Abstract
Introduction
The physical properties of proton beam radiation may offer advantages for treating patients with non-small cell lung cancer (NSCLC). However, its utility for the treatment of medically inoperable stage I NSCLC patients with Stereotactic Body Radiation Therapy (SBRT) is unknown.
Methods
Outcomes for patients with medically inoperable stage I NSCLC treated with proton SBRT were retrospectively analyzed. Proton SBRT was selected as the treatment modality based on pulmonary co-morbidities (n=5), prior chest radiation or/and multiple primary tumors (n=7), or other reasons (n=3). Treatments were administered using 2-3 proton beams. Treatment toxicity was scored according to CTCAE version 4 criteria.
Results
Fifteen consecutive patients and 20 tumors were treated with proton SBRT to 42-50 Gy(RBE) in 3-5 fractions between July 2008 and September 2010. Treatments were well tolerated with only one case of grade 2 fatigue, one case of grade 2 dermatitis, three cases of rib fracture (maximum grade 2), and one case of grade 3 pneumonitis in a patient with severe COPD. With a median follow up of 24.1 months, 2-year overall survival and local control rates were 64% (95% confidence limits, 34-83%) and 100% (83-100%), respectively.
Conclusions
We conclude that proton SBRT is effective and well tolerated in this unfavorable group of patients. Prospective clinical trials testing the utility of proton SBRT in stage I NSCLC are warranted.
Keywords: SBRT, Proton therapy, NSCLC
Stereotactic Body Radiation Therapy (SBRT), or Stereotactic Ablative Body Radiotherapy, is a specialized type of radiation therapy characterized by a higher (“ablative”) dose per fraction compared to conventional fractionated radiation therapy, a highly conformal dose distribution with a sharp dose gradient between tumor and normal tissues, and measures that ensure precise radiation delivery such as daily image guidance, fiducial tracking, or stereotactic body frame use. SBRT with 3-5 fractions has emerged as a standard therapy for medically inoperable patients with peripherally located stage I non-small cell lung cancer (NSCLC) 1,2.
However, there are several clinical settings in which the therapeutic benefit of photon radiation based SBRT might be limited, including the treatment of centrally located tumors, tumors close to the chest wall, and large tumors (> 5 cm) 3, 4. In addition, the utility of SBRT in patients with poor lung function (defined as FEV1 < 50% or DLCO < 40% of predicted, or oxygen dependence)5, multiple primary tumors, and prior chest radiation therapy has not been established. In these scenarios, the use of proton beam radiation may be beneficial for normal tissue sparing because, because proton beams have no exit dose and high conformality can be achieved with 2-3 beams instead of the 7-10 beams usually employed for photon SBRT. As a result, there is a significantly reduced low dose bath with protons compared to photons, thereby allowing sparing of non-targeted organs such as uninvolved lung and heart.
Excellent outcomes for hypofractionated proton therapy in early-stage NSCLC have been reported, however the treatment courses were still protracted by modern SBRT standards spanning 10-35 fractions 6-8. Theoretical dosimetric studies have shown that proton radiation can reduce the integral dose to normal lung consistent with the idea that patients with poor lung function may tolerate proton SBRT but not photon SBRT 9-11. However, the beam characteristics of proton therapy also result in a more homogeneous dose distribution, compared to photon SBRT where doses are typically prescribed to isodose lines of ~70-80%, raising the possibility of lower tumor control rates due to lack of hotspots in the tumor center.
Here, we report the outcomes for the first 15 consecutive patients treated with proton SBRT at the Francis H. Burr Therapy Center at Massachusetts General Hospital, which we believe is the first published cohort of patients treated in this fashion.
METHODS
Patient Selection
Patients with clinical stage I NSCLC were treated with proton SBRT between July 2008 and September 2010. All consecutively treated patients in this time period were included in the current analysis. Patients typically had severe COPD or interstitial lung disease, multiple primary tumors, and/or prior radiation to the chest (Table 1). No re-irradiation cases (failure in a prior irradiated site) were included. All patients were staged by PET/CT at the time of diagnosis. A retrospective database was constructed using the electronic medical record and by extracting data from the treatment planning system. An Institutional Review Board approved the current study.
Table 1. Patient characteristics (n =15).
| Sub ject |
Rationale for Protons | Ag e |
Gender | PS | Pack years smoking |
FEV1 (%) / DLCO (%) |
Oxygen requirement (L/min) |
Prior Cancer History |
Tumors treated with proton SBRT |
Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | COPD, oxygen dependence | 70 | M | 2 | 80 | 24 / 42 | 4 -6 | Lung | 2 | Pneumonia |
| 2 | COPD; prior chest RT; multiple primary tumors | 82 | F | 2 | 47 | 55 / 37 | 2 | Lung, breast | 2 | Alive |
| 3 | COPD | 73 | F | 2 | 22 | 38 / 22 | 3 | Lung | 1 | Alive |
| 4 | COPD; SLE; prior chest RT | 66 | F | 1 | 50 | N/A | 0 | Lung | 1 | Lung cancer |
| 5 | COPD; multiple primary tumors | 80 | F | 2 | 100 | 62 / 29 | 2* | Lung, breast | 3 | Alive |
| 6 | COPD | 86 | M | 1 | 30 | 45 / 25 | 0 | Colon, AML | 1 | AML |
| 7 | ILD; COPD | 82 | F | 1 | 80 | 99 / 34 | 0 | - | 1 | Alive |
| 8 | Adjacent ICD/pacemaker | 79 | M | 0 | 40 | 56/ 62 | 0 | - | 1 | Cardiac |
| 9 | COPD, S/P intubation | 63 | F | 2 | 80 | N/A | 0 | - | 1 | Alive |
| 10 | Prior chest RT; multiple primary tumors | 79 | F | 1 | 30 | N/A | 0 | - | 1 | Alive |
| 11 | Prior chest RT; multiple primary tumors | 70 | F | 1 | 150 | 97 / 74 | 0 | Lung, NHL | 1 | Respiratory |
| 12 | Prior chest RT | 62 | F | 1 | 60 | 19 / N/A | 0 | Lung, larynx | 1 | Lung cancer |
| 13 | Central tumor; poor PS | 72 | F | 3 | 0 | N/A | 0 | - | 1 | Alive |
| 14 | N/A | 89 | F | 1 | 25 | N/A | 0 | Breast | 1 | Alive |
| 15 | Two ipsilateral primary tumors | 78 | F | 1 | 50 | N/A | 0 | Lung | 2 | Alive |
With ambulation. COPD=Chronic obstructive pulmonary disease; RT=radiation therapy; ILD=interstitial lung disease; ICD=internal cardiac defibrillator; S/P=status post; M=male, F=female; PS=ECOG performance status; N/A=not available; AML=Acute myeloid leukemia; NHL=Non Hodgkin Lymphoma
Simulation and treatment planning
Patients were positioned supine on a wing board and asked to breathe freely while undergoing 4D CT imaging without iv contrast and respiratory monitoring using the real time positioning management system from Varian Medical Systems. Image contouring was performed on 2.5 mm CT slices using the AdvantageSim MD version 4.4 (GE Healthcare Systems). Gross target volume (GTV) delineation was typically performed on the 30% phase CT set according to the mid-ventilation approach 12. No margin was added for microscopic disease.
Proton treatment plans were created by CMS Xio (version 4.2) following our published approach12, 13. Treatments were designed with 2-3 passively scattered coplanar beams, depending on tumor size and location of the tumor. For each beam, the 95% isodose line was chosen to conform around the GTV before applying aperture expansion and range compensator smearing to account for set-up error (5 mm) and individual respiratory motion. Because of range uncertainty, 3.5% + 2 mm of the proximal and distal ranges were added as margin proximally and distally to the GTV.
Target doses and organs at risk dose constraints were adapted from RTOG protocols 14. Fractionation was chosen so that a biological effective dose (BED) of at least 100 Gy(RBE) to the tumor was achieved 15. The relative biological effectiveness (RBE) factor for proton planning was 1.1.
Radiation Treatment
Treatments were delivered at the Francis H. Burr Proton Therapy Center. The accuracy of SBRT with protons requires dosimetric and geometric accuracy as established and confirmed through various quality assurance (QA) procedures. Dosimetry of proton fields in our system has been well established with an accurate dosimetry prediction model 16 and is maintained by a weekly QA procedure to track the stability of the model parameters. Weekly QA also tracks stability of range better than +/−0.5 mm. The lung geometry is particularly complex for proton treatments as a consequence of the large tissue inhomogeneities. Overall dosimetry accuracy in patient is therefore verified by Monte Carlo calculations 17 and confirms the applicability of our dose algorithm in patient in this complex anatomy.
Daily treatment verification relies on geometric repositioning of the patient using the Digital Imaging Position System (DIPS). Dual orthogonal X-rays and treatment portals for each field were taken pre-treatment to match multiple bony landmarks to digitally reconstructed radiographs from the planning CT. All fields were treated every day. Total treatment time for each fraction was routinely 20-25 minutes. Treatments were given on consecutive weekdays, 24 hours apart.
Follow-up
Follow-up began on the day of completion of proton therapy and continued until last observation or death through September of 2011. Follow-up consisted of evaluations 4-6 weeks after the completion of radiation treatment and every 3-4 months for 2 years, and every 6 months thereafter. Each evaluation consisted of a complete history and physical and a diagnostic chest CT. PFTs and PET scans were obtained as clinically indicated. Local failure was defined as a 20% increase in the longest tumor diameter on CT scan and either biopsy confirmation of recurrence or associated FDG avidity on two consecutive PET scans that was of similar intensity as on the pretreatment staging scan. Regional failure was defined as intra-lobar relapse outside of a 2 cm margin around the GTV or in the hilar, mediastinal, or supraclavicular lymph nodes. Distant failure was defined as intrathoracic relapse elsewhere or hematogenous spread. Toxicity grading was according to the Common Toxicity Criteria for Adverse Events (CTCAE) v4.03. Grading for chest wall pain was adapted from the CTCAE criteria for general pain.
Statistical methods
Time to overall survival and regional, distant and local failure was assessed from the date of completion of radiation therapy. Local failure was defined as recurrence within 2 cm of the treated GTV. Regional failure was defined as lymph node recurrence within the hilum, mediastinum, supraclavicular fossa or as intra-lobar recurrence distant to the treated GTV. The method of Kaplan and Meier was used to estimate and characterize all cause mortality and regional, distant, and local failure 18. Analysis was performed with GraphPad Prism Version 5.04.
RESULTS
The median age was 78 years (range, 62-89) and six of the 15 patients (40%) had an ECOG performance status of 2-3 (Table 1). Severe COPD or interstitial lung disease was present in eight patients and five patients had at least two primary tumors. In five cases, prior thoracic radiation therapy, and in one case central location of the tumor, were also factors in the selection of protons as treatment modality. The median tumor size was 15 mm (range, 10-31 mm) (Table 2). Four patients did not undergo biopsy because they either refused or were judged to be at unacceptably high procedural risk. Bronchoscopy or mediastinoscopy to assess nodal stations were not performed in any patients prior to SBRT. Eleven tumors were within 1 cm of the chest wall, and two were within 2 cm of the mediastinal pleura. The average respiratory tumor motion was 2 mm (range, 0-10 mm).
Table 2. Tumor characteristics (n = 20).
| Characteristic n | (%) |
| AJCC T-Stage | |
| T1a (≤2 cm) | 16 (80) |
| T1b (>2-3 cm) | 2 (10) |
| T2a (>3-5 cm) | 2 (10) |
| Location | |
| Right | 11 (55) |
| Left | 9 (45) |
| Lobe | |
| Upper | 13 (65) |
| Middle | 4 (20) |
| Lower | 3 (15) |
| Histology | |
| Adenocarcinoma | 9 |
| NSCLC NOS | 4 |
| Squamous cell carcinoma | 3 |
| No biopsy | 4 |
| Distance to chest wall | |
| ≤ 10 mm | 11 (55) |
| > 10 mm | 9 (45) |
| Distance to proximal bronchial tree or mediastinal pleura | |
| ≤ 20 mm | 2 (10) |
| > 20 mm | 18 (90) |
| Respiratory tumor motion, peak to peak | |
| > 5 mm | 2 (10) |
AJCC=American Joint Committee on Cancer; NSCLC NOS = Non small cell lung cancer not otherwise specified
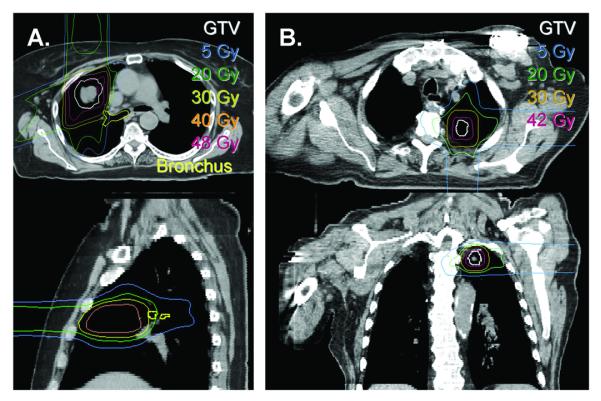
The median total dose was 45 Gy(RBE) (range, 42-50 Gy(RBE)) and the median fraction size was 14 Gy(RBE) (range, 10-16 Gy(RBE)) corresponding to a BED10 of at least 100 Gy(RBE). The number of fractions was 3 for 17 out of 20 tumors. Four patients were treated with two or more courses of therapy, each for separate and distinct tumors (Table 1). The average V5 and V20 for the ipsilateral lung for these patients were 20.5% (range, 5-39%) and 9.5% (range, 2-27%) respectively. The ipsilateral mean lung dose was 5.12 Gy(RBE) (range, 1.37 – 12.56). The contralateral lung in all cases received no dose due to the lack of exit dose of protons. An example dose distribution is shown in Figure 1a. One patient had a pacemaker at the same level as his lung lesion and dose to this area was avoided by employing only two beams (see Figure 1b).
Figure 1.
Sample proton SBRT dose distributions. (A) Limitation of dose to central structures. Radiation dose to the right bronchial tree (yellow) was limited by using a two beam arrangement. The dose falloff of the anterior beam results in a relatively small dose deposition for the major airways immediately posterior. (B) Limitation of dose to a pace maker/defibrillator. A similar strategy to that shown in (A) was used to limit dose to a pacemaker, only in this case dose falloff of a posterior beam was critical.
Treatments were well tolerated with only one case of grade 2 dermatitis (Table 3). There was one case of grade 3 pneumonitis in a patient with severe COPD which responded to prednisone. This patient had an ipsilateral mean lung dose of 3.36 Gy(RBE). One patient had a symptomatic rib fracture and two had asymptomatic fractures. For these patients, chest wall V30 and maximum doses were 11.1-24.9 cc and 44.3-48.6 Gy(RBE), respectively. No grade 4 or 5 toxicities were observed.
Table 3. Radiation associated toxicity (n = 20).
| Graded Toxicity | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Chest wall pain | 0 | 1 | 0 |
| Dermatitis | 3 | 1 | 0 |
| Dyspnea | 0 | 0 | 0 |
| Fatigue | 1 | 1 | 0 |
| Pneumonitis | 6 | 0 | 1 |
There were six deaths (Table 1). Of these, two were attributable to lung cancer progression while the other four were due to other causes. Two patients developed both regional and distant metastases, one developed only regional metastases, and one developed only distant metastases. No local failures within the radiation field were observed. With a median follow-up time of 24.1 months, 2-year Kaplan Meier estimates of overall survival, local control, regional control, and distant control were 64% (95% confidence limits, 34-83%), 100% (83-100%), 78% (38-93%), and 86% (56-95%), respectively. Three patients developed metachronous primary tumors.
DISCUSSION
There is great interest in testing the utility of proton beam radiation in patients with both locally advanced stage III disease and early-stage NSCLC 8, 19. While standard photon SBRT achieves excellent local tumor control with low toxicity in the majority of patients of stage I patients, proton SBRT may be advantageous in cases that involve central tumors, tumors located close to the chest wall, multiple tumors, previous chest irradiation, and poor pulmonary function.
To this end, we find that proton SBRT was well tolerated in a cohort of patients with adverse factors such as pulmonary co-morbidities, prior chest irradiation, or multiple primary tumors (80% of cases) (Table 1). In general, COPD and larger low dose regions have been correlated with higher rates of radiation pneumonitis 20-22. We hypothesize that the low pulmonary toxicity seen in our study may be a result of lower integral dose to surrounding lung due to the unique properties of protons and the use of only 2-3 beams. Notably, we only observed one case of grade 2 dermatitis even though protons are typically associated with higher skin doses. A recently reported proton dose escalation trial from the M.D. Anderson Cancer Center administered 87.5 Gy(RBE) at 2.5 Gy(RBE)/fraction for stage I NSCLC, which was associated with grade 2 and 3 dermatitis in 67% and 17% of patients, respectively 8. Our results are similar to the dermatitis rate seen in the RTOG 0236 trial of photon SBRT where 7% of patients developed grade 2 or higher dermatitis 23.
It is also noteworthy that we did not observe any local recurrence with a median follow-up of approximately 2 years, even though proton radiation is not associated with the typical large intratumoral hostpots that are produced by photon SBRT. Prior dosimetric comparisons of proton and photon SRBT have generally shown that target coverage is similar between both techniques, although with more generous allowances for range uncertainty the conformity index for protons can be larger 3, 11. It should be noted that most tumors in our cohort were located in the upper lobes and tumor motion was therefore low (average of 2 mm) and resulting in less additional margin around the GTV.
In conclusion, this clinical experience with proton SBRT may be regarded as an important preliminary confirmation of the theoretical advantages of proton beam radiation that have been highlighted by several groups 3, 9-11. We hypothesize that in appropriate clinical settings proton SBRT has the potential for normal tissue sparing that cannot be achieved with conformal photon techniques. In addition, for larger tumors particular in difficult locations, protons may allow for escalation of dose to levels that cannot be safely achieved with photons. Prospective clinical trials testing the safety and efficacy of proton SBRT in comparison to photon SBRT are warranted.
ACKNOWLEDGMENTS
The project was supported by the Federal Share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest relating to the work reported in this manuscript.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Robinson CG, Bradley JD. The treatment of early-stage disease. Semin Radiat Oncol. 2010;20:178–185. doi: 10.1016/j.semradonc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Lo SS, Fakiris AJ, Chang EL, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7:44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 3.Georg D, Hillbrand M, Stock M, et al. Can protons improve SBRT for lung lesions? Dosimetric considerations. Radiother Oncol. 2008;88:368–375. doi: 10.1016/j.radonc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 6.Bush DA, Slater JD, Shin BB, et al. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest. 2004;126:1198–1203. doi: 10.1378/chest.126.4.1198. [DOI] [PubMed] [Google Scholar]
- 7.Nihei K, Ogino T, Ishikura S, et al. High-dose proton beam therapy for Stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:107–111. doi: 10.1016/j.ijrobp.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Chang JY, Komaki R, Wen HY, et al. Toxicity and patterns of failure of adaptive/ablative proton therapy for early-stage, medically inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;80:1350–1357. doi: 10.1016/j.ijrobp.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoppe BS, Huh S, Flampouri S, et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: a dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother Oncol. 2010;97:425–430. doi: 10.1016/j.radonc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald OK, Kruse JJ, Miller JM, et al. Proton beam radiotherapy versus three-dimensional conformal stereotactic body radiotherapy in primary peripheral, early-stage non-small-cell lung carcinoma: a comparative dosimetric analysis. Int J Radiat Oncol Biol Phys. 2009;75:950–958. doi: 10.1016/j.ijrobp.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Seco J, Panahandeh HR, Westover K, et al. Treatment of Non-Small-Cell Lung Cancer Patients with Proton Beam-Based Stereotactic Body Radiotherapy: Dosimetric Comparison with Photon Plans Highlights Importance of Range Uncertainty. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2011.05.062. in press. [DOI] [PubMed] [Google Scholar]
- 12.Engelsman M, Rietzel E, Kooy HM. Four-dimensional proton treatment planning for lung tumors. Int J Radiat Oncol Biol Phys. 2006;64:1589–1595. doi: 10.1016/j.ijrobp.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Engelsman M, Kooy HM. Target volume dose considerations in proton beam treatment planning for lung tumors. Med Phys. 2005;32:3549–3557. doi: 10.1118/1.2126187. [DOI] [PubMed] [Google Scholar]
- 14.Timmerman R, Galvin J, Michalski J, et al. Accreditation and quality assurance for Radiation Therapy Oncology Group: Multicenter clinical trials using Stereotactic Body Radiation Therapy in lung cancer. Acta Oncol. 2006;45:779–786. doi: 10.1080/02841860600902213. [DOI] [PubMed] [Google Scholar]
- 15.Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623–1631. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 16.Kooy HM, Rosenthal SJ, Engelsman M, et al. The prediction of output factors for spread-out proton Bragg peak fields in clinical practice. Phys Med Biol. 2005;50:5847–5856. doi: 10.1088/0031-9155/50/24/006. [DOI] [PubMed] [Google Scholar]
- 17.Paganetti H, Jiang H, Lee SY, et al. Accurate Monte Carlo simulations for nozzle design, commissioning and quality assurance for a proton radiation therapy facility. Med Phys. 2004;31:2107–2118. doi: 10.1118/1.1762792. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 19.Chang JY, Komaki R, Lu C, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011 doi: 10.1002/cncr.26080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rancati T, Ceresoli GL, Gagliardi G, et al. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol. 2003;67:275–283. doi: 10.1016/s0167-8140(03)00119-1. [DOI] [PubMed] [Google Scholar]
- 21.Hernando ML, Marks LB, Bentel GC, et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–659. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 22.Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol. 2009;4:838–844. doi: 10.1097/JTO.0b013e3181a99ff6. [DOI] [PubMed] [Google Scholar]
- 23.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. Jama. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]