Abstract
Subunit vaccines are significantly more expensive to produce than traditional vaccines because they are based primarily on recombinant proteins that must be purified from the expression system. Despite the increased cost, subunit vaccines are being developed because they are safe, effective, and can elicit antibodies that confer protection against diseases that are not currently vaccine-preventable. Algae are an attractive platform for producing subunit vaccines because they are relatively inexpensive to grow, genetically tractable, easily scaled to large volumes, have a short generation time, and are devoid of inflammatory, viral, or prion contaminants often present in other systems. We tested whether algal chloroplasts can produce malaria transmission blocking vaccine candidates, Plasmodium falciparum surface protein 25 (Pfs25) and 28 (Pfs28). Antibodies that recognize Pfs25 and Pfs28 disrupt the sexual development of parasites within the mosquito midgut, thus preventing transmission of malaria from one human host to the next. These proteins have been difficult to produce in traditional recombinant systems because they contain tandem repeats of structurally complex epidermal growth factor-like domains, which cannot be produced in bacterial systems, and because they are not glycosylated, so they must be modified for production in eukaryotic systems. Production in algal chloroplasts avoids these issues because chloroplasts can fold complex eukaryotic proteins and do not glycosylate proteins. Here we demonstrate that algae are the first recombinant system to successfully produce an unmodified and aglycosylated version of Pfs25 or Pfs28. These antigens are structurally similar to the native proteins and antibodies raised to these recombinant proteins recognize Pfs25 and Pfs28 from P. falciparum. Furthermore, antibodies to algae-produced Pfs25 bind the surface of in-vitro cultured P. falciparum sexual stage parasites and exhibit transmission blocking activity. Thus, algae are promising organisms for producing cysteine-disulfide-containing malaria transmission blocking vaccine candidate proteins.
Introduction
Vaccines have transformed medicine and dramatically improved human health. However, traditional vaccines that consist of attenuated or killed pathogens are not always practical or effective. A more modern strategy is to present specific immunogenic subunits from a pathogen to the immune system [1], [2]. Antibodies raised to these subunit vaccines, which are generally recombinant proteins or synthesized peptides, recognize native proteins from the appropriate pathogen and confer protection to the vaccinated individual. This reductionist approach is generally safer than traditional vaccines because there is no risk of a recombinant protein becoming virulent, and protein subunits promote a more directed immune response because of decreased antigenic competition. In the United States, vaccines cost $10–120 per dose (Center for Disease Control; [3]) with subunit vaccines being the most expensive. Though impoverished nations would benefit the most from potential subunit vaccines for malaria, dengue, and other diseases for which traditional vaccines do not exist, the cost of recombinant subunit vaccines is too expensive for use in these areas [4]. Thus, cheaper methods for producing subunit vaccines should be a priority if we are to reduce the heavy disease burdens found in low income countries.
Every system used to produce recombinant proteins has its own advantages and disadvantages with respect to cost, protein folding, yield, ease of manipulation, and scalability, which must be carefully weighed against the specific application and structure of the antigen. One strategy for reducing the cost of subunit vaccines is to use plants as the expression system [5], [6]. Photosynthetic growth, combined with high recombinant protein yields and economy of scale, result in a relatively inexpensive production platform [7]. In plants, both the nuclear and plastid genome can be transformed with genes that encode subunit vaccines. Indeed, several plant-made pharmaceuticals are currently in clinical trials [8]. Microalgae, which are essentially single-celled water-borne plants, are easily manipulated, have much shorter generation times than terrestrial plants, and can be cultured in complete containment at a relatively low cost. The development of algal biofuels has also spurred interest in potential co-products that could be produced concurrently, thus driving down the cost of both the fuel and any associated algae-produced protein. The chloroplast of the eukaryotic green microalgae, Chlamydomonas reinhardtii, is genetically tractable and has been used to produce human monoclonal antibodies [9], [10], human protein therapeutics [11], and subunit vaccines [12], [13]. However, the diversity of recombinant proteins that can be produced in algal chloroplasts is still not well-explored. We tested whether algal chloroplasts can produce malaria transmission blocking vaccines (TBV) by producing the well-studied structurally complex malaria TBV candidates, Plasmodium falciparum surface protein 25 (Pfs25) and 28 (Pfs28).
The Plasmodium parasite that is the causative agent of malaria can only be transmitted via the female Anopheles mosquito. There it undergoes the sexual stages of development that eventually lead to the infectious sporozoites that reside in the mosquito salivary gland [14]. Several conserved proteins involved in this process, in particular Pfs25, are of great interest because antibodies raised to these proteins block parasite maturation leading to sporozoites, thus preventing subsequent transmission to a human host [15]. A TBV would complement current vector control strategies and increase the efficacy of the protective RTS/S vaccine [16], [17], but a safe and effective TBV has not been realized despite its initial conception in 1976 [18], [19].
Pfs25 and Pfs28 are structurally complex aglycosylated outer membrane proteins that contain four tandem epidermal growth factor-like (EGF) domains, each with several disulfide bonds. The biological limitations of traditional recombinant systems have made it difficult to reproduce the complex structures of these proteins. Attempts to produce conformationally correct Pfs25 in Escherichia coli failed [20], and yeast-produced Pfs25 has multiple conformations [21], [22] and caused an allergic reaction during human clinical trials [23]. More recently, Pfs25 was produced in a tobacco transient expression system and is a promising TBV candidate [24], but like yeast, requires mutations in the nucleotide sequence to prevent post-translational glycosylation. Furthermore, transient expression mediated by Agrobacterium infiltration may not be as suitable for large scale production as algae. Like yeast and tobacco, the chloroplast of green algae can fold complex proteins and make disulfide bonds, but lacks the machinery for glycosylation. Here we describe the physical and immunological characteristics of algae-produced Pfs25 and Pfs28.
Results
Production and purification of recombinant Pfs25 and Pfs28 in algal chloroplasts
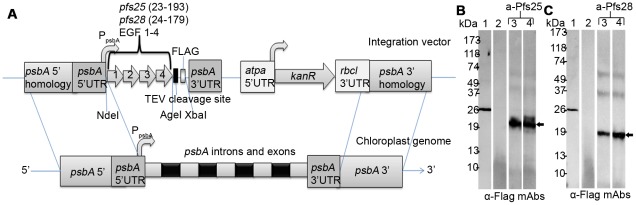
We synthesized genes encoding pfs25(Ala22 – Thr193) and pfs28(Val24 – Pro179) with a codon bias that resembles the C. reinhardtii chloroplast codon usage (hereafter referred to as a-pfs25 and a-pfs28). Codon optimization of heterologous genes for expression in the chloroplast was previously shown to increase protein yields [25]. Synthetic a-pfs25 and a-pfs28 each contain four EGF-like domains and a C-terminal FLAG tag for ease of detection and purification, but lack the native signal sequence and GPI-anchor sequence. The expected codon adaptation index (eCAI), which quantitates the codon bias of a transgene against a reference set, is 0.878 (p<.01) and 0.892 (p<. 01) for a-pfs25 and a-pfs25 respectively [26]. CAI values range from zero to one where a score of one indicates that every instance of an amino acid is encoded by the most common codon in the reference codon table [27]. A-pfs25 and a-pfs28 were synthesized and separately cloned into a chloroplast expression cassette that replaces endogenous psbA through homologous recombination such that transgene expression is controlled by the psbA promoter and 5′ and 3′ untranslated regions (UTRs; Fig. 1A). C. reinhardtii chloroplasts were transformed by particle bombardment and a-pfs25 and a-pfs28 were detected by PCR (data not shown). Transformed C. reinhardtii were screened for a-Pfs25 (Fig. 1B – arrow) and a-Pfs28 (Fig. 1C – arrow) protein by Western blot. A-Pfs25 and a-Pfs28 accumulate in chloroplasts at 0.5% and 0.2% total soluble protein, respectively, as determined by ELISA (see materials and methods).
Figure 1. Diagram of chloroplast transformation vector and Western blots of C. reinhardti transformed with vectors containing codon optimized pfs25 or pfs28.
(A) The codon optimized nucleotide sequences corresponding to EGF domains 1–4 of pfs25 and pfs28 were separately cloned into an open reading frame that is upstream of a TEV protease site and FLAG epitope. Transgenes were integrated at the psbA locus by homologous recombination. Gene expression is driven by the psbA promoter and mRNA is stabilized by the psbA 5′ and 3′ untranslated regions (UTRs). (B) Western blot analysis of purified algae-produced HMGB1 containing a FLAG epitope (lane 1), lysate of untransformed parental strain (lane 2), lysate of C. reinhardtii containing a-pfs25 total protein (lane 3) and soluble protein (lane 4) probed with anti-FLAG mAbs. (C) Western blot using anti-FLAG mAbs of purified algae-produced HMGB1 containing a FLAG epitope (lane 1), lysate of untransformed parental strain (lane 2), lysate of C. reinhardtii containing a-pfs28 total protein (lane 3) and soluble protein (lane 4) probed with anti-FLAG mAbs.
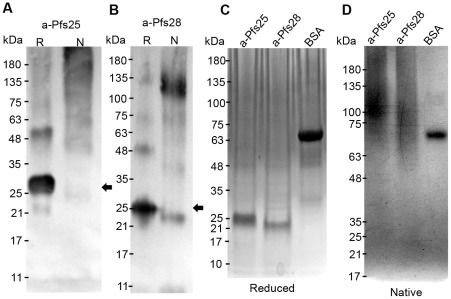
A-Pfs25 and a-Pfs28 were affinity purified using anti-FLAG M2 affinity resin (see materials and methods), positively identified by mass spectrometry (data not shown), and analyzed by Western blot (Fig. 2A–B). The predominant band in reduced a-Pfs25 and a-Pfs28 migrated near their predicted sizes of 21.4 kDa (Fig. 2A – arrow) and 20.2 kDa (Fig. 2B – arrow), respectively. The sizes of the larger bands suggest that a-Pfs25 and a-Pfs28 could be running as dimers. The monomeric form of a-Pfs25 and a-Pfs28 is diminished in unreduced samples and appears at larger, less well-defined molecular species. Similar to Western blot analysis, Coomassie-blue staining of reduced samples resolved by SDS-PAGE shows a single predominant band with few obvious impurities for both a-Pfs25 and a-Pfs28 (Fig. 2C). Similar results were obtained with silver staining (Fig. S1). A-Pfs25 and a-Pfs28 appear as larger less defined molecular weight complexes when analyzed by native-PAGE (Fig. 2D). The apparent larger size of a-Pfs25 and a-Pfs28 in the non-reduced and native gels could be the result of multimerization that form because both proteins are rich in β-strand secondary structures that are known to interact [28]. Indeed, multimerization of recombinant Plasmodium surface proteins was recently observed [29], [30]. Thus, algal chloroplasts produce a single protein that is the appropriate size for a-Pfs25 and a-Pfs28, and those monomers appear to assemble into higher molecular weight aggregates that could have multiple conformations.
Figure 2. Immunoblot and Coomassie-blue stain of algae-produced Pfs25 and Pfs28 analyzed by SDS and Native-PAGE.
Five micrograms of reduced and non-reduced affinity purified (A) a-Pfs25 and (B) a-Pfs28 were resolved by SDS-PAGE, transferred to nitrocellulose, and detected with anti-FLAG mAbs. (C) Five micrograms of reduced a-Pfs25, a-Pfs28, and BSA were resolved by SDS-PAGE and stained with Coomassie-blue. (D) Five micrograms of non-reduced a-Pfs25, a-Pfs28, and BSA were resolved by Native-PAGE and stained with Coomassie-blue. (R – reduced, N – non-reduced).
Structural characterization of a-Pfs25 and a-Pfs28
A-Pfs25 and a-Pfs28 were analyzed for the presence of epitopes found in native Pfs25 and Pfs28 by Western blot using transmission blocking monoclonal antibodies 4B7 (Pfs25, [31]) and 2D8 (Pfs28, a gift from David Narum, NIAID [32]), which were previously shown to recognize epitopes only present on properly folded Pfs25 and Pfs28, respectively(Fig. 3). Anti-Pfs25-4B7 mAbs recognize a β-hairpin epitope within the ILDTSNPVKT peptide sequence of the third EGF-like domain of native Pfs25 [33], [34]. Anti-Pfs25-4B7 mAbs binds a-Pfs25 as a band that migrates between 100 and 130 KDa in non-reduced samples (Fig. 3A). Unlike anti-FLAG mAbs (Fig. 2), anti-Pfs25-4B7 does not recognize reduced a-Pfs25, which is consistent with recognition being conformationally dependent. Similarly, anti-Pfs28-2D8 mAbs recognize a-Pfs28 in non-reduced, but not reduced samples, as a larger molecular weight species (Fig. 3B). Thus, a-Pfs25 and a-Pfs28 contain epitopes present on native Pfs25 and Pfs28, respectively.
Figure 3. Immunoblot of reduced and non-reduced algae-produced Pfs25 and Pfs28 with monoclonal transmission blocking antibodies.
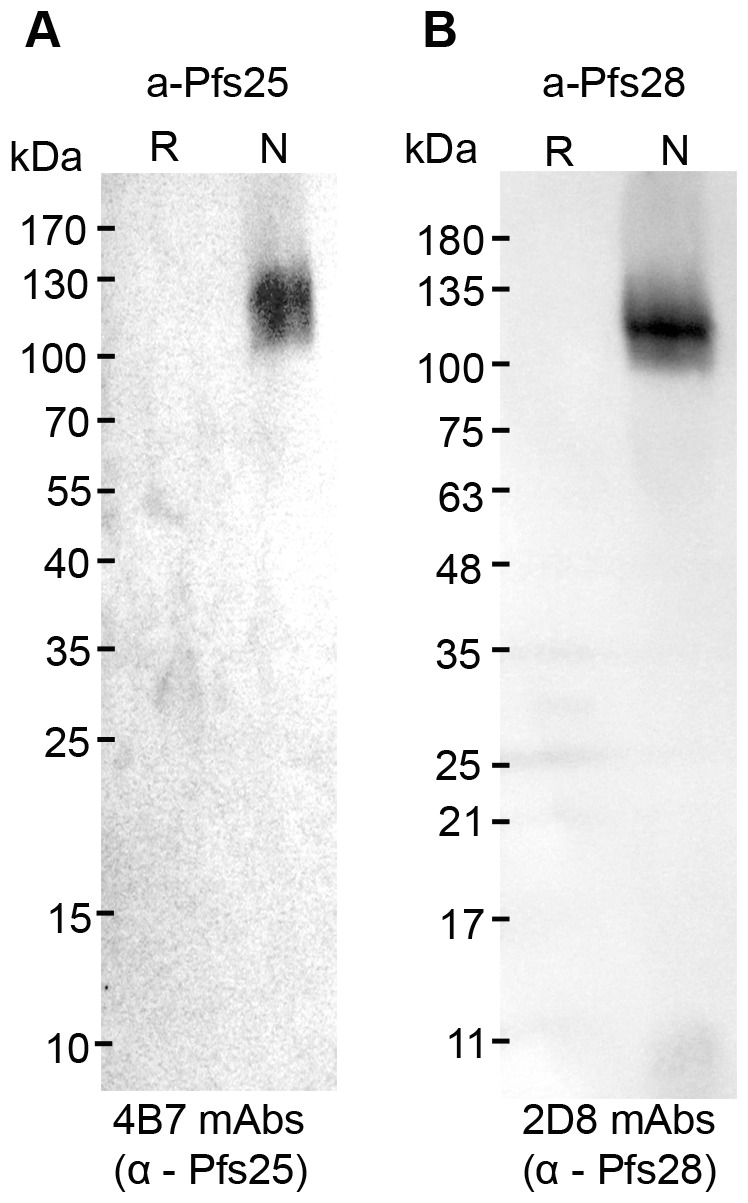
Reduced and non-reduced a-Pfs25 and a-Pfs28 were resolved by SDS-PAGE, transferred to nitrocellulose, and detected with (A) anti-Pfs25 4B7 mAb and (B) anti-Pfs28-2D8 mAbs, respectively.
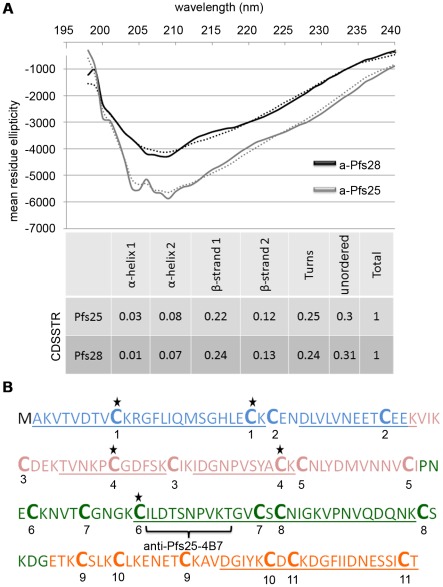
We assessed the secondary structure of purified a-Pfs25 and a-Pfs28 using circular dichroism (CD) spectroscopy(Fig. 4A). The crystal structure of yeast-produced Plasmodium vivax surface protein 25 (Pvs25), a homologue of Pfs25, has two central β-strands separated by a turn [35]. Bioinformatic analysis of Pfs25 and Pfs28 suggest their structures are similar [33]. Deconvolution of CD spectra with CDSSTR [36], [37], which estimates the contribution of each secondary structure to the overall spectrum, predicts that a-Pfs25 and a-Pfs28 are composed of nearly 60% β-strands and turns, 10% α-helix, and 30% unordered peptide. Thus, the CD spectra reveal that a-Pfs25 and a-Pfs28 have comparable secondary structures that are predicted to be primarily β-strands and turns, which is consistent with the crystal structure of Pvs25.
Figure 4. Structural analysis of algae-produced Pfs25 and Pfs28.
(A) Far-UV circular dichroism spectra of algae-produced Pfs25 (Gray) and Pfs28 (Black). Spectra shown are measured mean residue ellipticity (solid lines) and best fit (dotted lines) using CDSSTR. The contribution of each secondary structure to the overall spectra as predicted by CDSSTR is shown as fractions. (B) Analysis of protected cysteines in a-Pfs25 by tandem mass spectrometry. The peptide sequence of Pfs25 from Ala22 to Thr193 is shown with EGF-like domain 1 (blue), 2(pink), 3(green), 4(orange). Peptides detected by mass spectrometry are underlined and protected cysteines are marked by stars. Predicted disulfide linkages between cysteines are marked numerically (i.e. the two cysteines labeled 1 form a dilsulfide linkage and so on). The epitope recognized by anti-Pfs25 4B7 mAb is indicated.
We analyzed free cysteine residues in a-Pfs25 by mass spectrometry to determine which residues are bridged by disulfide bonds (see materials and methods). Briefly, a-Pfs25 was treated with iodoacetamide to carboxymethylate free cysteines followed by trypsin digestion. Tryptic peptides were analyzed by high pressure liquid chromatography (HPLC) coupled to tandem mass spectrometry (LC-MS-MS) using nanospray ionization. Presumably, cysteines that remained unmodified after treatment with iodoacetamide were protected from modification because they were in disulfide bonds. The detected peptides covered approximately 64% of the Pfs25 sequence (Fig. 4B – underlined). A-Pfs25 digested with GluC and chymotrypsin did not add to the total coverage (data not shown). When compared to the disulfide bonds identified in crystallized yeast-produced Pvs25, we found that disulfide bonds 1, 4, and 6 are intact while 2, 7, 8, 10, and 11 may not be completely formed in a-Pfs25. Peptides for the remaining disulfide bonds were not detected. Hence, C. reinhardtii chloroplasts form disulfide bonds in a-Pfs25, but it may not be as extensively bridged by disulfide bonds as yeast-produced Pfs25.
Antibodies from mice vaccinated with a-Pfs25 or a-Pfs28 recognize native parasite proteins
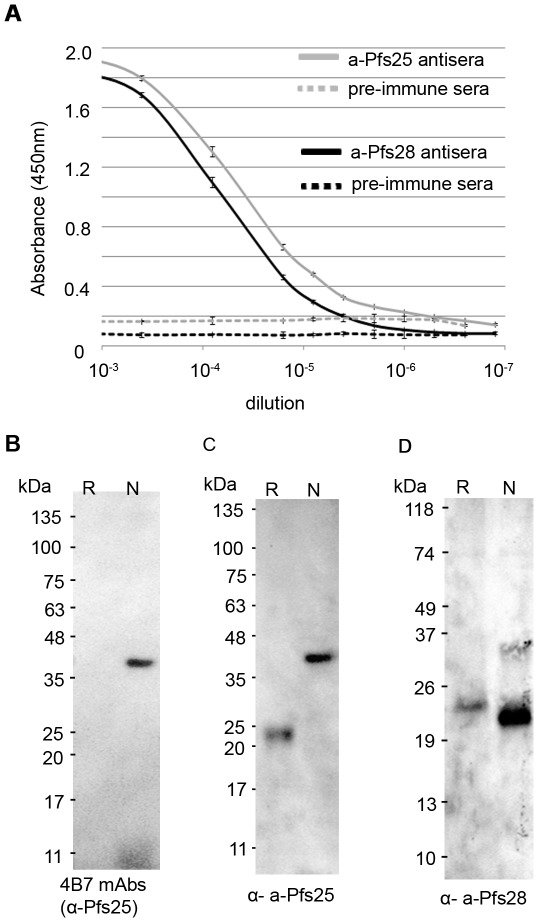
Balb/c mice were immunized with affinity purified a-Pfs25 or a-Pfs28 using complete Freund's adjuvant for the first vaccination and incomplete Freund's adjuvant for subsequent vaccinations (see materials and methods). Antibody titers were measured by ELISA against affinity purified a-Pfs25 or a-Pfs28 (Fig. 5A). Sera from mice immunized with a-Pfs25 and a-Pfs28 contained high titers of antibodies for a-Pfs25 and a-Pfs28 respectively. Pre-immune sera showed no IgG response to either of these proteins.
Figure 5. Analysis of antibodies from mice immunized with algae-produced Pfs25 or Pfs28.
(A) ELISA titers of mouse anti-sera elicited by algae-produced Pfs25 and Pfs28. Mice were immunized with affinity purified a-Pfs25 or a-Pfs28 using complete Freund's adjuvant followed by boosters with incomplete Fruend's adjuvant by intraperitoneal injection. Pooled sera was serially diluted and tested in triplicate against the corresponding algae-produced Pfs antigen; error bars are one standard deviation. Prebleed sera were tested as a negative control; error bars are four standard deviations. (B–D) Western blot analysis of P. falciparum mixed sexual stage lysates with anti-Pfs25-4B7 mAbs, a-Pfs 25 antisera, or a-Pfs28 antisera. Reduced and non-reduced sexual stage lysates were resolved by SDS-PAGE and transferred to nitrocellulose. Blots were probed with (B) anti-Pfs25-4B7 mAbs, (C) antibodies raised to a- Pfs25, and (D) antibodies raised to a-Pfs28. (R – reduced, N – non-reduced).
We tested the specificity of antibodies raised to algae produced Pfs antigens to native Pfs25 or Pfs28 in sexual stage parasite lysates. Reduced and non-reduced P. falciparum sexual stage lysates were probed with anti-Pfs25-4B7 mAbs, anti-Pfs28-2D8 mAbs, and with serum from mice injected with a-Pfs25 or a-Pfs28. As expected, anti-Pfs25-4B7 recognized a band in the non-reduced sample, but not the reduced sample, which is consistent with anti-Pfs25-4B7 recognizing only conformationally-correct Pfs25 (Fig. 4B). Sera from mice injected with a-Pfs25 recognized a 25 kDa band in reduced P. falciparum lysates, which is consistent with molecular weight of native Pfs25, and an approximately 40 KDa band that is identical in size to the band recognized by anti-Pfs25-4B7 in non-reduced lysates (Fig. 5B & C). Antibodies raised to a-Pfs28 recognized an approximately 22 KDa band in both reduced and non-reduced parasite lysates (Fig. 5D), but required ten-fold more parasite lysate than a-Pfs25 antisera for detection. This could be due to lower Pfs28 protein abundance or due to lower affinity antibodies. We did not detect Pfs28 with anti-Pfs28-2D8 mAbs (data not shown) indicating Pfs28 is indeed less abundant than Pfs25 in our in vitro cultured parasites. Importantly, the size of the protein recognized by a-Pfs28 antisera is consistent with antibodies that were previously shown to recognize Pfs28 in sexual stage parasites [38]. Thus, antisera to both a-Pfs25 and a-Pfs28 recognize native parasite proteins of the appropriate size and a-Pfs25 antisera recognize a band that identical to the transmission blocking anti-Pfs25 4B7 mAbs in non-reduced samples.
Pfs25 and Pfs28 are outer membrane proteins that are produced in P. falciparum sexual stage parasites [39]. We therefore tested the affinity of a-Pfs25 and a-Pfs28 antisera as well as anti-Pfs25-4B7 mAbs and sera from unvaccinated isogenic mice for in-vitro cultured parasites. Binding of a-Pfs25 antisera was predominantly confined to the outer membrane(Fig. 6A; additional images Fig. S2). Staining with anti-Pfs25-4B7 was identical to a-Pfs25 antisera (Fig. 6B & Fig. S2), which suggests antibodies to a-Pfs25 also bind Pfs25. Binding of a-Pfs28 antisera was significantly weaker than a-Pfs25 antisera and often not detectable above background levels seen with sera from unvaccinated isogenic mice (Fig. S2). Similar to Western blot analysis of parasite lysates, this suggests that antibody titers to Pfs28 are low or that Pfs28 is not as abundant as Pfs25 in our in-vitro cultured parasites.
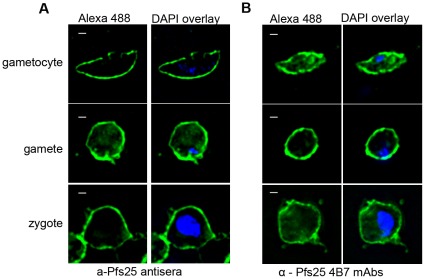
Figure 6. Indirect immunofluorescence using immune sera from mice immunized with algae-produced Pfs25 or Pfs28 on in-vitro cultured P. falciparum gametocytes, gametes, and zygotes.
DNA was stained using DAPI (blue) and antibody binding was visualized using Alexa Fluor 488-conjugated rabbit anti-mouse IgG (green) for (A) a-Pfs25 antisera and (B) anti-Pfs25 4B7 mAbs. Scale bars, 1 µm.
Evaluation of transmission blocking activity of a-Pfs25 and a-Pfs28 antisera
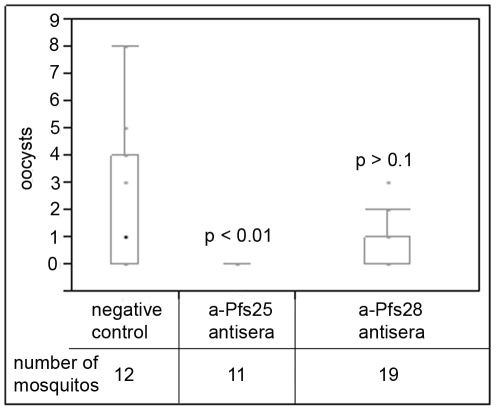
Transmission blocking activity was measured by the reduction of oocysts in mosquito midguts by a standard membrane feed assay (SMFA). Briefly, P. falciparum NF 54 gametocytes and heat inactivated antisera from mice immunized with a-Pfs25, a-Pfs28, or sera from isogenic mice were fed to female Anopheles stephensi mosquitoes. Mosquitos were dissected nine days later and analyzed for oocysts (Fig. 7). Oocyst counts were reduced in mosquitos that were fed a-Pfs28 antisera, but transmission blocking activity did not reach significance using a nonparametric comparison test (see materials and methods). Antibodies to a-Pfs25 completely blocked transmission as indicated by the absence of oocysts in all dissected mosquitos. These results are consistent with previous data that suggests that antibodies to Pfs25 more efficiently block malaria transmission than antibodies to Pfs28 [21], [40], and that the algal expressed a-Pfs25 protein elicits an antibody response most suitable for vaccine development.
Figure 7. Standard membrane feeding assay with sera from mice immunized with algae-produced Pfs25 or Pfs28.
Mosquitos midguts were dissected and analyzed for the presence of oocysts following SMFA. Oocyst numbers are presented as a boxplot. The total number of mosquitos analyzed is listed. Statistics were calculated with a single-tailed Wilcoxon nonparametric comparison.
Discussion
In this study, we demonstrated that algae are a robust platform for producing malaria subunit vaccines by characterizing Pfs25 and Pfs28, two structurally complex malaria transmission blocking vaccine candidates, made in C. reinhardtii chloroplasts. Algae are the only recombinant system to date that has successfully produced unmodified aglycosylated recombinant Pfs25 or Pfs28. The algae-produced recombinant proteins are similar in structure to native Pfs25 and Pfs28 and are recognized by transmission blocking monoclonal antibodies that only bind conformationally correct Pfs antigens. Analysis of free cysteines in a-Pfs25 revealed that disulfide bonds are formed by algal chloroplasts in this protein, but these may not be as extensively formed as in the yeast-produced homologue, Pvs25 from P. vivax [35], and this may be an area where the algae system can be improved. However, the disulfide linkages in native Pfs25 are not known, nor have they been characterized in any other recombinant system. The disulfide bonds are likely similar to the native protein because both a-Pfs25 and a-Pfs28 elicit antibodies in mice that recognize native proteins in P. falciparum sexual stage lysates. A-Pfs25, but not a-Pfs28, elicited antibodies with significant levels of transmission blocking activity, which is consistent with previous observations [21], [40]. The structural analysis of a-Pfs25 and a-Pfs28 suggests these antigens resemble the native proteins and are of similar conformational quality. The apparent difference in transmission blocking activity between a-Pfs25 and a-Pfs28 is likely due to the delayed appearance of Pfs28 in ookinete development compared to the earlier appearance of Pfs25 in zygote development, rendering any antibodies directed against the later-expressed Pfs28 protein on the parasite superfluous. For these reasons, interest in Pfs28 as a TBV candidate has diminished in recent years compared to Pfs25, which remains the lead TBV candidate.
TBVs and other subunit vaccines must be produced at a cost that is appropriate for low income countries if they are to be implemented. Indeed, financial constraints are already limiting the dissemination of effective meningococcal, pneumococcal, and rotavirus vaccines [41]. Algal biomass could be a low cost source for recombinant protein, especially because recent interest in algal biofuels is driving research in large scale algae cultivation, which is certain to reduce the price of algal biomass production. Recombinant proteins could be separated from lipids used for fuel production, which would drive down the cost of both. Alternatively, fusing mucosal adjuvants to vaccine candidates might allow for oral delivery and eliminate the need for injection and cold chain storage [6], [13], both of which significantly contribute to vaccine cost. Unicellular green algae like C. reinhardtii are generally regarded as safe by the U.S. Food and Drug administration because they do not harbor endotoxins, human pathogens, or other known toxic compounds. Therefore, algae may be an ideal platform for producing low-cost subunit vaccines.
Materials and Methods
Plasmid construction
The peptide sequences of Pfs25(Ala22 – Thr193) (Genbank accession: AAD55785) and Pfs28(Val24 – Pro179) (Genbank accession: AAG27295) were reverse translated with the gene designer algorithm from DNA2.0 (Menlo, CA, USA) using the C. reinhardtii complete codon usage table as a reference set (http://www.kazusa.or.jp). The codon bias of the sequence was validated by computing the expected codon adaptation index (eCAI) [26]. The codon optimized algal-pfs25 (a-pfs25) and algal-pfs28 (a-pfs28) were synthesized by GeneArt®(now Life Technologies Carlsbad, CA, USA) and cloned into the pD1-KanR [11], which contains 5′ and 3′ psbA homology to direct homologous recombination to the psbA locus and a kanamycin resistance cassette for selection. The resulting plasmids contain a-pfs25 (pJAG9) or a-pfs28 (pJAG15) fused to a 3′ TEV protease recognition sequence followed by a FLAG affinity tag. Synthetic pfs genes are downstream of the psbA promoter and 5′ UTR and upstream of the psbA 3′ UTR. Each construct was verified by sequencing (Retrogen, San Diego, CA).
C. reinhardtii transformation
C. reinhardtii strain W1.1 [42] was grown in TAP (Tris-acetate-phosphate) medium at 23°C on a rotary shaker to mid log phase and harvested by centrifugation. Approximately 5×107 cells were plated on TAP agar with 100 ug/ml kanamycin and transformed by particle bombardment [43]. Briefly, 1 mg of gold particles (S550d, Seashell technologies, San Diego) coated with 10 ug of plasmid DNA were shot with the PDS-1000/He system (Biorad, Hercules, CA) under vaccum at a distance of 9 cm at 1350 psi. Transformants were propagated on TAP agar with 150 ug/mL kanamycin and screened for the presence of a-pfs25 or a-pfs28 using gene specific primers and for homoplasmicity as previously described [44].
Western blotting, Silver Staining, and affinity purification of a-Pfs25 and a-Pfs28
Initial screens for Pfs protein were performed on homoplasmic strains in 250 ml TAP cultures grown on a rotary shaker. Cultures were grown in low light to mid log phase and then switched to high light (∼5000 lux) conditions. Samples were harvested at various timepoints post-light shift and resuspended in 1 ml lysis buffer per 0.1 g wet algal paste (50 mM Tris pH 8.0, 400 mM NaCl, 0.1% Tween 20, protease inhibitor cocktail (Roche – Mannheim, Germany)). Cells were lysed by sonication and cleared by centrifugation at 30,000×G for 15 minutes. Total and soluble protein samples were prepared in SDS buffer with urea and DTT at a final concentration of 50 mM Tris, 2% SDS, 10% glycerol, 2 M urea, and 100 mM DTT. Samples were heated at 37°C for 10 min then resolved on RUNBLUE precast 12% SDS-PAGE gels (Expedeon – San Diego, CA) and transferred to nitrocellulose membranes. Blots were probed with mouse anti-FLAG primary mAb (Sigma Aldrich – St. Louis, MO) and alkaline phosphatase (AP)-conjugated goat anti-mouse IgG secondary Ab (Sigma-Aldrich) and visualized using nitro-blue tetrazolium (NBT) and 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt (BCIP) in alkaline phosphatase buffer.
A-Pfs25 and A-Pfs28 were purified from 20 liters of TAP medium grown in photobioreactors. Photobioreactors were constructed from Nalgene carboys (part# – 2251-0050) fitted with Nunc bulkhead adapters (part# – 6149-002) for bubbling air through a 0.1 micron filter. Photobioreactors were inoculated with 250 ml starter cultures at late log phase and grown in the dark until reaching an approximate density of 1×106 cells/mL and then shifted to high light (∼5000 lux). Optimal yields were reached when harvesting 5–8 hrs post-light induction. Cells were harvested using a Lavin L2 continuous flow centrifuge (AML ind. – Hatboro, PA) fed by a peristaltic pump. Total soluble protein was isolated as described above. M2 anti-FLAG resin (Sigma Aldrich) was added to the cleared lysate and rotated end over end at 4°C for 2 hrs. Resin was washed with 20 volumes of lysis buffer twice and once with lysis buffer without Tween 20. Resin was then collected by filtration in a Bio-rad Econo-pac column and the protein was eluted using 100 mM glycine pH 3.5, 400 mM NaCl and neutralized with Tris pH 8.0 to a final concentration of 50 mM. Eluted fractions were resolved by SDS-PAGE and analyzed by Western blot as described above. All fractions containing protein were then combined and buffer exchanged using Vivaspin 6 centrifugal concentrators (GE Healthcare) with a 10 kDa molecular weight cutoff into PBS. The concentration of purified protein was determined using BioRad protein Assay (Biorad). Reduced samples were prepared in SDS buffer with 10% β-mercapotoethanol (BME) and heated to 90°C for 10 min. Non-reduced samples were prepared using 4X Native Buffer (Expedeon). Both reduced and non-reduced samples were resolved on 16% RUNBLUE SDS-PAGE precast gels (Expedeon) for analysis by Western blot. For gel staining, samples were resolved on 4–20% gradient SDS-PAGE precast gels (Biorad) and stained using silver stain plus (Biorad) or Coomassie-blue. Samples for Native-PAGE analysis were prepared with 4X Native Buffer (Expedeon) and resolved by 10% Native-PAGE precast gel (Expedeon) then stained with Coomassie-blue.
Anti-Pfs25 4B7 monoclonal antibody was prepared from mouse hybridoma cell line 4B7.1.1 (ATCC – HB-12575). Cells were grown in hybridoma-SFM media (Gibco, 12045) supplemented with 10% FBS (Gemini) and antibodies were harvested after seven days. Reduced and non-reduced a-Pfs25 and a-Pfs28 were prepared as above. Both were resolved on RUNBLUE 16% SDS-PAGE precast gels (Expedeon) and transferred to a nitrocellulose membrane. The blot was probed using filtered lysate containing anti-Pfs25 4B7 mAbs for a-Pfs25 or anti-Pfs28-2D8. Antibody binding was detected using alkaline phosphatase-conjugated goat anti-mouse secondary (Sigma-Aldrich) and visualized using NBT and BCIP in alkaline phosphatase buffer.
Circular Dichroism
Far-UC CD spectra were recorded from 190–260 nm with a 0.1 nm data pitch using a Jasco J-815 spectropolarimeter and 1 mm path length. The cuvette chamber temperature was maintained at 20°C by a Jasco PFD-425S/15 temperature unit. Samples were at 21 µM and 27 µM in PBS for Pfs28 and Pfs25, respectively. Spectra were acquired at 1 nm band width, 4 second response time, and a scan speed of 100 nm/min. The results were calculated after subtracting the PBS baseline spectra and reported as mean residue ellipticity. Helical, β-strand, and turn content were predicted using CDSSTR on Dichroweb [36], [37].
Mass Spectrometry
Pfs25 treated with 0.5 mg/mL of iodoacetamide for 30 min at 37°C to carboxymethylate the cysteine residues that were not in cysteine cross-link bonds. The reaction was quenched with addition of 2 mM DDT followed by buffer exchange using 10 kDa cutoff membrane in 1X TNE buffer (50 mM Tris pH 8.0, 100 mM NaCl, 1 mM EDTA). RapiGest SF reagent (Waters Corp.) was added to the mix to a final concentration of 0.1% and samples were boiled for 5 min. TCEP (Tris (2-carboxyethyl) phosphine) was added to 1 mM (final concentration) and the samples were incubated at 37°C for 30 min. Proteins samples prepared as above were digested with trypsin (trypsin∶protein ratio – 1∶50) overnight at 37°C. RapiGest was degraded and removed by treating the samples with 250 mM HCl at 37°C for 1 h followed by centrifugation at 14000 rpm for 30 min at 4°C. The soluble fraction was then added to a new tube and the peptides were extracted and desalted using Aspire RP30 desalting columns (Thermo Scientific). Trypsin-digested peptides were analyzed by high pressure liquid chromatography (HPLC) coupled with tandem mass spectroscopy (LC-MS/MS) using nano-spray ionization. The nanospray ionization experiments were performed using a TripleTOF 5600 hybrid mass spectrometer (ABSCIEX) interfaced with nano-scale reversed-phase HPLC (Tempo) using a 10 cm-180 micron ID glass capillary packed with 5-µm C18 Zorbax™ beads (Agilent Technologies, Santa Clara, CA). Peptides were eluted from the C18 column into the mass spectrometer using a linear gradient (5–60%) of ACN (Acetonitrile) at a flow rate of 250 µL/min for 1 h. The buffers used to create the ACN gradient were: Buffer A (98% H2O, 2% ACN, 0.2% formic acid, and 0.005% TFA) and Buffer B (100% ACN, 0.2% formic acid, and 0.005% TFA). MS/MS data were acquired in a data-dependent manner in which the MS1 data was acquired at m/z of 400 to 1250 Da and the MS/MS data was acquired from m/z of 50 to 2,000 Da. Finally, the collected data were analyzed using MASCOT® (Matrix Sciences) and Protein Pilot 4.0 (ABSCIEX) for peptide identifications.
Preparation of mouse antisera
Animal experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of California. Balb/c mice were vaccinated by intraperitoneal injection with 25 µg of affinity purified a-Pfs25 or a-Pfs28 emulsified in complete Freund's adjuvant (Sigma-Aldrich). Four boosters were given at two week intervals with 15 µg of affinity purified a-Pfs25 or a-Pfs28 using incomplete Fruend's adjuvant (Sigma-Aldrich). Blood was collected prior to immunization and one week following the final immunization.
Enzyme-linked immunosorbent assay
The percent of a-Pfs25 and a-Pfs28 of the soluble protein fraction was measured by ELISA. The soluble protein lysates from JAG9 (a-Pfs25) and JAG15 (a-Pfs28) were compared to soluble protein from the untransformed parental strain (W1.1) with a known amount of affinity purified a-Pfs25 or a-Pfs28 as follows. Soluble protein fractions were prepared by sonication followed by centrifugation at 20,000× g. Affinity purified a-Pfs25 or a-Pfs28 was mixed with soluble protein from W1.1 at a 1∶50 ratio, or 2% recombinant protein, and diluted to a final concentration of 0.5 mg/mL in PBS. A standard curve was then prepared using two-fold serial dilutions with a 0.5 mg/ml solution of W1.1 soluble protein. MaxiSorpTM plates (Nunc – Rochester, NY) were coated in triplicate with 50 µg of soluble protein from JAG9, JAG15, or the prepared standard curve and incubated overnight at 4°C on a rocker. Wells were washed three times with PBS with 0.1% Tween (PBS-T) and blocked with 5% milk in PBS-T for 2 hrs at room temperature. A-Pfs25 and a-Pfs28 were detected with M2 anti-FLAG mAbs diluted 1∶4000 in PBS-T and goat anti-mouse horse radish peroxidase (Thermo Scientific – Rockford, IL) at 1∶5000. Antibody binding was detected using the TMB substrate kit (Thermo Scientific) and read with an Infinite M200 pro plate reader (Tecan – Switzerland) at 450 nm. Readings from JAG9 and JAG15 soluble protein were compared to the standard curves prepared with purified protein.
IgG titers in the mouse antisera against a-Pfs25 and a-Pfs28 were measured by ELISA as follows. A-Pfs25 and a-Pfs28 were diluted to 1 µg/ml in PBS and used to coat 96-well MaxiSorp™ plates (Nunc – Rochester, NY) and incubated overnight at 4°C on a rocker. Wells were washed three times with PBS-T and blocked with 5% milk in PBS-T for 2 hours at room temperature. Serum samples were prepared by 1∶5 serial dilutions from 1∶100 to 1∶62500, followed by 1∶2 dilutions from 1∶65000 to 8×107, plated in triplicate, and incubated overnight at 4°C. IgG titers were detected using AP-conjugated goat anti-mouse IgG (Sigma-Aldrich) at 1∶4000 in PBS-T for two hours at room temperature and visualized using p-Nitrophenylphosphate (Enzo Life Sciences – Farmingdale, NY) as the substrate. Absorbances were measured at 450 nm using Infinite M200 pro plate reader (Tecan – Switzerland).
Western Blotting of parasite lysates
Plasmodium falciparum strain NF54 was maintained in vitro in continuous cultivation. Gametocytes, macrogametes, zygotes, and ookinetes were cultured and purified as previously described [45]. For antibodies raised to a-Pfs25, 2 µg of reduced and nonreduced parasite lysate was prepared as described above and resolved on RUNBLUE 12% SDS-PAGE precast gels (Expedeon). Resolved proteins were transferred to nitrocellulose and probed with antisera raised to a-Pfs25 at a 1∶100 dilution. Identical blots were prepared and probed with anti-Pfs25 4B7 mAbs or preimmune sera. For antibodies raised to a-Pfs28, 20 µg was prepared in an identical manner and probed with a-Pfs28 antisera at a 1∶100 dilution or preimmune sera. All membranes were visualized using AP-conjugated goat anti-mouse IgG antibodies.
Indirect Immunofluorescence
Fixed, permeabilized gametocytes, macrogametes, and zygotes were subjected to IFA. Parasites were heat-fixed onto 10-well glass slides (Fisher Scientific). Fixed cells were permeabilized by incubation in PBS containing 3% bovine serum albumin and 0.1% Triton X-100 at room temperature for 15 minutes followed by blocking for 2 hrs at room temperature in 3% BSA in PBS-T. The preparations were incubated with a-Pfs25 or a-Pfs28 antisera (1∶100 dilution) or 4b7 mAbs overnight at 4°C followed by Alexa Fluor 488 goat anti-mouse IgG (1∶500 dilution; Molecular Probes) at room temperature for 1 hr. Parasite nuclei were stained with 2 µg/mL 4′-6-Diamidino-2-phenylindole (DAPI; Molecular Probes) at room temperature for 5 min. Slides were mounted with SlowFade® anti-fade kit (Molecular Probes)and images were acquired using an Applied Precision Spectris microscope and deconvolved using Softworx software (Applied Precision – Issaquah, Washington). Images were adjusted for contrast in Softworx and exported as tiffs and assembled with Adobe Photoshop.
Standard Membrane Feeding Assay
Transmission blocking activity was assessed by standard membrane feeding [46]. Anopheles stephensi STE2 was maintained at 27°C and 80% relative humidity on a 12 hr day/night light cycle. Larvae were fed a diet of powdered fish food (Tetramin) mixed with yeast. Adults were provided a 10% sugar solution. Four to six day-old female A. stephensi mosquitoes were fed with P. falciparum NF 54 gametocytes in the presence of heat inactivated control or immune sera or mAbs using a membrane feeding apparatus. Heat inactivation was performed at 56°C for 45 min. After 15 min of feeding, un-engorged mosquitoes were removed and engorged mosquitoes were maintained in the insectary under standard conditions [47]. Midguts were dissected 9 days after the infectious bloodmeal, stained with 0.1% mercurochrome and the number of oocysts in each preparation counted. Uninfected bloodmeals were provided to wild-type control mosquitoes following the membrane feeding. Infected female mosquitoes were dissected for oocyst counts nine days after infection. Statistics were calculated in JMP ver. 9.0.2 (SAS – Cary, NC) using a single tailed Wilcoxon nonparametric comparison.
Supporting Information
Silver stain analysis of algae-produced Pfs25 and Pfs28. Increasing amounts of affinity purified a-Pfs25 and a-Pfs28 (100, 200, and 400 ng) and 200 ng of BSA were resolved on 16% SDS-PAGE and total protein was detected using silver stain.
(TIF)
Indirect immunofluorescence using immune sera from mice injected with algae-produced Pfs25 or Pfs28 on in-vitro cultured P. falciparum gametocytes, gametes, and zygotes. DNA was stained using DAPI (blue) and antibody binding was visualized using Alexa Fluor 488-conjugated rabbit anti-mouse IgG (green) for a-Pfs25 antisera, a-Pfs28 antisera, anti-Pfs25 4B7 mAbs, and sera from isogenic unvaccinated mice.
(TIF)
Acknowledgments
We would like to thank Kurt Wüthrich for the use of his spectropolarimeter and Christine Beuck for instruction. We thank Kit Pogliano for the use of her microscope and Majid Ghassemian with the UCSD biomolecular and proteomics mass spectrometry facility. We would also like to thank David Narum for antibodies and Beatriz Herrera for insectary work and mosquito husbandry.
Footnotes
Competing Interests: Stephen Mayfield has a financial interest in Sapphire Energy. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This research was supported by funding from the San Diego Foundation, the California energy commission (500-10-039), and from the U.S. Public Health Service grant from the National Institute of Allergy and Infectious Diseases (AI45999). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov. 2007;6:404–414. doi: 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- 2.Patarroyo ME, Patarroyo MA. Emerging rules for subunit-based, multiantigenic, multistage chemically synthesized vaccines. Acc Chem Res. 2008;41:377–386. doi: 10.1021/ar700120t. [DOI] [PubMed] [Google Scholar]
- 3.Center for Disease Control. CDC Vaccine Price List. Vaccines and Immunizations. 2011.
- 4.Ravallion M, Chen S, Sangraula P. The World Bank; 2008. Dollar a day revisited. [Google Scholar]
- 5.Rigano MM, Walmsley AM. Expression systems and developments in plant-made vaccines. Immunol Cell Biol. 2005;83:271–277. doi: 10.1111/j.1440-1711.2005.01336.x. [DOI] [PubMed] [Google Scholar]
- 6.Daniell H, Singh ND, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009;14:669–679. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dove A. Uncorking the biomanufacturing bottleneck. Nat Biotechnol. 2002;20:777–779. doi: 10.1038/nbt0802-777. [DOI] [PubMed] [Google Scholar]
- 8.Yusibov V, Streatfield SJ, Kushnir N. Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Hum Vaccin. 2011;7:313–321. doi: 10.4161/hv.7.3.14207. [DOI] [PubMed] [Google Scholar]
- 9.Mayfield SP, Franklin SE, Lerner RA. Expression and assembly of a fully active antibody in algae. Proc Natl Acad Sci U S A. 2003;100:438–442. doi: 10.1073/pnas.0237108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran M, Zhou B, Pettersson PL, Gonzalez MJ, Mayfield SP. Synthesis and assembly of a full-length human monoclonal antibody in algal chloroplasts. Biotechnol Bioeng. 2009;104:663–673. doi: 10.1002/bit.22446. [DOI] [PubMed] [Google Scholar]
- 11.Rasala BA, Mayfield SP. The microalga Chlamydomonas reinhardtii as a platform for the production of human protein therapeutics. Bioeng Bugs. 2011;2:50–54. doi: 10.4161/bbug.2.1.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dauvillee D, Delhaye S, Gruyer S, Slomianny C, Moretz SE, et al. Engineering the chloroplast targeted malarial vaccine antigens in Chlamydomonas starch granules. PLoS One. 2010;5:e15424. doi: 10.1371/journal.pone.0015424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreesen IA, Charpin-El Hamri G, Fussenegger M. Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J Biotechnol. 2010;145:273–280. doi: 10.1016/j.jbiotec.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh AK, Jacobs-Lorena M. Plasmodium sporozoite invasion of the mosquito salivary gland. Curr Opin Microbiol. 2009;12:394–400. doi: 10.1016/j.mib.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradel G. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology. 2007;134:1911–1929. doi: 10.1017/S0031182007003381. [DOI] [PubMed] [Google Scholar]
- 16.malERA Consultative Group on Diagnosis and Diagnostics. A research agenda for malaria eradication: vaccines. PLoS Med. 2011;8:e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan CJ, Hill AV. What is the efficacy of the RTS, S malaria vaccine? BMJ. 2011;343:d7728. doi: 10.1136/bmj.d7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter R, Chen DH. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature. 1976;263:57–60. doi: 10.1038/263057a0. [DOI] [PubMed] [Google Scholar]
- 19.Gwadz RW. Successful immunization against the sexual stages of Plasmodium gallinaceum. Science. 1976;193:1150–1151. doi: 10.1126/science.959832. [DOI] [PubMed] [Google Scholar]
- 20.Kaslow DC, Bathurst IC, Isaacs SN, Keister DB, Moss B, et al. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant Pfs25. Mem Inst Oswaldo Cruz. 1992;87(Suppl 3):175–177. doi: 10.1590/s0074-02761992000700028. [DOI] [PubMed] [Google Scholar]
- 21.Gozar MM, Price VL, Kaslow DC. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect Immun. 1998;66:59–64. doi: 10.1128/iai.66.1.59-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaslow DC, Shiloach J. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Biotechnology (N Y) 1994;12:494–499. doi: 10.1038/nbt0594-494. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One. 2008;3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrance CE, Chichester JA, Musiychuk K, Shamloul M, Rhee A, et al. Hum Vaccin 7 Suppl; 2011. Antibodies to plant-produced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. pp. 191–198. [DOI] [PubMed] [Google Scholar]
- 25.Franklin S, Ngo B, Efuet E, Mayfield SP. Development of a GFP reporter gene for Chlamydomonas reinhardtii chloroplast. Plant J. 2002;30:733–744. doi: 10.1046/j.1365-313x.2002.01319.x. [DOI] [PubMed] [Google Scholar]
- 26.Puigbo P, Bravo IG, Garcia-Vallve S. E-CAI: a novel server to estimate an expected value of Codon Adaptation Index (eCAI). BMC Bioinformatics. 2008;9:65. doi: 10.1186/1471-2105-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharp PM, Li WH. The codon Adaptation Index–a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987;15:1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson JS, Richardson DC. Natural beta-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc Natl Acad Sci U S A. 2002;99:2754–2759. doi: 10.1073/pnas.052706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyata T, Harakuni T, Tsuboi T, Sattabongkot J, Kohama H, et al. Plasmodium vivax ookinete surface protein Pvs25 linked to cholera toxin B subunit induces potent transmission-blocking immunity by intranasal as well as subcutaneous immunization. Infect Immun. 2010;78:3773–3782. doi: 10.1128/IAI.00306-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mlambo G, Kumar N, Yoshida S. Functional immunogenicity of baculovirus expressing Pfs25, a human malaria transmission-blocking vaccine candidate antigen. Vaccine. 2010;28:7025–7029. doi: 10.1016/j.vaccine.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, et al. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian F, Aebig JA, Reiter K, Barnafo E, Zhang Y, et al. Enhanced antibody responses to Plasmodium falciparum Pfs28 induced in mice by conjugation to ExoProtein A of Pseudomonas aeruginosa with an improved procedure. Microbes Infect. 2009;11:408–412. doi: 10.1016/j.micinf.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma B. Structure and mechanism of a transmission blocking vaccine candidate protein Pfs25 from P. falciparum: a molecular modeling and docking study. In Silico Biol. 2008;8:193–206. [PubMed] [Google Scholar]
- 34.Stura EA, Satterthwait AC, Calvo JC, Stefanko RS, Langeveld JP, et al. Crystallization of an intact monoclonal antibody (4B7) against Plasmodium falciparum malaria with peptides from the Pfs25 protein antigen. Acta Crystallogr D Biol Crystallogr. 1994;50:556–562. doi: 10.1107/S0907444994001782. [DOI] [PubMed] [Google Scholar]
- 35.Saxena AK, Singh K, Su HP, Klein MM, Stowers AW, et al. The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms. Nat Struct Mol Biol. 2006;13:90–91. doi: 10.1038/nsmb1024. [DOI] [PubMed] [Google Scholar]
- 36.Sreerama N, Woody RW. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem. 2000;287:252–260. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
- 37.Sreerama N, Venyaminov SY, Woody RW. Estimation of protein secondary structure from circular dichroism spectra: inclusion of denatured proteins with native proteins in the analysis. Anal Biochem. 2000;287:243–251. doi: 10.1006/abio.2000.4879. [DOI] [PubMed] [Google Scholar]
- 38.Duffy PE, Kaslow DC. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun. 1997;65:1109–1113. doi: 10.1128/iai.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dechering KJ, Kaan AM, Mbacham W, Wirth DF, Eling W, et al. Isolation and functional characterization of two distinct sexual-stage-specific promoters of the human malaria parasite Plasmodium falciparum. Mol Cell Biol. 1999;19:967–978. doi: 10.1128/mcb.19.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gozar MM, Muratova O, Keister DB, Kensil CR, Price VL, et al. Plasmodium falciparum: immunogenicity of alum-adsorbed clinical-grade TBV25-28, a yeast-secreted malaria transmission-blocking vaccine candidate. Exp Parasitol. 2001;97:61–69. doi: 10.1006/expr.2000.4580. [DOI] [PubMed] [Google Scholar]
- 41.Greenwood BM, Targett GA. Malaria vaccines and the new malaria agenda. Clin Microbiol Infect. 2011;17:1600–1607. doi: 10.1111/j.1469-0691.2011.03612.x. [DOI] [PubMed] [Google Scholar]
- 42.Manuell AL, Beligni MV, Elder JH, Siefker DT, Tran M, et al. Robust expression of a bioactive mammalian protein in Chlamydomonas chloroplast. Plant Biotechnol J. 2007;5:402–412. doi: 10.1111/j.1467-7652.2007.00249.x. [DOI] [PubMed] [Google Scholar]
- 43.Boynton JE, Gillham NW, Harris EH, Hosler JP, Johnson AM, et al. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988;240:1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- 44.Rasala BA, Muto M, Lee PA, Jager M, Cardoso RM, et al. Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol J. 2010;8:719–733. doi: 10.1111/j.1467-7652.2010.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bounkeua V, Li F, Vinetz JM. In vitro generation of Plasmodium falciparum ookinetes. Am J Trop Med Hyg. 2010;83:1187–1194. doi: 10.4269/ajtmh.2010.10-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isaacs AT, Li F, Jasinskiene N, Chen X, Nirmala X, et al. Engineered resistance to Plasmodium falciparum development in transgenic Anopheles stephensi. PLoS Pathog. 2011;7:e1002017. doi: 10.1371/journal.ppat.1002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benedict M. Chapman and Hall, London; 1997. Care and maintenance of anopheline mosquito colonies. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Silver stain analysis of algae-produced Pfs25 and Pfs28. Increasing amounts of affinity purified a-Pfs25 and a-Pfs28 (100, 200, and 400 ng) and 200 ng of BSA were resolved on 16% SDS-PAGE and total protein was detected using silver stain.
(TIF)
Indirect immunofluorescence using immune sera from mice injected with algae-produced Pfs25 or Pfs28 on in-vitro cultured P. falciparum gametocytes, gametes, and zygotes. DNA was stained using DAPI (blue) and antibody binding was visualized using Alexa Fluor 488-conjugated rabbit anti-mouse IgG (green) for a-Pfs25 antisera, a-Pfs28 antisera, anti-Pfs25 4B7 mAbs, and sera from isogenic unvaccinated mice.
(TIF)