Abstract
Background
Many debilitating symptoms arise from cancer and its treatment that are often unrelieved by established methods. Pranayama, a series of yogic breathing techniques, may improve cancer-related symptoms and quality of life, but it has not been studied for this purpose.
Objectives
A pilot study was performed to evaluate feasibility and to test the effects of pranayama on cancer-associated symptoms and quality of life.
Design
This was a randomized controlled clinical trial comparing pranayama to usual care.
Setting
The study was conducted at a university medical center.
Subjects
Patients receiving cancer chemotherapy were randomized to receive pranayama immediately or after a waiting period (control group).
Interventions
The pranayama intervention consisted of four breathing techniques taught in weekly classes and practiced at home. The treatment group received pranayama during two consecutive cycles of chemotherapy. The control group received usual care during their first cycle, and received pranayama during their second cycle of chemotherapy.
Outcome measures
Feasibility, cancer-associated symptoms (fatigue, sleep disturbance, anxiety, depression, stress), and quality of life were the outcomes.
Results
Class attendance was nearly 100% in both groups. Sixteen (16) participants were included in the final intent-to-treat analyses. The repeated-measures analyses demonstrated that any increase in pranayama dose, with dose measured in the number of hours practiced in class or at home, resulted in improved symptom and quality-of-life scores. Several of these associations—sleep disturbance (p=0.04), anxiety (p=0.04), and mental quality of life (p=0.05)—reached or approached statistical significance.
Conclusions
Yoga breathing was a feasible intervention among patients with cancer receiving chemotherapy. Pranayama may improve sleep disturbance, anxiety, and mental quality of life. A dose–response relationship was found between pranayama use and improvements in chemotherapy-associated symptoms and quality of life. These findings need to be confirmed in a larger study.
Introduction
The experience of cancer chemotherapy is often characterized by symptoms of sleep disturbance, stress, anxiety, fatigue, and impaired quality of life, imposed on a background of usual life challenges. Despite numerous advances in cancer treatment, effective symptom management interventions have lagged. While pharmacologic interventions have been studied for symptoms such as cancer-related fatigue, these drugs are frequently expensive, ineffective, and often associated with their own side-effects.1,2 Behavioral interventions, such as pranayama, are generally free of side-effects and inexpensive.
The ancient practice of yoga, meaning “union,” is a spiritual practice that begins by strengthening the union of mind and body and aims toward spiritual evolution.3 In approximately 200 bce, Patanjali's Yoga Sutras codified eight parts, called ashtanga, to the path of yoga: yama (rules of conduct), niyama (rules for self-purification), asana (physical postures), pranayama (breathing techniques), pratyahara (controlling the senses), dharana (single-pointed concentration), dhyana (meditation), and samadhi (liberation). As the initial steps of this process, asana and pranayama were concerned with the health of mind and body. Pranayama, which works with the mind and the organs of respiration, is both a physical practice and a form of meditation. Prana means spirit, life force, or breath, and yama means expansion, control, or regulation. Pranayama is the regulation and expansion of breath. Though originally designed as a way to quiet the mind, these breathing techniques have also shown health benefits, especially in the area of mind–body illness.4–16 Studies have suggested that pranayama practices create a relaxed state by enhancing parasympathetic tone.11,13,16
Several studies have investigated the efficacy of a multimodality yoga intervention, which includes breathing, postures, and/or meditation, in a population of patients with cancer.17–29 However, conclusive results are lacking.30 Many studies have small and mixed samples (i.e., included both patients who were receiving cancer treatment and those post-treatment) and others have methodological limitations leading to challenges in drawing definitive conclusions.30 All published studies have investigated multimodality yoga interventions, making it difficult to determine which component of yoga is most beneficial.30 There are no studies that have evaluated yoga breathing as a single modality to improve symptoms among oncology patients. A yoga breathing intervention is appealing because it is relatively easy to learn, can be done without equipment, and can be done at any time, even during the infusion of chemotherapy.
This study aimed to assess the feasibility of practicing yoga breathing techniques among patients receiving chemotherapy, and to test the efficacy of these techniques in alleviating common chemotherapy-associated symptoms (fatigue, sleep disturbance, stress, anxiety, and depression) and improving quality of life.
Materials and Methods
The study was approved by the University of California San Francisco institutional review board. Written informed consent was obtained from all participants. All clinical investigations in this study were conducted in accordance with the 1964 Declaration of Helsinki.
Participants were randomized 1:1 in blocks of four. The allocation sequence was generated by the study statistician and then transferred to sealed numbered envelopes. The study staff enrolled participants and implemented the allocation sequence, which was concealed from the study staff until study assignment. Blinding of participants to study group assignment was not possible due to the nature of the intervention. The treatment group received the pranayama intervention, which consisted of a 60-minute class once a week and twice daily home practice that totaled 20–30 minutes per day, along with usual care during two consecutive cycles of chemotherapy (cycle A and B). The control group received only usual care during the initial cycle of chemotherapy (cycle A) and the pranayama intervention along with usual care during the second cycle of chemotherapy (cycle B).
Participants
The study enrollment occurred over a 1-year period beginning in October of 2008. Inclusion criteria were as follows: receiving intravenous chemotherapy for a cancer diagnosis; had visual analog scale score for fatigue of at least a 4 out of 10; and had a Karnofsky Performance Status (KPS)31 of 60 or greater. The KPS measures overall performance status and is rated on a scale from 0 (death) to 100 (normal). Exclusion criteria were the following: an ongoing yoga practice, severe chronic obstructive pulmonary disease, class III or IV heart failure, Childe C cirrhosis, end-stage renal disease, or having received greater than three prior chemotherapy regimens.
Pranayama intervention
The pranayama protocol consisted of four breathing practices, which were chosen by the principal investigator for their ease of use and potential for efficacy after extensive discussion with the study yoga therapists. These breathing practices were taught and practiced during weekly 60-minute sessions by two certified yoga instructors, who had special training in therapeutic yoga and over 25 years of combined experience teaching yoga to patients with cancer. The majority of the sessions were conducted with 1 teacher and 1–2 students. The participants were expected to practice the techniques at least 10–15 minutes twice a day at home. The class attendance was measured by study staff and home practice time was measured by self-report in participant daily log books. The yoga instructor adherence to the protocol was assessed by the principal investigator using a yoga instructor log book and random recordings of sessions. All four breathing techniques were taught and practiced at each session.
The first breathing practice was a breath observation technique. In this exercise, participants focused on their natural breath with the goal of maintaining continuity of awareness. The second technique was a form of ujjayi breathing. The instruction in this breath focused on abdominal breathing, slow deep rhythmic inhalation and exhalation, extended exhalation, and expanding the lungs (upper and lower). A partially closed glottis was used to facilitate this practice. The third technique was a forced abdominal exhalation, kapalabhati pranayama. In this technique, the participant was instructed to inhale gently, retain briefly, and then exhale forcefully. The duration and intensity of this instruction was tailored to the individual strength of the patient. The final breathing technique was alternate nostril breathing, nadi shodhana. This technique involved moving air through alternate nostrils by placing the fingers in a hand position called a mudra.
Measurements
The measurements were obtained at baseline, between the first and second cycles of chemotherapy, and at the end of the study. The outcomes were measured in the same way in both groups. The symptom and quality of life measures were self-administered. Feasibility was assessed based on ease of accrual, retention of subjects, attendance in classes, completion of home practice, and completion of study measures. The participants kept a daily diary recording the amount of time spent practicing pranayama. The data on adverse effects were collected from participants by the yoga instructors at each class and in the study exit questionnaire.
Fatigue was measured using the revised Piper Fatigue Scale, which is a 22-item instrument that has four subscales: behavioral/severity, affective meaning, sensory, and cognitive/mood. Each quantitative item is rated on a 0–10 numeric rating scale. The instrument also includes four open-ended items. The Cronbach α for the entire scale is 0.97 in a population of patients with breast cancer.32
Sleep disturbance was measured using the General Sleep Disturbance Scale33 (GSDS), which contains 21 items that rate the frequency of individual sleep problems over the past week from 0 (not at all) to 7 (every day). The GSDS total score is the sum of the seven subscale scores: quality of sleep, quantity of sleep, sleep onset latency, midsleep awakenings, early awakenings, medications for sleep, and excessive daytime sleepiness. The scale yields a total score that ranges from 0 to 147. Good and poor sleepers are distinguished by cutoff scores of 43 for the total scale and 3 on any one subscale. The GSDS has well-established validity and reliability in an oncology setting.34
Anxiety and depression were measured using the 14-item Hospital Anxiety and Depression Scale 35 (HADS). A subscale score for both anxiety and depression is obtained from the HADS. The Cronbach's α for the anxiety subscale range from 0.80 to 0.93 and 0.81 to 0.9 for the depression subscale. The scale is thought to be sensitive to change.36
Stress was measured using the Perceived Stress Scale,37 which is a 10-item scale that asks participants to rate the frequency of feelings and thoughts from 0 (never) to 4 (very often). The original studies of the scale revealed a Cronbach's α above 0.8.37
The SF-12 version one was used to measure quality of life (QOL). The SF-12 physical and mental component summary scores correlate well with the corresponding scores on the SF-36 with R2 scores greater than 0.9 for both subscales.38
Analysis
An intention-to-treat analysis was employed in which all available data were analyzed. Patient characteristics were analyzed using t-tests for continuous variables and χ2 tests for categorical variables to determine between-group differences. The primary goal of this pilot study was to test feasibility and to inform a larger randomized controlled trial.
Repeated-measures analyses were done on the symptom measures and QOL using multilevel mixed-effects linear regression. The models used the individual scale scores as the outcome variables and the pranayama dose (the number of hours in class combined with the number of hours of home practice) as the predictor variable. The pranayama dose variable accounts for group assignment (i.e., the dose variable is coded as 0 for control participants during cycle A [prior to receiving the pranayama intervention]). This analytic approach was chosen because it allows all available data for all participants in this study design to be analyzed (i.e., includes control participant's data from cycle A [the period in which they receive usual care only] as control and control participant's data from cycle B [the period in which they received pranayama] as treatment) and includes data at all three study time points. As there were no statistically significant differences between the groups in terms of known confounding variables, covariates were not added to the regression models. Due to the small sample size, possible non-normality, and influential observations, the results were checked with bootstrapped standard errors and confidence intervals and were found to be consistent.
Results
Participants
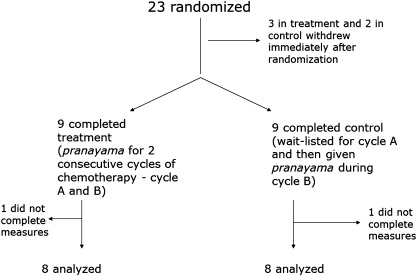
Forty-six (46) participants were screened and 23 were enrolled and randomized (Fig. 1). Five (5) participants withdrew immediately after randomization, either because their illness worsened or they changed their minds about participation. These individuals did not attend any classes or complete any measures. All remaining participants completed study treatment, and class attendance was nearly 100% in both groups (Table 1). The participants completed a substantial amount of daily home practice of pranayama (Table 1), averaging 3.2 hours per week (1134 total hours) in the treatment group and 2.4 hours per week (345 total hours) in the control group during cycle B. Sixteen (16) out of 18 participants completed all of the study measures, and were therefore included in the final analyses. Two (2) out of 18 participants, 1 from each group, did not complete any study measures despite repeated reminders. There were no adverse effects reported due to the study intervention.
FIG. 1.
Enrollment, patient flow, and attrition of the randomized controlled clinical trial.
Table 1.
Baseline Characteristics of Participants According to Group
| Characteristic | Control group (n=8) | Treatment group (n=8) | p-Value |
|---|---|---|---|
| Age–mean years (±SD) | 56.0 (11.9) | 52.4 (14.6) | 0.6 |
| Karnofsky Performance Status | 76.3 (7.4) | 71.3 (8.3) | 0.2 |
| Sex–% (n) | 0.5 | ||
| Female | 100 (8) | 75 (6) | |
| Male | 0 (0) | 25 (2) | |
| Race–% (n) | 1.0 | ||
| White | 62.5 (5) | 62.5 (5) | |
| Nonwhite | 37.5 (3) | 37.5 (3) | |
| Cancer type–% (n) | 0.6 | ||
| Breast | 62.5 (5) | 37.5 (3) | |
| Othera | 37.5 (3) | 62.5 (5) | |
| Cancer stage–% (n) | 1.0 | ||
| Nonmetastatic | 62.5 (5) | 50 (4) | |
| Metastatic | 37.5 (3) | 50 (4) | |
| Prior treatment–% (n) | |||
| Chemotherapy | 37.5 (3) | 50 (4) | 1.0 |
| Radiation | 0 (0) | 12.5 (1) | 1.0 |
| Surgery | 50 (4) | 37.5 (3) | 1.0 |
| Working % (n) | 50 (4) | 37.5 (3) | 1.0 |
| Percent class attendance | 100b | 97 | 0.6 |
| Average daily home practice–hours per week (SD) | 2.4 (1.3)b | 3.2 (2.8) | 0.5 |
Other=gastrointestinal cancer, gynecologic cancer, lymphoproliferative disease, lung cancer.
Control group/delayed treatment: received pranayama during cycle B (after crossover).
SD, standard deviation.
There were no statistically significant differences in demographic characteristics between the treatment and control groups (Table 1). However, differences in demographic characteristics may have been found to be nonsignificant due to the small sample size. The majority of the participants had a diagnosis of breast cancer. There were no differences between groups in terms of type, dose, or schedule of chemotherapy. All of the participants were using other forms of complementary and alternative medicine (CAM) during the study. The most common forms of CAM reported were biologically based therapies, mind–body therapies, and alternative medical systems. There were no between-group differences found in soporific, antidepressant, or anxiolytic medication use.
Symptoms and quality of life
There were no statistically significant between-group differences in any of the baseline symptom or QOL scores (Table 2). The control group worsened during the control phase (cycle A) and improved during the treatment phase (cycle B) for sleep disturbance, anxiety, depression, and mental quality of life. The treatment group showed gradual improvements in stress, sleep disturbance, anxiety, and mental quality of life at both the midpoint and study endpoint. For fatigue and depression, the treatment group initially worsened during cycle A, followed by improvements during cycle B. The between-group comparisons at the study midpoint did not reach statistical significance.
Table 2.
Mean Scale Scores at the Three Study Time Points
| Scale | Baseline | Midpoint | Study end |
|---|---|---|---|
| Fatigue (PFS) | |||
| Controla (SD) | 5.5 (2.2) | 4.8 (2.5) | 4.4 (2.0) |
| Treatment (SD) | 5.0 (2.6) | 5.6 (2.1) | 4.7 (2.3) |
| Sleep Disturbance (GSDS) | |||
| Controla (SD) | 57.3 (27.3) | 61.1 (23.1) | 43.5 (16.8) |
| Treatment (SD) | 70.5 (15.2) | 66.2 (13.2) | 60.0 (21.4) |
| Stress (PSS) | |||
| Controla (SD) | 17.6 (9.2) | 16.8 (10.2) | 16.6 (8.3) |
| Treatment (SD) | 18.5 (10.0) | 18.5 (9.9) | 15.1 (8.1) |
| Anxiety and Depression (HADS) | |||
| Total | |||
| Controla (SD) | 12.1 (7.8) | 14.9 (7.6) | 11.6 (8.5) |
| Treatment (SD) | 13.4 (5.6) | 14.8 (8.4) | 11.1 (7.5) |
| Anxiety Subscale | |||
| Controla (SD) | 5.6 (4.2) | 7.0 (3.5) | 5.5 (3.4) |
| Treatment (SD) | 8.4 (2.7) | 8.3 (3.5) | 5.6 (3.8) |
| Depression Subscale | |||
| Controla (SD) | 6.5 (4.4) | 7.9 (4.8) | 6.1 (5.2) |
| Treatment (SD) | 5.0 (3.5) | 6.5 (5.4) | 5.5 (4.3) |
| Quality of Life (SF-12) | |||
| Mental Component Score | |||
| Controla (SD) | 53.1 (27.5) | 43.8 (25.9) | 52.8 (29.2) |
| Treatment (SD) | 43.8 (23.6) | 52.8 (29.7) | 56.3 (20.3) |
| Physical Component Score | |||
| Controla (SD) | 50.0 (18.9) | 52.3 (25.2) | 53.9 (25.2) |
| Treatment (SD) | 47.7 (16.0) | 46.1 (31.1) | 52.3 (22.4) |
Control group/delayed treatment: control group participants received the intervention during cycle B (between midpoint and study end).
PFS, revised Piper Fatigue Scale; SD, Standard Deviation; GSDS, General Sleep Disturbance Scale; PSS, Perceived Stress Scale; HADS, Hospital Anxiety and Depression Scale; SF-12, 12-Item Short-Form Health Survey.
Any increase in the amount of yoga practice (either in class or home practice) was associated with improvement in symptoms and QOL (Table 3). This finding was consistent across all measures as represented by negative coefficients for all the symptom scores and positive coefficients for the QOL scores. Statistically significant improvements in sleep disturbance, and anxiety were seen with increased yoga practice.
Table 3.
Repeated-Measures Models for Pranayama Dose on Individual Scale Scores
| Outcome variable | Coefficient for pranayama dosea(95% CI) | p-Value |
|---|---|---|
| Fatigue (PFS) | −0.02 (−0.06, 0.01) | 0.29 |
| Sleep Disturbance (GSDS) | −0.39 (−0.70, −0.08) | 0.04 |
| Stress (PSS) | −0.08 (−0.18, 0.03) | 0.23 |
| Anxiety and Depression (HADS) | ||
| Total | −0.08 (−0.17, 0.009) | 0.14 |
| Anxiety Subscale | −0.07 (−0.12, −0.01) | 0.04 |
| Depression Subscale | −0.009 (−0.06, 0.05) | 0.79 |
| Quality of Life (SF-12) | ||
| Mental Component Score | 0.43 (0.07, 0.79) | 0.05 |
| Physical Component Score | 0.15 (−0.18, 0.47) | 0.46 |
This variable is a combination of class time and home practice time in hours and accounts for group assignment.
CI, confidence interval; PFS, revised Piper Fatigue Scale; GSDS, General Sleep Disturbance Scale; PSS, Perceived Stress Scale; HADS, Hospital Anxiety and Depression Scale; SF-12, 12-Item Short-Form Health Survey.
Discussion
The repeated-measures analyses of the yoga breathing dose effect revealed that any increase in the amount of yoga breathing practice (either in-class practice or home practice) was associated with improved symptom and QOL scores. This finding was consistent across all measures as evidenced by the favorable direction of the coefficients for all scale scores in the repeated-measures analyses in Table 3. The interpretation of these coefficients is that for each 1-hour increase in pranayama practice, the scale scores improved by the amount of the coefficient. Several of the associations (i.e., for sleep disturbance scale, anxiety, and the mental component of QOL) reached or approached statistical significance.
The directionality of the associations derived from the repeated measures analyses, as in any such analyses, cannot be certain. It is possible that those who were more adherent to the pranayama intervention (and therefore achieved improved symptom scores and QOL) may also be those who are generally more “health oriented.” Such “health oriented” people may be predisposed to do better in general. However, the strength of the repeated-measures analyses is that they analyze all the study data by including the control group data after the wait-list period with the treatment group. The normal trajectory of most symptoms during chemotherapy is gradual worsening. Had the control group participants not received the intervention during cycle B, it would be expected that their symptoms would continue to worsen.39,40 It could then be speculated that a longer intervention would result in greater differences between groups. Overall, these data suggest that a longer or more intensive yoga breathing intervention could have greater beneficial effects on symptoms and QOL. A prior study suggested a similar dose– response relationship between hatha yoga, lower body–mass index, and reduced medication use.41
The primary efficacy endpoint of fatigue showed no difference between groups. Possible explanations for this finding include that yoga breathing does not have an effect on fatigue or that such a benefit was not found due to the variability in this symptom in this small sample size. An absence of an effect for depression may also be explained with these same reasons. The levels of depression in this sample were not high, and this may also have precluded finding an effect.
It is important to note that no prior study has focused exclusively on yoga breathing among patients with cancer, and only a few studies have focused on patients receiving chemotherapy. In a study by Cohen and colleagues,19 31 patients with lymphoma were randomized to either a Tibetan yoga intervention or a wait-list control group. The intervention, which included breathwork and mindfulness components, consisted of seven weekly yoga sessions. Although no differences were found in anxiety, depression, and fatigue, the treatment group showed an improvement in sleep disturbance compared to control. The Tibetan yoga intervention had a strong mindfulness component, which may be the common thread between that and the present study in terms of the improvements in sleep disturbance. Rao and colleagues evaluated an integrated yoga program that included a breathwork component among women with breast cancer receiving treatment.25 The intent-to-treat analysis from this study suggested improvements in state anxiety favoring the yoga group. Danhauer and colleagues conducted a randomized pilot study of a restorative yoga intervention, which included a breathwork component.21 Forty-four (44) women with breast cancer were randomized to yoga or a wait-list control. The restorative yoga intervention lasted for 10 weeks and included gentle poses that were supported by props. Statistically significant differences were shown favoring the yoga group for the mental component of QOL, depressive symptoms, positive affect, and spirituality. Two (2) articles by Kim and colleagues42,43 reported on an exercise intervention with a strong breathwork component for patients undergoing stem cell transplantation. These studies showed a benefit in fatigue, anxiety, and depressive symptoms among these patients.
A distinction of the present study, compared to prior work in this area, is that greater feasibility, as exemplified by high rates of class attendance and completion of home practice, was demonstrated. Several other studies in the literature had lower rates of class attendance.19,21,23,28 This finding may be due to the fact that this pranayama intervention was designed for its ease of practice. This attribute of pranayama makes it an attractive intervention for further study in the cancer chemotherapy setting.
This study, performed at a single institution, is limited by the small sample size. The potential for selection bias introduced by those who would elect to participate in a study of this kind also exists. The inactive control group, lack of blinding to group assignment, which is a challenge with behavioral interventions such as the one used in this study, and the reliance on self-reported outcomes were other limitations of this study. Efficacy results need to be confirmed in a larger study.
Conclusions
This first study of a pure pranayama intervention in a population of patients with cancer successfully demonstrated that yoga breathing is feasible and can be safely recommended for patients with cancer receiving chemotherapy. Any increase in the yoga breathing practice was correlated with improvements in both cancer chemotherapy associated symptoms and QOL. Pranayama may be helpful for improving sleep disturbance, anxiety, and mental QOL among patients undergoing chemotherapy. Definitive conclusions on efficacy await further study.
Acknowledgments
The assistance of the yoga therapists on the project and the contributions of the participants in this study were greatly appreciated. This research was supported by a grant from the Mount Zion Health Fund (Grant #20070658). Dr. Dhruva is funded through the National Institutes of Health Mentored Patient-Oriented Research Career Development Award (K23 AT005340-01). Dr. Miaskowski is funded by the American Cancer Society as a Clinical Research Professor. There are no conflicts of interest to report. The funding agency did not have any role in study design; in the collection, analysis, interpretation, or presentation of the information; or in the decision to publish. Trial registration: clinicaltrials.gov Identifier: NCT00982748.
Disclosure Statement
All authors declare that no competing financial interests exist.
References
- 1.Bruera E. El Osta B. Valero V, et al. Donepezil for cancer fatigue: A double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2007;25:3475–3481. doi: 10.1200/JCO.2007.10.9231. [DOI] [PubMed] [Google Scholar]
- 2.Bruera E. Valero V. Driver L, et al. Patient-controlled methylphenidate for cancer fatigue: A double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2006;24:2073–2078. doi: 10.1200/JCO.2005.02.8506. [DOI] [PubMed] [Google Scholar]
- 3.Iyengar B. Light on Pranayama. London: Harper Collins; 1992. [Google Scholar]
- 4.Mohan M. Saravanane C. Surange SG, et al. Effect of yoga type breathing on heart rate and cardiac axis of normal subjects. Indian J Physiol Pharmacol. 1986;30:334–340. [PubMed] [Google Scholar]
- 5.Bhargava R. Gogate MG. Mascarenhas JF. Autonomic responses to breath holding and its variations following pranayama. Indian J Physiol Pharmacol. 1988;32:257–264. [PubMed] [Google Scholar]
- 6.Singh V. Wisniewski A. Britton J. Tattersfield A. Effect of yoga breathing exercises (pranayama) on airway reactivity in subjects with asthma. Lancet. 1990;335:1381–1383. doi: 10.1016/0140-6736(90)91254-8. [DOI] [PubMed] [Google Scholar]
- 7.Telles S. Nagarathna R. Nagendra HR. Breathing through a particular nostril can alter metabolism and autonomic activities. Indian J Physiol Pharmacol. 1994;38:133–137. [PubMed] [Google Scholar]
- 8.Bhattacharya S. Pandey US. Verma NS. Improvement in oxidative status with yogic breathing in young healthy males. Indian J Physiol Pharmacol. 2002;46:349–354. [PubMed] [Google Scholar]
- 9.Pal GK. Velkumary S. Madanmohan Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. Indian J Med Res. 2004;120:115–121. [PubMed] [Google Scholar]
- 10.Jovanov E. On spectral analysis of heart rate variability during very slow yogic Breathing. Conf Proc IEEE Eng Med Biol Soc. 2005;3:2467–2470. doi: 10.1109/IEMBS.2005.1616968. [DOI] [PubMed] [Google Scholar]
- 11.Upadhyay Dhungel K. Malhotra V. Sarkar D. Prajapati R. Effect of alternate nostril breathing exercise on cardiorespiratory functions. Nepal Med Coll J. 2008;10:25–27. [PubMed] [Google Scholar]
- 12.Martarelli D. Cocchioni M. Scuri S. Pompei P. Diaphragmatic breathing reduces exercise-induced oxidative stress. Evid Based Complement Altern Med. 2009;2011:1–10. doi: 10.1093/ecam/nep169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pramanik T. Sharma HO. Mishra S, et al. Immediate effect of slow pace bhastrika pranayama on blood pressure and heart rate. J Altern Complement Med. 2009;15:293–295. doi: 10.1089/acm.2008.0440. [DOI] [PubMed] [Google Scholar]
- 14.Saxena T. Saxena M. The effect of various breathing exercises (pranayama) in patients with bronchial asthma of mild to moderate severity. Int J Yoga. 2009;2:22–25. doi: 10.4103/0973-6131.53838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vialatte FB. Bakardjian H. Prasad R. Cichocki A. EEG paroxysmal gamma waves during Bhramari Pranayama: A yoga breathing technique. Conscious Cogn. 2009;18:977–988. doi: 10.1016/j.concog.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Pramanik T. Pudasaini B. Prajapati R. Immediate effect of a slow pace breathing exercise Bhramari pranayama on blood pressure and heart rate. Nepal Med Coll J. 2010;12:154–157. [PubMed] [Google Scholar]
- 17.Banerjee B. Vadiraj HS. Ram A, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6:242–250. doi: 10.1177/1534735407306214. [DOI] [PubMed] [Google Scholar]
- 18.Carson JW. Carson KM. Porter LS, et al. Yoga for women with metastatic breast cancer: Results from a pilot study. J Pain Symptom Manage. 2007;33:331–341. doi: 10.1016/j.jpainsymman.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Cohen L. Warneke C. Fouladi RT, et al. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253–2260. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 20.Culos-Reed SN. Carlson LE. Daroux LM. Hately-Aldous S. A pilot study of yoga for breast cancer survivors: Physical and psychological benefits. Psychooncology. 2006;15:891–897. doi: 10.1002/pon.1021. [DOI] [PubMed] [Google Scholar]
- 21.Danhauer SC. Mihalko SL. Russell GB, et al. Restorative yoga for women with breast cancer: Findings from a randomized pilot study. Psychooncology. 2009;18:360–368. doi: 10.1002/pon.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danhauer SC. Tooze JA. Farmer DF, et al. Restorative yoga for women with ovarian or breast cancer: Findings from a pilot study. J Soc Integr Oncol. 2008;6:47–58. [PubMed] [Google Scholar]
- 23.Moadel AB. Shah C. Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: Effects on quality of life. J Clin Oncol. 2007;4:4. doi: 10.1200/JCO.2006.06.6027. [DOI] [PubMed] [Google Scholar]
- 24.Raghavendra RM. Nagarathna R. Nagendra HR, et al. Effects of an integrated yoga programme on chemotherapy-induced nausea and emesis in breast cancer patients. Eur J Cancer Care (Engl) 2007;16:462–474. doi: 10.1111/j.1365-2354.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 25.Rao MR. Raghuram N. Nagendra HR, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: A randomized controlled trial. Complement Ther Med. 2009;17:1–8. doi: 10.1016/j.ctim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Vadiraja HS. Raghavendra RM. Nagarathna R, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: A randomized controlled trial. Integr Cancer Ther. 2009;8:37–46. doi: 10.1177/1534735409331456. [DOI] [PubMed] [Google Scholar]
- 27.Vadiraja HS. Rao MR. Nagarathna R, et al. Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: A randomized controlled trial. Complement Ther Med. 2009;17:274–280. doi: 10.1016/j.ctim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Chandwani KD. Thornton B. Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8:43–55. [PubMed] [Google Scholar]
- 29.Carson JW. Carson KM. Porter LS, et al. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: Results from a randomized trial. Support Care Cancer. 2009;17:1301–1309. doi: 10.1007/s00520-009-0587-5. [DOI] [PubMed] [Google Scholar]
- 30.Smith KB. Pukall CF. An evidence-based review of yoga as a complementary intervention for patients with cancer. Psychooncology. 2009;18:465–475. doi: 10.1002/pon.1411. [DOI] [PubMed] [Google Scholar]
- 31.Karnofsky D. Performance Scale. New York: Plenum Press; 1977. [Google Scholar]
- 32.Piper BF. Dibble SL. Dodd MJ, et al. The revised Piper Fatigue Scale: Psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25:677–684. [PubMed] [Google Scholar]
- 33.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15:493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 34.Miaskowski C. Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: A pilot study. J Pain Symptom Manage. 1999;17:320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 35.Zigmond AS. Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 36.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale: A review of validation data and clinical results. J Psychosom Res. 1997;42:17–41. doi: 10.1016/s0022-3999(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 37.Cohen S. Kamarck T. Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 38.Ware J., Jr Kosinski M. Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Prue G. Rankin J. Allen J, et al. Cancer-related fatigue: A critical appraisal. Eur J Cancer. 2006;42:846–863. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Palesh OG. Roscoe JA. Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2009;28:292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moliver N. Mika E. Chartrand M, et al. Increased Hatha yoga experience predicts lower body mass index and reduced medication use in women over 45 years. Int J Yoga. 2011;4:77–86. doi: 10.4103/0973-6131.85490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SD. Kim HS. Effects of a relaxation breathing exercise on fatigue in haemopoietic stem cell transplantation patients. J Clin Nurs. 2005;14:51–5. doi: 10.1111/j.1365-2702.2004.00938.x. [DOI] [PubMed] [Google Scholar]
- 43.Kim SD. Kim HS. Effects of a relaxation breathing exercise on anxiety, depression, and leukocyte in hemopoietic stem cell transplantation patients. Cancer Nurs. 2005;28:79–83. doi: 10.1097/00002820-200501000-00012. [DOI] [PubMed] [Google Scholar]