Abstract
Background
Maternal plasma lipids, including total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), increase during pregnancy, remaining elevated over prepregnancy levels through the immediate postpartum period. Triglycerides decrease rapidly to prepregnancy levels after delivery. Few data on postpartum lipid levels are available, and levels in postpartum women with depression have not been evaluated. We sought to determine the cross-sectional levels of total cholesterol, LDL-C, HDL-C, and triglycerides between 1 and 14 weeks postpartum in postpartum women with DSM-4 diagnoses of major depression and determine if they are similarly elevated to published levels in other postpartum populations.
Methods
As part of screening for a randomized controlled trial comparing treatments for postpartum depression (PPD), women (n=120) had postpartum fasting lipid levels determined. Linear regression models were used to assess the association between time postpartum and lipid levels. Analysis of covariance models (ANCOVA) assessed the association of baseline characteristics with lipids.
Results
Total cholesterol levels were >200 mg/dL in 45% of the sample at baseline. Mean baseline total cholesterol was 196±39 mg/dL. There was an inverse linear relationship between postpartum week and total cholesterol, with cholesterol values decreasing an average of 4.5 mg/dL per week. Similarly, LDL-C and HDL-C trended down over time. Triglycerides were stable and within the normal range during the observation period.
Conclusions
Total cholesterol, HDL-C, and LDL-C are significantly elevated in the early postpartum period and do not return to <200 mg/dL until 6 weeks postpartum in women with PPD. The magnitude and duration of elevation are consistent with the sparse published data on nondepressed women.
Introduction
Pregnancy alters maternal lipid metabolism. Total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride levels rise throughout the second and third trimester, peaking just before term at approximately 36 weeks of gestational age.1–3 Previous studies4–6 have noted that lipid levels, except for triglycerides, remain elevated above prepregnancy values after delivery. The average duration of lipid elevation and the time of return to prepregnancy levels, if that is achieved, is not well studied. Pregnancy may permanently alter or reset the lipid profile in women. For healthy, nonpregnant women, optimal values for total cholesterol are <200 mg/dL, for LDL-C<100 mg/dL, and for HDL-C between 40 and 59 mg/dL.7 Elevated total cholesterol is associated with increased risk for cardiovascular disease (CVD) and early death.8
Multiple large, well-designed studies have conclusively shown that elevated cholesterol is an independent risk factor for coronary heart disease (CHD) and early death.9,10 Epidemiologic data have been used to set guidelines for optimal lipid levels, as outlined in the Adult Treatment Panel (ATP) III.7 These data underlie the recommendation for a target total cholesterol level ≤200 mg/dL.7 These studies excluded women who were pregnant or breastfeeding.
Postpartum depression (PPD) affects 14.5% women in the United States,11 has long-lasting negative effects on parenting and child development, and is infrequently diagnosed and inadequately treated.12 Risk factors for PPD include lower socioeconomic status, a history of prior depression, and stressful life events. Major depression is a risk factor for future CVD,8,13 and pregnant women with major depression are more likely to be overweight and obese.14,15 To our knowledge, there are no published data on postpartum lipid profiles in women with PPD; postpartum women with depression may have lipid levels higher than nondepressed postpartum women because of modifiable behavioral or genetic risk factors. This could, in turn, further increase their future risk for CVD and early death.
We sought to determine the cross-sectional levels of total cholesterol, LDL-C, HDL-C, and triglycerides between 1 and 14 weeks postpartum in postpartum women with DSM-4 diagnoses of major depression and determine if they are similarly elevated to published levels in other postpartum populations. Women with PPD who sought evaluation for a randomized controlled trial (RCT) comparing treatments for PPD had baseline plasma measurements, including total cholesterol, HDL-C, LDL-C, and triglyceride levels, obtained at enrollment to the clinical trial within 99 days (14.1 weeks) postpartum and analyzed to account for multiple demographic and health risk factors.
Materials and Methods
This study was reviewed and approved the University of Pittsburgh Institutional Review Board and registered at www.clinicaltrials.gov (NCT00744328). Women (n=120) were screened for the RCT. Eligible women met the inclusion criteria: aged 18–45 years, able to provide written informed consent in English, met criteria for major depressive disorder (MDD) by Structured Clinical Interview for DSM-4 Disorders (SCID) research interview,16 had normal laboratory evaluations (including thyroid, hematologic and hepatic functions, and negative urine pregnancy tests), and were within 14.1 weeks (99 days) of delivery. Participants were recruited from the community, three maternity hospitals, and physicians' offices in Western Pennsylvania. Women with psychosis were excluded. Women had to appear for evaluation between delivery and 99 days (14.1 weeks) postpartum; therefore, the baseline values are distributed though this time frame. Fasting blood was collected at the baseline assessment visit. Total cholesterol, HDL-C, LDL-C, and triglycerides were measured at a single laboratory facility using the Siemens Dimension clinical chemistry system (Deerfield, IL). Postpartum fasting lipids were measured on 120 subjects who met inclusion criteria and are included in this analysis.
Baseline characteristics, including age, ethnicity, education, marital status, and obstetric history, were obtained by interview. Infant feeding method (exclusively breastfeeding, breast milk and formula, exclusively formula feeding) was determined by participant self-report as well as feedings observed by study staff.
Summary statistics, means and standard deviations (SD) for continuous variables, and percentages for discrete variables were used to describe the sample. Linear regression models were used to assess the association between time and cholesterol levels. Separate analysis of covariance (ANCOVA) models were used to assess the association of baseline characteristics with cholesterol, HDL-C, LDL-C, and triglycerides. All multivariable results were adjusted for time since delivery.
Results
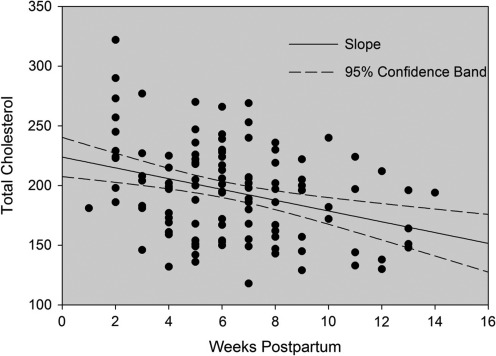
Characteristics of the sample at baseline are presented in Table 1; 61% of the sample were white, 35% African American, and 4% other. The mean age was 25.8 years. Mean gravidity was 2.6 and parity was 2.0. Prepregnancy mean body mass index (BMI) was available for 89 of 120 subjects (77%) and was 29.1 kg/m2. The results are summarized in Figure 1 and Table 2. At baseline, 45% of subjects had total cholesterol ≥200 mg/dL. Total cholesterol decreased linearly with week postpartum (Fig. 1). On average, total cholesterol decreased 4.5 mg/dL per week over the 14-week study period.
Table 1.
Sample Characteristics (n=120)
| Characteristic | Mean±SD n (%)a |
|---|---|
| Age | 25.8±5.6 |
| Ethnicity | |
| White | 70 (61.4) |
| African American | 40 (35.1) |
| Other | 4 (3.7) |
| Education | |
| <High school | 12 (12.6) |
| High school | 28 (29.5) |
| Some college | 36 (37.2) |
| College | 15 (15.8) |
| Postgraduate | 4 (4.2) |
| Marital status | |
| Married/partner | 46 (46.9) |
| Single | 52 (53) |
| Gravidity | 2.6±1.7 |
| Parity | 2.0±1.1 |
| BMI (prepregnancy) | 29.1±8.5 |
| Completed weeks gestation | 38.9±1.7 |
| Mode of delivery | |
| Vaginal (including operative) | 67 (65.7) |
| Cesarean section | 35 (34.3) |
| Infant feeding method | |
| Breast | 29 (25.0) |
| Formula | 68 (58.6) |
| Both | 19 (16.4) |
| Pregnancy complications | |
| GDM | 4 (3.4) |
| HTN | 13 (11.2) |
| Preeclampsia | 8 (6.9) |
| Days postpartum at baseline blood draw | 47.2±19.9 |
| Baseline HDL-C (mg/dL) | 50.4±14.0 |
| Baseline LDL-C (mg/dL) | 125±34 |
| Baseline total cholesterol (mg/dL) | 196±39 |
| Total cholesterol >200 mg/dL | 54 (45) |
All descriptive statistics are based on available data.
BMI, body mass index, defined as weight (kg)/height(m)2; GDM, gestational diabetes; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension during pregnancy; LDL-C, low density lipoprotein cholesterol.
FIG. 1.
Total cholesterol (mg/dL) by weeks postpartum.
Table 2.
Multivariable Results: Beta Coefficients and Associated p Values by Lipid and Measure
| |
Total cholesterol |
High-density-lipoprotein cholesterol |
Low-density-lipoprotein cholesterol |
Triglycerides |
||||
|---|---|---|---|---|---|---|---|---|
| Measure | coefficienta | p | coefficient | p | coefficient | p | coefficient | p |
| Age | 2.0491 | 0.0005 | 0.8516 | <0.0001 | 0.7552 | 0.1725 | 0.0095 | 0.3283 |
| Race (Reference: white) | 0.2568 | 0.0268 | 0.9039 | 0.7951 | ||||
| Black | −12.1145 | −7.0949 | 0.0077 | −2.8356 | −0.0713 | |||
| Other | −5.7358 | 1.7218 | 0.8048 | −3.1781 | −0.1172 | |||
| Education level (Reference:<HS) | 0.0466 | 0.1270 | 0.4090 | 0.1941 | ||||
| High school | 13.6378 | 0.2796 | 3.3208 | 1.4485 | 0.3835 | |||
| Some college | 29.0213 | 0.0175 | 7.7548 | 13.5201 | 0.3317 | |||
| College | 19.8883 | 0.1597 | 12.2631 | 6.0686 | 0.0985 | |||
| Graduate school | 54.5697 | 0.0098 | 8.3635 | 28.1669 | 0.5407 | |||
| Marital status (Reference: single) | 0.5358 | 0.0687 | 0.8869 | 0.8994 | ||||
| Married/cohabiting | 8.5027 | 4.0723 | 3.0658 | −0.0558 | ||||
| Divorced/separated | −1.1925 | −8.6024 | −2.2533 | −0.0003 | ||||
| Prepregnancy BMI | 0.2806 | 0.5400 | −0.4551 | 0.0048 | 0.3470 | 0.4025 | 0.0159 | 0.0309 |
| During most recent pregnancy | ||||||||
| Diabetes | 14.4835 | 0.4470 | 7.8184 | 0.2712 | 4.9044 | 0.7715 | 0.2418 | 0.4239 |
| High BP | −14.9235 | 0.1680 | −6.9407 | 0.0851 | −7.9786 | 0.4075 | 0.0626 | 0.7178 |
| Preeclampsia | −19.4453 | 0.1488 | −2.9471 | 0.5615 | −15.3994 | 0.1972 | −0.0418 | 0.8464 |
| Vaginal delivery | 8.3138 | 0.2910 | −2.2526 | 0.4381 | 9.0991 | 0.1872 | 0.1147 | 0.3490 |
| Gestational age at delivery (weeks) | 1.1676 | 0.5749 | −0.2856 | 0.7067 | 1.2190 | 0.4962 | −0.0039 | 0.9025 |
| Infant feeding method (Reference: breast) | 0.9931 | <0.0001 | 0.6100 | 0.0004 | ||||
| Formula | −0.8219 | −14.9185 | <0.0001 | 7.2950 | 0.4790 | <0.0001 | ||
| Both | 0.0239 | −14.2676 | <0.0001 | 4.8129 | 0.5100 | 0.0017 | ||
| Parity | 3.4616 | 0.2968 | −0.6994 | 0.5717 | 1.2154 | 0.6846 | 0.0864 | 0.0977 |
| Parity (Reference: 1) | 0.2811 | 0.9966 | 0.4242 | 0.3353 | ||||
| 2 | −2.4821 | 0.2352 | −5.2320 | 0.0564 | ||||
| 3+ | 11.4416 | 0.1953 | 5.2312 | 0.2106 | ||||
| Gravidity | 2.1312 | 0.3035 | −1.0169 | 0.2021 | 0.9493 | 0.6069 | 0.0684 | 0.0393 |
All models adjusted for time since delivery.
For continuous measures, such as age, the coefficient indicates the amount of change in the dependent variable (e.g., total cholesterol) for each unit increase in the dependent variable. For dichotomous measures, such as vaginal delivery, the coefficient indicates the mean difference in the dependent variable for vaginal delivery=yes compared to vaginal delivery=no. For categorical measures, such as race, the coefficient indicates the mean difference in the dependent variable for race=black compared to race=white.
BP, blood pressure; HS, high school.
Older age was associated with higher total cholesterol and higher HDL-C. African American race was associated with lower HDL-C. Lower HDL-C levels also were associated with higher prepregnancy BMI and any formula feeding. Higher BMI was associated overall with a less favorable lipid profile, including higher triglycerides in addition to lower HDL-C. Triglyceride levels were higher in formula-feeding women than in exclusively breastfeeding women.
Discussion
In this cohort of 120 postpartum women with major depression, plasma lipids were observed to be elevated between 1 and 14 weeks postpartum. The very high early postpartum total cholesterol and LDL-C levels we found in postpartum women with depression are consistent with results in the sparse literature published more than three decades ago on general populations of postpartum women. In 1979, Potter and Nestel5 reported that plasma lipids were elevated postpartum. They followed 43 women through pregnancy and the first year after delivery and found total cholesterol elevated at an average just above 250 mg/dL at 6 weeks and still slightly higher than prepregnancy baseline at 52 weeks. Darmody and Postle17 studied 34 women and showed a similar pattern and values for total cholesterol at approximately 250 mg/dL at 6 weeks, but they reported a return to baseline by 40 weeks.
Van Stiphout et al.6 compared total cholesterol and HDL-C in 831 Dutch women and reported that women who had ever been pregnant had slightly higher total cholesterol and lower HDL-C levels than similar never pregnant women. Sixty women studied from early pregnancy through 40 days postpartum by Jimenez et al.1 showed persistently elevated total cholesterol at 40 days, with a mean of 240 mg/day. As in our study, women who breastfed had more favorable lipid profiles postpartum than had their formula-feeding peers.17,18 Physiologically, this is plausible, based on alterations in lipid metabolism required for lactation. It is also possible that breastfeeding is a marker for overall better health, as women who breastfeed tend to be healthier, with higher socioeconomic status and educational attainment, than formula-feeding women. The potential positive effect of breastfeeding on lipids could represent a reduction in long-term risk and is an additional reason to promote breastfeeding to improve public health.
Both older age and higher BMI have been shown to be associated with elevated total cholesterol and LDL-C, as in our study.19 Although African Americans have higher rates of CVD, race has not been consistently shown to be associated with differences in lipid profiles.20 Previous studies of postpartum lipids have shown no association with maternal age1,5,17,18,21 or weight.1,17,21 Several studies, however, either did not examine these covariants, did not report their significance, or only examined them in relation to change in lipids over time rather than for absolute lipid levels. Because of this, as well as the multiethnic nature of our sample and the increasing rate of obesity in the United States since some of the older studies were published, it is difficult to accurately compare these results.
HDL-C has been studied more recently. In two published reports from the Coronary Artery Risk Development in Young Adults (CARDIA) study,21,22 postpartum HDL-C was found to be decreased compared to prepregnancy levels for a period of 10 years after the first pregnancy, consistent with van Stiphout's findings.6 This is suggestive of long-term lipid alterations produced by pregnancy.
The strengths of this study include the relatively large sample of postpartum women who provided standardized, fasting measures of plasma lipids done in the same laboratory. Laboratory assessments for thyroid function, complete blood count (CBC), full metabolic screens, and detailed information on breastfeeding status were available for all participants. The data (Fig. 1) provide a visual representation of the decline in total cholesterol by postpartum week to aid interpretation of laboratory values obtained in postpartum women. We established the average time of return of cholesterol to the desired value of <200 mg/dL to be 6 weeks after birth. Study participants were recruited from a diverse community, which increases generalizability. Finally, PPD was diagnosed objectively with DSM-4 diagnostic interviews.
Although the sample size allows a reasonable evaluation of postpartum lipids, it is too small to identify contributions from pregnancy complications, such as preeclampsia or gestational diabetes. Likewise, it would be preferable to have prepregnancy BMI on all subjects, and some are missing from this sample. Because of methodologic differences, we are unable to directly compare the results from our depressed population with the populations previously studied. Finally, prepregnancy lipid levels for comparison would allow us to make interperson comparisons that we are unable to do with our current data.
The possible long-term effects of elevated postpartum lipids on health, including potential interaction with depression as well as cardiovascular health, are an area for further study. In depressed patients, lipoprotein structure is changed toward increasing LDL-C and higher atherogenic potential, and remission is associated with improvement of the LDL-C/HDL-C ratio.23 Longitudinal assessment to determine if subgroups of postpartum women at risk (such as those with depression) have sustained serum cholesterol elevations are also needed. The women in this RCT will be evaluated for lipid profiles related to both treatment assignment and remission status at study completion. People with severe mental illness, including refractory depression, are at increased risk of early death.24 Women with depression are more likely to be obese and may already be at increased risk of death from CVD. Additional studies on the effects of reproductive life, including pregnancy and lactation, are required to understand the cumulative burden of risk factors throughout life and their role in future health. This study is the first step in understanding whether physiologic changes in lipid metabolism caused by pregnancy may affect long-term health outcomes in women with depression.
Acknowledgments
We thank Heather Eng, Andrea Confer, Rebecca Zoretich, Mary McShea, Michelle Costantino, David Rizzo, and Carolyn Hughes This work was funded by the National Institutes of Health's National Institute of Child Health and Human Development (5K 12HD043441-09, B.A.P.) and National Institutes of Health's National Institute of Mental Health (R01 MH057102, S.R.W., J.F.L., D.S., K.L.W.).
Disclosure Statement
K.L.W. served on one Advisory Board Meeting for Eli Lilly and received donated estradiol and placebo patches from Novartis for an NIMH-funded randomized clinical trial.
References
- 1.Jimenez DM. Pocovi M. Ramon-Cajal J. Romeroa MA. Martinez H. Grande F. Longitudinal study of plasma lipids and lipoprotein cholesterol in normal pregnancy and puerperium. Gynecol Obstet Invest. 1988;25:158–164. doi: 10.1159/000293765. [DOI] [PubMed] [Google Scholar]
- 2.Piechota W. Staszewsi A. Reference ranges of lipids and apolipoproteins in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1992;45:27–35. doi: 10.1016/0028-2243(92)90190-a. [DOI] [PubMed] [Google Scholar]
- 3.Martin U. Davies C. Hayavi S. Hartland A. Dunne F. Is normal pregnancy atherogenic? Clin Sci. 1999;96:421–425. doi: 10.1042/cs0960421. [DOI] [PubMed] [Google Scholar]
- 4.Reboud P. Groulade J. Groslambert P. Colomb M. The influence of normal pregnancy and the postpartum state on plasma proteins and lipids. Am J Obstet Gynecol. 1963;86:820–828. doi: 10.1016/s0002-9378(16)35200-0. [DOI] [PubMed] [Google Scholar]
- 5.Potter JM. Nestel PJ. The hyperlipemia of pregnancy in normal and complicated pregnancies. Am J Obstet Gynecol. 1979;133:165–170. doi: 10.1016/0002-9378(79)90469-1. [DOI] [PubMed] [Google Scholar]
- 6.Van Stiphout WAHJ. Hofman A. de Bruijn AM. Serum lipids in young pregnant women before, during, and after pregnancy. Am J Epidemiol. 1987;126:922–928. doi: 10.1093/oxfordjournals.aje.a114729. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM. Becker D. Clark LT National Institutes of Health; on behalf of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2002. www.nhlbi.nih.gov/guidelines/cholesterol/index.htm. [Jun 30;2011 ]. www.nhlbi.nih.gov/guidelines/cholesterol/index.htm [PubMed]
- 8.Wulsin LR. Evans JC. Ramachandran SV. Murabito JM. Kelly-Hayes M. Benjamin EJ. Depressive symptoms, coronary heart disease, and overall mortality in the Framingham Heart Study. Psychosom Med. 2005;67:697–702. doi: 10.1097/01.psy.0000181274.56785.28. [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB. Castelli WP. Gordon T. Mcnamara PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. Ann Intern Med. 1971;74:1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- 10.Yaari S. Goldbourt U. Evan-Zohar S. Neufeld HN. Associations of serum high density lipoprotein and total cholesterol with total, cardiovascular, and cancer mortality in a 7-year prospective study of 10,000 men. Lancet. 1981;1:1011–1015. doi: 10.1016/s0140-6736(81)92184-x. [DOI] [PubMed] [Google Scholar]
- 11.Gaynes BN. Gavin N. Meltzer-Brody S, et al. Perinatal depression: Prevalence, screening accuracy, and screening outcomes summary. Evid Rep Technol Assess (Summ) 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vesga-Lopez O. Blanco C. Keyes K. Olfson M. Grant BF. Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65:805–815. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carney RM. Freedland KE. Jaffe AS. Depression as a risk factor for coronary heart disease mortality. Arch Gen Psychiatry. 2001;58:229–230. doi: 10.1001/archpsyc.58.3.229. [DOI] [PubMed] [Google Scholar]
- 14.Bodnar LM. Wisner KL. Moses-Kolko E. Sit D. Hanusa BH. Prepregnancy body mass index, gestational weight gain, and the likelihood of major depressive disorder during pregnancy. Clin Psychiatry. 2009;70:1290–1296. doi: 10.4088/JCP.08m04651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams LJ. Pasco JA. Henry MJ, et al. Lifetime psychiatric disorders and body composition: A population-based study. J Affect Disord. 2009;118:173–179. doi: 10.1016/j.jad.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 16.First MB. Spitzer RL. Gibbon M. Williams JBW. Washington, DC: American Psychiatric Press, Inc.; 1996. Structured Clinical Interview for DSM-IV Axis I Disorders, clinician version (SCID-CV) [Google Scholar]
- 17.Darmady JM. Postle AD. Lipid metabolism in pregnancy. Br J Obstet Gynaecol. 1982;89:211–215. doi: 10.1111/j.1471-0528.1982.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi IA. Xi XR. Limbu YR. Chen MI. Hyperlipidaemia during normal pregnancy, parturition and lactation. Ann Acad Med Singapore. 1999;28:217–221. [PubMed] [Google Scholar]
- 19.Howard BV. Ruotolo G. Robbins DC. Obesity and dyslipidemia. Endocrinol Metab Clin N Am. 2003;32:855–867. doi: 10.1016/s0889-8529(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 20.Cossrow N. Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab. 2004;89:2590–2594. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 21.Gunderson EP. Lewis CE. Murtaugh MA. Quesenberry CP. Smith West D. Sidney S. Long-term plasma lipid changes associated with a first birth: The Coronary Artery Risk Development in Young Adults study. Am J Epidemiol. 2004;159:1028–1039. doi: 10.1093/aje/kwh146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis CE. Funkhouser E. Raczynski JM. Sidney S. Bild DE. Howard BV. Adverse effect of pregnancy on high density lipoprotein (LDL) cholesterol in young adult women: The CARDIA study. Am J Epidemiol. 1996;144:247–254. doi: 10.1093/oxfordjournals.aje.a008919. [DOI] [PubMed] [Google Scholar]
- 23.Hummel J. Westphal S. Weber-Hamann B, et al. Serum lipoproteins improve after successful pharmacologic antidepressant therapy: A randomized, open-lablel propesctive trial. J Clin Pscyhiatry. 2011;72:885–891. doi: 10.4088/JCP.09m05853blu. [DOI] [PubMed] [Google Scholar]
- 24.Morden NE. Mistler LA. Weeks WB. Bartels SJ. Health care for patients with serious mental illness: Family medicine's role. J Am Board Fam Med. 2009;22:187–195. doi: 10.3122/jabfm.2009.02.080059. [DOI] [PMC free article] [PubMed] [Google Scholar]