.Hunter AM. Muthen BO. Cook IA. Leuchter AF. Antidepressant response trajectories and quantitative electroencephalography (qEEG) biomarkers in major depressive disorder. J Psychiatr Res. 2010;44:90–98. doi: 10.1016/j.jpsychires.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leuchter AF. Cook IA. DeBrota DJ, et al. Changes in brain function during administration of venlafaxine or placebo to normal subjects. Clin EEG Neurosci. 2008;39:175–181. doi: 10.1177/155005940803900405. [DOI] [PubMed] [Google Scholar]
- 11.Hunter AM. Cook IA. Leuchter AF. The promise of the quantitative electroencephalogram as a predictor of antidepressant treatment outcomes in major depressive disorder. Psychiatr Clin North Am. 2007;30:105–124. doi: 10.1016/j.psc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Cook IA. Leuchter AF. Morgan M, et al. Early changes in prefrontal activity characterize clinical responders to antidepressants. Neuropsychopharmacology. 2002;27:120–131. doi: 10.1016/S0893-133X(02)00294-4. [DOI] [PubMed] [Google Scholar]
- 13.Leuchter AF. Cook IA. Mena I, et al. Assessment of cerebral perfusion using quantitative EEG cordance. Psychiatry Res. 1994;55:141–152. doi: 10.1016/0925-4927(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 14.Leuchter AF. Cook IA. Witte EA, et al. Changes in brain function of depressed subjects during treatment with placebo. Am J Psychiatry. 2002;159:122–129. doi: 10.1176/appi.ajp.159.1.122. [DOI] [PubMed] [Google Scholar]
- 15.Owen D. Principles and Practice of Homeopathy: The Therapeutic and Healing Process. London, UK: Churchill Livingstone; 2007. [Google Scholar]
- 16.Lancet. The end of homeopathy. Lancet. 2005;366:690. [Google Scholar]
- 17.Witt C. Albrecht H. New Directions in Homeopathy Research. Essen, Germany: KVC Verlag; 2009. [Google Scholar]
- 18.Rey L. Thermoluminescence of ultra-high dilutions of lithium chloride and sodium chloride. Physica A: Statistical mechanics and its applications. 2003;323:67–74. [Google Scholar]
- 19.Chikramane PS. Suresh AK. Bellare JR. Kane SG. Extreme homeopathic dilutions retain starting materials: A nanoparticulate perspective. Homeopathy. 2010;99:231–242. doi: 10.1016/j.homp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Calabrese EJ. Jonas WB. Evaluating homeopathic drugs within a biomedical framework. Hum Exp Toxicol. 2010;29:545–549. doi: 10.1177/0960327110369775. [DOI] [PubMed] [Google Scholar]
- 21.Fisher P. Does homeopathy have anything to contribute to hormesis? Hum Exp Toxicol. 2010;29:555–560. doi: 10.1177/0960327110369776. [DOI] [PubMed] [Google Scholar]
- 22.Van Wijk R. Wiegant FA. Postconditioning hormesis and the homeopathic similia principle: Molecular aspects. Hum Exp Toxicol. 2010;29:561–655. doi: 10.1177/0960327110369860. [DOI] [PubMed] [Google Scholar]
- 23.Wiegant F. Van Wijk R. The similia principle: Results obtained in a cellular model system. Homeopathy. 2010;99:3–14. doi: 10.1016/j.homp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Ives JA. Jonas WB. Frye JC. Do serial dilutions really dilute? Homeopathy. 2010;99:229–230. doi: 10.1016/j.homp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Buzea C. Pacheco II. Robbie K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases. 2007;2:MR17–MR71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 26.Iavicoli I. Calabrese EJ. Nascarella MA. Exposure to nanoparticles and hormesis. Dose Response. 2010;8:501–517. doi: 10.2203/dose-response.10-016.Iavicoli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahnemann S. Organon of the Medical Art. 6th. Redmond, WA: Birdcage Books; 1843. [Google Scholar]
- 28.Möllinger H. Schneider R. Walach H. Homeopathic pathogenetic trials produce specific symptoms different from placebo. Forsch Komplementmed. 2009;16:105–110. doi: 10.1159/000209386. [DOI] [PubMed] [Google Scholar]
- 29.Walach H. Möllinger H. Sherr J. Schneider R. Homeopathic pathogenetic trials produce more specific than non-specific symptoms: Results from two double-blind placebo controlled trials. J Psychopharmacol. 2008;22:543–552. doi: 10.1177/0269881108091259. [DOI] [PubMed] [Google Scholar]
- 30.Sherr J. The Dynamics and Methodology of Homeopathic Provings. 2nd. Malvern, UK: Dynamis Books; 1994. [Google Scholar]
- 31.Wiegant FA. Prins HA. Van Wijk R. Postconditioning hormesis put in perspective: An overview of experimental and clinical studies. Dose Response. 2011;9:209–224. doi: 10.2203/dose-response.10-004.Wiegant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks AJ. Bell IR. Howerter A, et al. Effects of homeopathic medicines on mood of adults with histories of coffee-related insomnia. Forsch Komplementarmed Klass Naturheilkd. 2010;17:250–257. doi: 10.1159/000320952. [DOI] [PubMed] [Google Scholar]
- 33.Bertani S. Lussignoli S. Andrioli G, et al. Dual effects of a homeopathic mineral complex on carrageenan-induced oedema in rats. Br Homoeopath J. 1999;88:101–105. doi: 10.1054/homp.1999.0310. [DOI] [PubMed] [Google Scholar]
- 34.Davidson J. Psychiatry and homeopathy: Basis for a dialogue. Br Homoeopath J. 1994;83:78–83. [Google Scholar]
- 35.Antelman SM. Caggiula AR. Oscillation follows drug sensitization: Implications. Crit Rev Neurobiol. 1996;10:101–117. doi: 10.1615/critrevneurobiol.v10.i1.50. [DOI] [PubMed] [Google Scholar]
- 36.Antelman SM. Caggiula AR. Gershon S, et al. Stressor-induced oscillation: A possible model of the bidirectional symptoms in PTSD. Ann N Y Acad Sci. 1997;821:296–304. doi: 10.1111/j.1749-6632.1997.tb48288.x. [DOI] [PubMed] [Google Scholar]
- 37.Wiegant FA. Spieker N. van Wijk R. Stressor-specific enhancement of hsp induction by low doses of stressors in conditions of self- and cross-sensitization. Toxicology. 1998;127:107–119. doi: 10.1016/s0300-483x(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 38.Bell IR. Koithan M. Models for the study of whole systems. Integr Cancer Ther. 2006;5:293–307. doi: 10.1177/1534735406295293. [DOI] [PubMed] [Google Scholar]
- 39.Ware J., Jr Kosinski M. Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 40.van Haselen RA. Cinar S. Fisher P. Davidson J. The Constitutional Type Questionnaire: Validation in the patient population of the Royal London Homoeopathic Hospital. Br Homoeopath J. 2001;90:131–137. doi: 10.1054/homp.1999.0493. [DOI] [PubMed] [Google Scholar]
- 41.Bell IR. Baldwin CM. Schwartz GE. Davidson JR. Homeopathic constitutional type questionnaire correlates of conventional psychological and physical health scales: Individual difference characteristics of young adults. Homeopathy. 2002;91:63–74. doi: 10.1054/homp.2002.0003. [DOI] [PubMed] [Google Scholar]
- 42.Szarek MJ. Bell IR. Schwartz GE. Validation of a brief screening measure of environmental chemical sensitivity: The chemical odor intolerance index. J Environ Psychol. 1997;17:345–351. [Google Scholar]
- 43.Bell IR. Baldwin CM. Stoltz E. Walsh BT. Schwartz GE. EEG ß 1 oscillation and sucrose sensitization in fibromyalgia with chemical intolerance. Int J Neurosci. 2001;108:31–42. doi: 10.3109/00207450108986503. [DOI] [PubMed] [Google Scholar]
- 44.Bell IR. Schwartz GE. Hardin EE, et al. Differential resting qEEG α patterns in women with environmental chemical intolerance, depressives, and normals. Biol Psychiatry. 1998;43:376–388. doi: 10.1016/s0006-3223(97)00245-x. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez M. Bell IR. Schwartz GE. EEG sensitization during chemical exposure in women with and without chemical sensitivity of unknown etiology. Toxicol Ind Health. 1999;15:305–312. doi: 10.1177/074823379901500304. [DOI] [PubMed] [Google Scholar]
- 46.Stub T. Salamonsen A. Alraek T. Is it possible to distinguish homeopathic aggravation from adverse effects? A qualitative study. Forsch Komplementmed. 2012;19:13–19. doi: 10.1159/000335827. [DOI] [PubMed] [Google Scholar]
- 47.Morrison R. Carbon: Organic and Hydrocarbon Remedies in Homeopathy. Nevada City, CA: Hahnemann Clinic Publishing; 2006. [Google Scholar]
- 48.Bell IR. Brooks AJ. Howerter A, et al. Short term effects of repeated olfactory administration of homeopathic Sulphur or Pulsatilla on electroencephalographic α power in healthy young adults. Homeopathy. 2011;100:203–211. doi: 10.1016/j.homp.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunter AM. Leuchter AF. Cook IA, et al. Brain functional changes and duloxetine treatment response in fibromyalgia: A pilot study. Pain Med. 2009;10:730–738. doi: 10.1111/j.1526-4637.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 50.Cook IA. Hunter AM. Abrams M, et al. Midline and right frontal brain function as a physiologic biomarker of remission in major depression. Psychiatry Res. 2009;174:152–157. doi: 10.1016/j.pscychresns.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook IA. Leuchter AF. Prefrontal changes and treatment response prediction in depression. Semin Clin Neuropsychiatry. 2001;6:113–120. doi: 10.1053/scnp.2001.21844. [DOI] [PubMed] [Google Scholar]
- 52.Hunter AM. Ravikumar S. Cook IA. Leuchter AF. Brain functional changes during placebo lead-in and changes in specific symptoms during pharmacotherapy for major depression. Acta Psychiatr Scand. 2009;119:266–273. doi: 10.1111/j.1600-0447.2008.01305.x. [DOI] [PubMed] [Google Scholar]
- 53.Leuchter AF. Morgan M. Cook IA, et al. Pretreatment neurophysiological and clinical characteristics of placebo responders in treatment trials for major depression. Psychopharmacology (Berl) 2004;177:15–22. doi: 10.1007/s00213-004-1919-2. [DOI] [PubMed] [Google Scholar]
- 54.Vickers A. Altman DG. Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothman K. Modern Epidemiology. New York: Brown, Little, and Co.; 1986. [Google Scholar]
- 56.Morrison R. Desktop Guide to Keynotes and Confirmatory Symptoms. Nevada City, CA: Hahnemann Clinic Publishing; 1993. [Google Scholar]
- 57.Boericke W. Pocket Manual of Homeopathic Materia Medica. Santa Rosa, CA: Boericke and Tafel; 1927. [Google Scholar]
- 58.Kreutzer R. Neutra RR. Lashuay N. Prevalence of people reporting sensitivities to chemicals in a population-based survey. Am J Epidemiol. 1999;150:1–12. doi: 10.1093/oxfordjournals.aje.a009908. [DOI] [PubMed] [Google Scholar]
- 59.Leuchter AF. Cook IA. Lufkin RB, et al. Cordance: A new method for assessment of cerebral perfusion and metabolism using quantitative electroencephalography. Neuroimage. 1994;1:208–219. doi: 10.1006/nimg.1994.1006. [DOI] [PubMed] [Google Scholar]
- 60.Rao M. Roy R. Bell IR. Characterization of the structure of ultra dilute sols with remarkable biological properties. Mater Lett. 2008;62:1487–1490. doi: 10.1016/j.matlet.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao ML. Roy R. Bell IR. The defining role of structure (including epitaxy) in the plausibility of homeopathy. Homeopathy. 2007;96:175–182. doi: 10.1016/j.homp.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 62.Rey L. Can low-temperature thermoluminescence cast light on the nature of ultra-high dilutions? Homeopathy. 2007;96:170–174. doi: 10.1016/j.homp.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Hyland ME. Lewith GT. Oscillatory effects in a homeopathic clinical trial: An explanation using complexity theory, and implications for clinical practice. Homeopathy. 2002;91:145–149. doi: 10.1054/homp.2002.0025. [DOI] [PubMed] [Google Scholar]
- 64.Lewith GT. Watkins AD. Hyland ME, et al. Use of ultramolecular potencies of allergen to treat asthmatic people allergic to house dust mite: Double blind randomised controlled clinical trial. BMJ. 2002;324:520–523. doi: 10.1136/bmj.324.7336.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baumgartner S. The state of basic research on homeopathy. In: Witt C, editor; Albrecht H, editor. New Directions in Homeopathy Research. Essen, Germany: KVC Verlag; 2009. pp. 107–130. [Google Scholar]
- 66.Sukul NC. Bala SK. Bhattacharyya B. Prolonged cataleptogenic effects of potentized homoeopathic drugs. Psychopharmacology. 1986;89:338–339. doi: 10.1007/BF00174371. [DOI] [PubMed] [Google Scholar]
- 67.Ruiz-Vega G. Poitevin B. Pérez-Ordaz L. Histamine at high dilution reduces spectral density in δ band in sleeping rats. Homeopathy. 2005;94:86–91. doi: 10.1016/j.homp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Antelman SM. Time-dependent sensitization in animals: A possible model of multiple chemical sensitivity in humans. Toxicol Ind Health. 1994;10:335–342. [PubMed] [Google Scholar]
- 69.Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 70.Antelman SM. Levine J. Gershon S. Time-dependent sensitization: The odyssey of a scientific heresy from the laboratory to the door of the clinic. Mol Psychiatry. 2000;5:350–356. doi: 10.1038/sj.mp.4000721. [DOI] [PubMed] [Google Scholar]
- 71.Antelman SM. Caggiula AR. Edwards DJ, et al. Long-term oscillation of corticosterone following intermittent cocaine. J Neural Transm. 2000;107:369–375. doi: 10.1007/s007020050031. [DOI] [PubMed] [Google Scholar]
- 72.Antelman SM. Caggiula AR. Kiss S, et al. Neurochemical and physiological effects of cocaine oscillate with sequential drug treatment: Possibly a major factor in drug variability. Neuropsychopharmacology. 1995;12:297–306. doi: 10.1016/0893-133X(94)00094-G. [DOI] [PubMed] [Google Scholar]
- 73.Caggiula AR. Antelman SM. Kucinski BJ, et al. Oscillatory-sensitization model of repeated drug exposure: Cocaine's effects on shock-induced hypoalgesia. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:511–521. doi: 10.1016/s0278-5846(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 74.Antelman SM. Kocan D. Knopf S, et al. One brief exposure to a psychological stressor induces long-lasting, time-dependent sensitization of both the cataleptic and neurochemical responses to haloperidol. Life Sci. 1992;51:261–266. doi: 10.1016/0024-3205(92)90084-3. [DOI] [PubMed] [Google Scholar]
- 75.Antelman SM. Caggiula AR. Knopf S, et al. Amphetamine or haloperidol 2 weeks earlier antagonized the plasma corticosterone response to amphetamine: Evidence for the stressful/foreign nature of drugs. Psychopharmacology. 1992;107:331–336. doi: 10.1007/BF02245157. [DOI] [PubMed] [Google Scholar]
- 76.Kucinski BJ. Antelman SM. Caggiula AR, et al. Oscillatory effects of repeated morphine on shock-induced hypoalgesia and ß-endorphin. Synapse. 1998;30:30–37. doi: 10.1002/(SICI)1098-2396(199809)30:1<30::AID-SYN4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 77.Abraham WC. Metaplasticity: Tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 78.Calabrese EJ. Jonas WB. Homeopathy: Clarifying its relationship to hormesis. Hum Exp Toxicol. 2010;29:531–536. doi: 10.1177/0960327110369857. [DOI] [PubMed] [Google Scholar]
- 79.Jonas WB. Ives JA. Should we explore the clinical utility of hormesis? Hum Exp Toxicol. 2008;27:123–127. doi: 10.1177/0960327108090754. [DOI] [PubMed] [Google Scholar]
- 80.Dhond RP. Yeh C. Park K, et al. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136:407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bell IR. Baldwin CM. Schwartz GE. Translating a nonlinear systems theory model for homeopathy into empirical tests. Altern Ther Health Med. 2002;8:58–66. [PubMed] [Google Scholar]