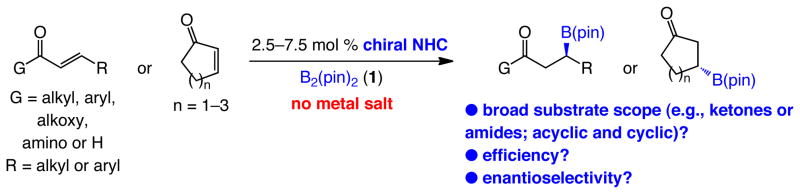
Abstract
The first broadly applicable metal-free enantioselective method for boron conjugate addition (BCA) to α,β-unsaturated carbonyls is presented. The C–B bond forming reactions are promoted in the presence of 2.5–7.5 mol % of a readily accessible C1-symmetric chiral imidazolinium salt, which is converted, in situ, to the catalytically active diastereo- and enantiomerically pure N-heterocyclic carbene (NHC) by the common organic base 1,8-diazabicyclo[5.4.0]undec-7-ene (dbu). In addition to the commercially available bis(pinacolato)diboron [B2(pin)2], and in contrast to reactions with the less sterically demanding achiral NHCs, the presence of MeOH is required for high efficiency. Acyclic and cyclic α,β-unsaturated ketones, as well as acyclic esters, Weinreb amides and aldehydes can serve as suitable substrates; the desired β-boryl carbonyls are isolated in up to 94% yield and >98:2 enantiomer ratio (er). Transformations are often carried out at ambient temperature. In certain cases, such as when the relatively less reactive unsaturated amides are used, elevated temperatures are required (50–66 °C); nonetheless, reactions remain highly enantioselective. The utility of the NHC-catalyzed method is demonstrated through comparison with the alternative Cu-catalyzed protocols; in cases involving a polyfunctional substrate, unique profiles in chemoselectivity are exhibited by the metal-free approach (e.g., conjugate addition vs reaction with an alkyne, allene or aldehyde).
1. Introduction
We recently reported the discovery of the first metal-free catalytic protocol for the formation of C–B bonds.1 We demonstrated that N-heterocyclic carbenes (NHCs), a class of organic molecules commonly used to catalyze C–C bond formation,2 promote reactions between bis(pinacolato)diboron [B2(pin)2; 1] and an assortment of cyclic and acyclic α,β-unsaturated ketones and esters. Such processes deliver β-boryl carbonyls, which are versatile intermediates in chemical synthesis.3,4 The NHC-catalyzed (pinacolato)boron conjugate addition (BCA) reactions were shown to be mechanistically distinct, allowing them to be complementary to the more extensively examined Cu-catalyzed variants5 (e.g., distinct chemoselectivity profiles, see below). A subsequent study focused on the use of chiral phosphines to promote enantioselective BCA.6 Additions to a limited number of acyclic esters and ketones were performed at 70 °C, affording products in 76:24–97.5:2.5 enantiomer ratio (er); reaction with cyclohexenone was shown to proceed to 62% conversion (16 h, 70 °C), generating the cyclic β-boryl ketone in 68:32 er. In our initial disclosure,1a we proposed that the metal-free reactions are initiated by formation of an NHC•B2(pin)2 complex, wherein the B–B bond is activated due to association of the Lewis basic heterocycle;7 support for the aforementioned hypothesis and the associated structural changes has more recently been provided through X-ray crystallography.8
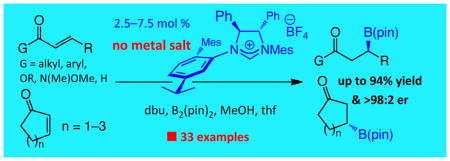
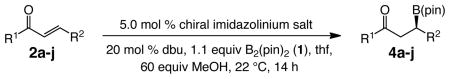
Herein, we report the enantioselective version of the NHC-catalyzed BCA process. From the outset, our goal was to develop an efficient catalytic C–B bond forming process that is applicable to a wide substrate range. Accordingly, as illustrated in Scheme 1, the method described can be performed with acyclic α,β-unsaturated ketones, esters, Weinreb amides or aldehydes; in some cases, NHC-catalyzed variants have not been previously reported (including with achiral catalysts; e.g., additions to amides and enals). We show that metal-free catalytic BCA may be performed with cyclic enones of different ring sizes. Conjugate additions proceed in the presence of 2.5–7.5 mol % of a readily accessible C1-symmetric chiral imidazolinium salt, a common organic base and commercially available B2(pin)2 (1), delivering a wide range of products in up to 94% yield and >98:2 er. The NHC-catalyzed BCA reactions furnish chemoselectivity profiles that are unavailable through the use of alternative Cu-catalyzed variants, and thus represent a valuable addition to the arsenal of transformations that generate organoborons in high enantiomeric purity.
Scheme 1. NHC-Catalyzed Enantioselective BCA to Unsaturated Carbonyls.
2. Results and Discussion
2.1. Identification of optimal conditions and catalysts
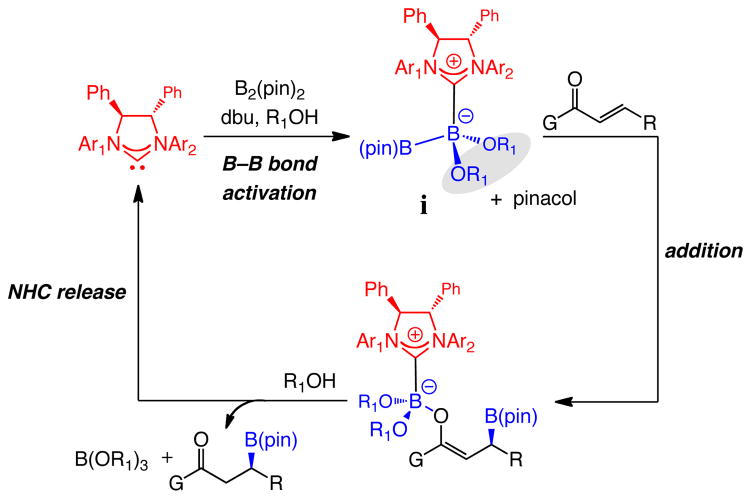
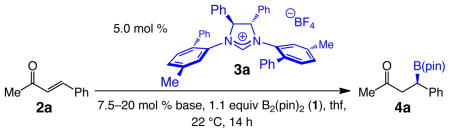
We began by probing the ability of the NHC derived from chiral imidazolinium salt 3a to effect reactions of unsaturated carbonyls with B2(pin)2 (1). The basis for such a starting point was the finding that the latter heterocyclic carbene is effective in promoting the corresponding enantioselective silyl conjugate additions with (dimethylphenylsilyl)boronic acid pinacol ester.9 Under conditions that result in efficient transformations when achiral NHCs are utilized,1a as illustrated in entry 1 of Table 1, there is hardly any product generated in the presence of 3a and NaOt-Bu after 14 hours at ambient temperature. Inefficient BCA is not due to the inability of the relatively more sterically congested chiral NHC (vs the smaller achiral variants) to associate with 1;10 as with the previously illustrated cases involving achiral heterocyclic carbenes,1,8,9 spectroscopic studies point to rapid (within 15 min) generation of NHC•1 complexes. Ongoing studies aimed at elucidating the exact nature of the catalytically active species indicate that in reactions promoted by chiral NHCs, formation of a less sterically demanding complex (i in Figure 1), generated by the exchange of a pinacolato unit with a common alcohol, can facilitate catalysis through the pathway originally proposed in 2009.1a,11 Accordingly, we chose to probe the effect of MeOH as an additive.
Table 1.
Study of Reaction Conditions with 3a as the Chiral NHCa
 | ||||
|---|---|---|---|---|
| entry | base; mol % | additive; equiv | conv (%)b | erc |
| 1 | NaOt-Bu; 7.5 | none | <5 | na |
| 2 | NaOt-Bu; 7.5 | MeOH; 20 | 24 | 86.5:13.5 |
| 3 | dbu; 10 | MeOH; 20 | 31 | 90:10 |
| 4 | dbu; 20 | MeOH; 20 | 47 | 92:8 |
Reactions were performed under N2 atmosphere.
Determined through analysis of 400 MHz 1H NMR spectra of unpurified mixtures (±5%).
Determined by HPLC analysis (±2%); see the Supporting Information for details. na = not applicable; dbu = 1,8-diazabicyclo[5.4.0]undec-7-ene.
Figure 1.
Catalytic cycle proposed for NHC-catalyzed BCA; exchanging a B(pin) with smaller alkoxides could lead to a faster rate of reaction.
With 20 equivalents of the alcohol present (entry 2, Table 1), there is indeed 24% conversion to the desired β-boryl ketone 4a, which is generated in 86.5:13.5 er. Use of the more robust and user-friendly organic base 1,8-diazabicyclo[5.4.0]undec-7-ene (dbu; vs NaOt-Bu) leads to similar reactivity and enantioselectivity (31% conv, 90:10 er; entry 3). There is enhancement in BCA efficiency when the amount of dbu is increased to 20 mol % (47% conv, 92:8 er; entry 4). The positive influence of the larger concentration of dbu could arise from a more efficient generation of chiral NHC and/or it might be because exchange of pinacol with MeOH is base-catalyzed (cf. formation of i in Figure 1). Such issues are the subjects of continuing investigations designed to clarify these and other mechanistic intricacies of the metal-free process. It is nonetheless worthy of note that, in contrast to the formerly reported NHC-catalyzed silyl conjugate additions,9 water cannot be utilized as the additive (vs MeOH), and only a complex mixture of products is generated under such conditions (data not shown).
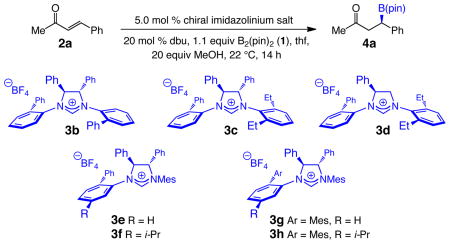
We thus took advantage of the facility with which chiral imidazolinium salts can be structurally modified,12 and began search for a chiral NHC that can furnish higher conversion and promote the C–B bond forming reaction enantioselectively. Since studies aimed at elucidating the identity of the active chiral catalyst, required for outlining an appropriate stereochemical model, remain in progress, we turned to the systematic screening of C2- and C1-symmetric imidazolinium salts. Representative findings, depicted in Table 2, demonstrate that alteration of the structure of the chiral NHC has a notable influence on the reaction outcome. Comparison of the activity of 3b and 3c–d demonstrates that similar conversion and er values can be obtained with C2-and C1-symmetric carbenes, with the catalyst bearing two phenyl heterocyclic substituents affording somewhat higher er values (3c vs 3d, entries 2–3, Table 2). Overall, the NHC derived from C1-symmetric 3h, bearing a sizeable mesityl at its dissymmetric N–Ar moiety furnishes the highest reactivity and enantioselectivity (Table 2, entry 7; 81% conv, 95:5 er). Catalytic BCA with 3f (entry 5), an imidazoliumum salt that carries a smaller phenyl unit at the same site, is less discriminating (76:24 vs 95:5 er). Subsequent optimization studies led us to establish that the presence of 60 equivalents of MeOH results in optimal reactivity and selectivity (>98% conv and 95:5 er); such a requirement points to the possible significance of the requisite ligand exchange mentioned above (pinacol with MeOH; cf. i, Figure 1).
Table 2.
Initial Examination of Chiral NHC Catalystsa
 | |||
|---|---|---|---|
| entry | imidazolinium salt | conv (%)b | erc |
| 1 | 3b | 35 | 82:18 |
| 2 | 3c | 37 | 81:19 |
| 3 | 3d | 30 | 69:31 |
| 4 | 3e | 10 | 56:44 |
| 5 | 3f | 78 | 76:24 |
| 6 | 3g | 30 | 94:6 |
| 7 | 3h | 81 | 95:5 |
See Table 1. Mes = 2,4,6-(Me)3C6H2.
An additional attribute of C1-symmetric catalysts merits mention. Although it is possible that the C2-symmetric chiral variants of certain highly hindered imidazolinium salts such as 3h could give rise to improved enantioselectivity, synthesis of such sterically encumbered heterocycles is typically inefficient; this shortcoming is largely due to minimal yields obtained for the formation of the C–N bond required for installment of the second hindered N–Ar moiety. The more readily accessible C1-symmetric NHCs that contain a single sterically demanding dissymmetric aryl unit, can be more easily synthesized and thus offer an attractive option.
2.2. NHC-Catalyzed Enantioselective BCA
2.2.1 Reactions with aryl- and alkyl-substituted acyclic α,β-unsaturated ketones
Under the optimal conditions, the NHC derived from 3a, optimal for catalytic silyl conjugate additions, promotes BCA to enone 2a (cf. Table 1), affording 4a in 82% yield and 92:8 er (entry 1, Table 3). However, when 3h serves as the catalyst precursor (Table 3, entry 2), the reaction is more facile (>98% conv and 92% yield vs 87% conv and 82% yield), and the desired product is obtained with higher enantiomeric purity (96:4 vs 92:8 er).13 As the additional data in Table 3 illustrate, an array of acyclic α,β-unsaturated ketones can be used as substrates. Enantioselective formation of 4b (Table 3, entry 3), in spite of the presence of a sterically demanding o-tolyl substituent is worthy of note. Synthesis of enantiomerically enriched 4b (entry 3), however, necessitates that the BCA is performed at 50 °C; ~15% conversion is observed when the NHC-catalyzed reaction is carried out at 22 °C. The findings in Table 3 indicate that, in general, electron-rich enones are superior substrates; indeed, as illustrated in entry 4 (Table 3), enantioselective addition to 2c can be performed with 2.5 mol % 3h and 10 mol % dbu under otherwise identical conditions with little or no loss of efficiency (90% conv, 80% yield, 94:6 er).
Table 3.
NHC-Catalyzed Enantioselective BCA to Acyclic Enonesa
 | ||||||
|---|---|---|---|---|---|---|
| entry | R1; R2 | imidazolinium salt | conv(%)b | yield (%)c | erd | |
| 1 | Me, Ph | 2a | 3a | 87 | 82 | 92:8 |
| 2 | Me; Ph | 2a | 3h | >98 | 92 | 96:4 |
| 3 | Me; o-MeC6H4e | 2b | 3h | 95 | 90 | 85.5:14.5 |
| 4 | Me; p-MeOC6H4f | 2c | 3h | 90 | 80 | 94:6 |
| 5 | Me; p-BrC6H4g | 2d | 3h | 60 | 43 | 92:8 |
| 6 | Me; 2-furyl | 2e | 3h | 75 | 73 | 92:8 |
| 7 | Me; n-pentyl | 2f | 3h | >98 | 94 | 94.5:5.5 |
| 8 | Me; i-Pr | 2g | 3h | >98 | 93 | 94:6 |
| 9 | n-Bu; Ph | 2h | 3h | 81 | 76 | 91:9 |
| 10 | i-Pr; Phh | 2i | 3h | 94 | 90 | 90:10 |
| 11 | Ph; Phi | 2j | 3h | 82 | 72 | 93.5:6.5 |
Reactions were performed under N2 atmosphere.
Conversion determined through analysis of 400 MHz 1H NMR spectra of unpurified mixtures (±5%).
Yields of isolated purified products (±2%).
Enantiomeric ratio (er) determined by GLC or HPLC analysis (±2%); see the Supporting Information for details.
Reaction performed at 50 °C.
Reaction performed with 2.5 mol % 3h and 10 mol % dbu.
In addition, ~10% proto-deboration (saturated ketone) product is formed (determined by analysis of 400 MHz 1H NMR spectrum of the unpurified mixture).
Reaction performed with 7.5 mol % 3h and 30 mol % dbu.
Reaction time = 18 h.
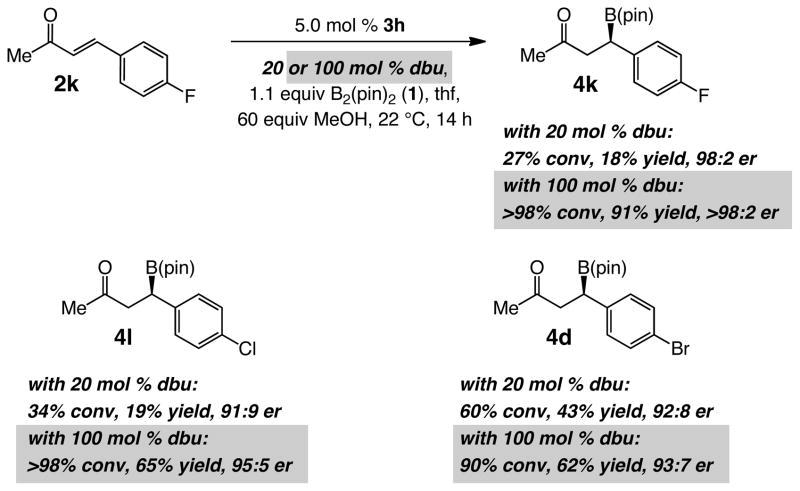
Catalytic BCA with bromophenyl-substituted ketone 2d (entry 5, Table 3) affords 4d in 43% yield (60% conv, 92:8 er). As the findings in Scheme 2 indicate, the relative inefficiency corresponds to a variety of halogen-substituted substrates. Thus, reaction of p-fluoro-substituted 2k, under the conditions employed for the transformations in Table 2, although exceedingly enantioselective (98:2 er), results in only 27% conversion after 14 hours; NHC-catalyzed BCA of chloro-substituted 2l is similarly inefficient.14 Our attempts to address the above complications led to the finding that increasing the amount of the organic base (dbu) from 20 mol % to one equivalent leads to substantial improvements in efficiency with similar or higher enantioselectivity (Scheme 2). Such an advance was made based on the assumption that a more efficient generation of the NHC catalyst or the aforementioned methanol/pinacol exchange (cf. i, Figure 1) might demand larger amounts of base, thus allowing the enantioselective pathway to become more competitive.15,16
Scheme 2. Influence of Amine Base on BCA Efficiency.
The transformations in entries 7–8 show that alkyl-substituted α,β-unsaturated ketones, including those that bear a relatively hindered moiety (e.g., i-Pr in 2g) are suitable BCA substrates. Enones with an n-alkyl, an i-propyl or an aryl substituent at the carbonyl carbon can be efficiently converted to the β-boryl ketones through the NHC-catalyzed transformation (Table 3, entries 9–11). Some of the more sterically demanding electrophiles require 7.5 mol % 3h (Table 3, entry 10; 68% conv, 66% yield, 90:10 er with 5 mol %) or somewhat longer reaction times (entry 11). It should be pointed out that the positive influence of stoichiometric quantities of dbu is not limited to the catalytic transformations depicted in Scheme 2. As illustrated through improved yields obtained for BCA processes that give rise to the formation of furyl-substituted β-boryl methyl ketone 4e and phenyl-substituted phenylketone 4j, the same strategy can be applied to other types of substrates as well.

2.2.2 Reactions with aryl- and alkyl-substituted acyclic α,β-unsaturated esters
α,β-Unsaturated esters can be used in NHC-catalyzed BCA (Table 4); the desired products are generated in 62–87% yield and 94:6–98:2 er. In certain cases, such as those involving an electron-deficient aryl substituent (entry 2) and the sizeable t-butyl-ester (entry 3), additions require elevated temperatures (43% conv and 96.5:3.5 er and 57% conv and 98:2 er respectively, at 22 °C). In spite of the more forcing conditions in entries 2–3 (Table 4), enantioselectivities remain high (94:6–98:2 er).17
Table 4.
NHC-Catalyzed Enantioselective BCA to Acyclic Unsaturated Estersa
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | R1; R2 | mol % 3h | time (h); temp (°C) | conv (%)b | yield (%)c | erd | |
| 1 | Me, Ph | 5a | 5.0 | 14; 22 | >98 | 87 | 98:2 |
| 2 | Me; p-BrC6H4[e] | 5b | 7.5 | 10; 50 | >98 | 62 | 94:6 |
| 3 | t-Bu; Ph | 5c | 5.0 | 14; 50 | 88 | 80 | 98:2 |
| 4 | Me; npentyl | 5d | 5.0 | 14; 22 | 83 | 80 | 95:5 |
See Table 3.
Approximately 30% of the proto-deboration byproduct is generated (determined by analysis of 400 MHz 1H NMR spectrum of the unpurified mixture).
2.2.3 Reactions with aryl- and alkyl-substituted acyclic α,β-unsaturated amides
α,β-Unsaturated amides and aldehydes are two substrate classes that have received relatively scant attention in connection with catalytic enantioselective BCA. There are no reported examples of reactions with the aforementioned substrate types under any metal-free catalytic conditions. Accordingly, we set out to investigate their respective NHC-catalyzed BCA processes.
We first examined the transformations involving the versatile Weinreb amides, for which only one example of Cu-catalyzed enantioselective BCA has thus far been reported.18 As the data in Table 5 indicate, reactions with amides require relatively higher catalyst loadings (7.5 mol %), elevated temperatures (50–66 °C) and longer reaction times (up to 36 h); nonetheless, aryl- and alkyl-substituted β-boryl amides are obtained in 60–92% yield after purification and high enantioselectivities are observed in most cases (86.5:13.5–95:5 er). It is noteworthy that sterically demanding substrates 7e and 7f (Table 5, entries 5–6) are converted to the desired products in 60–73% yield and 95:5 er. In the case of electron-deficient amide 7b (Table 5, entry 2), shorter reaction times and reduced amounts of MeOH are preferred (30 vs 60 equiv), since the undesired proto-deboration14 (cf. 6b, entry 2, Table 4) as well as formation of the corresponding β-boryl methyl ester is otherwise significantly more competitive19 (~70% byproduct formation with 60 equiv MeOH). Control experiments indicate that it is the β-boryl Weinreb amide that is subsequently converted to the ester (vs generation of the unsaturated ester directly from the unsaturated amide followed by BCA); the resident Lewis acidic β-boron likely activates the amide group towards reaction with MeOH.20
Table 5.
NHC-Catalyzed Enantioselective BCA to Acyclic Unsaturated Amidesa
 | ||||||
|---|---|---|---|---|---|---|
| entry | R | time (h); temp (°C) | conv (%)b | yield (%)c | erd | |
| 1 | Phe | 7a | 36; 50 | 86 | 61 | 86.5:13.5 |
| 2 | p-BrC6H4e | 7b | 10; 50 | >98 | 70 | 94:6 |
| 3 | n-pentyl | 7c | 14; 50 | 77 | 74 | 95:5 |
| 4 | (CH2)2OTBS | 7d | 24; 66 | 96 | 92 | 93:7 |
| 5 | i-Bu | 7e | 24; 66 | 78 | 73 | 95:5 |
| 6 | i-Pr | 7f | 24; 50 | 67 | 60 | 95:5 |
See Table 3.
Reaction performed with 30 equivalents of MeOH.
2.2.4 Reactions with alkyl-substituted α,β-unsaturated aldehydes
α,β-Unsaturated aldehydes are another set of substrates that have been less extensively examined in the context of Cu-catalyzed BCA. Part of the complication with NHC–Cu-catalyzed BCA reactions involving aldehydes is that the corresponding 1,2-addition to the carbonyl group can be efficient.21 In one study, synthesis and isolation of an (achiral) NHC-Cu–OMe complex proved necessary, since the presence of base had to be avoided if efficient generation of β-boryl aldehydes were to be achieved.22 In another example, an NHC–Cu-catalyzed BCA of cinnamaldehyde is shown to afford the desired product in 70:30 er (86% conv; yield of isolated product was not reported).23 Finally, a protocol for Cu-catalyzed enantioselective BCA to enals was reported most recently; transformations proceed through in situ-generated chiral α,β-unsaturated iminiums formed from reaction with an enantiomerically pure amine (20 mol %).24 Although high efficiency and enantioselectivities can be achieved through the latter method, and reactions can be carried out with aryl- as well as alkyl-substituted enals may, use of air- and moisture-sensitive Cu(OTf)2 and 10 mol % of a Brønsted acid (o-FC6H4CO2H) are required. Furthermore, the β-boryl aldehydes obtained were not isolated, but were directly functionalized (e.g., Wittig olefin synthesis).
The NHC derived from imidazolinium salt 3h promotes enantioselective BCA to alkyl-substituted enals, as indicated by the examples shown in Table 6. As illustrated in entry 1, when C2-symmetric 3a is used in the reaction, under otherwise identical conditions, 10a is obtained in 81% yield (>98% conv) and 84:16 er; catalytic BCA with 3h delivers 10a in 72% yield and the desired product is generated in significantly higher enantioselectivity (95:5 vs 84:16 er; entry 2, Table 6). Thus, reactions generally proceed to ≥95% conversion, are often performed at 22 °C, and afford the desired β-boryl aldehydes in 63–72% yield after isolation and purification and in 91:9–95:5 er.
Table 6.
NHC-Catalyzed Enantioselective BCA to Unsaturated Aldehydesa
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | R | imidazolinium salt; mol % | time (h); temp (°C) | conv (%)b | yield (%)c | erd | |
| 1 | n-propyl | 9a | 3a; 5.0 | 14; 22 | >98 | 81 | 84:16 |
| 2 | n-propyl | 9a | 3h; 5.0 | 14; 22 | 95 | 72 | 95:5 |
| 3 | (CH2)2Phe | 9b | 3h; 7.5 | 7.0; 50 | >98 | 65 | 95:5 |
| 4 | i-Bu | 9c | 3h; 5.0 | 14; 22 | 95 | 63 | 94:6 |
| 5 | i-Pr | 9d | 3h; 5.0 | 14; 22 | >98 | 72 | 91:9 |
See Table 3.
In addition, 18% of the derived β-methoxy aldehyde is obtained (determined by analysis of the 400 MHz 1H NMR spectrum of the unpurified mixture).
Attempts to carry out catalytic conjugate addition with aryl-substituted variants resulted in the formation of a complex product mixture. Alternative pathways that might render transformations of aryl-substituted enals inefficient are addition of the NHC to an enal, which can lead to substrate homocoupling or generation of the derived saturated ester,25 reaction with a β-boryl aldehyde product, or methoxide conjugate addition26 (a minor byproduct generated in the reaction shown in entry 2 of Table 6).
2.2.5 Reactions with cyclic α,β-unsaturated carbonyls
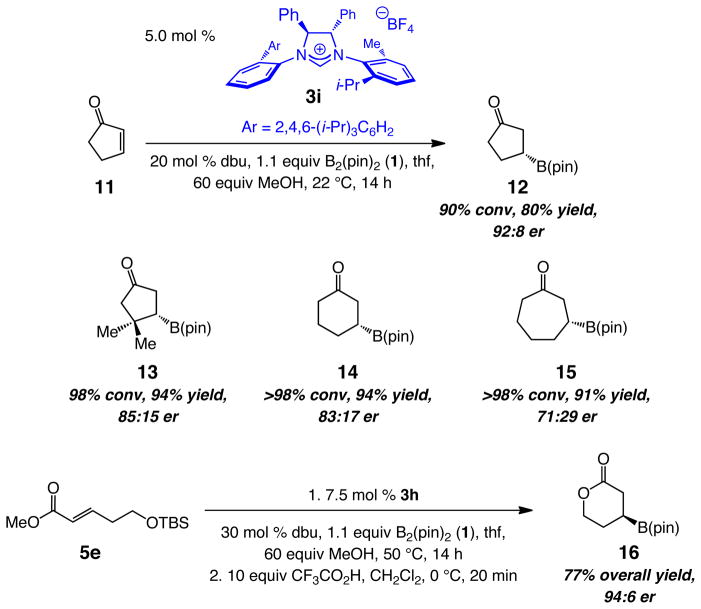
The final class of substrates examined involved cyclic enones.5c–d As already mentioned, only one case of a phosphine-catalyzed BCA has been reported (cyclohexenone, 68:32 er).6 Preliminary studies indicated that NHCs derived from 3a and 3h promote additions to cyclopentenone efficiently (93% conv, 79% yield and >98% conv, 95% yield, respectively), but with diminished enantioselectivity; for example, β-boryl cyclopentanone 12 (Scheme 3) is obtained in 67:33 and 78.5:21.5 er with 3a and 3h, respectively. The relatively low stereoselectivity is consistent with the fact that BCA to Z-2a delivers 4a in 55:45 er with 3h serving as the NHC precursor (vs 96:4 for the E isomer; see entry 2, Table 3).27 Catalyst screening led us to establish that use of imidazolinium salt 3i (Scheme 3) promotes the BCA with similar efficiency (90% conv, 80% yield) but with improved enantioselectivity (92:8 er). Several additional examples, involving enones of different ring sizes are provided in Scheme 3.28 Enantioselectivities are lower than those obtained with acyclic enones; future studies, supported by a better appreciation of the mechanistic nuances of the catalytic cycle, will be aimed at identification of catalysts that furnish higher er. Nevertheless, efficient formation of 13, involving the reaction of sterically congested cyclopentenone, is particularly noteworthy, since a previous report involving the use of chiral Cu–phosphine catalysts indicates <2% conversion with a similarly substituted cyclohexenone.5c Additions to lactones are hampered by adventitious cleavage of the heterocyclic ring (MeOH/dbu) and formation of various byproducts. Nonetheless, as the example regarding the conversion of 5e to 16 indicates (Scheme 3), such cyclic β-boryl esters can be readily accessed by means of BCA of the acyclic unsaturated ester, followed by an efficient cyclization.
Scheme 3. NHC-Catalyzed Enantioselective BCA to Cyclic Enones.
2.3. Comparison with Cu-Catalyzed Variants. Complementarity in Chemical Synthesis
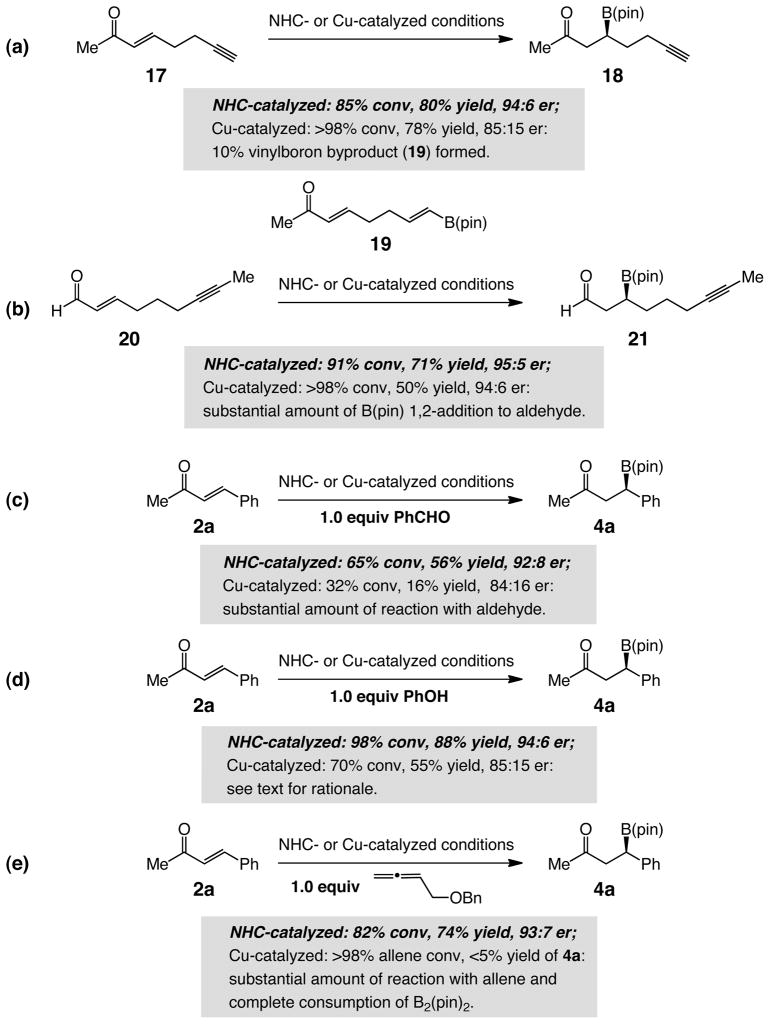
An impetus for performing the present studies was to develop a catalytic protocol that offers distinct advantages to the more established Cu-catalyzed versions.1a,5 Such an expectation has been based on the principle that, since metal-free BCA reactions proceed through an entirely different pathway compared to the processes promoted by organocopper complexes, they can deliver certain complementarities that would otherwise remain unavailable. Certain distinctions, above all in connection with reactions of enals, have been already mentioned. Additional revealing examples, underlining some of the distinct attributes of the NHC-catalyzed processes are illustrated in Scheme 4. The chiral catalyst used as representative of Cu-based approaches was introduced in previous studies (with S,R-Josiphos as the chiral ligand).5a–b,f
Scheme 4. Functional Group Tolerance: Differences Between NHC- and Cu-Catalyzed Protocolsa.
aNHC-catalyzed conditions: Reactions (a), (b), and (d): 5.0 mol % 3h, 20 mol % dbu, 1.1 equiv B2(pin)2, thf, 60 equiv MeOH, 22 °C, 14 h; reactions (c) and (e): 7.5 mol % 3h, 30 mol % dbu, 1.1 equiv B2(pin)2, thf, 60 equiv MeOH, 50 °C, 14 h. Cu-catalyzed conditions: Reactions (a), (b), and (d): 5.0 mol % Josiphos, 5.0 mol % CuCl, 5.0 mol % NaOt-Bu, 1.1 equiv B2(pin)2, thf, 1.0 equiv MeOH, 22 °C, 14 h; reactions (c) and (e): 7.5 mol % Josiphos, 7.5 mol % CuCl, 7.5 mol % NaOt-Bu, 1.1 equiv B2(pin)2, thf, 1.2 equiv MeOH, 50 °C, 14 h. Conversion values relate to the percent starting material consumed and were determined through analysis of the 400 MHz 1H NMR spectra of the unpurified mixtures (±5%). Yield values refer to isolated and purified β-boryl products (±2%). See the Supporting Information for details.
Whereas NHC-catalyzed BCA to alkyne-substituted enone 17 proceeds with complete chemoselectivity to afford 18 in 95% yield and 94:6 er (Scheme 4a), under Cu-catalyzed conditions, 10% of β-vinylboron product (19),29 derived from Cu–B addition addition/protonation of the alkyne, is observed as well. A more notable dissimilarity manifests itself in the context of reactions with alkyne-substituted enal 20 (Scheme 4b). In contrast to the NHC-catalyzed reaction, which affords β-boryl aldehyde 21 in 71% yield, when Josiphos–Cu complex is employed, the desired product is isolated in 50% yield; the diminished efficiency is likely the result of competitive 1,2-addition.1a The greater degree of chemoselectivity that is feasible through NHC-catalyzed BCA is further illustrated by the reaction of unsaturated ketone 2a in the presence of one equivalent of benzaldehyde (Scheme 4c). Although the efficiency of the NHC-catalyzed process is somewhat diminished, probably as a result of reversible NHC addition to the aldehyde30 resulting in partial sequestration of the catalytically active heterocycle, the negative impact on the Cu-catalyzed reaction is significantly more severe (32% conv and 16% yield vs 65% conv and 56% yield under NHC-catalyzed conditions).31,32 As further illustrated in Scheme 4d, the presence of phenol (1.0 equiv) leads to a more significant rate reduction in the BCA promoted by the Cu-based catalyst (70% conv and 55% yield vs 98% conv and 88% yield with the NHC-catalyzed process); due to reduced Lewis basicity of the oxygen atom (vs an alkoxide), the intermediate Cu–OPh is likely more reluctant to undergo reaction with B2(pin)2 to regenerate the active Cu–B(pin) complex. Finally, whereas Cu-catalyzed processes involving an allene33 appear to be significantly more favored (Scheme 4e), leading to complete consumption of the available B2(pin)2 and the formation of byproducts, the NHC-catalyzed BCA proceeds readily to afford the desired 4a in 74% yield (vs <5% yield).34 It should be mentioned that, in certain aspects, the state-of-the-art in Cu-catalyzed BCA is superior. The latter class of metal-catalyzed reactions typically require lower catalyst loading and shorter reaction times and the corresponding additions to cyclic enones are generally more enantioselective.5 Nonetheless, the examples provided in Scheme 4 clearly indicate that the availability of the NHC-catalyzed enantioselective BCA can serve as a set of transformations that provide a useful complement to the highly effective metal-catalyzed variants.
3. Conclusions
The present account puts forth the first general metal-free protocol that can be used for enantioselective synthesis of a variety of β-boryl carbonyls. These studies emphasize the substantially higher activity of NHCs as BCA catalysts (vs phosphines6), as indicated in the initial disclosure.1a A significantly wider range of substrates participate in the C–B bond forming reactions, including those that contain sterically demanding substituents (e.g., 2g in Table 3, 7f in Table 5, 9d in Table 6, 13 in Scheme 3). Conjugate additions are more facile (e.g., elevated temperatures are not needed for acyclic enones35) and generally proceed with superior enantioselectivity with a chiral NHC serving as the catalyst than when phospines are utilized.6
The metal-free catalytic method possesses attributes that are complementary to the Cu-catalyzed alternatives:5 in a polyfunctional molecule, highly chemoselective boron addition is more likely under the Cu-free conditions. In certain cases, such as reactions with alkyl-substituted enals (cf. Table 6) or sterically hindered cyclic enones (cf. Scheme 3), the NHC-catalyzed protocol offers a milder, more efficient and/or selective alternative to the Cu-catalyzed approach.
A critical finding disclosed in this report relates to the central role of methanol, as otherwise there is minimal transformation when sterically hindered chiral NHC are used (vs the originally disclosed achiral variants1a). Studies to shed light on various mechanistic aspects of the enantioselective metal-free process will be assisted by such findings, as well as by the variations in the levels and profiles in reactivity and selectivity observed during the screening of a wide range of chiral NHCs. Thus, a mechanistic scenario must provide a rationale as to why the aforementioned conditions are required for achieving efficient and highly enantioselective BCA reactions; it must also account for the dependence of the levels of reactivity and enantioselectivity on specific steric and electronic attributes of the chiral NHC catalysts.
Details of ongoing mechanistic investigations regarding NHC-catalyzed BCA will be reported in due course. The design and development of other NHC-catalyzed processes and applications to total synthesis of complex molecules are additional goals of future investigations.
Supplementary Material
Acknowledgments
This paper is dedicated to the memory of Professor Robert J. Silbey. Financial support was provided by the NIH (GM-57212) and the NSF (CHE-1111074). We thank Dr. K-s. Lee and Dr. F. Haeffner for helpful discussions and Frontier Scientific for gifts of bis(pinacolato)diboron.
Footnotes
Supporting Information Available. Experimental procedures and spectral data for substrates and products (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
Footnotes and References
- 1.(a) Lee K-s, Zhugralin AR, Hoveyda AH. J Am Chem Soc. 2009;131:7253–7255. doi: 10.1021/ja902889s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee K-s, Zhugralin AR, Hoveyda AH. J Am Chem Soc. 2010;132:12766. [Google Scholar]
- 2.For representative reviews on NHC-catalyzed processes in chemical synthesis, see: Enders D, Balensfiefer T. Acc Chem Res. 2004;37:534–541. doi: 10.1021/ar030050j.Enders D, Niemeier O, Henseler A. Chem Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z.
- 3.For a review regarding the significance of enantioselective conjugate additions with B- and Si-based nucleophiles, see: Hartmann E, Vyas DJ, Oestreich M. Chem Commun. 2011;47:7917–7932. doi: 10.1039/c1cc10528k.
- 4.For an application of enantioselective Cu-catalyzed (pinacolato)boron conjugate addition to synthesis of a biologically active molecule, see: Chea H, Sim HS, Yun J. Adv Synth Catal. 2009;351:855–858.For a related example, see: Marcus AP, Sarpong R. Org Lett. 2010;12:4560–4563. doi: 10.1021/ol1018536.
- 5.For representative examples of Cu-catalyzed enantioselective (pinacolato)boron conjugate additions, see: Lee JE, Yun J. Angew Chem, Int Ed. 2008;47:145–147. doi: 10.1002/anie.200703699.Sim HS, Feng X, Yun J. Chem Eur J. 2009;15:1939–1943. doi: 10.1002/chem.200802150.Feng X, Yun J. Chem Commun. 2009:6577–6579. doi: 10.1039/b914207j.Chen I-H, Yin L, Itano W, Kanai M, Shibasaki M. J Am Chem Soc. 2009;131:11664–11665. doi: 10.1021/ja9045839.O’Brien JM, Lee K-s, Hoveyda AH. J Am Chem Soc. 2010;132:10630–10633. doi: 10.1021/ja104777u.Feng X, Yun J. Chem Eur J. 2010;16:13609–13612. doi: 10.1002/chem.201002361.Chen IH, Kanai M, Shibasaki M. Org Lett. 2010;12:4098–4101. doi: 10.1021/ol101691p.Park JK, Lackey HH, Rexford MD, Kovnir K, Shatruk M, McQuade DT. Org Lett. 2010;12:5008–5011. doi: 10.1021/ol1021756.Moure AL, Arrayás RG, Carretero JC. Chem Commun. 2011;47:6701–6703. doi: 10.1039/c1cc11949d.Lee JCH, McDonald R, Hall DG. Nature Chem. 2011;3:894–899. doi: 10.1038/nchem.1150.
- 6.Bonet A, Gulyás H, Fernández E. Angew Chem, Int Ed. 2010;49:5130–5134. doi: 10.1002/anie.201001198. [DOI] [PubMed] [Google Scholar]
- 7.For a review on NHC–borane complexes, see: Curran DP, Solovyev A, Makhlouf Brahmi M, Fensterbank L, Malacria M, Lacôte E. Angew Chem, Int Ed. 2011;50:10294–10317. doi: 10.1002/anie.201102717.
- 8.Kleeberg C, Crawford AG, Batsanov AS, Hodgkinson P, Apperley DC, Cheung MS, Lin Z, Marder TB. J Org Chem. 2012;77:785–789. doi: 10.1021/jo202127c. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien JM, Hoveyda AH. J Am Chem Soc. 2011;133:7712–7715. doi: 10.1021/ja203031a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For example, treatment of imidazolinium salt 3h (see Table 2) with B2(pin)2 at 22 °C leads to the clean formation of a new complex (11B NMR: δ 33.57 ppm and δ 12.20 ppm vs δ 30.10 ppm for 1 in d8-thf at 22 °C). See the Supporting Information for details.
- 11.Details of the pathways that lead to the proposed NHC•diboron complex i and the role of such an intermediate in the context of a catalytic cycle and the reasons for its higher reactivity will be the subject of a detailed upcoming disclosure.
- 12.For a study that highlights the significance of easily modifiable C1-symmetric chiral N-heterocyclic carbenes in enantioselective synthesis, see: Lee K-s, Hoveyda AH. J Org Chem. 2009;75:4455–4462. doi: 10.1021/jo900589x.For application of such entities in other catalytic enantioselective processes, see: (b) Ref 5e. (c) Ref 9.
- 13.Although C2-symmetric imidazolinium salt 3a typically gives rise to less efficient and enantioselective (pinacolato)boron conjugate addition than the C1-symmetric 3h, it can be prepared by a shorter synthesis route (three vs five steps in 25–40% yield). See the Supporting Information for details.
- 14.Proto-deboration of the β-boryl ketone products can occur when the substrates carry an aryl group with a relatively strong electron withdrawing substituent. As an example, formation of p-bromophenyl-substituted 4d is accompanied with ~10% of the saturated β-aryl ketone (Table 3, entry 5). Indeed, re-subjection of pure β-boryl ketone 4d to the reaction conditions (22 °C, 14 h) leads to >98% conversion to the saturated ketone. It is plausible that protonation of the C–B bond occurs via the borate derived from addition of MeOH, followed by intramolecular protonation of the C–B bond. Thus, the more electrophilic boron atoms of a (pinacolato)boron unit adjacent to an electron-withdrawing aryl substituent, where polarization inherent in the protonation process might be better stabilized, are expected to be more prone towards participating in this undesired reaction pathway. In support of the significance of an electron-withdrawing unit to the degree of proto-deboration, BCA leading to the formation of p-fluoroaryl 4k is typically accompanied with <2% of the saturated ketone (stronger hyperconjugative electron donation by F vs Br). Finally, it is also possible that a small amount (corresponding to the catalyst loading) of proto-deboration is caused by the tetrafluoroboron counter-ion of the imidazolinium salt. See: Nave S, Sonawane RP, Elford TG, Aggarwal VK. J Am Chem Soc. 2010;132:17096–17098. doi: 10.1021/ja1084207.Lennox AJJ, Lloyd-Jones GC. J Am Chem Soc. 2012 doi: 10.1021/ja300236k. doi:10/1021/ja300236k.
- 15.Use of excess dbu, while substantially improving the yield of isolated β-boryl ketones, in some cases increases the amount of proto-deboration product as well (e.g., 4% vs 28% and 10 vs 21% protodeboration for 4l and 4d with 20 mol % and 100 mol % dbu, respectively). The latter observation is congruent with the aforementioned proposal regarding the role of MeOH in causing the protonation of the C–B bond, since borate formation is expected to be more facile under more basic conditions.
- 16.Performing the reactions shown in Scheme 2 with 20 mol % dbu but at 50 °C (vs 22 °C) also leads to a more rapid rate of substrate consumption. The product mixture, however, contains larger amounts of the proto-deboration product. For example, under such conditions, 4d is obtained in 40% yield and 89:11 er along with ~60% of the saturated ketone.
- 17.Unlike acyclic enones, when 100 mol % dbu is used with reactions of α,β-unsaturated esters, somewhat lower conversion levels are observed. For instance, in the reaction to generate 6b, there is 87% conversion to the desired product along with 27% of the saturated carboxylic ester (vs >98% conv and a similar degree of proto-deboration).
- 18.Hirsch-Weil D, Abboud KA, Hong S. Chem Commun. 2010;46:7525–7527. doi: 10.1039/c0cc02211j.For one example of catalytic enantioselective (pinacolato)boron conjugate addition to an α,β-unsaturated N,N-dimethylamide, see: (b) Ref 4a.
- 19.As with the unsaturated esters, when 100 mol % dbu is used, the desired β-boryl amides are formed with less efficiency (larger degree of ester formation and similar amounts of proto-deboration).
- 20.For examples of chelation between a carbonyl group and a β-boron, influencing the course of a catalytic process, see: Sandrock DL, Jean-Gerard L, Chen CY, Dreher SD, Molander GA. J Am Chem Soc. 2010;132:17108–17110. doi: 10.1021/ja108949w.Ohmura T, Awano T, Suginome M. J Am Chem Soc. 2010;132:13191–13193. doi: 10.1021/ja106632j.(c) Ref 5j.
- 21.Laitar DS, Tsui EY, Sadighi JP. J Am Chem Soc. 2006;128:11036–11037. doi: 10.1021/ja064019z. [DOI] [PubMed] [Google Scholar]
- 22.Bonet A, Lillo V, Ramírez J, Mar Diaz-Requejo M, Fernández E. Org Biomol Chem. 2009;7:1533–1535. doi: 10.1039/b901767d. [DOI] [PubMed] [Google Scholar]
- 23.Lillo V, Prieto A, Bonet A, Mar Díaz-Requejo M, Ramírez J, Pérez PJ, Fernández E. Organometallics. 2009;28:659–662. [Google Scholar]
- 24.Ibrahem I, Breistein P, Córdova A. Angew Chem, Int Ed. 2011;50:12036–12041. doi: 10.1002/anie.201105458. [DOI] [PubMed] [Google Scholar]
- 25.For examples of NHC-catalyzed homocoupling reaction, see: Burstein C, Glorius F. Angew Chem, Int Ed. 2004;43:6205–6208. doi: 10.1002/anie.200461572.Sohn SS, Rosen EL, Bode JW. J Am Chem Soc. 2004;126:14370–14371. doi: 10.1021/ja044714b.For NHC-catalyzed conversion of unsaturated aldehydes to the saturated ester derivatives, see: Chan A, Scheidt KA. Org Lett. 2005;7:905–908. doi: 10.1021/ol050100f.For examples of both types of processes, see: Sohn SS, Bode JW. Org Lett. 2005;7:3873–3876. doi: 10.1021/ol051269w.
- 26.For a recent study regarding NHC-catalyzed conjugate addition of alcohols to α,β-unsaturated ketones, see: Phillips EM, Riedrich M, Scheidt KA. J Am Chem Soc. 2010;132:13179–13181. doi: 10.1021/ja1061196.
- 27.In addition, control experiments, with chiral NHC excluded, indicate that BCA of the relatively more strained cyclic enones is promoted more readily by dbu (e.g., ~25% conv with cyclopentenone under the conditions used to synthesize 12 in Scheme 3). In contrast, there is ≤10% conversion under the same conditions with the acyclic substrates.
- 28.The absolute sense of enantioselectivity in NHC–catalyzed silyl conjugate additions (ref 9) is opposite to those illustrated in Scheme 3. The basis for this reversal of selectivity will be addressed in the upcoming account regarding the mechanism of these classes of transformations.
- 29.For catalytic hydroborations of alkynes, which involve Cu–B additions, see: Takahashi K, Ishiyama T, Miayura N. J Organomet Chem. 2001;625:47–52.Lee JE, Kwon J, Yun J. Chem Commun. 2008;44:733–734. doi: 10.1039/b716697d.Kim HR, Jung IG, Yoo K, Jang K, Lee ES, Yun J, Son SU. Chem Commun. 2010;46:758–760. doi: 10.1039/b919515g.Kim HR, Yun J. Chem Commun. 2011;47:2943–2945. doi: 10.1039/c0cc04496b.Jang H, Zhugralin AR, Lee Y, Hoveyda AH. J Am Chem Soc. 2011;133:7859–7871. doi: 10.1021/ja2007643.
- 30.DiRocco DA, Oberg KM, Rovis T. J Am Chem Soc. 2012;134:6143–6145. doi: 10.1021/ja302031v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.With 5.0 mol % catalyst loading (1.1 equiv B2(pin)2, 60 equiv MeOH, 22 °C, 14 h), the NHC-catalyzed reaction affords the desired β-boryl ketone product in 48% yield (54% conv) and in 95:5 er. The Josiphos–Cu-catalyzed BCA, under the same conditions, proceeds to 22% conversion to afford 4a in 15% yield and 85:15 er.
- 32.Treatment of benzaldehyde to the BCA conditions (with 5.0 mol % 3h without an α,β-unsaturated carbonyl in otherwise identical conditions as described for reaction is Table 3) leads to <5% conversion.
- 33.For Cu-catalyzed hydroborations of allenes, see: Jung B, Hoveyda AH. J Am Chem Soc. 2012;134:1490–1493. doi: 10.1021/ja211269w.
- 34.With 5.0 mol % catalyst loading (1.1 equiv B2(pin)2, 60 equiv MeOH, 22 °C, 14 h), the NHC-catalyzed reaction affords the desired β-boryl ketone product in 44% yield (52% conv) and in 95:5 er. The Josiphos–Cu-catalyzed BCA, under the same conditions, proceeds to >98% conversion to afford 4a in 15% yield and 85:15 er.
- 35.In one case involving a sterically demanding o-tolyl-substituted substrate (enone 2b in entry 3, Table 3), NHC-catalyzed BCA is performed at 50 °C for optimal results. However, with 100 mol % dbu (vs 20 mol % used in the reaction in Table 3), this transformation can be performed at ambient temperature to afford 4b in 93% yield (>98% conv) and 88:12 er.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.