Abstract
Background
We sought to evaluate perinatal outcomes by race/ethnicity among women with gestational diabetes mellitus (GDM).
Methods
We conducted a 14-year retrospective cohort study of women with GDM. Selected perinatal outcomes were examined. Unadjusted and adjusted prevalence ratios (PR, aPR) of perinatal outcomes, comparing Hispanic and African American women with Caucasian women, were calculated.
Results
Hispanics comprised 54% of the 1018 woman cohort. Half received medical management of GDM (52%), more than African Americans (45%) or Caucasians (39%)(p<0.05). Compared with Caucasians, Hispanics had fewer deliveries <37 weeks (aPR 0.58, 95% confidence interval [CI] 0.42-0.81), less gestational hypertension (aPR 0.43, 95% CI 0.22-0.83), larger median birth weight infants (3453 g vs 3291 g, p<0.05), and greater risk of shoulder dystocia (aPR 3.52, 95% CI 1.30-9.50). Hispanic women had fewer adverse outcomes overall compared with Caucasian or African American women.
Conclusions
We report differences between Hispanic and Caucasian women with GDM. Treatment to achieve glycemic control and reduce adverse outcomes may differ by race/ethnicity.
Introduction
Gestational diabetes mellitus (GDM) complicates 4%–7% of all pregnancies.1 Untreated or poorly controlled GDM is associated with adverse perinatal outcomes, including large for gestational age (LGA), shoulder dystocia, cesarean delivery, and hypertensive disorders of pregnancy.2,3 Strict glycemic control through diet or medical treatment lowers the risks of these adverse outcomes.4 Prevalence of GDM varies by race/ethnicity. Hispanic women in the United States have a higher prevalence of GDM compared with Caucasian or African American women.5–7 In a large North Carolina cohort, Hispanic women were 50% more likely to have GDM compared with Caucasian or African American women.8
Differences in risk of adverse perinatal outcomes by ethnicity, however, are less clear. Among those without GDM, Hispanic women are less likely to deliver low birth weight (LBW) infants, experience lower neonatal mortality,9,10 and have had a lower reported prevalence of preeclampsia compared with Caucasians and African Americans.8 Nonpregnant Hispanics are less likely to develop hypertension at a given body mass index (BMI) compared with non-Hispanic white or blacks women and East Asians.11 Ethnic differences in insulin resistance, as well as protective social factors, may contribute to these unexpected better health outcomes among Hispanics despite their overall socioeconomic disadvantage, which typically has been associated with poorer health outcomes.12
Some data specific to women with GDM have suggested that adverse outcome risk may also vary by race/ethnicity.13–15 Findings conflict, however, and recent data suggest the magnitude of racial/ethnic differences is small, particularly when individual, more narrowly defined racial/ethnic groups are evaluated.16,17 The association of race/ethnicity with complications of GDM and the role GDM treatment may play thus remain unclear.
Hispanics are the largest ethnic minority in the United States and are projected to be nearly 30% of the population by 2050.18 With an overall growing prevalence of GDM and a disproportionate burden among Hispanic women, understanding racial/ethnic differences in GDM treatment and perinatal outcomes is an important step toward potentially tailoring treatment to improve perinatal health. In this retrospective 14-year cohort of women with GDM, we, therefore, measured the association between race/ethnicity and GDM management and adverse outcomes among Hispanic, African American, and Caucasian women with GDM.
Materials and Methods
Study cohort
We conducted a retrospective cohort study of all women diagnosed with GDM who delivered at University of North Carolina (UNC) Womens' Hospital, Chapel Hill, NC, between April 1, 1996, and May 31, 2010. Women were excluded if they delivered prior to 24 weeks' gestation, had pregestational diabetes, or did not have a documented GDM screening and diagnostic test result. All other women who delivered at UNC Womens' Hospital during the study period were included in the study cohort. Neonatal data for the firstborn were used in multiple gestations. UNC Institutional Review Board approval was obtained for this study.
Gestational diabetes diagnosis
At our institution, we perform universal screening for GDM between 24 and 28 weeks' gestation with a 50-g, 1-hour oral glucose load. Women who screened positive with plasma glucose values ≥140 mg/dL during the study period underwent diagnostic testing with a 100-g, 3-hour oral glucose tolerance test (OGTT). Women diagnosed with GDM based solely on the results of their 1-hour oral glucose load (plasma glucose ≥200 mg/dL or at the discretion of provider) were not included in the analysis. During the study period, National Diabetes Data Group (NDDG) diagnostic criteria were followed (fasting, ≥105 mg/dL; 1 hour, ≥190 mg/dL; 2 hour, ≥165 mg/dL; 3 hour, ≥145 mg/dL), and women with two or more OGTT values above thresholds were diagnosed with GDM.19
Women were cared for by attending physicians and fellows and residents under attending supervision, using the following protocol: after diagnosis, women received nutritional counseling with instruction for glucose self-monitoring. Diet recommendations initially follow the American Dietetic Association regarding carbohydrate intake, based on body mass index mg/kg2 (BMI), and were tailored to the individual patient as indicated.20 Capillary blood glucose goals were fasting <105 mg/dL and 1-hour postprandial <140 mg/dL or 2-hour postprandial <130 mg/dL. Patient report of glucose self-monitoring was reviewed at each clinic visit, and adequate glycemic control was defined as ≥50% of blood glucose levels at goal levels. Medical therapy was initiated (subcutaneous insulin, also oral glyburide as of 2004) if these goal levels were not achieved with diet alone.
Data abstraction
We abstracted maternal demographic data and pregnancy diagnoses from the UNC Perinatal Database (PD) to create our research database. Clinical providers prospectively record perinatal data from all deliveries at UNC, and trained abstractors enter information into and maintain the PD. Women who did not deliver at UNC, regardless of any care received at this institution, were not included in the PD. Before analysis, outliers and clinically implausible values were identified by exploratory analysis and corrected by review of original paper charts and electronic medical records (EMRs). A random sample of 200 records was cross-referenced with original paper charts and EMRs to ascertain accuracy of key variables.
Race/ethnicity was recorded during prenatal care as Caucasian, African American, Hispanic, Asian, or other/not reported. These data were abstracted from the UNC PD for analysis. As the Hispanic population in North Carolina was 63% of Mexican origin and 37% of non-Mexican origin in 2009 and has consistently increased throughout our study period, self-reported race/ethnicity reflects the nonhomogeneous nature of this group.21
Perinatal outcomes
We assessed perinatal outcomes found to improve with treatment of mild GDM in randomized controlled trials.2,4,22 Birth weight in grams and gestational age at delivery were abstracted as continuous variables and further considered as categorical variables. Other abstracted categorical variables included mode of delivery (spontaneous vaginal, operative vaginal, cesarean), 3rd or 4th degree perineal laceration, preeclampsia, gestational hypertension, shoulder dystocia, neonatal intensive care unit (NICU) admission, and NICU stay length >24 hours. We classified GDM management as diet control if a woman did not receive any medical management (insulin or glyburide) during pregnancy and as medical management if the medical record reflected any administration of glyburide or insulin during pregnancy.
Statistical analysis
We analyzed maternal demographic variables and pregnancy diagnoses among women with GDM by three self-reported racial/ethnic groups: Caucasian, African American, and Hispanic. Bivariate analysis included one-way analysis of variance (ANOVA) and Kruskal-Wallis for continuous variables and Pearson's chi-square for categorical variables, with p<0.05 statistically significant. Means with standard deviations (SD) and medians with interquartile ranges (IQR) were reported for continuous variables with normal and nonnormal distributions, respectively.
In multivariate analyses of adverse perinatal outcomes, African American and Hispanic women were individually compared with the reference group, Caucasian women. We compared the prevalence of each dichotomous adverse outcome, fitting Poisson regression models with robust standard errors (SE) to account for women with more than one pregnancy in our database.23 Variables significant in bivariate analysis or identified in published literature as clinical risk factors for the outcome of interest were considered for inclusion in adjusted models. All final Poisson regression models included 1-hour oral glucose load results as a continuous variable and tobacco use in pregnancy as a dichotomous variable. Gestational age at delivery was not included in models, as adjusting for this intermediate in the causal pathway between GDM and selected outcomes can produce biased estimates of direct effects.24 We reported unadjusted and adjusted prevalence ratios (PR, aPR) with 95% confidence intervals (CI), and CIs that excluded the null were statistically significant. Stata 10.0 (StataCorp, College Station, TX) was used for all analyses.
Results
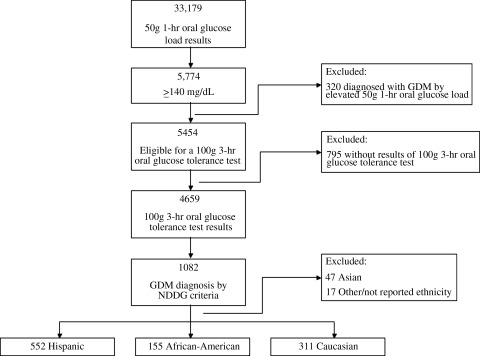
From April 1, 1996, to May 31, 2010, 33,179 women of any race/ethnicity were screened for GDM with a 50-g 1-hour oral glucose load and met initial study inclusion criteria. Overall disease prevalence among the 33,179 women screened for GDM was 3.3 % (1,082 of 33,179). GDM prevalence was greatest among Hispanic women, 4.9% (552 of 11,201), followed by African American women at 2.6% (155 of 5,877). Caucasian women had the lowest GDM prevalence, 2.2% (311 of 14,412). Figure 1 illustrates our study sample from eligibility through GDM diagnosis; 320 women classified as GDM and referred for treatment based solely on an elevated screening test (≥200 mg/dL or at the discretion of the primary provider) were not included in further analysis. Compared with the 1,018 women who comprised our final sample for analysis, these 320 women not included were less likely to be Hispanic (44% vs. 54%, p<0.05), more likely to be African American (22% vs. 15%, p<0.05), and equally likely to be Caucasian (34% vs. 31%, p≥0.05). Compared with screen-positive women who underwent a 3-hour OGTT result, the 15% who did not have 3-hour OGTT results available (795 of 5,454) were less likely to be Hispanic (37% vs. 45%, p<0.05) and more likely to be Caucasian (40% vs. 36%, p<0.05) or African American (18% vs. 12%) (p<0.001). This 15%, or 795 women, had 1-hour glucose load values of 150 mg/dL (IQR 144–162) compared with median values of 153 mg/dL (IQR 146–165) among women who completed a 3-hour OGTT but did not meet diagnostic criteria for GDM.
FIG. 1.
Flow of gestational diabetes mellitus (GDM) cohort, from eligibility through sample for analysis. NDDG, National Diabetes Data Group.
Of the 4,659 women who underwent diagnostic testing, 23% (1,082 of 4,659) were diagnosed with and treated for GDM by NDDG criteria. After excluding women of Asian and other or unknown (n=64), our final sample of 1,018 women with GDM was 54% Hispanic (552 of 1,018), 31% Caucasian (311 of 1,018), and 15% African American (155 of 1,018). Fifty-three percent (543 of 1,018) of the study sample of diagnosed and treated women achieved goal glucose thresholds with nutritional counseling and dietary management alone. The remaining 47% (475 of 1018) were treated with medical management with either glyburide or insulin. Hispanic women were more likely to be treated with medical management than either Caucasian (52% vs. 39%, p<0.001) or African American (52% vs. 45%, p=0.002) women. Maternal demographic and pregnancy data are shown in Table 1.
Table 1.
Characteristics by Race/Ethnicity
| |
Mean (SD), median (IQR), or no (%)a |
||
|---|---|---|---|
| Hispanic (n=552) | Caucasian (n=311) | African American (n=155) | |
| Maternal age at delivery (years) | 30.5 (5.8) | 31.1 (5.8) | 30.1 (6.6) |
| Maternal age at delivery ≥35 years | 111 (20) | 83 (27) | 37 (24) |
| 1-Hour oral glucose load screening test (mg/dL)* | 171 (157–191) | 162 (151–179) | 174 (156–195) |
| 3-Hour glucose load diagnostic test (mg/dL), fasting (n=1,004) | 95 (85–107) | 94 (84–106) | 96 (86–109) |
| Medical management (vs. diet-control only)* | 285 (52) | 120 (39) | 70 (45) |
| Any tobacco use reported in pregnancy* | 3 (0.5) | 54 (17) | 20 (13) |
| Multiparity* | 420 (76) | 163 (52) | 95 (61) |
| Chronic hypertension* | 23 (4) | 37 (12) | 28 (18) |
| Multiple gestation | 12 (2) | 13 (12) | 4 (3) |
| History of preeclampsia* | 26 (5) | 29 (9) | 9 (6) |
| History of gestational diabetes | 16 (3) | 14 (5) | 9 (6) |
| Placenta previa diagnosis | 12 (2) | 3 (1) | 1 (1) |
| Induction of labor | 208 (38) | 114 (37) | 59 (18) |
| Prior cesarean delivery | 120 (22) | 51 (16) | 28 (18) |
| Breech presentation* | 9 (2) | 14 (5) | 3 (2) |
| Placental abruption | 6 (1) | 4 (1) | 2 (1) |
| Preterm premature rupture of membranes* | 28 (5) | 36 (12) | 22 (14) |
Numbers may not add up to 100 because of rounding.
p<0.05.
SD, standard deviation; IQR, interquartile range; no, number.
As shown in Table 2, in unadjusted bivariate analysis, Hispanic women delivered infants of greater birth weight and at greater median gestational ages and had fewer preterm births <37 and <34 weeks compared with Caucasian women. African American women were more likely to have each of these measured outcomes (p<0.05 for each comparison) compared with Caucasian women.
Table 2.
Perinatal Outcomes Among Women Diagnosed with Gestational Diabetes Mellitus by 3-Hour Oral Glucose Tolerance Test (n=1018), by Three Categories of Race/Ethnicity
| |
Median (IQR) or n (%)a |
||
|---|---|---|---|
| Variable | Hispanic (n=552) | Caucasian (n=311) | African American (n=155) |
| Birth weight (grams)* | 3,453 (3,050–3,812) | 3,291 (2,869–3,743) | 3,260 (2,850–3,750) |
| Macrosomia >4,000 g | 90 (16) | 37 (12) | 15 (10) |
| Low birth weight <2,500g* | 43 (8) | 44 (14) | 23 (15) |
| Very low birth weight <1,500g* | 2 (0.4) | 6 (2) | 7 (5) |
| Cesarean (vs. spontaneous vaginal delivery, n=957) | 198 (38) | 117 (41) | 66 (45) |
| Operative (vs. spontaneous vaginal delivery, n=637) | 27 (8) | 27 (14) | 7 (8) |
| 3rd or 4th degree laceration | 16 (3) | 15 (5) | 3 (2) |
| Gestational age at delivery (weeks)* | 39.0 (38.1–39.9) | 38.9 (37.3–39.9) | 38.7 (37.1–39.6) |
| Preterm delivery <37 weeks* | 67 (12) | 66 (21) | 32 (21) |
| Preterm delivery <34 weeks* | 19 (3) | 20 (6) | 13 (8) |
| Preeclampsia | 36 (7) | 24 (8) | 19 (12) |
| Gestational hypertension* | 16 (3) | 23 (7) | 10 (6) |
| Shoulder dystocia* | 28 (5) | 5 (2) | 7 (5) |
| NICU admission* | 160 (29) | 123 (40) | 51 (33) |
| >24-hour NICU stay (if admitted)* | 118 (74) | 105 (89) | 44 (90) |
Numbers may not add up to 100 because of rounding.
p<0.05, significant differences among groups by one-way ANOVA, Kruskal-Wallis tests.
NICU, neonatal intensive care unit; IQR, interquartile range; no, number.
Adjusted maternal and infant outcomes are shown in Table 3. Hispanic and African American women were each compared with the reference group, Caucasian women. Adverse outcomes that were significantly different in unadjusted analyses remained significant in final adjusted models. Adjusting for 1-hour oral glucose load results and tobacco use in pregnancy did not substantially change the magnitude or precision of results. Hispanic women had lower likelihood of LBW infant (aPR 0.54, 95% CI 0.36-0.82) and very low birth weight (VLBW) infant (aPR 0.20, 95% CI 0.04-1.01) compared with Caucasian women. Macrosomia risk, however, was 21% greater (aPR 1.21, 95% CI 0.83-1.76) among Hispanic women compared with Caucasian women. Shoulder dystocia was statistically more common among Hispanic women compared with Caucasian women (aPR 3.52, 95% CI 1.30-9.50). Hispanic women were significantly less likely to deliver preterm <37 weeks (aPR 0.58, 95% CI 0.42-0.81) and <34 weeks (aPR 0.51, 95% CI 0.27-0.96), compared with Caucasian women.
Table 3.
Unadjusted and Adjusted Prevalence Ratios of Perinatal Outcomes Among Hispanics and African Americans Compared to Reference Group, Caucasians
| |
Hispanic vs. Caucasian |
African American vs. Caucasian |
||
|---|---|---|---|---|
| Perinatal outcomes | PR (95% CI)a | aPR (95% CI)a | PR (95% CI) | aPR (95% CI) |
| Medical management (vs. diet control only) | 1.36 (1.15-1.60) | 1.31 (1.10-1.56) | 1.18 (0.94-1.48) | 1.09 (0.87-1.37) |
| Macrosomia <4,000 g | 1.36 (0.95-1.96) | 1.21 (0.83-1.76) | 0.83 (0.47-1.46) | 0.80 (0.42-1.37) |
| Low birth weight <2,500 g | 0.55 (0.37-0.82) | 0.54 (0.36-0.82) | 1.05 (0.66-1.67) | 0.95 (0.60-1.51) |
| Very low birth weight <1,500 g | 0.19 (0.04-0.95) | 0.20 (0.04-1.01) | 2.34 (0.80-6.84) | 2.05 (0.70-6.03) |
| Cesarean section (vs. spontaneous vaginal delivery) | 0.91 (0.76-1.08) | 0.88 (0.73-1.06) | 1.08 (0.86-1.36) | 1.07 (0.85-1.35) |
| Operative (vs. spontaneous vaginal delivery) | 0.53 (0.32-0.88) | 0.45 (0.27-0.77) | 0.57 (0.26-1.25) | 0.52 (0.24-1.15) |
| 3rd or 4th degree perineal laceration | 0.60 (0.30-1.20) | 0.52 (0.26-1.04) | 0.40 (0.12-1.37) | 0.38 (0.11-1.34) |
| Preterm delivery <37 weeks | 0.58 (0.43-0.80) | 0.58 (0.42-0.81) | 1.00 (0.68-1.45) | 0.92 (0.63-1.35) |
| Preterm delivery <34 weeks | 0.54 (0.29-0.99) | 0.51 (0.27-0.96) | 1.30 (0.67-2.55) | 1.15 (0.59-2.27) |
| Preeclampsia | 0.86 (0.52-1.42) | 0.89 (0.51-1.54) | 1.61 (0.91-2.88) | 1.49 (0.82-2.70) |
| Gestational hypertension | 0.39 (0.21-0.73) | 0.43 (0.22-0.83) | 0.86 (0.42-1.77) | 0.91 (0.45-1.86) |
| Shoulder dystocia | 3.13 (1.22-8.00) | 3.52 (1.30-9.50) | 2.76 (0.89-8.56) | 2.46 (0.78-7.80) |
| NICU admission (n=326) | 0.73 (0.60-0.88) | 0.72 (0.59-0.88) | 0.83 (0.63-1.08) | 0.80 (0.61-1.05) |
| >24-hour NICU stay (if admitted) | 0.83 (0.75-0.93) | 0.81 (0.72-0.90) | 1.01 (0.90-1.13) | 1.00 (0.89-1.13) |
All adjusted models include 1-hour oral glucose load results modeled as a continuous variable and any tobacco use in pregnancy as a dichotomous variable.
aPR, adjusted prevalence rate; CI, confidence interval; PR, prevalence ratio.
The prevalence of hypertensive disorders of pregnancy was also considered in multivariate analysis. Hispanic women were less than half as likely to be diagnosed with gestational hypertension in both unadjusted and adjusted models (aPR 0.43, 95% CI 0.22-0.83) compared with Caucasian women. However, the prevalence of preeclampsia (mild or severe) was similar between the two racial/ethnic groups in both adjusted and unadjusted models (Table 3).
NICU admission and length of stay were examined. Compared with infants of Caucasian women, infants of Hispanic mothers were nearly 30% less likely to be admitted to the NICU (aPR 0.72, 95% CI 0.59-0.88). Among infants admitted to the NICU, Hispanic infants were 0.81 times as likely to be admitted for >24 hours (aPR 0.81, 95% CI 0.72-0.90) as Caucasian infants (Table 3).
In contrast to comparisons between Hispanic and Caucasian women, outcomes were similar between African American and Caucasian women for most measured outcomes. In unadjusted analyses, African American women were not more likely to experience our measured adverse perinatal outcomes compared with Caucasian women. In adjusted models, controlling for 1-hour glucose load results and tobacco use in pregnancy, African American women remained equally likely as Caucasian women to be diagnosed with other adverse outcomes (Table 3).
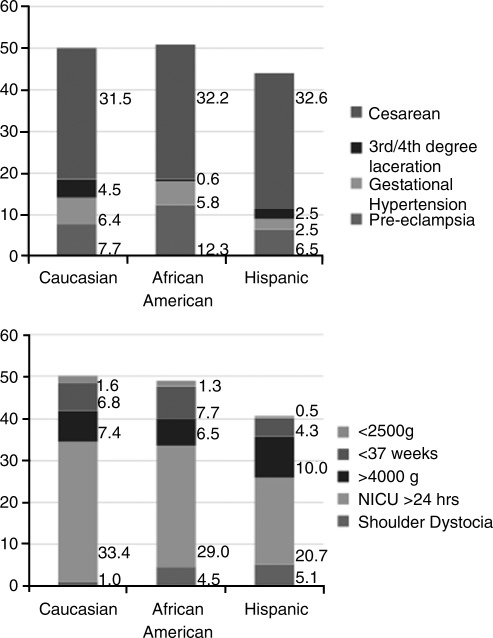
Overall, Hispanic women were least likely to experience one of our measured maternal or neonatal adverse outcomes. Figure 2 shows the distribution of these outcomes. Half of all Caucasian and African American women, compared with 44% of Hispanic women, had at least one maternal adverse outcome (p<0.05). Similarly, half of all Caucasian and African American women, compared with 41% of Hispanic women, had at least one adverse perinatal outcome (p<0.05).
FIG. 2.
Adverse maternal and neonatal outcomes are more common among Caucasian and African American women than among Hispanic women (p<0.05): among maternal outcomes, Caucasian 50% (95% CI 44%-56%), African American 51% (95% CI 43%-59%), and Hispanic 44% (95% CI 40%-48%); among neonatal outcomes, Caucasian 51% (95% CI 45%-57%), African American 49% (95% CI 39%-55%), Hispanic 41% (95% CI 37%-45%).
Discussion
In a large retrospective cohort study of women in North Carolina, GDM prevalence was approximately twice as common among Hispanic women compared with Caucasian and African American women. Hispanic women with GDM were more likely to be prescribed medical therapy compared with Caucasian women. Hispanic women were at lower risk for preterm delivery, hypertensive disorders of pregnancy, and NICU admission compared with Caucasian women but were at greater risk of shoulder dystocia. In contrast to Hispanic women, outcomes among African American women were similar to those of Caucasian women. Overall, Hispanic women had fewer maternal and neonatal adverse outcomes. The overall prevalence of at least one measured adverse outcome, however, remained high in all racial/ethnic groups.
Our findings differ from other studies measuring GDM outcomes between Hispanic and non-Hispanic women. Among an ethnically diverse California population of women with GDM, Hispanic women were just as likely as Caucasian women to require medical management of GDM and to deliver preterm at <37 or <34 weeks.15 Hispanic women in our cohort were nearly half as likely to deliver a preterm infant at <37 or <34 weeks or to deliver an LBW or VLBW, although they were more likely to be prescribed medical management of their GDM. Less preterm birth among Hispanic women with GDM follows a phenomenon known as the Hispanic Paradox, describing some better than expected health outcomes in this racial/ethnic group.12 We report a nonstatistical increased risk of macrosomia (>4,000 g) among Hispanic women compared with Caucasian women, whereas other studies found that Hispanic women were statistically more likely to deliver an infant <4,000 g.13, 14 As overall prevalence of macrosomia was small in each retrospective analysis, however, low power to detect true differences may explain disparate findings.
Several strengths of our study exist. We examined a large, clinically representative sample of all women delivered at our institution who were diagnosed with GDM. Our 14-year database of pregnancy diagnoses and pertinent adverse outcomes avoids selection bias that exists when individuals are recruited and enrolled in research studies. Further, using GDM treatment data, we reported that Hispanic women in our study were more likely to be prescribed medical treatment compared with Caucasian women. These results suggest that there are racial/ethnic differences in overall glycemic control. These treatment differences may explain differences in perinatal outcomes compared with prior studies. Finally, differences in analytic approach may explain some differences in our findings compared to findings in other work. We used Poisson regression modeling and did not adjust for intermediates, such as gestational age at delivery, that might otherwise have introduced bias.24
Our findings must be interpreted in the context of the retrospective study design. Although GDM treatment with glyburide or insulin was initiated and increased based on established glucose thresholds in our prenatal clinic, we cannot definitively report the percentage of women who achieved adequate vs. poor glycemic control. Maternal height and prepregnancy weight were not recorded in the UNC PD throughout our study period. As we were unable to use these variables, gestational weight gain, or BMI data in our analysis, this may bias our findings, as obesity is associated with pregnancy complications, including GDM, cesarean delivery, and macrosomia.25
The observed associations between race/ethnicity and perinatal outcomes may not reflect causal effects but rather noncausal associations that arise in the subpopulation of women with a diagnosis of GDM.26 As in any observational study, we cannot determine causal associations. In addition, measures of nativity or acculturation or proxies, such as language preference, were not available in this cohort but have been shown to impact perinatal outcomes9,27,28 and should be included in future work examining ethnic differences in health outcomes. Although our study cohort likely reflects the demographics of Hispanic women in North Carolina, the majority of whom are of Mexican origin, race/ethnicity still describes a heterogeneous group. Caution should be used if extrapolating our results by race/ethnicity to other Hispanic groups.
Finally, we excluded women who were diagnosed based on elevated 1-hour oral glucose load results or at provider discretion and excluded women who did not undergo the recommended 3-hour OGTT. If Hispanic women with more severe glucose intolerance were more likely to be treated presumptively or were more likely to not undergo the 3-hour OGTT, this could have biased our results. However, Hispanic women were actually least likely to be excluded and, among our final sample, had the greatest prevalence of GDM. This reduces our potential for bias. In fact, our data may underestimate the potential differences in perinatal outcomes, but we a priori determined and maintained our analysis inclusion criteria, including standard of care GDM screening and diagnostic tests.
Interestingly, Hispanic women had fewer measured maternal and neonatal adverse outcomes overall compared with Caucasian and African American women, although not all outcomes were less common. Hispanic women were more likely to be prescribed medical treatment of GDM and were less likely to have gestational hypertension, a diagnosis known to improve with glycemic control. However, they were more likely to have a shoulder dystocia at delivery, with an infant birth weight approximately 150 g greater than that of Caucasian infants born at similar gestational ages.
Although we know that strict glycemic control translates to lower risk of GDM-associated adverse outcomes, perhaps racial/ethnic differences exist in etiologies of hyperglycemia in pregnancy. If, for instance, GDM among Hispanic women is related to poor beta cell function, the need for medical management to improve glycemic control is logical. If inflammatory pathophysiology plays a greater role, however, among African American or Caucasian women compared with Hispanic women, those two racial/ethnic groups may be more responsive to diet control. Differences in risk of hypertension by ethnicity have been demonstrated outside of pregnancy, and underlying mechanisms are the focus of ongoing research.11,29 Our data illustrate different needs in GDM management and associated outcomes, but we do not yet know how specific racial/ethnic groups would benefit from tailored treatment. How we should adequately measure and achieve glycemic control to optimize perinatal outcomes may differ by race/ethnicity. These plausible but largely unstudied etiologies of racial/ethnic differences in achieving glycemic control should be the focus of ongoing and future research.
Conclusions
Our data demonstrate that racial/ethnic differences exist among women with GDM, particularly when Hispanic women are compared with Caucasian women. These differences may indicate a need for more aggressive treatment of GDM in Hispanic women coupled with improved measures of glycemic control. Hispanic groups are the largest and fastest growing ethnic minority in the United States,18 and efforts that improve perinatal outcomes among this group can have further reaching positive public health implications. Further studies are needed to determine the mechanisms underlying our finding of lower rates of preterm birth and hypertensive complications but higher rates of macrosomia-related outcomes among Hispanic women.
Acknowledgments
E.K.B. is supported, in part, by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grant number T32 HD30672-01 as a clinical Training in Epidemiology and Clinical Trials (TECT) fellow, Department of Obstetrics and Gynecology, University of North Carolina at Chapel Hill. M.J.F. is supported by the Agency for Healthcare Research and Quality through a career development award (K02 HS17950) and has received salary support from GlaxoSmithKline through the UNC Center for Excellence in Pharmacoepidemiology & Public Health.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Dabelea D. Snell-Bergeon JK. Hartsfield CL, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28:579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 2.Metzger BE. Lowe LP. Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 3.Langer O. Yogev Y. Most O. Xenakis EM. Gestational diabetes: The consequences of not treating. Am J Obstet Gynecol. 2005;192:989–997. doi: 10.1016/j.ajog.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Crowther CA. Hiller JE. Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 5.Getahun D. Nath C. Ananth CV. Chavez MR. Smulian JC. Gestational diabetes in the United States: Temporal trends 1989 through 2004. Am J Obstet Gynecol. 2008;198(525):e1–5. doi: 10.1016/j.ajog.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Thorpe LE. Berger D. Ellis JA, et al. Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990–2001. Am J Public Health. 2005;95:1536–1539. doi: 10.2105/AJPH.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara A. Hedderson MM. Quesenberry CP. Selby JV. Prevalence of gestational diabetes mellitus detected by the National Diabetes Data Group or the Carpenter and Coustan plasma glucose thresholds. Diabetes Care. 2002;25:1625–1630. doi: 10.2337/diacare.25.9.1625. [DOI] [PubMed] [Google Scholar]
- 8.Brown HL. Chireau MV. Jallah Y. Howard D. The “Hispanic paradox”: An investigation of racial disparity in pregnancy outcomes at a tertiary care medical center. Am J Obstet Gynecol. 2007;197(197):e1–7. doi: 10.1016/j.ajog.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 9.McGlade MS. Saha S. Dahlstrom ME. The Latina paradox: An opportunity for restructuring prenatal care delivery. Am J Public Health. 2004;94:2062–2065. doi: 10.2105/ajph.94.12.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung JH. Boscardin WJ. Garite TJ. Lagrew DC. Porto M. Ethnic differences in birth weight by gestational age: At least a partial explanation for the Hispanic epidemiologic paradox? Am J Obstet Gynecol. 2003;189:1058–1062. doi: 10.1067/s0002-9378(03)00848-2. [DOI] [PubMed] [Google Scholar]
- 11.Stommel M. Schoenborn CA. Variations in BMI and prevalence of health risks in diverse racial and ethnic populations. Obesity (Silver Spring) 2010;18:1821–1826. doi: 10.1038/oby.2009.472. [DOI] [PubMed] [Google Scholar]
- 12.Gallo LC. Penedo FJ. Espinosa de los Monteros K. Arguelles W. Resiliency in the face of disadvantage: Do Hispanic cultural characteristics protect health outcomes? J Pers. 2009;77:1707–1746. doi: 10.1111/j.1467-6494.2009.00598.x. [DOI] [PubMed] [Google Scholar]
- 13.Saldana TM. Siega-Riz AM. Adair LS. Savitz DA. Thorp JM., Jr. The association between impaired glucose tolerance and birth weight among black and white women in central North Carolina. Diabetes Care. 2003;26:656–661. doi: 10.2337/diacare.26.3.656. [DOI] [PubMed] [Google Scholar]
- 14.Dunne FP. Brydon PA. Proffitt M, et al. Fetal and maternal outcomes in Indo-Asian compared to Caucasian women with diabetes in pregnancy. Q J Med. 2000;93:813–818. doi: 10.1093/qjmed/93.12.813. [DOI] [PubMed] [Google Scholar]
- 15.Esakoff TF. Caughey AB. Block-Kurbisch I. Inturrisi M. Cheng YW. Perinatal outcomes in patients with gestational diabetes mellitus by race/ethnicity. J Matern Fetal Neonatal Med. 2010 doi: 10.3109/14767058.2010.504287. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Mocarski M. Savitz DA. Ethnic differences in the association between gestational diabetes and pregnancy outcome. Matern Child Health J. 2011 doi: 10.1007/s10995-011-0760-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg TJ. Garbers S. Lipkind H. Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: Differences among 4 racial/ethnic groups. Am J Public Health. 2005;95:1545–1551. doi: 10.2105/AJPH.2005.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statistical portrait of Hispanics in the United States, 2006. Washington, DC: Pew Hispanic Center; 2008. [Google Scholar]
- 19.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 20.American Dietetic Association. 2011. www.eatright.org/ [Feb 1;2011 ]. www.eatright.org/
- 21.Statistical portrait of Hispanics in the United States, 2009. Washington, DC: Pew Hispanic Center; 2011. [Google Scholar]
- 22.Landon MB. Spong CY. Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton P. Gurrin L. Sly P. Extending the simple linear regression model to account for correlated responses: An introduction to generalized estimating equations and multi-level mixed modelling. Stat Med. 1998;17:1261–1291. doi: 10.1002/(sici)1097-0258(19980615)17:11<1261::aid-sim846>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 24.Robins JM. Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3:143–155. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Weiss JL. Malone FD. Emig D, et al. Obesity, obstetric complications and cesarean delivery rate—A population-based screening study. Am J Obstet Gynecol. 2004;190:1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 26.Hernan MA. Hernandez-Diaz S. Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 27.Cobas JA. Balcazar H. Benin MB. Keith VM. Chong Y. Acculturation and low-birthweight infants among Latino women: A reanalysis of NHANES data with structural equation models. Am J Public Health. 1996;86:394–396. doi: 10.2105/ajph.86.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landale NS. Oropesa RS. Gorman BK. Migration and infant death: Assimilation or selective migration among Puerto Ricans? Am Sociol Rev. 2000;65:888–909. [Google Scholar]
- 29.Foy CG. Hsu FC. Haffner SM, et al. Visceral fat and prevalence of hypertension among African Americans and Hispanic Americans: Findings from the IRAS family study. Am J Hypertens. 2008;21:910–916. doi: 10.1038/ajh.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]