Abstract
Objectives
To examine the associations of depressive symptoms, antidepressant use, and duration of use with incident frailty three years later in nonfrail women ≥ age 65.
Design
Secondary analysis of the Women’s Health Initiative Observational Study (WHI-OS), a prospective cohort study.
Setting
WHI-OS was conducted in 40 U.S. clinical centers.
Participants
Women aged 65-79, not frail at baseline.
Measurements
Antidepressant use was assessed through medication container inspection at baseline. We created four groups according to baseline use and Burnam depression screen (range 0-1, 0.06 cut-off): antidepressant non-users without depressive symptoms (referent group), antidepressant non-users with depressive symptoms, antidepressant users without depressive symptoms, and antidepressant users with depressive symptoms. Frailty components included slowness/weakness, exhaustion, low physical activity, and unintended weight loss, ascertained through self-report and physical measurements at baseline and year 3.
Results
Among 27652 women at baseline, 4.9% (n=1350) were antidepressant users and 6.5% (n=1794) were categorized depressed. At year 3, 14.9% (n=4125) were frail. All groups had an increased risk for incident frailty compared to the referent group. Odds ratios ranged from 1.73 (95% Confidence Interval (CI) =1.41-2.12) among non-depressed antidepressant users to 3.63 among depressed antidepressant users (95% CI = 2.37-5.55). All durations of use were associated with incident frailty (<1 year OR = 1.95, 95% CI = 1.41-2.68; 1 to 3 years OR = 1.99, 95% CI = 1.45-2.74; > 3 years OR = 1.60, 95% CI = 1.20-2.14).
Conclusion
In older adult women, depressive symptoms and antidepressant use were associated with frailty after 3 years follow-up.
Keywords: Antidepressant Use, Frailty, Depression, Women’s Health Initiative
INTRODUCTION
Frailty is characterized by a lack of physiological reserve, an increased vulnerability to stressors and a subsequent risk for adverse health outcomes.(1) In the past several years, a standard and measurable definition of frailty has emerged, paving the way for an expansion of frailty-related research. Recent epidemiological studies define frailty as a syndrome consisting of involuntary weight loss, exhaustion, low activity level, weakness, and slow gait.(2, 3) Older frail adults are at increased risk for various adverse health outcomes, including disability, falls, fractures, hospitalizations, death, and depression.(2-4)
Depression is a significant public health problem in older adults. Approximately 15-20% of community-dwelling elders are estimated to suffer from depressive symptoms.(5-8) These estimates are likely low, however, as mental disorders in older adults often go under recognized and under diagnosed.(9) In older adults, depressive symptoms are associated with numerous adverse outcomes, including social stressors, risk for and worsening of concomitant medical problems, disability, frailty, and death.(2, 3, 8, 10-15) A significantly higher proportion of frail elders experience depressive symptoms compared to nonfrail older adults. Furthermore, nonfrail older adults experiencing depressive symptoms are more likely to become frail after 3 years than those without depressive symptoms.(2, 3)
Because previous studies examining the relationship between frailty and depression excluded individuals prescribed antidepressant medication (2, 3) it is unknown what role, if any, antidepressants play in mitigating, or exacerbating, the risk for frailty in older adults. We are unaware of any study examining antidepressant use in relation to frailty development. It is reasonable to postulate that effective treatment of depressive symptoms with pharmacotherapy could protect from becoming frail by reducing the burden of depression. However, the negative consequences of antidepressant treatment, including risk of falls and fracture, are well established.(16-21) Using longitudinal assessments of women aged 65 years and older who were not frail at baseline, this study examines the associations of depression, antidepressant use, and duration of antidepressant use at baseline with incident frailty three years later using data from the Women’s Health Initiative Observational Study (WHI-OS), a large prospective cohort study.
METHODS
Study sample
We used data from the WHI-OS, a prospective study of 93,676 postmenopausal women aged 50-79 years recruited from 40 clinical centers throughout the United States between 1993 and 1998. Women enrolled in any of the WHI clinical trials were not eligible for the WHI-OS. Details about the WHI-OS study design, recruitment, and data collection methods have been previously published.(22-24) Human subjects review committees at each participating institution reviewed and approved the study, and all women gave written informed consent.
The present analysis includes women aged 65 to 79 years who were not frail at baseline (n=33,324). Women were excluded if they reported Parkinson’s disease or medications for Parkinson’s disease at baseline, which could manifest as frailty, or if they were missing information on the Burnam depression screen. The frailty outcome could not be determined in 4564 women missing information on one of the frailty components, leaving a final sample of 27652 participants.
Frailty
The definition of frailty was based on criteria used in the Cardiovascular Health Study.(2) This definition has been associated with future disability, hospitalization, hip fracture, and mortality in older adult women in the WHI-OS.(3) The four components of frailty were muscle weakness or slowness, exhaustion, low physical activity, and unintentional weight loss. Frailty was assessed at year three.
Muscle weakness or slowness
Muscle weakness or slowness was measured by the RAND-36 Physical Function Scale.(25) This scale includes 10 items asking how much (a lot, a little, not at all) current health limits a variety of activities, including walking; climbing stairs; lifting or carrying groceries; bending, kneeling, or stooping; and moderate and vigorous activities. Scores range from 0 to 100, with higher scores indicating better physical function. A score in the lowest quartile of this scale was highly associated with poor grip strength and slow walking speed in the WHI clinical trial.(3)
Exhaustion
Poor endurance or exhaustion was measured by the RAND-36 Vitality Scale (range 0-100). This scale includes 4 items pertaining to how the participant felt in the previous four weeks: “Did you… feel worn-out? …feel tired? …feel full of pep? …have a lot of energy?”
Physical activity
Low physical activity was assessed using items on a questionnaire that asks the frequency and duration of four walking speeds and activities in the prior week.(26, 27) Energy expended in a week (kcal) on leisure activities was calculated as a metabolic equivalent task hours score = kcal/wk × kg.(28)
Unintentional weight loss
Unintentional weight loss was based on weight measured at clinic visits at baseline and year 3 as well as a self-reported item at year 3 asking whether recent weight loss was intentional. Unintentional weight loss was defined as weight loss of more than 5% of body weight in the previous 2 years that was not reported as intentional.
Frailty classification
A frailty component was classified as present if a participant scored in the lowest quartile of distribution for that component or had unintentional weight loss. Participants were given 1 point for each frailty component present, except muscle weakness or slowness. To align scoring with the convention of Fried(2), a participant was given 2 points if they had muscle weakness or slowness because the physical function scale measured both muscle strength and walking ability. The individual frailty component points were summed, and a participant was classified as frail for ≥ 3 points, intermediate frail for 1 or 2 points, and non-frail for 0 points.
Antidepressant use
Participants were asked to bring all current medications to their baseline interview. Medications used for at least 2 weeks were recorded by clinic interviewers who entered medication names and strengths directly from containers into a database that assigned drug codes using Medi-Span software (First DataBank, Inc., San Bruno, CA). Duration of use was recorded from self-report. We categorized women as users or non-users of antidepressant medications based on baseline medication use only. Duration was categorized as use for one year or less, use for one to three years, or use for greater than three years. We did not consider trazodone an antidepressant in our analysis, based on its more common use as a sedative/hypnotic. Antidepressants were categorized based on mechanism of action into selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), or other or multiple antidepressants.
Depressive symptoms
Depressive symptoms were assessed at baseline using the Burnam 8-item depression screening instrument.(29) The Burnam screen comprises six items from the Center for Epidemiologic Studies Depression Scale (CES-D) about the frequency of depressive symptoms during the past week and two items from the National Institute of Mental Health’s Diagnostic Interview Schedule (DIS) about the duration of symptoms. Specifically, participants indicated how often they felt depressed, had restless sleep, enjoyed life, had crying spells, felt sad, and felt that people disliked them in the past week, scored from 0 (less than 1 day) to 3 (5-7 days). Duration questions are scored yes or no and ask 1.) if individuals felt sad, blue, or depressed or lost pleasure in things for two weeks or more in the past year and 2.) if individuals felt depressed or sad much of the time in the past year. The normative groups for the screener were drawn from two population-based studies.(29) In both studies, respondents were classified as either primary care outpatients or mental health patients. Scores for each item are weighted ranging from -0.280 to 2.712. The scoring algorithm, using a prediction equation developed by Burnam et al (29), gives a composite score between 0 and 1 which represents the probability of having a depressive disorder. We used the standard cut point of ≥ 0.06 to dichotomize the continuous scale into depressive and not depressive. (29, 30) This cut-point had an 86% sensitivity and a 90-95% specificity for detecting a current depressive episode in primary care patients.(29) In addition, we created a 3-category depressive-symptoms scale, using a more sensitive cut-point of 0.009 (≤ 0.009, 0.009 to 0.06, ≥ 0.06), to indicate low, medium, and high depressive symptoms. The more sensitive cut-point of 0.009 improves the sensitivity of the Burnam for detecting depressive symptoms in those with a lifetime mood disorder.(29, 30) The Burnam screen does not include items about fatigue or energy, reducing the risk of operational confounding between the measures of depression and definition used for frailty.
Covariates
We examined several covariates that could be potential confounders. Demographic information, medical history, and health behaviors were based on self-report at baseline. Smoking status was classified as current, past, or never. Weekly alcohol consumption was estimated from a food frequency questionnaire, and categorized based on self-reported drinks per week. Hormone therapy use was classified as current, past, or never based on reported use of estrogen with or without progestin. Baseline medical conditions included a physician diagnosis of hypertension (taking high blood pressure medications and/or a blood pressure of ≥140/90 mmHg), diabetes mellitus treatment and self-report of the following: emphysema, hip fracture after the age of 55, a history of at least 2 falls in the previous year, arthritis, cancer, and stroke. A history of coronary heart disease (CHD) was based on self-reported physician diagnosis of myocardial infarction, angina, or coronary artery bypass graft (CABG), or percutaneous transluminal coronary angioplasty procedures (PTCA). Body mass index (BMI) was measured using baseline height and weight and defined as weight (kg) divided by height (m2). Activities of daily living (ADL) disability was measured by asking participants about the amount of help (no help, some help, totally dependent) needed to eat, dress and undress, get in and out of bed, and take a bath or shower. ADL disability was defined as needing assistance with one or more ADL.
Statistical analysis
We created four distinct groups based on baseline antidepressant use and depressive symptoms: 1. Antidepressant non-users who were not depressed; 2. Antidepressant users who were not depressed; 3. Antidepressant non-users who were depressed; and 4. Antidepressant users who were depressed. Baseline characteristics were compared across these four groups using chi-square tests for categorical variables and ANOVA for continuous variables. Multinomial logistic regression models were used to examine the association between frailty, antidepressant use, and depressive symptoms. Frailty and intermediate frailty were the two outcomes and the four distinct depressive symptoms-antidepressant use groups were the predictors of interest, adjusting for covariates known to be independent predictors of frailty.(3) To examine the association between length of antidepressant use, severity of depressive symptoms, and antidepressant class with incident frailty, we conducted additional analyses with frailty as the outcome and the 3-category depressive symptoms, 4-category length of antidepressant use, and 4-category antidepressant class as predictors of interest, adjusting for covariates. We report odds ratios and corresponding 95% confidence intervals from these logistic models. All data were analyzed using STATA SE version 10.1 (StataCorp LP, College Station, Texas).
RESULTS
At baseline, 1794 women (6.5%) were categorized as depressed. Only 4.9% of women (n=1350) were using an antidepressant (Table 1), of whom 18.5% (n=250) were experiencing depressive symptoms. Tricyclic antidepressant use was more common than the other categories of antidepressants, with 44.5% (n=601) of women using TCAs, 41.3% (n=557) using SSRIs, and 14.2% (n=192) using other or multiple antidepressants. Out of the 192 other or multiple antidepressant users, 23.3% were using multiple antidepressants, which mainly consisted of concurrent SSRI and TCA use. Baseline depression was associated with lower income, less education, living alone, obesity, and being a current smoker (Table 1). Women who were depressed were more likely to self-report poorer health, and to have diabetes, a history of CHD, and a history of COPD. A greater proportion of antidepressant users at baseline were younger in age, white, current hormone therapy users, and had hypertension; a history of falls, arthritis, and stroke; and at least two chronic diseases.
Table 1.
Baseline Characteristics by Depressive Symptom Status and Antidepressant Use (n=27652)
| Not depressive* | Depressive | ||||
|---|---|---|---|---|---|
| Non-user N=24758 | User N=1100 | Non-user N=1544 | User N=250 | p-value+ | |
| Depressive status | |||||
| Burnam score, mean (SD) | 0.005 (0.009) | 0.009 (0.013) | 0.275 (0.221) | 0.313 (0.231) | <0.001 |
| Demographics | |||||
| Age, yr | <0.001 | ||||
| 65-69 | 12563 (50.7) | 607 (55.2) | 831 (53.8) | 155 (62.0) | |
| 70-79 | 12195 (49.3) | 493 (44.8) | 713 (46.2) | 95 (38.0) | |
| Income, $ | <0.001 | ||||
| <20,000 | 3666 (16.1) | 165 (15.8) | 366 (26.2) | 56 (24.6) | |
| 20,000 – 34,999 | 6593 (28.9) | 313 (30.1) | 392 (28.1) | 74 (32.5) | |
| 35,000 – 49,999 | 5038 (22.1) | 220 (21.1) | 290 (20.8) | 39 (17.1) | |
| 50,000 – 74,999 | 4209 (18.4) | 195 (18.7) | 208 (14.9) | 39 (17.1) | |
| ≥75,000 | 3309 (14.5) | 148 (14.2) | 140 (10.0) | 20 (8.8) | |
| Education | <0.001 | ||||
| ≤ High school | 5024 (20.4) | 232 (21.2) | 436 (28.5) | 59 (24.0) | |
| School after high school | 9028 (36.7) | 417 (38.1) | 590 (38.6) | 103 (41.9) | |
| ≥College | 10557 (42.9) | 445 (40.7) | 504 (32.9) | 84 (34.1) | |
| Race | <0.001 | ||||
| White | 22037 (89.2) | 1025 (93.6) | 1312 (85.4) | 234 (94.0) | |
| Black | 1047 (4.2) | 22 (2.0) | 104 (6.8) | 3 (1.2) | |
| Hispanic | 437 (1.8) | 21 (1.9) | 61 (4.0) | 5 (2.0) | |
| American Indian | 54 (0.2) | 3 (0.3) | 8 (0.5) | 2 (0.8) | |
| Asian/Pacific Islander | 858 (3.5) | 14 (1.3) | 26 (1.7) | 1 (0.4) | |
| Unknown | 257 (1.0) | 10 (0.9) | 26 (1.7) | 4 (1.6) | |
| Living alone | 7628 (31.0) | 372 (34.0) | 665 (43.4) | 105 (42.2) | <0.001 |
| Health Status | |||||
| Body mass index (BMI), kg/m2 | <0.001 | ||||
| Underweight (<18.5) | 346 (1.4) | 12 (1.1) | 19 (1.2) | 2 (0.8) | |
| Normal (18.5-24.9) | 11178 (45.4) | 451 (41.2) | 631 (41.2) | 96 (38.7) | |
| Overweight (25.0-29.9) | 8807 (35.8) | 430 (39.3) | 566 (36.8) | 100 (40.3) | |
| Obese (≥ 30) | 4269 (17.3) | 201 (18.4) | 320 (20.8) | 50 (20.2) | |
| Self-reported health | <0.001 | ||||
| Excellent | 4707 (19.1) | 136 (12.4) | 151 (9.8) | 18 (7.2) | |
| Very good | 11705 (47.5) | 441 (40.4) | 593 (38.7) | 97 (39.0) | |
| Good | 7369 (29.9) | 437 (40.0) | 645 (42.1) | 105 (42.2) | |
| Fair/poor | 862 (3.5) | 78 (7.1) | 144 (9.4) | 29 (11.6) | |
| ADL Disability (>= 1 ADL affected) | 242 (1.0) | 16 (1.5) | 28 (1.9) | 3 (1.2) | 0.008 |
| Health Behaviors | |||||
| Smoking | <0.001 | ||||
| Never | 13350 (54.6) | 493 (45.4) | 789 (51.7) | 115 (46.2) | |
| Past | 10145 (41.5) | 546 (50.3) | 653 (42.8) | 112 (45.0) | |
| Current | 933 (3.8) | 46 (4.2) | 83 (5.4) | 22 (8.8) | |
| Alcohol consumption, drinks/wk | <0.001 | ||||
| 0/past drinker | 9276 (37.5) | 455 (41.4) | 652 (42.3) | 120 (48.2) | |
| <1 | 4946 (20.0) | 220 (20.0) | 342 (22.2) | 39 (15.7) | |
| 1-14 | 9308 (37.7) | 369 (33.6) | 470 (30.5) | 85 (34.1) | |
| ≥14 | 1178 (4.8) | 54 (4.9) | 77 (5.0) | 5 (2.0) | |
| Hormone therapy use | <0.001 | ||||
| Never | 8434 (34.6) | 210 (19.4) | 505 (33.1) | 60 (24.1) | |
| Past | 5891 (24.2) | 248 (23.0) | 412 (27.0) | 67 (26.9) | |
| Current | 10060 (41.2) | 622 (57.6) | 609 (39.9) | 122 (49.0) | |
| Comorbidities | |||||
| Hypertension (on medications or high blood pressure) | 8505 (34.9) | 455 (42.0) | 603 (39.8) | 99 (40.6) | <0.001 |
| Diabetes Mellitus treatment | 696 (2.8) | 43 (3.9) | 65 (4.2) | 12 (4.8) | 0.001 |
| History coronary heart disease (CHD) | 1844 (7.4) | 109 (9.9) | 156 (10.1) | 32 (12.8) | <0.001 |
| History chronic obstructive pulmonary disease (COPD) | 712 (2.9) | 58 (5.3) | 84 (5.5) | 14 (5.7) | <0.001 |
| History hip fracture at age ≥ 55 | 210 (0.9) | 11 (1.1) | 16 (1.2) | 2 (0.9) | 0.826 |
| History falls (≥ 2 in past year) | 2468 (10.1) | 191 (17.5) | 201 (13.1) | 48 (19.4) | <0.001 |
| History arthritis | 12713 (51.7) | 712 (65.4) | 907 (59.1) | 161 (64.9) | <0.001 |
| History cancer | 3502 (14.2) | 182 (16.6) | 253 (16.5) | 46 (18.8) | 0.003 |
| History stroke | 317 (1.3) | 22 (2.0) | 26 (1.7) | 10 (4.0) | <0.001 |
| At least 2 chronic diseases | 10996 (44.4) | 629 (57.2) | 837 (54.2) | 150 (60.0) | <0.001 |
Depression status based on Burnam score: < 0.06 = not depressed, score ≥ 0.06 = depressed.
Comparisons made using ANOVA for continuous variables and chi2 for categorical variables.
At the three year follow-up, 8653 women (31.3%) met the criteria for intermediate frailty and 4125 (14.9%) were frail. Depressive symptoms were associated with an increased risk for becoming intermediate frail and frail, adjusting for antidepressant use and other important covariates. Women with high depressive symptom scores had the highest risk for incident frailty (OR=2.19, 95% Confidence Interval (CI) = 1.86-2.59), but those with intermediate depressive symptoms still had an increased risk (OR = 1.31, 95% CI = 1.14-1.50) compared to women without depressive symptoms (Figure 1). All durations of antidepressant use were associated with similar increased odds of becoming intermediate frail and frail at year 3, adjusting for depressive symptoms and other covariates (Figure 1). Results for intermediate frailty were not as strong, but showed a similar pattern.
Figure 1. Adjusted Odds Ratios (95% CI) Relating Depressive Symptoms (Burnam Scale Score)* and Antidepressant Duration of Use+ to Incident Frailty at 3 Years Follow-Up.
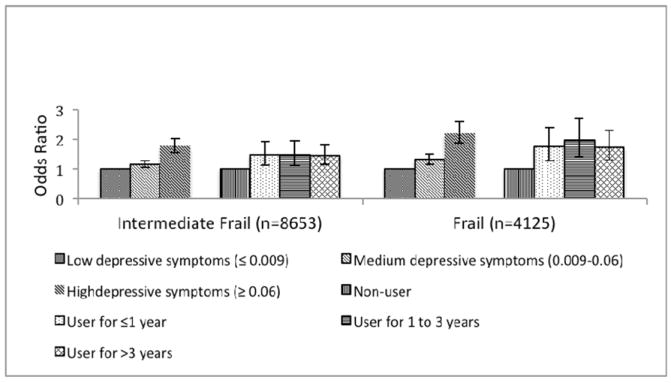
*Adjusted for age, income, education, race, living alone, body mass index (BMI), self-reported health, ADL disability, smoking, alcohol consumption, hormone therapy use, hypertension, diabetes, hx coronary heart disease (CHD), hx chronic obstructive pulmonary disease (COPD), hx hip fracture at age >=55, at least 2 falls in previous year, history arthritis, history cancer, history stroke, presence of at least 2 chronic diseases, and antidepressant use.
+Adjusted for age, income, education, race, living alone, BMI, self-reported health, ADL disability, smoking, alcohol consumption, hormone therapy use, hypertension, diabetes, hx CHD, hx COPD, hx hip fracture at age >=55, at least 2 falls in previous year, history arthritis, history cancer, history stroke, presence of at least 2 chronic diseases, and Burnam depressive symptom score.
Compared to non-users of antidepressants without depressive symptoms, all three groups had an increased risk for incident frailty, even after controlling for important covariates (Table 2). Specifically, antidepressant users exhibiting depressive symptoms were 3.63 times as likely to develop frailty as non-depressed, non-users (95% CI = 2.37-5.55); non-users with depressive symptoms were 2.05 times as likely (95% CI = 1.71-2.46); and non-depressed users were 1.73 times as likely (95% CI = 1.41-2.12). As with the other analyses, the strength of the associations with intermediate frailty was weaker. Additional analyses (results not shown) excluding users of other psychoactive medications (anxiolytics, hypnotics, and anti-psychotics) did not change the results to any appreciable extent.
Table 2.
Adjusted* Odds Ratios (95% Confidence Interval (CI)) Relating Depressive Symptoms / Antidepressant Use to Incident Frailty at 3 Years Follow-Up
| Not Frail (n= 14874) | Intermediate Frailty (n=8653) | Frail (n= 4125) | ||||
|---|---|---|---|---|---|---|
| n | n | Odds Ratio (95% CI) | n | Odds Ratio (95% CI) | p-value | |
| Not depressive | ||||||
| Antidepressant non-user | 13808 | 7547 | 1.00 | 3403 | 1.00 | <0.001 |
| Antidepressant user | 446 | 396 | 1.49 (1.27-1.76) | 258 | 1.79 (1.47-2.19) | |
| Depressive | ||||||
| Antidepressant non-user | 555 | 610 | 1.75 (1.52-2.03) | 379 | 2.11 (1.77-2.52) | |
| Antidepressant user | 65 | 100 | 2.28 (1.56-3.32) | 85 | 3.64 (2.41-5.53) | |
Adjusted for age, income, education, race, living alone, body mass index (BMI), self-reported health, ADL disability, smoking, alcohol consumption, hormone therapy use, hypertension, diabetes, hx coronary heart disease (CHD), hx chronic obstructive pulmonary disease (COPD), hx hip fracture at age >=55, at least 2 falls in previous year, history arthritis, history cancer, history stroke, and presence of at least 2 chronic diseases.
CI = Confidence Interval, OR = Odds Ratio
All three antidepressant classes were associated with increased risk of incident frailty compared to no antidepressant use. TCA users were 1.52 times as likely to become frail (95% CI =1.17-1.96) and SSRI users were 1.86 times as likely (95% CI=1.40-2.48). The odds ratio for other or multiple antidepressant use was highest at 2.94 (95% CI = 1.85-4.66). The use of tricyclic antidepressants was not significantly associated with intermediate frailty, after controlling for covariates, including depressive symptoms (OR=1.21, 95% CI=0.97-1.51). However, use of SSRIs or other / multiple antidepressants did reach statistical significance (intermediate frailty OR=1.64, 95% CI = 1.31-2.06 and OR=2.06, 95% CI = 1.23-3.46, respectively).
DISCUSSION
Our data support a strong association between depression and incident frailty in older adult women. We also found, in those free from frailty at baseline, any amount of antidepressant use is associated with frailty development at three years follow-up. All comparison groups had an increased risk for incident frailty, even when controlling for confounders, including chronic medical co-morbidities. However, antidepressant users also exhibiting depressive symptoms appeared to have the highest risk of becoming frail. The reasons for these findings warrant exploration.
To our knowledge, this is the first study examining antidepressant medication as treatment for depression in relation to frailty development. Prior studies have linked depressive symptoms, as opposed to treatment for depression, to current (prevalent) frailty, as well as to new onset (incident) frailty.(2, 3, 15) In their landmark study using data from the Cardiovascular Health Study, Fried and colleagues reported that 31% of frail older adults had a CES-D score of at least 10, suggestive of depression, compared with 14% of intermediate frail older adults, and only 3% of nonfrail elders (trend p-value <0.001).(2) Using data from the Women’s Health Initiative, Woods and colleagues reported that nonfrail older adults experiencing depressive symptoms were 2.2 times as likely to become frail over 3 years than older adults without depressive symptoms (p-value <0.001).(3) Older adults taking antidepressants were excluded from both the Fried and the Woods study, so results cannot be generalized to individuals not receiving pharmacologic treatment for depression.
The strong association between frailty and depression is not surprising given the overlap of frailty characteristics with depressive symptoms. Both frailty and depression are associated with inactivity, weight loss, reduced physical activity, and exhaustion, in addition to negative long-term health consequences, such as physical disability, hospitalization, and mortality. (2, 3, 31-35) The role of antidepressants is less clear. Amongst the women taking antidepressants who had few depressive symptoms, it is reasonable to postulate a similar risk for frailty as for non-users with few depressive symptoms. Similarly, it is reasonable to postulate that all depressed older adults might have a similar risk of frailty regardless of antidepressant use. Instead, we found that, even in the absence of depressive symptoms, antidepressant users had an increased risk for developing frailty.
We offer the following possible explanations for our seemingly incongruent findings. First, antidepressant adverse effects may have contributed to frailty risk. Second, individuals receiving medication treatment for depressive symptoms may differ from those who do not receive pharmacotherapy in ways we did not measure. Third, and most likely, antidepressant users, especially those still experiencing depressive symptoms, may suffer from a more severe, recurrent, or chronic form of depression.
All antidepressants are associated with adverse effects. For example, antidepressants are associated with increase risk for falls and fractures, which are in turn associated with frailty development.(19, 21, 36, 37) Adverse effects and tolerability vary by antidepressant class due to class specific pharmacologic actions. Despite this variability, our results indicate that all antidepressant classes are associated with an increased risk for frailty. We were limited in our ability to meaningfully compare frailty risk between individual classes, however. Instead, we compared SSRI, TCA, and other or multiple antidepressant use to no use and found that users of other or multiple antidepressants appeared to have the highest risk of becoming frail. Direct antidepressant class comparisons warrant future exploration.
Antidepressant users experiencing depressive symptoms scored higher on the Burnam scale, on average, than the other groups in our study, suggesting more severe depression. Additionally, we found that women with greater depressive symptoms had the highest risk of becoming frail. In a sensitivity analysis, we excluded users of other psychoactive medications in an attempt to exclude those with worse mental health or with co-occurring mental health diagnoses. Results did not change. Users with depressive symptoms also suffered from significantly poorer health – they were more likely to rate their health as fair or poor and to suffer from chronic co-morbidities. Co-morbid illness is a predictor of both depression and inadequate response to antidepressant treatment. (38-41) Our results suggest the overlap of depressive symptoms with disease burden may promote frailty development, despite antidepressant treatment.
We did not have access to information about length of the current depressive episode or history of depression. However, recurrent and treatment resistant depression would be expected to result in longer durations of antidepressant use. We examined the association between duration of antidepressant use as a proxy for length of depressive symptoms and incident frailty. Risk did not seem to vary with duration of antidepressant use, suggesting no differences in short-term and long-term treatment. These findings must be interpreted with caution, however, and future studies should explore how depression severity and treatment resistance relates to frailty.
Strengths of our study include its prospective design, the inclusion of more than 1300 antidepressant users, the objective collection of antidepressant use, and the availability of information on health behaviors, health status, and co-morbidities. We were able to control for a large number of covariates related to frailty. Additionally, unlike many other frailty studies, our frailty definition was distinct from our measure of depressive symptoms.
Limitations include the lack of information on antidepressant indication, dose, and treatment adherence. Antidepressants are commonly used for conditions other than depression, including anxiety, sleep, and pain.(42-45) While we were able to determine that an antidepressant had been prescribed, we did not have access to clinician diagnoses, nor did we have dosing information to help elucidate treatment adequacy or indication. Further, we could not determine that an antidepressant medication was actually taken, though misclassification of antidepressant use could be expected to bias our results toward the null. During the 3-year follow-up period, we were not able to ascertain antidepressant initiation or discontinuation in relation to frailty development. The prevalence of depression in our sample is lower than that reported in other WHI studies, which could be due to our exclusion of women who were frail at baseline. Also, we were not able to directly examine whether the effect of antidepressant use on incident frailty differs between women who did and did not experience depressive symptoms at baseline. Finally, despite attempts to control for potential confounding, our results are subject to confounding by indication biases.
Confounding by indication threatens the validity of all pharmacologic observational studies. In general terms, confounding by indication occurs when the indication for a medication being studied is associated with the outcome of interest. Depression is a known strong predictor of frailty development, and the primary indication for antidepressant use. Confounding by indication bias could conceal any beneficial effects of antidepressant treatment on incident frailty. We attempted to control for this source of bias by creating four mutually exclusive groups based on antidepressant use and depressive symptoms, by controlling for potential confounders in our multivariate adjustment, and by using multinomial logistic regression models. However, we cannot exclude the possibility that we were unable to completely account for baseline differences between antidepressant users and nonusers. Only a randomized controlled trial can completely control for this potential bias.
In conclusion, in this large, prospective observational study of more than 27,000 women, depressive symptoms and antidepressant use were strongly related to increased risks for incident frailty over three years. Even in the absence of depressive symptoms, antidepressant use was associated with becoming frail. While we cannot exclude the possibility of residual confounding due to inadequate measurement of depressive symptoms, our results highlight the importance of depression screening in older adults and suggest that pharmacotherapy alone for treatment of depression does not reduce an older depressed woman’s risk for becoming frail. Depression treatment trials in older adults should include frailty as an outcome measure. Further research is needed to elucidate the possible role of antidepressants in the depression-frailty relationship.
Acknowledgments
Funding Source: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. This work was supported by the T32 AG027677 fellowship sponsored by the National Institute of Aging.
The authors would like to thank the WHI investigators and staff for their dedication and commitment.
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Haleh Sangi-Haghpeykar; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence S. Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Erin LeBlanc; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA)
Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael S. Simon.
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Sponsor’s Role: None
Footnotes
Author Contributions: Each author contributed significantly to the study concept and design, acquisition of subjects and/or data, or analysis and interpretation of data. All authors contributed significantly to the preparation of the manuscript.
Data were presented, in part, at the Gerontological Society of America 62nd Annual Scientific Meeting, Atlanta, Georgia, November 18-22, 2009.
Conflict of Interest: In the past 3 years, Dr. Smoller has consulted to Eli Lilly, the Herman Dana Trust, and RTI International, Inc. There are no other conflicts of interest to report.
References
- 1.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: An emerging research and clinical paradigm--issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 4.Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: The study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 6.Beekman AT, Deeg DJ, van Tilburg T, et al. Major and minor depression in later life: A study of prevalence and risk factors. J Affect Disord. 1995;36:65–75. doi: 10.1016/0165-0327(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 7.Gallo JJ, Lebowitz BD. The epidemiology of common late-life mental disorders in the community:Themes for the new century. Psychiatr Serv. 1999;50:1158–1166. doi: 10.1176/ps.50.9.1158. [DOI] [PubMed] [Google Scholar]
- 8.Lyness JM, Kim J, Tang W, et al. The clinical significance of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry. 2007;15:214–223. doi: 10.1097/01.JGP.0000235763.50230.83. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services. Mental Health: A Report of the Surgeon General. Rockville MD: US Dept of Health and Human Services; 1999. [Google Scholar]
- 10.Beekman AT, Penninx BW, Deeg DJ, et al. The impact of depression on the well-being, disability and use of services in older adults: A longitudinal perspective. Acta Psychiatrica Scandinavica. 2002;105:20–27. doi: 10.1034/j.1600-0447.2002.10078.x. [DOI] [PubMed] [Google Scholar]
- 11.Bellino S, Patria L, Ziero S, et al. Clinical features of dysthymia and age: A clinical investigation. Psychiatry Res. 2001;103:219–228. doi: 10.1016/s0165-1781(01)00274-8. [DOI] [PubMed] [Google Scholar]
- 12.Brenes GA, Penninx BW, Judd PH, et al. Anxiety, depression and disability across the lifespan. Aging Ment Health. 2008;12:158–163. doi: 10.1080/13607860601124115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kivela SL, Pahkala K. Dysthymic disorder in the aged in the community. Soc Psychiatry Psychiatr Epidemiol. 1989;24:77–83. doi: 10.1007/BF01788630. [DOI] [PubMed] [Google Scholar]
- 14.Lyness JM, King DA, Cox C, et al. The importance of subsyndromal depression in older primary care patients: Prevalence and associated functional disability. J Am Geriatr Soc. 1999;47:647–652. doi: 10.1111/j.1532-5415.1999.tb01584.x. [DOI] [PubMed] [Google Scholar]
- 15.Andrew MK, Rockwood K. Psychiatric illness in relation to frailty in community-dwelling elderly people without dementia: A report from the Canadian Study of Health and Aging. Can J Aging. 2007;26:33–38. doi: 10.3138/8774-758w-702q-2531. [DOI] [PubMed] [Google Scholar]
- 16.Darowski A, Chambers SA, Chambers DJ. Antidepressants and falls in the elderly. Drugs Aging. 2009;26:381–394. doi: 10.2165/00002512-200926050-00002. [DOI] [PubMed] [Google Scholar]
- 17.Ginzburg R, Rosero E. Risk of fractures with selective serotonin-reuptake inhibitors or tricyclic antidepressants. Ann Pharmacother. 2009;43:98–103. doi: 10.1345/aph.1L264. [DOI] [PubMed] [Google Scholar]
- 18.Kerse N, Flicker L, Pfaff JJ, et al. Falls, depression and antidepressants in later life: A large primary care appraisal. PLoS One. 2008;3:e2423. doi: 10.1371/journal.pone.0002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169:1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 20.Wu Q, Liu J, Gallegos-Orozco JF, et al. Depression, fracture risk, and bone loss: A meta-analysis of cohort studies. Osteoporos Int. 2010;21:1627–1635. doi: 10.1007/s00198-010-1181-x. [DOI] [PubMed] [Google Scholar]
- 21.Ziere G, Dieleman JP, van der Cammen TJ, et al. Selective serotonin reuptake inhibiting antidepressants are associated with an increased risk of nonvertebral fractures. J Clin Psychopharmacol. 2008;28:411–417. doi: 10.1097/JCP.0b013e31817e0ecb. [DOI] [PubMed] [Google Scholar]
- 22.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 23.Langer RD, White E, Lewis CE, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 24.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 25.Hays RD, Prince-Embury S, Chen H. RAND-36 Health Status Inventory. San Antonio, Tex: Psychological Corp; 1998. [Google Scholar]
- 26.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 27.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 28.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Burnam MA, Wells KB, Leake B, et al. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;26:775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Tuunainen A, Langer RD, Klauber MR, et al. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res. 2001;103:261–270. doi: 10.1016/s0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- 31.Geerlings SW, Beekman AT, Deeg DJ, et al. The longitudinal effect of depression on functional limitations and disability in older adults: An eight-wave prospective community-based study. Psychol Med. 2001;31:1361–1371. doi: 10.1017/s0033291701004639. [DOI] [PubMed] [Google Scholar]
- 32.Schulz R, Beach SR, Ives DG, et al. Association between depression and mortality in older adults: The Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 33.Schulz R, Drayer RA, Rollman BL. Depression as a risk factor for non-suicide mortality in the elderly. Biol Psychiatry. 2002;52:205–225. doi: 10.1016/s0006-3223(02)01423-3. [DOI] [PubMed] [Google Scholar]
- 34.Wulsin LR, Vaillant GE, Wells VE. A systematic review of the mortality of depression. Psychosom Med. 1999;61:6–17. doi: 10.1097/00006842-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Rantanen T, Penninx BW, Masaki K, et al. Depressed mood and body mass index as predictors of muscle strength decline in old men. J Am Geriatr Soc. 2000;48:613–617. doi: 10.1111/j.1532-5415.2000.tb04717.x. [DOI] [PubMed] [Google Scholar]
- 36.Leipzig RM, Drugs Cumming RG, Tinetti ME. Drugs and falls in older people: A systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc. 1999;47:40–50. doi: 10.1111/j.1532-5415.1999.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 37.Vestergaard P, Rejnmark L, Mosekilde L. Anxiolytics, sedatives, antidepressants, neuroleptics and the risk of fracture. Osteoporos Int. 2006;17:807–816. doi: 10.1007/s00198-005-0065-y. [DOI] [PubMed] [Google Scholar]
- 38.Anderson RJ, Gott BM, Sayuk GS, et al. Antidepressant pharmacotherapy in adults with type 2 diabetes: Rates and predictors of initial response. Diabetes Care Mar. 33:485–489. doi: 10.2337/dc09-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iosifescu DV, Nierenberg AA, Alpert JE, et al. The impact of medical comorbidity on acute treatment in major depressive disorder. Am J Psychiatry. 2003;160:2122–2127. doi: 10.1176/appi.ajp.160.12.2122. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds CF, 3rd, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354:1130–1138. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- 41.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: A systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 42.Becker PM, Sattar M. Treatment of sleep dysfunction and psychiatric disorders. Curr Treat Options Neurol. 2009;11:349–357. doi: 10.1007/s11940-009-0039-z. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67:972–982. doi: 10.4088/jcp.v67n0615. [DOI] [PubMed] [Google Scholar]
- 44.Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 45.Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292:2388–2395. doi: 10.1001/jama.292.19.2388. [DOI] [PubMed] [Google Scholar]
- 46.Smoller JW, Allison M, Cochrane BB, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative Study. Arch Intern Med. 2009;169:2128–2139. doi: 10.1001/archinternmed.2009.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wassertheil-Smoller S, Shumaker S, Ockene J, et al. Depression and cardiovascular sequelae in postmenopausal women. Arch Intern Med. 2004;164:289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]


