Abstract
Oculofaciocardiodental syndrome (OFCD) is a rare genetic disorder affecting ocular, facial, dental and cardiac systems. The clinical diagnosis of OFCD can be challenging due to a wide variety of symptoms. OFCD is found only in females due to its X-linked inheritance pattern and embryonic lethality for males. Radiculomegaly of canines is the most consistent finding in these patients. In this report we present a female patient with characteristic facial features and a comprehensive overview of OFCD. Diagnosis of OFCD in this patient was verified by genetic analysis, during which we found a novel mutation in BCOR.
Keywords: oculofaciocardiodental syndrome, OFCD, BCOR, mutation analysis
Oculofaciocardiodental syndrome (OFCD) is an X-linked condition that is characterized by ocular, facial, cardiac, and dental abnormalities (Hayward, 1980). This syndrome has only been seen in heterozygous females and it is presumed to have lethal effects on affected males since there is no report of males developing this condition (Schulze et al., 1999). The phenotypic spectrum of OFCD (MIM# 300166) includes microphthalmia, congenital cataracts, as well as cardiac symptoms such as atrial septal defect and/or ventricular septal defect or mitral valve prolapse (Hayward, 1980; Schulze et al., 1999). The facial characteristics are frequently facial elongation with a high nasal bridge and broad nasal tip with separation of anterior cartilage (Hayward, 1980; Hedera and Gorski, 2003). Dental characteristics include delayed tooth eruption and prolonged retention of primary teeth. However, the most characteristic diagnostic symptom for OFCD is radiculomegaly, also known as dental root gigantism (Hayward, 1980; Marashi and Gorlin, 1990; Wilkie and Chambers, 1990). The roots of canines (in some cases incisors or lateral incisors) develop until they reach the cortical plate of the orbit or mandible (Oberoi et al., 2005; Wilkie and Chambers, 1990). The impact on the quality of life of OFCD patients depends on the organs that are affected. There is considerable variability in the expressivity of this disorder. Root gigantism may complicate orthodontic treatment, while eye defects can cause reduced vision or blindness and heart defects may have life-threatening consequences.
The genetic analysis of OFCD patients revealed mutations in the BCOR (BCL-6 interacting corepressor) gene (MIM# 300485) on chromosome Xp11.4 (Hilton et al., 2009; Jiang et al., 2009; Ng et al., 2004), which can be deletions, substitutions or splice site mutations and result in a frameshift that leads to a premature stop codon (Hilton et al., 2009; Ng et al., 2004). BCOR is expressed in many different tissues and has important and multiple functions during early embryogenesis (such as maintaining tissue homeostasis and gene silencing by epigenetic mechanisms), which explains in part the range of symptoms observed in OFCD patients (Fan et al., 2009).
In this report, we describe a patient with canine radiculomegaly and other clinical symptoms of OFCD who carries a novel mutation in BCOR and discuss the research results for BCOR that help to explain many of the diverse symptoms in patients.
MATERIALS AND METHODS
BCOR mutation analysis
Subjects for this study were recruited in accordance with an approved IRB protocol. Genomic DNA was isolated from saliva samples of the patient and her two daughters using a ORAgene saliva collection kit (DNA Genotek, Kanata, Ontario, Canada). Mutation analysis was performed by PCR-amplification of BCOR exons 4, 7, 9, 10, 11 and 13–14 (GenBank accession number BC114220.1) as described previously (Hilton et al., 2009). The initial denaturation step of 3 min at 95°C was followed by 35 cycles of amplification at 95°C for 30s, annealing at 60°C for 30s, and extension at 72°C for 90s, followed by final extension at 72°C for 10 min using GoTaq Flexi DNA polymerase (Promega). Unincorporated primers in PCR products were removed by ExoSAP-IT (USB Corp.) and samples were sequenced by an outside vendor (Agencourt Bioscience Corporation, Beverley, MA). Sequence analysis was conducted with Chromas sequence editor (Technelysium, Tewantin, QLD, Australia).
CASE REPORT
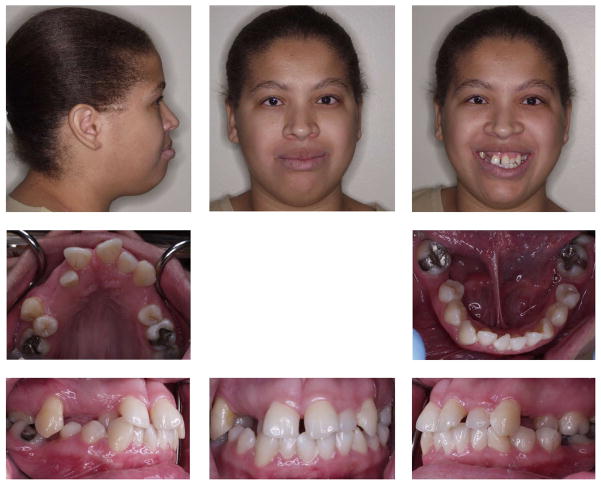
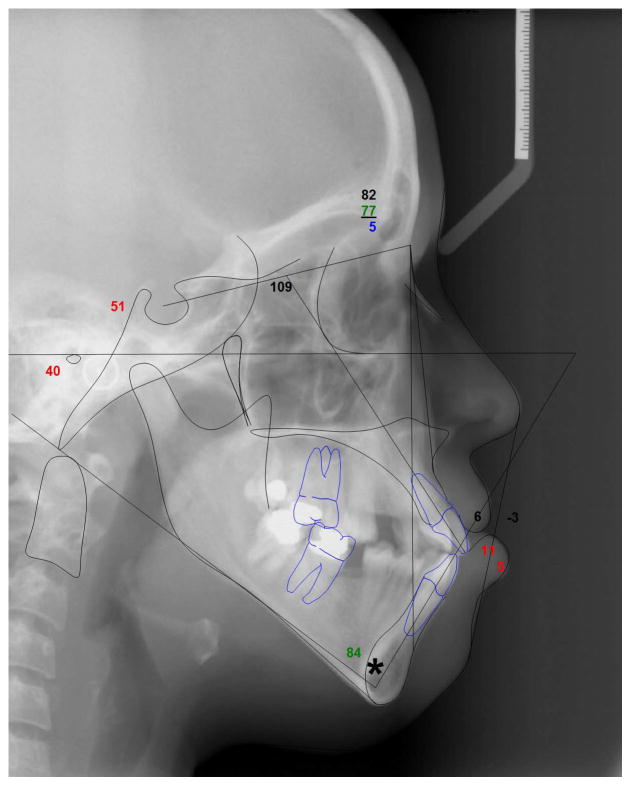
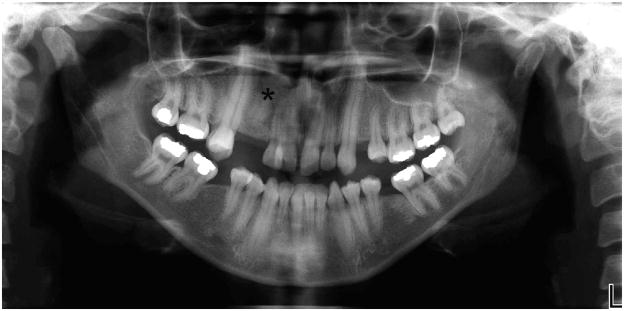
A 25-year-old Hispanic female patient presented to the Orthodontic Clinic at the University of Connecticut Health Center. Clinical examination revealed microphthalmia and microcornea of her right eye, a broad nasal tip and a long philtrum (Fig. 1). Intraoral examination revealed dental abnormalities such as agenesis of the maxillary right third molar and maxillary first premolars. Mandibular first molars had been extracted due to caries as well as severe malocclusion (Fig. 1). Cephalometric analysis confirmed that she had a long face with significantly increased lower facial height, a steep mandibular plane angle, convex profile and labially inclined incisors (Fig. 2; Table 1). A submucosal cleft palate was diagnosed upon examination. The cleft was diagnosed by palpation for the posterior nasal spine which was absent. Also, the patient had a mild velopharyngeal insufficiency during speech. Since this was a mild case of submucosal cleft, no surgery was indicated (Reiter et al., 2011). The upper and lower canines were elongated and root apices extended almost to the cortical plate of the orbit and mandible, respectively (Fig. 2–3). A panoramic radiograph of the patient showed radiculomegaly of maxillary and mandibular canines in addition to missing teeth (Fig. 3). These are typical facial and dental features of OFCD syndrome. Moreover, radiographic investigation identified a radio-opacity adjacent to the maxillary right canine which was diagnosed as an odontoma, which is likely the cause of the canine erupting distally to its proper location (Fig. 3). The odontoma was later removed by oral surgery. To further confirm the diagnosis of OFCD and to examine whether OFCD in this patient is inherited, her medical and family history was taken and genetic analysis was performed.
Figure 1.
Extraoral and intraoral photographs of the OFCD patient showing facial and dental characteristics of the OFCD syndrome. Note the broad nasal tip and long lower facial height as well as missing maxillary premolars and malalignment of upper and lower teeth. The patient had cataract surgery.
Figure 2.
Cephalometric radiograph showing elongated lower facial height, steep mandibular plane angle, convex profile, and labially inclined incisors. Mandibular canines almost reach dense cortical bone of the lower border of mandible as indicated by an asterisk.
Table 1.
Cephalometric analysis of OFCD patient.
| Patient | Normal | ||
|---|---|---|---|
| Skeletal | SNA (°) | 82 | 82 |
| SNB (°) | 77 | 80 | |
| ANB (°) | 5 | 2 | |
| SN-GoGn (°) | 51 | 32 | |
| FMA (°) | 40 | 24 | |
| Dentoalveolar | U1-SN (°) | 109 | 104 |
| IMPA (°) | 84 | 95 | |
| Soft Tissue | G′-Sn-Pg′ (°) | 6 | 12 |
| UL-E Plane | −3 | −4 | |
| LL-E Plane | 5 | −2 |
S:Sella, N: Nasion, A: Point A, Go: Gonion, Gn: Gnathion, FMA: Frankfort-Mandibular Plane Angle, U1: Upper incisor longitudinal axis, IMPA: Lower incisor mandibular plane angle, G: Glabella, Sn: Subnasale, Pg′: Soft tissue Pogonion, UL: Upper lip, E Plane: Esthetic plane (Pronasale-Soft tissue Pogonion), LL: Lower lip.
Figure 3.
Panoramic radiograph of patient with root gigantism of maxillary and mandibular canines. An odontoma between maxillary lateral incisor and canine on the right side is indicated by an asterisk. Maxillary first premolars and the maxillary right third molar are missing and the mandibular first molars had been extracted.
This patient was born to non-consanguineous parents with a birth weight of 7 lbs. The medical history revealed that she was born with congenital cataracts, which were surgically removed at age 6. However, there was no family history of cataracts. Upon cardiological examination, there was no indication of atrial septal defect (ASD) or ventricular septal defect (VSD). She had hammer-type flexion of toes 2 and 3 and clinodactyly of toes 4 and 5 on both feet. Dentally, she also reported delayed eruption of the secondary dentition. Retained primary dentition was removed by her dentist at age 12.
The patient reported that there were no ocular, cardiologic, dental or abnormal skeletal symptoms apparent in her parents and sibling. Her 8- and 3-year-old daughters were clinically evaluated. The radiograph of the older daughter was normal with canines not fully developed. She has no ocular or facial features that are characteristic for OFCD. The younger daughter was negative for medical or dental symptoms suggestive of OFCD.
RESULTS
During orthodontic treatment, all of the spaces in the mandible and in the maxillary left quadrant were closed. The maxillary midline was corrected and the upper right canine, which had erupted distally, was brought mesially to the first premolar position. Due to the fact that translating a canine with such long root would extend the treatment time, the canine was left at the first premolar position and a cantilever pontic was fabricated to restore the edentulous canine space. The outcome was a satisfactory correction of the malocclusion.
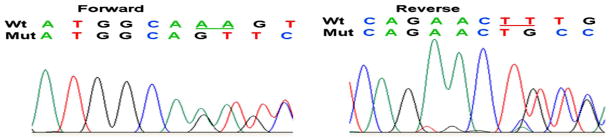
We sequenced exons 4, 7, 9, 10, 11 and 13–14 of BCOR where known mutations for OFCD had been identified. Forward and reverse sequencing of exon 4 showed a heterozygous deletion of two nucleotides at position 2858 and 2859 of the cDNA sequence (c.2858_2859delAA) (Fig. 4), which resulted in a frameshift at amino acid 593, changing a Lysine to a Serine (p.K593SfsX7). This frameshift deletion resulted in a premature stop codon 7 nucleotides further downstream in exon 4. This is the first time that this mutation has been reported in BCOR. We sequenced exon 4 of the patient’s two daughters, neither of whom carried the disease allele. The parents of the patient were not available for analysis.
Figure 4.
Sequencing results for BCOR from genomic DNA of OFCD patient. Sequencing of exon 4 revealed 2 bp deletion at position 2858–2859 (c.2858_2859delAA). Arrows indicate begin of sequence deviation and deleted nucleotides are underlined in the wild type sequence.
DISCUSSION
We have identified a novel mutation in exon 4 of BCOR in a patient with OFCD syndrome. Despite having undergone congenital cataract surgery at age 6, she had not been diagnosed with OFCD prior to her visit in our dental clinic at age 25. In our clinic the patient was diagnosed with possible OFCD due to her canine root gigantism. Radiculomegaly is the telltale sign for OFCD. In its absence, diagnosis may be more difficult as the occurrence of congenital heart anomalies and cataracts are variable. Radiculomegaly in OFCD patients has only been described in the secondary dentition and most often affects canines, but also incisors or lateral incisors (Horn et al., 2005). Enlarged roots are nearly always present in OFCD patients (Horn et al., 2005). and occasionally multiple teeth are affected (McGovern et al., 2006). The development of the dentition is often delayed and oligodontia or hypodontia is frequently observed.
Ocular abnormalities present mostly as congenital cataract or microphthalmia and sometimes both occur in the same patient. However, there appears to be a wide variability in other symptoms, such as facial characteristics (broad nasal tip, long narrow face, high nasal bridge, eyebrow curvature and orofacial clefting, high arched palate) and cardiac findings (atrial septal defect, ventricular septal defect, mitral valve prolapse), which can make diagnosis of an atypical patient more challenging. Other skeletal abnormalities like syndactyly, hammer-type flexion deformities and radioulnar synostosis have been mentioned in a number of patients. Mental retardation is rarely part of the OFCD spectrum opposed to several other syndromes that include microphthalmia. Hearing loss has been reported in one study (Hilton et al., 2009). OFCD, however, is the only disorder where radiculomegaly and microphthalmia or congenital cataracts are syndromic.
OFCD has been associated with mutations that cause premature stop codons in the X- chromosomal BCOR gene (Xp11.4). In two rare instances mutations in BCOR have been found in Lenz microphthalmia patients and in a microphthalmia patient with associated anomalies (MAA) (Hilton et al., 2009; Ng et al., 2004). Most mutations in BCOR that lead to premature stop codons are frameshift mutations in the form of small deletions. Other mutations include a small duplication or submicroscopic deletions that can involve several exons. Only the Lenz microphthalmia mutation constitutes a simple substitution leading to an amino acid change. The p.K593SfsX7 mutation found in our patient leads to a premature stop codon in BCOR that is between the ankyrin repeats and the BCL-6 binding region. BCOR haploinsufficiency is the most likely outcome of this mutation.
Recent progress in analyzing the role of BCOR during embryonic development resulted in interesting findings that help in part to explain the phenotype of OFCD. Studies utilizing Xenopus frog models have suggested that BCOR may play a role in left-right patterning, laterality determination, midline integrity and lens development (Hilton et al., 2007, 2009). Ablation of the homologous Xenopus BCOR protein (xtBcor) on one side of Xenopus embryos caused laterality defects and affected mainly gut patterning as well as cardiac orientation. On the other hand, mild repression of xtBcor resulted in more subtle asymmetry changes such as abnormal septa in hearts, which are a common finding in OFCD patients (Hilton et al., 2007). Blocking BCOR with short morpholinos (oligonucleotides that bind to the xtBcor mRNA and prevent translation of the protein) in frogs caused eye defects (microphthalmia and colobomas), which are reminiscent of the spectrum of eye defects found in OFCD patients. The xtBcor action on lens development is likely through the homeobox transcription factor xtPitx2. Interestingly, another member of the Ptx family, Ptx3, is known as an important regulator of lens development, however, it has not been shown whether it is regulated by BCOR (Rieger et al., 2001). Together, these functions of BCOR may explain why some OFCD patients also present with dextrocardia, asplenia, intestinal malrotation, septate nasal cartilage and are prone to various forms of oral clefting and high-arched palate formation. Some OFCD patients have also been diagnosed with bifid uvula (Hilton et al., 2009). Interestingly, BCOR is a regulator of the transcription factor AP-2 (Fan et al., 2009). Mutations or deletions in the AP-2_ gene TFAP2A cause branchiooculofacial syndrome (BOFS) (MIM# 113620) (Milunsky et al., 2008; Reiber et al., 2010), which includes orofacial clefting, ocular anomalies and other facial dysmorphisms. It is possible that the presence of the different forms of oral clefts in OFCD is regulated by functional polymorphisms in TFAP2A or in AP2-regulated genes such as IRF6 (Rahimov et al., 2008).
While those research results may partially explain the ocular and cardiac phenotype in OFCD patients, they do not address BCOR’s role in delayed secondary dentition, persisting primary teeth, radiculomegaly and absent teeth. There appears to be a role for Bcor in the regulation of gene expression in embryonic stem cells, which suggests that Bcor may be important in early tissue differentiation (Wamstad et al., 2008). In mice, the Bcor gene homologue is expressed in neural tube, the branchial arches and in tooth primordial (Wamstad and Bardwell 2007). In a recent study, Fan et al. (2009) investigated mesenchymal stem cells (MSC) from the apical papilla of an OFCD patient who underwent surgical root apex removal on a tooth with radiculomegaly. The patient had a confirmed BCOR mutation that led to the truncation of the protein. They showed that the patient stem cells from the apical papilla proliferated faster and had more osteogenic (i.e. mineral forming) potential compared to the control cultures. Furthermore, OFCD stem cells also had increased mRNA expression for extracellular matrix components of bone and dentin, such as osteocalcin and dentin sialoprotein. This finding could mean that BCOR functions as a repressor of osteogenic and dentinogenic differentiation.
Fan et al. (2009) identified a new functional pathway for BCOR by comparing stem cells from this rare disorder with other stem cell populations. Interestingly, the excessive mesenchymal stem cell proliferation appeared to be driven by mutant BCOR and to be mediated by the developmentally regulated transcription factor AP-2α (MIM# 107580), which is known to be involved in craniofacial development. In control cell cultures, BCOR binds to a demethylase (JHDM1B) and this complex regulates AP-2α transcription by blocking the methylation of histones. AP-2α is overexpressed in the absence of functional BCOR in OFCD cultures. High expression of the AP-2α protein is thought to be the cause for increased differentiation into osteogenic and dentinogenic cells but does not explain the increased proliferation of stem cells from the apical papilla of the patient. Based on these findings one might speculate that tooth agenesis in OFCD patients could be caused by dysregulation of an unspecified transcriptional activity in early tooth buds and the continuous growth of the roots by a yet unknown mechanism to regulate apical stem cell proliferation.
CONCLUSIONS
OFCD is one of the few rare craniofacial or dental disorders where the genetic cause is known and the mode of action of the mutant gene is fairly well studied. Recent progress in basic research on BCOR helped in part to explain the laterality and midline defects in OFCD and in turn, studying dental tissue from an OFCD patient helped to identify a new regulatory mechanism for dental stem cell differentiation. We believe that more can be learned about the role of BCOR during pattern formation, cell differentiation in various tissues, and especially during tooth development by studying OFCD. However, continued investigation will be required to identify additional genes and processes that mediate the phenotypic changes. This report exemplifies the important role of dentists in recognizing rare syndromic disorders with typical or unusual dental or intraoral phenotypes.
Acknowledgments
This study was supported by institutional funds and funding from NIH (M01RR006192) to the GCRC at UCHC.
We are indebted to the patient and her family for participating in this study. This study was supported by institutional funds and funding from NIH (M01RR006192) to the GCRC at UCHC. Written consent for publication was obtained from the patient.
References
- Fan Z, Yamaza T, Lee JS, Yu J, Wang S, Fan G, Shi S, Wang CY. BCOR regulates mesenchymal stem cell function by epigenetic mechanisms. Nature cell biology. 2009;11:1002–1009. doi: 10.1038/ncb1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward JR. Cuspid gigantism. Oral Surg Oral Med Oral Pathol. 1980;49:500–501. doi: 10.1016/0030-4220(80)90070-5. [DOI] [PubMed] [Google Scholar]
- Hedera P, Gorski JL. Oculo-facio-cardio-dental syndrome: skewed × chromosome inactivation in mother and daughter suggest X-linked dominant Inheritance. Am J Med Genet A. 2003;123A:261–266. doi: 10.1002/ajmg.a.20444. [DOI] [PubMed] [Google Scholar]
- Hilton E, Johnston J, Whalen S, Okamoto N, Hatsukawa Y, Nishio J, Kohara H, Hirano Y, Mizuno S, Torii C, et al. BCOR analysis in patients with OFCD and Lenz microphthalmia syndromes, mental retardation with ocular anomalies, and cardiac laterality defects. Eur J Hum Genet. 2009;17:1325–1335. doi: 10.1038/ejhg.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton EN, Manson FD, Urquhart JE, Johnston JJ, Slavotinek AM, Hedera P, Stattin EL, Nordgren A, Biesecker LG, Black GC. Left-sided embryonic expression of the BCL-6 corepressor, BCOR, is required for vertebrate laterality determination. Hum Mol Genet. 2007;16:1773–1782. doi: 10.1093/hmg/ddm125. [DOI] [PubMed] [Google Scholar]
- Horn D, Chyrek M, Kleier S, Luttgen S, Bolz H, Hinkel GK, Korenke GC, Riess A, Schell-Apacik C, Tinschert S, et al. Novel mutations in BCOR in three patients with oculo-facio-cardio-dental syndrome, but none in Lenz microphthalmia syndrome. Eur J Hum Genet. 2005;13:563–569. doi: 10.1038/sj.ejhg.5201391. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Fang P, Adesina AM, Furman P, Johnston JJ, Biesecker LG, Brown CW. Molecular characterization of co-occurring Duchenne muscular dystrophy and X-linked oculo-facio-cardio-dental syndrome in a girl. Am J Med Genet A. 2009;149A:1249–1252. doi: 10.1002/ajmg.a.32863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marashi AH, Gorlin RJ. Radiculomegaly of canines and congenital cataracts--a syndrome? Oral Surg Oral Med Oral Pathol. 1990;70:802–803. doi: 10.1016/0030-4220(90)90025-n. [DOI] [PubMed] [Google Scholar]
- McGovern E, Al-Mudaffer M, McMahon C, Brosnahan D, Fleming P, Reardon W. Oculo-facio- cardio-dental syndrome in a mother and daughter. Int J Oral Maxillofac Surg. 2006;35:1060–1062. doi: 10.1016/j.ijom.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Milunsky JM, Maher TA, Zhao G, Roberts AE, Stalker HJ, Zori RT, Burch MN, Clemens M, Mulliken JB, Smith R, et al. TFAP2A mutations result in branchio-oculo-facial syndrome. Am J Hum Genet. 2008;82:1171–1177. doi: 10.1016/j.ajhg.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D, Thakker N, Corcoran CM, Donnai D, Perveen R, Schneider A, Hadley DW, Tifft C, Zhang L, Wilkie AO, et al. Oculofaciocardiodental and Lenz microphthalmia syndromes result from distinct classes of mutations in BCOR. Nat Genet. 2004;36:411–416. doi: 10.1038/ng1321. [DOI] [PubMed] [Google Scholar]
- Oberoi S, Winder AE, Johnston J, Vargervik K, Slavotinek AM. Case reports of oculofaciocardiodental syndrome with unusual dental findings. Am J Med Genet A. 2005;136:275–277. doi: 10.1002/ajmg.a.30811. [DOI] [PubMed] [Google Scholar]
- Rahimov F, Marazita ML, Visel A, Cooper ME, Hitchler MJ, Rubini M, Domann FE, Govil M, Christensen K, Bille C, et al. Disruption of an AP-2alpha binding site in an IRF6 enhancer is associated with cleft lip. Nat Genet. 2008;40:1341–1347. doi: 10.1038/ng.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber J, Sznajer Y, Posteguillo EG, Muller D, Lyonnet S, Baumann C, Just W. Additional clinical and molecular analyses of TFAP2A in patients with the branchio-oculo-facial syndrome. Am J Med Genet A. 2010;152A:994–999. doi: 10.1002/ajmg.a.33331. [DOI] [PubMed] [Google Scholar]
- Reiter R, Brosch S, Wefel H, Schlomer G, Haase S. The submucous cleft palate: diagnosis and therapy. Int J Pediatr Otorhinolaryngol. 2011;75:85–88. doi: 10.1016/j.ijporl.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Rieger DK, Reichenberger E, McLean W, Sidow A, Olsen BR. A double-deletion mutation in the Pitx3 gene causes arrested lens development in aphakia mice. Genomics. 2001;72:61–72. doi: 10.1006/geno.2000.6464. [DOI] [PubMed] [Google Scholar]
- Schulze BR, Horn D, Kobelt A, Tariverdian G, Stellzig A. Rare dental abnormalities seen in oculo-facio-cardio-dental (OFCD) syndrome: three new cases and review of nine patients. Am J Med Genet. 1999;82:429–435. doi: 10.1002/(sici)1096-8628(19990219)82:5<429::aid-ajmg13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Wamstad JA, Bardwell VJ. Characterization of Bcor expression in mouse development. Gene Expr Patterns. 2007;7:550–557. doi: 10.1016/j.modgep.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamstad JA, Corcoran CM, Keating AM, Bardwell VJ. Role of the transcriptional corepressor Bcor in embryonic stem cell differentiation and early embryonic development. PLoS ONE. 2008;3:e2814. doi: 10.1371/journal.pone.0002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie GJ, Chambers IG. A very large maxillary cuspid. Oral Surg Oral Med Oral Pathol. 1990;70:159–160. doi: 10.1016/0030-4220(90)90110-e. [DOI] [PubMed] [Google Scholar]