In multicellular organisms, exchange between the cytoplasm of cells and the environment is facilitated by the vascular system in combination with the infolding of the surface epithelium to form the gastrointestinal, respiratory, and excretory (urinary) systems. These internalized, branching tubular pathways into the body of an organism are lined by continuous layers of cells, called epithelia, which preserve the boundary between the environment and the extracellular connective tissues and blood spaces. The epithelia lining these tubes are selectively permeable to molecules destined to be absorbed or secreted, by virtue of cellular transport systems that move these molecules through the cells between the inside and outside of the body. This route of transport of molecules through the epithelial cell cytoplasm is called the transcellular pathway. In addition to this route, molecules can move across epithelia by diffusing in between the cells [the so-called paracellular pathway (1)] (Fig. 1). Although the spaces between epithelial cells are very narrow, typically 20–30 nm, the paracellular pathway nonetheless represents a significant leak between the environment and the connective tissues that must be regulated for the epithelium to remain selectively permeable. Organisms also subdivide their internal spaces into separate physiological compartments that do not communicate with the external environment. These internal compartments include the blood and lymphatic systems, the thoracic, pericardial, and abdominal cavities, and the specialized matrix that accompanies peripheral nerves. These compartments are likewise delimited by epithelioid layers of cells, termed endothelium, mesothelium, and perineurium.
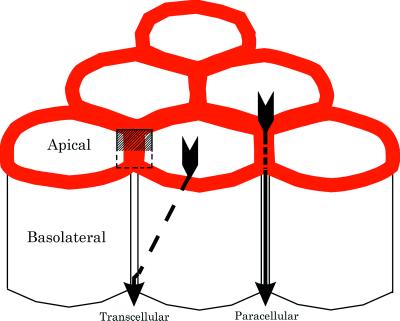
Figure 1.
A diagram of six epithelial cells packed into a portion of an epithelium. The apical and basolateral membranes are separated by tight junctions (shown as continuous red rings joining the cells). Selective epithelial permeability involves both the transcellular and paracellular routes. In the former, solutes are transported across the apical plasma membrane, diffuse through the cytoplasm (dashed arrow), and are then retransported out of the cell into the extracellular space beneath the tight junctions. In the paracellular route, solutes move through channels in the tight junction itself (solid arrow) and gain access to the paracellular spaces without entering the cytoplasm of the cells. The small box indicates the plane of section shown in diagram in Fig. 3.
The tight junction (zonula occludens) is the vertebrate gatekeeper of the paracellular pathway. Typically found at the most apical portion of epithelial cells’ lateral membranes, the tight junction is deployed as a belt around the circumference of each cell, joining all the cells, so that in the aggregate it forms a continuous, fishnet structure (Fig. 1, red). The structure of the tight junction has been characterized by using electron microscopy. Fig. 2 shows freeze-fracture and thin-section images of bile canaliculi in mouse liver, flanked by tight junctions. The membrane interactions appear as branching and anastomosing “strands” on the P fracture face (above the canaliculus) and complementary grooves on the E fracture face (below the canaliculus). In thin section, the tight junctions can be seen to deny an experimentally administered extracellular tracer (horseradish peroxidase) access to the canalicular lumen (2). Epithelia and endothelia differ greatly in their ability to regulate gradients across their paracellular pathways. Depending on the functional requirements of an epithelium, there may be small or large amounts of water and small solutes flowing passively through the tight junctions (3). The resistance of the paracellular pathway also is dynamically regulated under different physiological conditions (4) and is accompanied by substantive cytoskeletal reorganization (5). Differences in the resistances of paracellular pathways found between different epithelia have been correlated with the freeze-fracture appearance of the junctional membranes, with the depth and complexity of the freeze-fracture strands being directly (6) or indirectly (7) responsible for resistances in series. Although elements of this correlation may be true, it also is known that there are few observable differences in tight-junction structure evident in comparisons of two strains of the Madin–Darby canine kidney cell line that have different paracellular resistances (8), indicating that there must be biochemical heterogeneity of tight-junction strands. Of great importance to our understanding of the diversity and dynamics of tight junctions, the paper in this issue of the Proceedings (9) has identified a family of integral membrane proteins that compose the freeze-fracture strands.
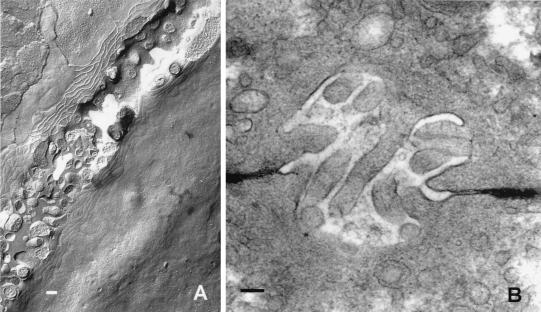
Figure 2.
Electron microscopy of tight junctions in liver from mouse bile canaliculi. (A) Freeze-fracture image of the canaliculus angles across the field of view from lower left to upper right. The tight junctions appear as branching and anastomosing strands on the P fracture face (above the canaliculus) and complementary grooves on the E fracture face (below the canaliculus). (B) Thin-section image in which the tight junctions can be seen flanking the microvillus-filled canaliculus. Horseradish peroxidase was introduced into the extracellular spaces via the vascular system and is seen to penetrate the extracellular spaces but is denied access to the canalicular lumen by the tight junctions. (Bar = 1 μm.)
Whereas several intracellular, membrane-associated proteins have been immunolocalized to the tight junction, only one integral membrane protein, occludin (10) has been shown to be part of the freeze-fracture strands (11), to interact with tight junction-associated cytoplasmic proteins (12), and to change the permeability properties of epithelia when mutated or overexpressed (13–15). Although justifiably hailed as the “holy grail” by researchers in the field (16), it was difficult to imagine how occludin alone could be responsible for the wide variety of observed epithelial permeability properties. It was subsequently shown that disruption of occludin by expression of mutant forms (13, 14), by targeted gene disruption (17), or by use of an extracellular peptide with dominant negative activity (18) did not result in the morphological loss of tight junctions, indicating that occludin contributed only a part of the structure. A second major breakthrough in the field, after the discovery of occludin, was accomplished by Tsukita and colleagues (19), who used an elegant biochemical approach to identify new proteins associated with occludin, called claudin-1 and claudin-2. Like occludin, these new proteins also are predicted to be four-pass integral membrane proteins and were shown to reside in tight-junctional strands. Tsukita and his associates (20) demonstrated that the claudins could form the tight-junctional strands when exogenously expressed in nontight junction-containing cells and that the claudins were able to recruit exogenously expressed occludin into the freeze-fracture structures. The present communication in the Proceedings (9) demonstrates that the claudins are a family of at least eight members.
The presence of a family of tight-junctional proteins offers an experimental paradigm to study and understand the different permeability properties of the paracellular pathways in different epithelia. Northern blot analysis (9) of eight mammalian tissues reveals a satisfying unique signature of distribution for each of the claudins studied (claudins 3–5 and 7–8). The kidney, an organ with known complexity and diversity in its component epithelial permeabilities along the length of its nephrons, shows expression of all the claudins tested, with claudin-8 mRNA in the highest abundance. Other nonepithelial tissues, such as brain, heart, and skeletal muscle, lack detectable signals for claudins-3, -4, and -7. Because these tissues (and all others tested) contain claudin-5, it is likely that that claudin-5 may be a universal component of endothelial tight junctions.
The discovery of the claudin family offers a molecular explanation for the finding reported in the literature that the freeze-fracture properties of tight-junctional strands vary between epithelia (6, 21, 22). In some cases, the strands seen on the fracture faces of the junctional membranes partition exclusively to the P fracture face, whereas strands in other epithelia partition to both the P and E fracture faces. Whereas claudin-1 induces freeze-fracture strands that are largely associated with the P face, claudin-2 strands partition between the two fracture faces (20). Although the fracture properties of the remainder of the claudin family are not yet known, it is clear from figure 6 in the Proceedings article (9) that claudin-3 also shows a tendency to present fragments of strands on the E fracture face. It will be of interest to understand whether these different fracture properties reflect the degree of interaction of each of the claudins with junction-associated proteins in the cytoplasm. Of equal interest will be an understanding of the localization of the claudins along the length of the strands, whether certain claudins are located at sites where the strands branch and anastomose in the plane of the membranes, and whether there are differences in the apical and basal strands in complex multistranded tight junctions.
It has been shown that the tight junction-associated proteins ZO-1 (23), ZO-2 (24–26), and ZO-3 (27) associate with each other and with the cytoplasmic C terminus of occludin (12, 28) (Fig. 3). The functional significance of these interactions is not known, nor are there any direct demonstrations of the functions of these and other tight junction-associated proteins in paracellular permeability (29–32). Studies have shown that the C-terminal half of ZO-1 can bind both to actin (28) and to stress fibers (33), creating a possible direct linkage between occludin and the cytoskeleton through the ZO complex of proteins. The N-terminal half of ZO-1 interacts with the Ras-binding domain of AF-6, and this interaction is inhibited by activated Ras (31). In non-tight junction-containing cells, Itoh et al. (33) have shown that the N-terminal half of ZO-1 can bind directly to α-catenin but not β-catenin or the cytoplasmic domain of E-cadherin. Thus, ZO-1 seems able to form additional linkages to the actin cytoskeleton, depending on the cell type. ZO-1 binds to α-spectrin and the second PDZ domain of ZO-1 binds to the C terminus of the gap junction protein connexin43 (34, 35), implicating an additional role for ZO-1 in gap junction-mediated intercellular communication.
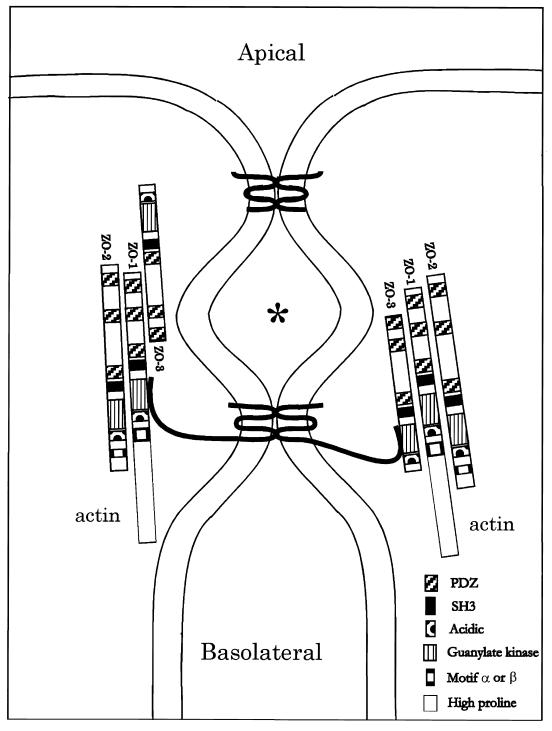
Figure 3.
A diagram of the vertical section (small box) in Fig. 1. The apical and basolateral membranes are separated by one or more membrane–membrane interactions extending in the plane normal to the figure to form the paracellular seal. In three dimensions, these interactions would anastomose in this plane and then branch, creating the weblike strand network seen in freeze-fracture images in Fig. 2. Two interactions are drawn here: apical, showing a fanciful bonding between two claudin proteins via their extracellular loops, and basal, showing a similar bonding between two occludin proteins, whose longer cytoplasmic C termini interact with the guanylate kinase domain of either ZO-1 or ZO-3. Actin is known to interact with the proline-rich tail of ZO-1. Each ZO-1 is shown interacting with ZO-2 via their second PDZ domains. The claudins and occludins are drawn as monomers; presumably they are oligomerized with themselves or with each other in the membrane. ∗ is placed in a chamber created between two membrane–membrane interactions, which may be isolated from both the apical and basolateral compartments.
There are as yet no known binding partners of the claudin family of proteins. Occludin has been shown to oligomerize when expressed in Xenopus embryos (15). Occludin and claudins-1 and -2 occupy vesicles with similar densities after sonication of membrane fractions from liver, and this relationship permitted the first identification of the claudins (19). However, it is not known whether occludin and claudins directly interact or co-oligomerize into larger structures. Given the relatively small molecular mass of the claudin family members, they would not be expected to present as a freeze-fracture particle in replicas, indicating that they probably are assembling into oligomeric assemblies not yet determined.
Although the identification of the claudin family of membrane proteins offers much excitement to the study of the regulation of epithelial permeability, the remarkable dynamics and variability of tight junctions in different epithelia suggests that a complete catalogue of the protein players may still be incomplete. Not only may the claudin family grow larger than eight, but additional, unknown proteins and isoforms of known proteins may reside in the deceptively homogenous tight-junction strands. Indeed, a splice variant of occludin has recently appeared in abstract form (36). Future work will need not only to complete the analysis of the proteins involved but also engage the difficult problem of studying the functions of each of the players individually and in supramolecular aggregates.
Acknowledgments
This work was supported by Grant GM18974 from the National Institutes of Health.
Footnotes
A commentary on this article begins on page 511.
References
- 1.Boulpaep E L, Seely J F. Am J Physiol. 1971;221:1084–1096. doi: 10.1152/ajplegacy.1971.221.4.1084. [DOI] [PubMed] [Google Scholar]
- 2.Goodenough D A, Revel J P. J Cell Biol. 1971;50:81–91. doi: 10.1083/jcb.50.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frömter E, Diamond J M. Nature (London) 1972;235:9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- 4.Pappenheimer J R. J Membr Biol. 1987;100:137–148. doi: 10.1007/BF02209146. [DOI] [PubMed] [Google Scholar]
- 5.Madara J L, Pappenheimer J R. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 6.Claude P, Goodenough D A. J Cell Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claude P. J Membr Biol. 1978;39:219–232. doi: 10.1007/BF01870332. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson B R, Anderson J M, Goodenough D A, Mooseker M S. J Cell Biol. 1988;107:2401–2408. doi: 10.1083/jcb.107.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita K, Furuse M, Fujimoto K, Tsukita S. Proc Natl Acad Sci USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita Sa, Tsukita Sh. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto K. J Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- 12.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy K M, Skare I B, Stankewich M C, Furuse M, Tsukita S, Rogers R A, Lynch R D, Schneeberger E E. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 14.Balda M S, Whitney J A, Flores C, Gonzalez S, Cereijido M, Matter K. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y-H, Merzdorf C, Paul D L, Goodenough D A. J Cell Biol. 1997;138:891–899. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gumbiner B M. J Cell Biol. 1993;123:1631–1633. doi: 10.1083/jcb.123.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong V, Gumbiner B M. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuse M, Sasaki H, Fujimoto K, Tsukita S. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friend D S, Gilula N B. J Cell Biol. 1972;53:758–776. doi: 10.1083/jcb.53.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staehelin L A. J Cell Sci. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson B R, Siliciano J D, Mooseker M S, Goodenough D A. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gumbiner B, Lowenkopf T, Apatira D. Proc Natl Acad Sci USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jesaitis L A, Goodenough D A. J Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beatch M, Jesaitis L A, Gallin W J, Goodenough D A, Stevenson B R. J Biol Chem. 1996;271:25723–25726. doi: 10.1074/jbc.271.42.25723. [DOI] [PubMed] [Google Scholar]
- 27.Haskins J, Gu L J, Wittchen E S, Hibbard J, Stevenson B R. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanning A S, Jameson B J, Jesaitis L A, Anderson J M. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 29.Keon B H, Schafer S, Kuhn C, Grund C, Franke W W. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Nature (London) 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K. J Cell Biol. 1997;139:785–795. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong Y T, Saitoh T, Minase T, Sawada N, Enomoto K, Mori M. J Cell Biol. 1993;120:477–483. doi: 10.1083/jcb.120.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itoh M, Nagafuchi A, Moroi S, Tsukita S. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giepmans B N G, Moolenaar W H. Curr Biol. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 35.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. J Biol Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- 36.Muresan Z, Paul D L, Goodenough D A. Mol Biol Cell. 1998;9:83. doi: 10.1091/mbc.11.2.627. (abstr.). [DOI] [PMC free article] [PubMed] [Google Scholar]