Abstract
Aim
To determine whether patient age contributed to the fracture resistance of teeth subjected to root canal treatment and post placement.
Methodology
Forty-five single-rooted, single-canal human teeth were mounted, instrumented, obturated and prepared for a post. The teeth were divided into young (18 ≤ age ≤ 35) and old (60 ≤ age) groups and subjected to cyclic loading until fracture; those reaching 200 000 cycles without undergoing failure were then subjected to static loading to fracture. Statistical differences between groups were examined using one-way ANOVAS, and correlations were identified using Pearson’s r; significance was established at P ≤ 0.05.
Results
There was no significant difference between the two age groups in terms of the number of cycles to fracture (P > 0.05) or the load to fracture (P > 0.05). However, there was a significant correlation (P ≤ 0.05) between the root fracture resistance and individual age, indicating that the susceptibility to root fracture increases significantly with increasing patient age. Also, the dentine thickness of roots that fractured was significantly less than those that did not (P = 0.04).
Conclusion
Vertical root fracture of teeth receiving root canal treatment with posts is more likely to occur in the teeth of older patients (60+) and particularly in those with low dentine thickness.
Keywords: age, cyclic loading, dentine thickness, vertical root fracture
Introduction
A vertical root fracture (VRF) is characterized as a longitudinal fracture of the root that initiates on the internal canal wall and propagates through the root dentine towards the external root surface (Pitts & Natkin 1983). They usually begin at the apex and propagate cervically. Previous studies attribute the incidence of VRF to excessive forces during obturation (Meister et al. 1980, Dang & Walton 1989), the wedging effect of spreaders during lateral condensation (Murgel & Walton 1990), excessive pressure during post cementation (Murgel & Walton 1990, Obermayr et al. 1991), a decrease in dentine thickness with instrumentation (Wilcox et al. 1997) and also post preparation (Murgel & Walton 1990).
Patient age and tooth type are also associated with VRFs, as most occur in posterior teeth of patients between 40 and 60 years old (Testori et al. 1993, Tamse 1988). Whilst the cause for age dependence in VRFs is unknown, there are changes in the mechanical properties of dentine with patient age that could reduce the damage tolerance of teeth. Indeed, laboratory studies show that the strength of dentine under both static and cyclic loading (Arola & Reprogel 2005, Kinney et al. 2005) as well as the fracture toughness (Koester et al. 2008, Nazari et al. 2009) decreases significantly with increasing patient age.
There have been many contributions to our understanding of VRFs. Previous investigations include laboratory studies implementing static loads on anterior teeth (Holcomb et al. 1987, Dang & Walton 1989, Murgel & Walton 1990, Wilcox et al. 1997), finite element simulations (Lertchirakarn et al. 2003, Sathorn et al. 2005, Versluis et al. 2006) and both retrospective and prospective clinical evaluations of VRF characteristics in various patient populations (Testori et al. 1993, Tamse 1988). Currently, no study has evaluated the effect of patient age on the occurrence of VRFs. Although VRFs may comprise <1% of all root filled teeth (Fouad & Burleson 2003), the incidence mostly leads to extraction (Pitts & Natkin 1983). An identification of characteristics associated with VRFs could aid in prevention. Therefore, the aim of the present study was to determine whether the age of the tooth structure (i.e. patient age) affects the incidence of VRF in teeth receiving root canal treatment with posts, which are subjected to cyclic loading.
Materials and methods
Tooth selection
Freshly extracted, single-rooted, single-canal, straight human teeth were acquired with record of patient age and tooth type. The teeth were obtained according to protocols approved by the Institutional Review Board of the University of Maryland Baltimore County. All of the collected samples were continuously hydrated in Hank’s Balanced Salt Solution® (Sigma-Aldrich Inc., St. Louis, MO, USA) (HBSS) at room temperature (23 °C). A total of 53 samples were prepared, and two experimental groups were evaluated according to age (i.e. young and old). With an N of 26 in each group, an effect size of 0.40, and a P ≤ 0.05, power was equal to 0.80. As the study progressed, mandibular incisors and teeth with craze lines, surface defects, or curved roots were excluded (N = 8), thereby lowering the power to 0.76. The young group then consisted of teeth from patients 18 to 35 years of age (n = 23), and the old group consisted of teeth from patients 60 years of age and older (n = 22).
Tooth preparation and mounting
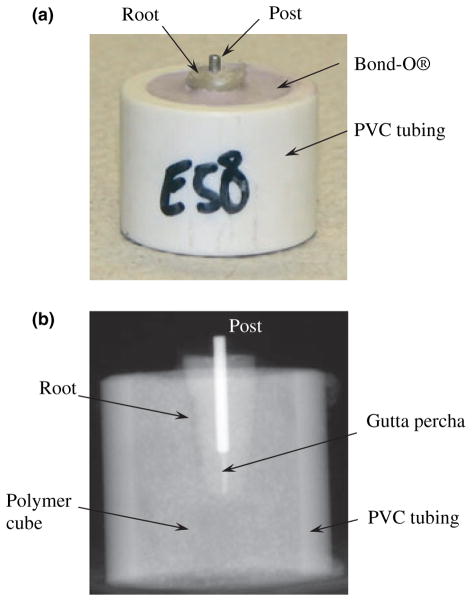
The teeth were decoronated using a Number 556 straight fissure crosscut bur (Dentsply International, York, PA, USA) to a standardized length of 13 mm of caries, and restoration-free root structure, resulting in a working length of approximately 12 mm. Care was taken to ensure that the process did not introduce damage (i.e. cracks) to the remaining root structure. The roots were wrapped in one layer of plastic (Glad Press’n Seal Plastic Wrap®; Clorox-First Brands Corp., Brooklyn, NY, USA) to simulate a periodontal ligament. These teeth were mounted within a cylindrical container and embodied by polymethyl methacrylate (Bond-O Mar-Hyde Corp, Cincinatti, OH, USA). The teeth were placed such that 2–3 mm of cervical root structure was visible above the cylinder as shown in Fig. 1a. The mounts were numbered and recorded along with the patient age of the corresponding tooth.
Figure 1.
Details of the instrumentation and prepared specimens. (a) An instrumented root with exposed root and post. (b) A digital radiograph of a specimen used to ensure post alignment and placement to within 1 mm of the Gutta-percha.
Instrumentation, obturation and post placement
The specimens (N = 45) were instrumented sequentially increasing from size 20, 0.04 taper to a size 40, 0.04 taper using ProFile 0.04 Taper ISO Rotary Instruments (Tulsa Dental Products, Tulsa, OK, USA). Saline was used after the passage of each file. The canals were dried with paper points, and lateral condensation was performed with size 40, 0.02 taper Gutta-percha point and Roth 801 sealer, using a ‘B’ finger spreader. The specimens were placed on a postal scale, and the spreader was used three to four times, at a maximum condensation load of 3 kg for 10 s each, to allow for the placement of three to four medium-fine accessory Gutta-percha cones. The post spaces were prepared using Gates Gliddens sizes 2 and 3, followed by the red ParaPost® drill (Coltene Whaledent, Cuyahoga Falls, OH, USA). Post spaces were prepared to a depth of 8 mm, 5 mm from the anatomic apex. Red ParaPosts® (stainless steel, 1.25 mm diameter, serrated surface) were cemented in each root with Fuji CEM® (GC America Inc., Alsip, IL, USA). Each specimen was imaged with digital radiography (Schick Technologies, Long Island, NY, USA) to ensure proper post alignment and placement to within 1 mm of the Gutta-percha (e.g. Fig. 1b). In addition, the roots were visually inspected after placement of the post to ensure that fracture did not occur; the teeth were removed from the mounts, and the roots were thoroughly examined using a stereomicroscope (Model SMZ 800; Nikon, Tokyo, Japan) at a magnification range from approximately 10× to 100×.
Testing methods
An Endura TEC ELF Model 3300 (Bose, Eden Prairie, MN, USA) testing system was used for loading (maximum load of 2250 N; sensitivity ± 0.1 N). The specimens were submerged in a hydration bath of HBSS® in an upright position, and loads were applied onto the post, down the long axis of the tooth. Cyclic loads were applied with sinusoidal waveform at a frequency of 5 Hz, with a maximum and minimum of 400 N and 40 N, respectively. The peak load is consistent with the range reported in evaluations of mastication (Hellsing 1980, Hagberg 1987, Lyons & Baxendale 1990, Tortopidis et al. 1998). Cyclic loading was conducted in 5000 cycle intervals up to 25 000 cycles, then 10 000 cycle intervals up to 75 000 cycles, then 25 000 cycle intervals up to 100 000 cycles and finally 50 000 cycle intervals up to 200 000 cycles. After each interval, the root was removed from the mount and visually inspected for fracture by eye and over a range of magnifications. A stereomicroscope (Model SMZ 800; Nikon) was used with transillumination at a magnification ranging from approximately 10× to 100×.
Fracture of the root was defined when identified at the external root surface. Roots that fractured were no longer subjected to cyclic loading. The mean of the lowest and highest number of cycles for the interval of fracture was recorded as the number of cycles to failure. Those roots that did not fracture were returned to the mounts and subjected to further cyclic loading. Specimens that did not fracture within 200 000 cycles were subjected to static loading. The static loads were applied on the post, down the long axis of the root at a rate of 10 N s−1 to a maximum of 1000 N or until fracture occurred. Fracture was identified by visual evidence or a discontinuation in the load carrying capacity of the specimen. Stereomicroscopic evaluation was also performed, and trans-illumination was used if necessary. The final load at fracture was recorded, and all fractures were qualitatively analysed for fracture patterns.
Dentine thickness evaluation
All specimens that fractured were sectioned down the fracture line using an Ultra Thin Multipurpose Abrasive Disk® (National Keystone Products Company, Cherry Hill, NJ, USA) on a straight handpiece. The dentine thickness was measured to the nearest 0.01 mm at three evenly distributed points along the fracture line using calipers. The average thickness was calculated and recorded. Specimens that did not fracture were sectioned longitudinally in two dimensions, mesiodistally and buccolingually. Measurements at the junction of the apical and middle thirds and the junction of the middle and cervical thirds from each dimension were taken and used in estimating the average thickness.
Statistical analysis
The average number of cycles to VRF (i.e. life) of the instrumented roots in each age group was determined. In addition, a normalized life was calculated using the cross-sectional geometry at the point of fracture and the fatigue life behaviour of dentine previously reported (Arola & Reprogel 2006). A normalization was necessary to account for the influence of tooth cross section on the cyclic stress. Specifically, the normalized life (N′) was conducted by dividing the cross-sectional area (A) at the point of fracture by the area of the control specimen (Ao) according to
| (1) |
where b is the stress life exponent for dentine (b = −0.06) (Arola & Reprogel 2006). The area of the control specimen was defined as the average cross-sectional area of all specimens tested. The normalized life accounted for the increase in cyclic stress that resulted from smaller dentine thickness and its importance to the fracture resistance. Additional details are reported in Mireku (2008).
Differences in the normalized value of the number of cycles to fracture by age, the maximum static load to fracture by age and the dentine thickness between specimens that fractured according to cyclic loads, static loads and/or did not fracture at all were analysed by one-way ANOVAS. Pearson’s r was used to evaluate the correlation between (i) the normalized number of cycles to fracture and the patient age in years and (ii) the static load required for fracture and the patient age in years. Statistical significance was established at P ≤ 0.05.
Results
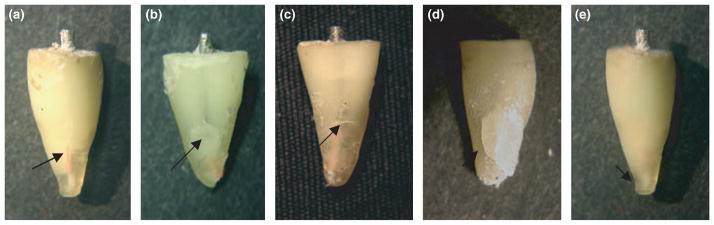
There were five basic fracture patterns exhibited (Fig. 2), which included longitudinal root fracture, lateral (push-out) root fracture, horizontal root fracture, crushing root fracture and apical root fracture. In nearly all specimens, the cracks that developed as a result of cyclic loading were visible to the naked eye. Of the 45 specimens subjected to cyclic loading, 27 fractured within 200 000 cycles including 13 (48%) young teeth and 14 (52%) old teeth. Of the remaining 18 specimens, nine fractured before reaching the maximum load of 1000 N [five (55%) young and four (44%) old]. There was a relatively large range in the number of cycles to failure (i.e. life) about the mean values. Outliers in the results of each age group were determined by identifying those responses with number of cycles to failure that were more than two standard deviations from the average life according to Norman & Streiner (2008). Two specimens from the old group and one from the young group were identified as outliers using this approach, and a new mean and standard deviation for cyclic loading was calculated excluding these responses. A summary of the results from cyclic loading (i.e. life) and static loading (load at fracture) for both age groups is listed in Table 1. No significant difference was found between the two age groups under either mode of loading (P > 0.05).
Figure 2.
The primary fracture patterns identified. (a) longitudinal root fracture, (b) lateral (push-out) root fracture, (c) horizontal root fracture, (d) crushing root fracture, (e) apical root fracture.
Table 1.
Comparisons between age and the number of cycles to fracture or the load at fracture
| Loading method | Old
|
Young
|
ANOVA
|
|||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | F | P | |
| Normalized numbers of cycles to fracture (outliers removed) | 12 | 198.4 ± 251.7 cycles | 12 | 3.768 ± 8.648 cycles | 2.13 | 0.16 |
| Load to fracture | 4 | 753 ± 48 newtons | 5 | 705 ± 108 newtons | 0.66 | 0.44 |
A Pearson’s correlation of −0.21 (P = 0.15) was found between age and the normalized number of cycles to fracture, and a Pearson’s correlation of 0.23 (P = 0.07) was found between age and the static load required for VRF. Neither of these correlations was significant (P > 0.05). However, when the outliers were removed, there was a significant correlation of −0.37 (P = 0.04) between age and the normalized number of cycles to fracture. Therefore, whilst the differences in average fatigue life of teeth from the two age groups were not significantly different, the results indicate that there was a significant reduction in the number of cycles to the incidence of VRF with increasing patient age. The measure of correlation accounts for the span in patient age within each group and the fatigue life of each individual tooth.
The mean dentine thickness along the line of fracture was calculated for the respective groups of specimens. There were no significant differences (P > 0.05) in the dentine thickness between the young and old specimens within all the groups evaluated (Table 2).
Table 2.
Mean dentin thickness (DT) and comparison of results between young and old specimens for all six groups
| Groups | N | Mean DT all (mm) | Old DT Mean ± SD (mm) | Young DT Mean ± SD (mm) | Old–young DT Mean (mm) | F | P |
|---|---|---|---|---|---|---|---|
| All specimens | 45 | 1.31 ± 0.35 | 1.34 ± 0.42 | 1.28 ± 0.28 | 0.06 | 0.29 | 0.60 |
| Fractured | 36 | 1.24 ± 0.35 | 1.30 ± 0.45 | 1.21 ± 0.20 | 0.09 | 0.62 | 0.44 |
| Cyclic fracture | 27 | 1.24 ± 0.28 | 1.25 ± 0.35 | 1.22 ± 0.20 | 0.03 | 0.09 | 0.77 |
| No cyclic fracture | 18 | 1.41 ± 0.42 | 1.48 ± 0.50 | 1.36 ± 0.35 | 0.12 | 0.39 | 0.55 |
| Static fracture | 9 | 1.31 ± 0.52 | 1.47 ± 0.77 | 1.18 ± 0.23 | 0.29 | 0.65 | 0.45 |
| No fracture | 9 | 1.52 ± 0.27 | 1.50 ± 0.10 | 1.54 ± 0.37 | −0.04 | 0.04 | 0.84 |
No significant difference was found between the dentine thickness of roots that fractured under cyclic loads and those that did not fracture under cyclic loads (F = 2.94; P = 0.09). Similarly, there was no significant difference between the dentine thickness of roots that fractured under cyclic loads, roots that fractured under static loads and roots that did not fracture at all (F = 2.39; P = 0.10). However, the dentine thickness of all specimens that fractured was significantly lower than that of all specimens that did not fracture (F = 4.54; P = 0.04). Dentine thickness was correlated with age and with number of cycles to fracture, but neither of these correlations were significant (P > 0.05).
Discussion
The present study was designed to determine whether the age of the tooth (i.e. defined by patient age) and the corresponding microstructure affects the incidence of VRF in teeth receiving root canal treatment with posts and subjected to cyclic loading. Whilst cyclic loading models have been adopted in the literature to evaluate fracture of post and core systems (Huysmans et al. 1992, Heydecke et al. 2002, Sahafi et al. 2005), they have not been used in examining the incidence of VRF and the importance of age. Cyclic loading of root filled treated teeth with posts is a method of evaluating root fracture resistance with a larger degree of clinical relevance than previous studies. Overall, 27 of the teeth fractured under cyclic loading. The mean number of cycles required to fracture these specimens (65 000 cycles) is approximately 3 months of oral function in vivo (Kelly 1997). The majority (~75%) of the teeth fractured in a clinically relevant manner. Four of the five fracture patterns were versions of clinical VRFs; the longitudinal VRF (Fig. 2a) occurred most frequently and was most akin to clinical VRFs. The lateral, horizontal and crushing VRFs (Fig. 2b–d) may occur clinically as a result of prolonged function on a malaligned post and may have resulted here from eccentric loads causing lateral movement of the post within the root. The apical fracture (Fig. 2e) has not been reported clinically, and may have occurred because of time-dependent deformation of the polymer embodying the tooth and consequent subsidence of the specimen within its foundation. Apical fractures did not occur with static loads because of the short period of loading (2 min or less per specimen).
There were no significant differences between the number of cycles required to fracture the young and old specimens when considering all results (raw or normalized). Similarly, the Pearson’s correlation between the normalized number of cycles required for fracture, and age was not significant. However, after removing outliers, there was a significant correlation (r = −0.37; P = 0.04) between the normalized number of cycles to fracture and patient age. Specifically, the normalized number of cycles required for fracture decreased as patient age increased. It is important to highlight that the young specimens exhibited a life two to three times greater (in terms of the number of cycles to failure) than the old specimens (Table 1). Previous case reports have shown that VRFs are associated with patient age (Testori et al. 1993, Tamse 1988). Also, laboratory studies adopting cyclic loading have reported that the endurance strength of dentine decreases with patient age (Arola & Reprogel 2005) and that the incremental crack growth rate in ‘old’ dentine is 100 times faster than that in ‘young’ dentine (Bajaj et al. 2006). Similar findings have been reported on the resistance to fracture of human dentine under quasi-static loading. There is also a significant decrease in the fracture toughness of aged dentine (Koester et al. 2008, Nazari et al. 2009). Results of these laboratory studies suggest that the reduction in resistance to VRF with age is attributed to the change in microstructure of dentine (i.e. onset and progression of sclerosis) and the corresponding lower resistance to damage initiation and propagation.
There was no significant difference in the results between the young and old teeth for static loading. Yet, it is important to highlight that results of static loading were limited by a relatively small sample size, which was a by-product of the study design; static loading was only performed on specimens that did not fail by cyclic loading and the majority of specimens failed as a result of fatigue. Furthermore, of all the different groups of specimens, those that fractured under static loads had the greatest difference in mean dentine thickness between the young and old specimens. The old specimens subjected to static loading were 20% thicker than the young specimens, which would serve to increase the load to fracture and potentially diminish the apparent influence of age on the incidence of fracture. Whilst not significant, the thicker dentine in the old teeth undoubtedly contributed to the weak and positive correlation between load to fracture and age. Nevertheless, the data reveal that age accounts for <14% of the total variance in resistance to root fracture (r = 0.37).
No significant difference was found between the dentine thickness of roots that fractured under cyclic loads and those that did not. There was also no significant difference in the dentine thickness between specimens that failed under cyclic loading, specimens that failed under static loading and specimens that did not fail. It is important to note that measurements of the dentine thickness were taken along the fracture line, which was not at the same location for all teeth and may have introduced variability. Nevertheless, both of these analyses yielded a trend showing that the resistance to VRF increased with increasing dentine thickness. A post hoc power analysis indicated that significant findings could be attained with a larger sample size (n = 27 for each fracture group). An evaluation of all teeth showed that those that did not fracture had a significantly greater thickness than those specimens that did. Therefore, the likelihood of VRF increases in regions, and in teeth, with low dentine thickness.
Whilst this study provides additional understanding of patient and tooth characteristics that are important to the incidence of VRF, there are recognized limitations. Cyclic loading was performed on the restored teeth to maximize consistency with the nature of loading imposed by mastication. Clinically, most root filled teeth that undergo VRF remain in function for ~5–10 years (~1–2 million cycles) before diagnosis (Testori et al. 1993). The period of cyclic loading for this investigation was limited to 200 000 cycles, which would represent <1 year of clinical function (Kelly 1997). Though the results cannot be directly extended to the clinical setting (most notably because of the lack of coronal coverage of the specimens in this study), there may be additional relevant findings achieved under a larger period of cyclic loading. Nevertheless, 75% of the specimens fractured because of cyclic loading. Future studies should consider extending the period of evaluation over a larger number of total cycles to achieve failures exclusively by fatigue.
Another concern is that both anterior and posterior teeth were included in the study but the ratio was not equivalent in the young (14 anterior/9 posterior) and old (19 anterior/3 posterior) groups. Inclusion of both anterior and posterior teeth allowed the sample size requirement to be met more readily, but potentially caused variation in the results. All teeth were subjected to the same cyclic loads down the axis of the root. That raises two issues. Posterior teeth withstand greater loads than anterior teeth in vivo (Okeson 1998), and most anterior teeth and premolars are loaded oblique to the long axis of the root. As such, variations in tooth type may have affected the results. Also, axial loading was performed directly on the post and without inclusion of core build-up or coronal coverage. That resulted in a more concentrated load transfer from the post to the tooth at the post floor, and may have contributed to the observed incidence of apical fractures (Fig. 2e). When a post is supported with core and coronal coverage, the loads become more distributed about the most coronal aspect of the preparation and there is a corresponding reduction in the level of stress towards the apical end of the post (Cailleteau et al. 1992, Dietschi et al. 2007, Sorrentino et al. 2007). However, the stress distribution achieved with core and coronal coverage becomes a larger function of tooth geometry and ‘fit’. That changes the focus of the study from properties of the coronal dentine to that of the post and core build-up, as well as issues related to material selection, placement and geometry of the tooth. Nevertheless, the aforementioned issues should be considered in future studies. Of equal note, metal posts were used rather than carbon fibre posts to maintain consistency with previous studies reported in the literature. Fibre reinforced posts possess mechanical properties closer to that of the natural tooth structure and have demonstrated less extensive fractures in endodontically restored teeth (Asmussen et al. 1999) and better resistance to fatigue failures (Dietschi et al. 2008). Thus, future studies evaluating fatigue of root filled restored teeth with posts should consider using carbon fibre posts.
Another concern is that irrigation was performed with saline rather than sodium hypochlorite, the standard for root canal treatment. Sodium hypochlorite has been reported to reduce the elastic modulus and flexure strength of dentine (Grigoratos et al. 2001, Sim et al. 2001), reduce the microhardness (Slutzky-Goldberg et al. 2004) and remove collagen from dentine (Arias et al. 2005, Guida 2006). However, these studies included complete immersion in sodium hypochlorite bath, excessively long exposure periods (10, 20, or 120 min), and/or high concentrations (6% and 10%) (Slutzky-Goldberg et al. 2004, Arias et al. 2005). No study has reported significant changes in the properties of dentine resulting from a clinically relevant exposure. Therefore, there is no expected limitation posed by the use of saline irrigation in place of sodium hypochlorite.
Conclusion
Within the limitations of this investigation, the findings indicate that the resistance to VRF of root filled teeth restored with posts decreases with increasing patient age and low dentine thickness. Future investigations of VRFs should implement cyclic loading and control for both age and dentine thickness.
Acknowledgments
Support for the following investigation was provided by the AAE Foundation and the National Institutes of Dental and Craniofacial Research (R01 DE016904).
References
- Arias VG, Bedran-de-Castro AK, Pimenta LA. Effects of sodium hypochlorite gel and sodium hypochlorite solution on dentine bond strength. Journal of Biomedical Materials Research B: Applied Biomaterials. 2005;72:339–44. doi: 10.1002/jbm.b.30160. [DOI] [PubMed] [Google Scholar]
- Arola D, Reprogel RK. Effects of aging on the mechanical behavior of human dentine. Biomaterials. 2005;26:4051–61. doi: 10.1016/j.biomaterials.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Arola DD, Reprogel RK. Tubule orientation and the fatigue strength of human dentine. Biomaterials. 2006;27:2131–40. doi: 10.1016/j.biomaterials.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Asmussen E, Peutzfeldt A, Heitmann T. Stiffness, elastic limit, and strength of newer types of endodontic posts. Journal of Dentistry. 1999;27:275–8. doi: 10.1016/s0300-5712(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Bajaj D, Sundaram N, Nazari A, Arola D. Age, dehydration and fatigue crack growth in dentine. Biomaterials. 2006;27:2507–17. doi: 10.1016/j.biomaterials.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Cailleteau JG, Rieger MR, Akin JE. A comparison of intracanal stresses in a post-restored tooth utilizing the finite element method. Journal of Endodontics. 1992;18:540–4. doi: 10.1016/S0099-2399(06)81210-0. [DOI] [PubMed] [Google Scholar]
- Dang DA, Walton RE. Vertical root fracture and root distortion: effect of spreader design. Journal of Endodontics. 1989;15:294–301. doi: 10.1016/S0099-2399(89)80050-0. [DOI] [PubMed] [Google Scholar]
- Dietschi D, Duc O, Krejci I, Sadan A. Biomechanical considerations for the restoration of endodontically treated teeth: a systematic review of the literature – Part 1. Composition and micro- and macrostructure alterations. Quintessence International. 2007;38:733–43. [PubMed] [Google Scholar]
- Dietschi D, Duc O, Krejci I, Sadan A. Biomechanical considerations for the restoration of endodontically treated teeth: a systematic review of the literature, Part II (Evaluation of fatigue behavior, interfaces, and in vivo studies) Quintessence International. 2008;39:117–29. [PubMed] [Google Scholar]
- Fouad AF, Burleson J. The effect of diabetes mellitus on endodontic treatment outcome: data from an electronic patient record. Journal of the American Dental Association. 2003;134:43–51. doi: 10.14219/jada.archive.2003.0016. [DOI] [PubMed] [Google Scholar]
- Grigoratos D, Knowles J, Ng YL, Gulabivala K. Effect of exposing dentine to sodium hypochlorite and calcium hydroxide on its flexural strength and elastic modulus. International Endodontic Journal. 2001;34:113–9. doi: 10.1046/j.1365-2591.2001.00356.x. [DOI] [PubMed] [Google Scholar]
- Guida A. Mechanism of action of sodium hypochlorite and its effects on dentine. Minerva Stomatologica. 2006;55:471–82. [PubMed] [Google Scholar]
- Hagberg C. Assessment of bite force: a review. Journal of Craniomandibular Disorders. 1987;1:162–9. [PubMed] [Google Scholar]
- Hellsing G. On the regulation of interincisor bite force in man. Journal of Oral Rehabilitation. 1980;7:403–11. doi: 10.1111/j.1365-2842.1980.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Heydecke G, Butz F, Hussein A, Strub JR. Fracture strength after dynamic loading of endodontically treated teeth restored with different post-and-core systems. Journal of Prosthetic Dentistry. 2002;87:438–45. doi: 10.1067/mpr.2002.123849. [DOI] [PubMed] [Google Scholar]
- Holcomb JQ, Pitts DL, Nicholls JI. Further investigation of spreader loads required to cause vertical root fracture during lateral condensation. Journal of Endodontics. 1987;13:277–84. doi: 10.1016/S0099-2399(87)80044-4. [DOI] [PubMed] [Google Scholar]
- Huysmans MC, van der Varst PG, Schafer R, Peters MC, Plasschaert AJ, Soltesz U. Fatigue behavior of direct post-and-core-restored premolars. Journal of Dental Research. 1992;71:1145–50. doi: 10.1177/00220345920710050301. [DOI] [PubMed] [Google Scholar]
- Kelly JR. Ceramics in restorative and prosthetic dentistry. Annual Review of Materials Science. 1997;27:443–68. [Google Scholar]
- Kinney JH, Nalla RK, Pople JA, Breunig TM, Ritchie RO. Age-related transparent root dentine: mineral concentration, crystallite size, and mechanical properties. Biomaterials. 2005;26:3363–76. doi: 10.1016/j.biomaterials.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Koester KJ, Ager JW, 3rd, Ritchie RO. The effect of aging on crack-growth resistance and toughening mechanisms in human dentine. Biomaterials. 2008;29:1318–28. doi: 10.1016/j.biomaterials.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Lertchirakarn V, Palamara JE, Messer HH. Finite element analysis and strain-gauge studies of vertical root fracture. Journal of Endodontics. 2003;29:529–34. doi: 10.1097/00004770-200308000-00009. [DOI] [PubMed] [Google Scholar]
- Lyons MF, Baxendale RH. A preliminary electromyographic study of bite force and jaw-closing muscle fatigue in human subjects with advanced tooth wear. Journal of Oral Rehabilitation. 1990;17:311–8. doi: 10.1111/j.1365-2842.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Meister F, Jr, Lommel TJ, Gerstein H. Diagnosis and possible causes of vertical root fractures. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 1980;49:243–53. doi: 10.1016/0030-4220(80)90056-0. [DOI] [PubMed] [Google Scholar]
- Mireku AS. An In Vitro Investigation of the Effect of Age on the Incidence of Vertical Root Fracture Resulting from Cyclic Loading. Baltimore: University of Maryland; 2008. [Google Scholar]
- Murgel CA, Walton RE. Vertical root fracture and dentine deformation in curved roots: the influence of spreader design. Endodontics and Dental Traumatology. 1990;6:273–8. doi: 10.1111/j.1600-9657.1990.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Nazari A, Bajaj D, Zhang D, Romberg E, Arola D. On the reduction in fracture toughness of human dentine with age. Journal of the Mechanical Behavior of Biomedical Materials. 2009;2:550–9. doi: 10.1016/j.jmbbm.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GR, Streiner DL. Biostatistics. Shelton, Connecticut: People’s Medical Publishing Company; 2008. p. 303. [Google Scholar]
- Obermayr G, Walton RE, Leary JM, Krell KV. Vertical root fracture and relative deformation during obturation and post cementation. Journal of Prosthetic Dentistry. 1991;66:181–7. doi: 10.1016/s0022-3913(05)80045-9. [DOI] [PubMed] [Google Scholar]
- Okeson JP. Management of Temporomandibular Disorders and Occlusion. 5. St Louis, MO: Mosby; 1998. [Google Scholar]
- Pitts DL, Natkin E. Diagnosis and treatment of vertical root fractures. Journal of Endodontics. 1983;19:338–46. doi: 10.1016/S0099-2399(83)80150-2. [DOI] [PubMed] [Google Scholar]
- Sahafi A, Peutzfeldt A, Ravnholt G, Asmussen E, Gotfredsen K. Resistance to cyclic loading of teeth restored with posts. Clinical Oral Investigations. 2005;9:84–90. doi: 10.1007/s00784-004-0299-7. [DOI] [PubMed] [Google Scholar]
- Sathorn C, Palamara JE, Palamara D, Messer HH. Effect of root canal size and external root surface morphology on fracture susceptibility and pattern: a finite element analysis. Journal of Endodontics. 2005;31:288–92. doi: 10.1097/01.don.0000140579.17573.f7. [DOI] [PubMed] [Google Scholar]
- Sim TP, Knowles JC, Ng YL, Shelton J, Gulabivala K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. International Endodontic Journal. 2001;34:120–32. doi: 10.1046/j.1365-2591.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- Slutzky-Goldberg I, Maree M, Liberman R, Heling I. Effect of sodium hypochlorite on dentine microhardness. Journal of Endodontics. 2004;30:880–2. doi: 10.1097/01.don.0000128748.05148.1e. [DOI] [PubMed] [Google Scholar]
- Sorrentino R, Aversa R, Ferro V, et al. Three-dimensional finite element analysis of strain and stress distributions in endodontically treated maxillary central incisors restored with different post, core and crown materials. Dental Materials. 2007;23:983–93. doi: 10.1016/j.dental.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Tamse A. Iatrogenic vertical root fractures in endodontically treated teeth. Endodontics and Dental Traumatology. 1988;4:190–6. doi: 10.1111/j.1600-9657.1988.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Testori T, Badino M, Castagnola M. Vertical root fractures in endodontically treated teeth: a clinical survey of 36 cases. Journal of Endodontics. 1993;19:87–91. doi: 10.1016/S0099-2399(06)81202-1. [DOI] [PubMed] [Google Scholar]
- Tortopidis D, Lyons MF, Baxendale RH, Gilmour WH. The variability of bite force measurement between sessions, in different positions within the dental arch. Journal of Oral Rehabilitation. 1998;25:681–6. doi: 10.1046/j.1365-2842.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- Versluis A, Messer HH, Pintado MR. Changes in compaction stress distributions in roots resulting from canal preparation. International Endodontic Journal. 2006;39:931–9. doi: 10.1111/j.1365-2591.2006.01164.x. [DOI] [PubMed] [Google Scholar]
- Wilcox LR, Roskelley C, Sutton T. The relationship of root canal enlargement to finger-spreader induced vertical root fracture. Journal of Endodontics. 1997;23:533–4. doi: 10.1016/S0099-2399(97)80316-0. [DOI] [PubMed] [Google Scholar]