Abstract
Tumor cell vasculogenic mimicry (VM) describes the functional plasticity of aggressive cancer cells forming de novo vascular networks, thereby providing a perfusion pathway for rapidly growing tumors -- transporting fluid from leaky vessels and/or connecting with endothelial-lined vasculature. The underlying induction of VM appears to be related to hypoxia, which may also promote the plastic, transendothelial phenotype of tumor cells capable of VM. Since its introduction in 1999 as a novel paradigm for melanoma tumor perfusion, many studies have contributed new insights into the underlying molecular pathways supporting VM in a variety of tumors, including melanoma, glioblastoma, carcinomas, and sarcomas. In particular, critical VM modulating genes are associated with vascular (VE-cadherin, EphA2, VEGFR1), embryonic/stem cell (Nodal, Notch4), and hypoxia-related (HIF, Twist1) signaling pathways. Each of these pathways warrants serious scrutiny as potential therapeutic, vascular targets and diagnostic indicators of plasticity, drug resistance and the aggressive metastatic phenotype.
Keywords: Vasculogenic Mimicry, Melanoma, Vascular Targets, Nodal, Notch
Background
Vasculogenic mimicry defined
Cancer deaths primarily result from metastases that are resistant to conventional therapies. Indeed, the accepted tenant underlying tumor survival has been that a blood supply is required to sustain growth and to metastasize (1). This important premise ignited the field of neoplastic angiogenesis research, which focused on targeting endothelial cells forming the neovasculature of growing tumors, and served as the major organizing principle for drug discovery and development, and clinical trials. However, the disappointing results of the angiogenesis inhibitor trials, together with new findings generated from sophisticated animal models of human tumor progression, have given us novel insights into the molecular mechanisms underlying the perfusion of tumors, particularly those expressing the aggressive metastatic phenotype. One of the new paradigms that has emerged, called “vasculogenic mimicry”, also referred to as “vascular mimicry” (VM), describes the de novo formation of perfusable, matrix-rich, vasculogenic-like networks by aggressive tumor cells in 3-D matrices in vitro, which parallels matrix-rich networks in patients’ aggressive tumors (2). The initial morphological and molecular characterization of VM was made in human melanoma where the tumor cells were shown to co-express endothelial and tumor markers, and formed channels, networks, and tubular structures that are rich in laminin, collagens IV and VI, and heparin sulfate proteoglycans, containing plasma and red blood cells -- indicating a perfusion pathway for rapidly growing tumors, as well as an escape route for metastasis (2–4). Interestingly, these findings agree with very early reports by others suggesting the perfusion of melanoma tumors via non-endothelial-lined channels (5). Since the introduction of VM, a plethora of studies have contributed mechanistic insights into the induction, formation and targeting of VM across a variety of cancers, including: melanoma; sarcomas (Ewing, mesothelial, synovial, osteosarcoma, alveolar rhabdomyosarcoma); carcinoma(s) of the breast, ovary, lung, prostate, bladder and kidney; gliomas, glioblastoma, and astrocytoma (reviewed in 6–8). From the extensive literature across this vast field, we now appreciate that the tumor vasculature is highly complex and can be derived from a variety of sources, including angiogenic vessels, co-option of pre-existing vessels, intussusceptive microvascular growth, mosaic vessels lined by both tumor cells and endothelium, post-natal vasculogenesis, and VM (9,10). Furthermore, recent studies have shown the tumor origin of endothelial-like cells in specific cancers (11,12), thus confounding our strategies for targeting a genetically unstable and heterogeneous vasculature.
Underlying plastic phenotype
Tumor cells capable of VM exhibit a high degree of plasticity indicative of a multipotent phenotype similar in many respects to embryonic stem cells (4, 13, 14). Molecular profiling of the tumor cell VM phenotype has revealed highly upregulated genes associated with embryonic progenitors, endothelial cells, vessel formation, matrix remodeling, and hypoxia; and downregulated genes generally associated with the respective, lineage-specific phenotype, such as in the case of melanoma where several melanocyte-lineage genes are suppressed (14). Confirmation of these genes was achieved by laser capture microdissection and microgenomics profiling of living melanoma cells versus endothelial cells forming vascular networks, where the expression of specific angiogenesis-related genes in melanoma resembled that of normal endothelial cells (15). In addition, we confirmed that plastic tumor cells express key pluripotent stem cell markers. However, unlike normal embryonic progenitors, these tumor cells lack major regulatory checkpoints resulting in the aberrant activation of embryonic signaling pathways -- such as Nodal and Notch, which underlies their stem cell-like phenotype, unregulated growth, and aggressive behavior (16).
Functional relevance of VM
The presence of VM in patients’ tumor tissues has been associated with a poor clinical outcome and suggests a possible advantage imparted by VM with respect to the survival of the aggressive tumor cell phenotype. Indeed, experimental evidence has shown a physiological perfusion of blood between endothelial-lined mouse vasculature and VM networks in human tumor xenografts using Doppler imaging of microbead circulation (17). Additional studies identified the anti-coagulant properties of tumor cells which line VM networks -- discussed under vascular pathways. Thus, VM can provide a functional perfusion pathway for rapidly growing tumors, by transporting fluid from leaky vessels and/or connecting with endothelial-lined vasculature. A remarkable example of VM functional plasticity was achieved by transplanting human metastatic melanoma cells into a circulation-deficient mouse limb, which resulted in the formation of a human melanoma-mouse endothelial chimeric neovasculature (18). Subsequent to the restoration of blood flow to the limb, the tumor cells formed a large tumor mass. Thus, this study highlighted the powerful influence of the microenvironment on the transendothelial differentiation of melanoma cells which reverted to a tumorigenic phenotype as the environmental cues changed.
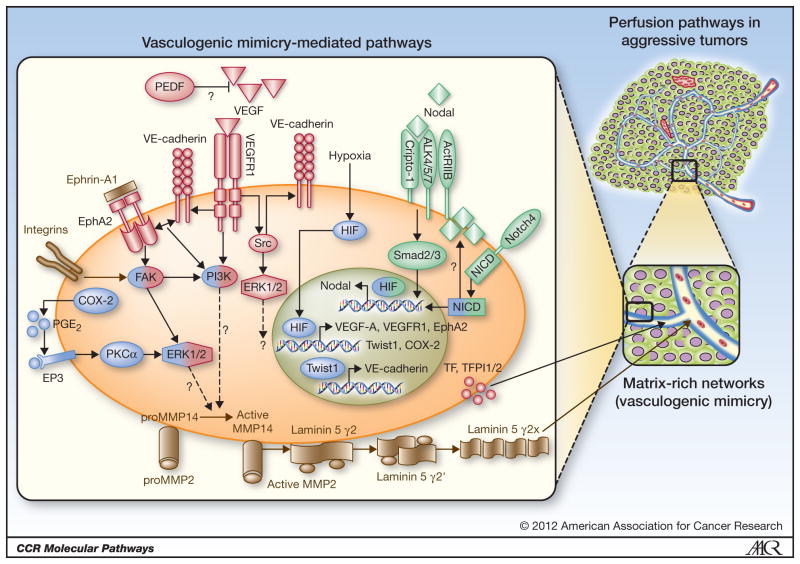
The multipotent phenotype underlying VM is supported by a complex co-option of signaling pathways that are normally restricted to developmental or cell-specific lineages. In particular, critical VM modulating genes can be categorized into pathways associated with vascular, embryonic/stem cell, and hypoxia signaling (Figure 1). Although a myriad of genes associated with VM have been reported, this review will focus on these three pathways that have overarching effects on the VM phenotype, are capable of modulating each other, and have the greatest potential for therapeutic intervention based on rigorous scientific scrutiny.
Figure 1.
Schematic model of signaling pathways implicated in tumor cell vasculogenic mimicry (VM). Only signaling molecules which have been specifically modulated using antisense oligonucletides, small inhibitory RNAs, blocking antibodies, small molecule inhibitors, or transient transfections are depicted -- demonstrating their ability to directly affect VM, and are categorized as vascular (red), embryonic/stem cell (green), and hypoxia signaling pathways (blue). Molecules shaded with two different colors demonstrate overlap between major VM signaling pathways. Proteins in grey play a quintessential role in VM formation and matrix remodeling (21). Question marks indicate the potential involvement of a protein and/or downstream effector proteins in modulating VM in aggressive cancer cells - - where the underlying signaling pathway(s) are not as yet clearly defined. (A portion of this schematic model was used in our previous publication; 9)
Vascular Signaling Pathways
One of the first vascular-associated genes shown to be involved in VM is VE-cadherin (CDH5). VE-cadherin is a transmembrane glycoprotein of the cadherin family that promotes homotypic cell-cell interactions and is considered specific for vascular endothelia and critical for vasculogenic events. Interestingly, VE-cadherin is expressed in aggressive, but not non-aggressive melanoma cells, and knockdown of VE-cadherin expression inhibits VM (19). VE-cadherin regulates erythropoietin-producing hepatocellular carcinoma-A2 (EphA2) activity by mediating its ability to become phosphorylated through interactions with its membrane bound ligand, Ephrin-A1 (20). Phosphorylated EphA2 subsequently activates PI3K, up-regulates matrix metalloproteinase (MMP)14 expression, and activates MMP2. Both MMP14 and MMP2 promote the cleavage of laminin 5γ2-chain into promigratory γ2′ and γ2x fragments which in turn stimulate migration, invasion, and VM in melanoma cells (21). In addition, VE-cadherin expression and activity are enhanced by binding of the transcription factor Twist1 to the VE-cadherin promoter; whereas downregulation of Twist1 expression leads to decreases in VE-cadherin, MMP2, and MMP9 expression and VM formation in human hepatocellular carcinoma cells (22).
Vascular endothelial growth factor (VEGF)-A is one of a family of five angiogenic growth factors that plays a crucial role in tumor angiogenesis by recruiting and stimulating the proliferation of endothelial cells in avascular regions of rapidly growing tumors. VM is dependent on the autocrine production of VEGF-A in melanoma (23); while, in ovarian cancer cells, VEGF-A treatment promotes VM by upregulating the expression of VE-cadherin, EphA2, and MMP2 and MMP9, demonstrating that VEGF-A can stimulate tumor cell plasticity (24). In mammary and pancreatic islet carcinoma cells, inhibiting EphA2 expression and/or activity leads to a decrease in VEGF expression and VEGF-induced angiogenesis in vivo (25, 26). This suggests that depending on the tumor cell type, VEGF signaling or EphA2 activation may provide the initiating event in VM formation, though further studies are needed to specifically address this possibility. In addition to EphA2, cyclooxygenase-2 (COX-2) can also upregulate VEGF expression in a variety of tumor cell types via activation of protein kinase C (PKC; 27). COX-2 catalyzes the conversion of arachadonic acid into prostaglandin H2, which is subsequently converted into primarily prostaglandin E2 (PGE2). COX-2 and PGE2 are up-regulated in aggressive cancers and are associated with a decrease in cellular apoptosis and an increase in tumor proliferation, invasion and angiogenesis. These processes are mediated by the family of prostanoid receptors (EP1-4), which activate epidermal growth factor receptor (EGFR)-mediated signaling, as well as PKC-dependent activation of ERK1/2 (28). Invasive breast cancer cells that express high levels of COX-2 form VM networks while knockdown of COX-2 expression or catalytic activity inhibits VM, which can be rescued with PGE2 (29, 30). Interestingly, EP3, but not EP4, has been shown to regulate VM network formation by aggressive inflammatory breast cancer cells; however, the underlying signaling pathway(s) by which COX-2/PGE2/EP3 promote(s) VM and possibly VEGF expression requires further study.
VEGF receptor tyrosine kinases (VEGFR1 and VEGFR2) bind VEGF-A in an autocrine or paracrine manner and demonstrate diverse signaling capacities (VEGF-A has a high binding affinity to VEGFR1, but weak kinase activity) and many VEGFR1 interacting signal transducers have been identified. For example, endothelial cell differentiation and organization into vascular tubes is thought to involve VEGFR1 activation of the PI3K/Akt pathway, whereas cancer cell invasion and migration have been shown to involve VEGFR1 activation of Src and ERK1/2 pathways (31). VEGFR1, but not VEGFR2, mediates VEGF-A induced VM in melanoma cells and it has been postulated that VM is mediated through the synergistic transduction of VEGF-A/VEGFR1/PI3K/PKCα and integrin signaling pathways (25). Moreover, a subpopulation of melanoma cells (ABCB5+ tumor-initiating cells) has been identified that preferentially expresses VEGFR1 and VEGFR1-mediated signaling, critical in VM, laminin production, and rapid tumor growth (32).
Pigment epithelium-derived factor (PEDF) is a multi-functional, secreted glycoprotein that is part of the non-inhibitory family of serine protease inhibitors. PEDF has direct (via suppressing growth by promoting tumor differentiation and apoptosis) and indirect (via suppressing angiogenesis by inducing apoptosis via FasL expression and inhibiting VEGF signaling through VEGFR1) anti-tumor effects (reviewed in 33). PEDF is expressed on melanocytes and non-aggressive melanoma cells, and is downregulated in aggressive melanoma (34). Knockdown of PEDF expression in non-aggressive melanoma cells induces VM, suggesting a linkage to molecular plasticity. Although the functional roles of PEDF are well characterized, the underlying signaling pathways, with respect to inhibiting VM, remain to be elucidated.
Tissue factor (TF), TF pathway inhibitor 1 (TFPI-1) and TFPI-2 are quintessential genes that initiate and regulate coagulation pathways, and all three of these genes are upregulated in aggressive melanoma cells (17). The procoagulant function of TF was shown to be regulated by TFPI-1 and is thought to contribute to the fluid-conducting potential in VM networks. However, while TFPI-2 did not inhibit TF coagulation, it was found to be required for VM tubular network formation, presumably through matrix remodeling, since inhibition of TFPI-2 expression suppressed MMP-2 activity. These studies suggest a novel mechanism for matrix-associated TFPI-2 in VM.
Embryonic/Stem Cell Pathways
Nodal signaling pathways are important regulators of human embryonic pluripotency and vertebrate embryonic development (35, 36). Nodal is a growth factor of the Transforming Growth Factor-β (TGFβ) superfamily that binds Cripto-1 and activates type I and type II activin-like kinase receptors (ALK4/5/7 and ActRIIB, respectively), which subsequently propagates canonical signaling via Smad2/3. This embryonic pathway has been shown to participate in tumor progression and aggressive tumor cell behavior, including VM (16, 37, 38). Treatment of aggressive melanoma cells with a function-blocking anti-Nodal antibody reduces their ability to engage in VM, implicating Nodal as an important regulator of tumor cell plasticity and the transendothelial phenotype (38). During embryonic development, Nodal expression is influenced by Notch (39). The Notch family consists of four transmembrane receptors (Notch1-4), whose signaling pathways are critical regulators of vertebrate embryogenesis. Notch signaling is initiated by binding of a Notch ligand, which induces a series of cleavages that generate the release of the Notch intracellular domain (NICD) that translocates to the nucleus and regulates the expression of a number of context-dependent targets, including Nodal (38–40). Inhibition of Notch4 function downregulates Nodal and VE-cadherin expression and impairs VM network formation by aggressive melanoma cells (16). Since Notch4 functions primarily in vascular development (40) and is enriched in the subpopulation of melanoma cells that form VM (16), Notch4-Nodal signaling may represent a master regulator of VM.
Hypoxia/Hypoxia-Reoxygenation Signaling Pathways
Hypoxia, either persistent or transient, is a hallmark of most tumors and has been shown to regulate pathways in the maintenance of the stem cell-like phenotype, cellular differentiation, invasion, metastasis, apoptotic resistance, genomic instability, angiogenesis and VM. Molecularly, protein stabilization and nuclear localization of HIF-1α/HIF-2α transcription factors and binding to hypoxia response elements (HREs) in promoter and enhancers of effector genes occurs in response to low oxygen, oncogenes or inactivated tumor suppressor genes (reviewed in 41). Hypoxia has been shown to induce VM in hepatocellular carcinoma (42), Ewing sarcoma (43), and melanoma (44). Moreover, hypoxia can induce a dedifferentiated phenotype in breast carcinoma (45). Pertinent to VM, hypoxia has been shown to either directly modulate VEGF-A, VEGFR, EphA2, Twist, Nodal, and COX-2 gene expression (via HIF-1/HRE binding), or indirectly modulate VE-cadherin and TF expression (via activation of an intermediary protein). Hypoxia can also modulate the expression of Notch-responsive genes; specifically, hypoxia stabilizes the NICD protein which interacts with HIF-1α and activates genes with Notch-responsive promoters, including Nodal (46, 47). This non-canonical crosstalk between HIF-1α and Notch signaling pathways is thought to promote an undifferentiated cell state, further illuminating the possible etiology of tumor cell plasticity underlying VM. Based on the numerous studies demonstrating hypoxia-induced VM and/or VM-associated genes, it is conceivable that therapeutic use of anti-angiogenic agents may promote tumor plasticity and metastatic progression.
Clinical-Translational Advances
The plastic phenotype of aggressive tumor cells has presented a significant challenge in the detection and targeting of the transdifferentiated endothelial phenotype -- manifested as VM. Although the premise of suppressing a tumor’s blood supply by targeting endothelial cells forming angiogenic vessels seems strategically viable, experimental evidence indicates that hypoxia induced by depriving tumors of oxygen promotes invasion and metastasis (48) as well as VM. Moreover, the lackluster results of cumulative clinical trials with angiogenesis inhibitors further demonstrate the need for new therapeutic strategies based on recent scientific breakthroughs. Other avenues of investigation have tested the effects of the classical angiogenesis inhibitors Endostatin and TNP-470 on melanoma VM in a side-by-side comparison with normal endothelial cells, and shown that these inhibitors do not suppress VM because the tumor cells lack the appropriate level of receptors for the inhibitors to act effectively (49). Furthermore, recent findings revealed that treatment of human breast cancer xenografts with Bevacizumab and Sunitinib resulted in intratumoral hypoxia and increased breast cancer stem cells (50). These basic observations should serve as a critical caveat in the development of new vascular-disrupting agents and/or combinatorial approaches. Certainly, the molecular pathways that have been experimentally identified as critically involved in VM serve as a strategic roadmap for drug development (shown in Figure 1), together with investigative observations generated from the testing of select FDA approved angiogenesis inhibitors (Table 1).
Table 1.
FDA approved angiogenesis inhibitors.
| Therapeutic Agents | Molecular Targets | Effect on VM |
|---|---|---|
| MAb Therapy | ||
| Bevacizumab (Avastin) | VEGF | No Effect* (12) |
| Cetuximab (Erbitux)) | EGFR | ND |
| Panitumumab (Vectibix) | EGFR | ND |
| Small Molecule TKIs | ||
| Sunitinib (Sutent) | VEGFRs, PDGFRβ, RET | ND |
| Sorafenib (Nevavar) | VEGFRs, PDGFRβ, Raf-1 | ND |
| Erlotinib (Tarceva) | EGFR | ND |
| Imatinib (Gleevec/Glivec) | TKI, Bcr-Abl | ND |
| Gefitinib (Iressa) | EGFR | ND |
| Pazopanib (Votrient, GW786034) | VEGFRs, PDGFRβ, c-Kit | ND |
| Lapantinib (Tykerb) | EGFR, HER2 | ND |
| Other Angiogenic Agents | ||
| Thalidomide (Thalomid) | TNFα, ROS producer | Inhibits VM (53) |
| TNP-470 (AGM-1470) | TKI | No Effect (49) |
| Endostatin (Endostar) | Integrin signaling | No Effect (49) |
| Rapamycin (Sirolimus) | mTOR, VEGF | Inhibits VM (54) |
Abbreviations: VM, vasculogenic mimicry; tyrosine kinase inhibitor, TKI; MAb, monoclonal antibody; ND, not determined
No effect on the ability of glioblastoma stem-like cells to transdifferentiate into endothelial-like precursors.
There is experimental evidence that specifically targeting pathways implicated in VM may have success in inhibiting tumor growth. Certainly, a handful of preclinical studies suggest that specific compounds affecting components of the previously described vascular, embryonic, or hypoxia pathways in tumor cells can inhibit VM formation in cancer models. For example, plant-derived compounds such as Genistein (an isoflavone found in soy) or Curcumin (derived from the spice turmeric) have been shown to reduce VM channel formation in melanoma cell lines in vitro and in vivo, concurrent with a downregulation of VE-cadherin, EphA2, or MMPs, depending on the agent (51, 52). Likewise, FDA approved angiogenesis inhibitors that have specifically suppressed tumor cell VM include: Thalidomide (a teratogenic sedative and a reactive oxygen species producer that targets TNFα) and Rapamycin (an inhibitor of the mTOR pathway and VEGF), coincident with a downregulation of VEGF, MMPs, and HIF-1α (53, 54).
Simultaneous targeting of the Notch and VEGF pathways may provide a more viable combinatorial approach to target cancer stem cells with anti-VEGF therapy. In glioblastoma, VM likely occurs independently of VEGF expression since VM network formation by mouse glioblastoma cell lines was not inhibited in vitro or in vivo by an anti-VEGF antibody (55). Similarly, treatment of glioblastoma cells with Bevacizumab had no effect on the ability of the stem-like subpopulation to transdifferentiate into endothelial-like progenitor cells, but the gamma-secretase inhibitor, DAPT, could block this phenomenon (12). Combined gamma-secretase and Bevacizumab treatment of xenografted glioblastoma and fibrosarcoma cell lines resulted in a significant inhibition of tumor growth compared with either treatment alone, predominantly through blockade of Dll4-Notch signaling (56). Since global gamma-secretase inhibitors can be toxic to intestinal cells, the use of antibodies to specific components of the Notch pathway may prove more desirable. Certainly, combined anti-Dll4 and anti-VEGF treatments in xenografted MV-522 cells had a greater impact on tumor angiogenesis and tumor growth than either alone (57). Our work has shown that treatment of Notch4-expressing aggressive melanoma cell lines with anti-Notch4 antibodies reduces VM formation in vitro, likely via regulation of Nodal expression (16). It is exciting to speculate that in vivo combinatorial therapy of anti-Notch4 or anti-Nodal plus VEGF inhibitors might simultaneously target tumor angiogenesis and VM in melanoma, and potentially other cancers. Certainly, as many have wisely advised, multiple signaling pathways should be targeted in a combinatorial manner to overcome tumor cell plasticity, drug resistance, neoplastic angiogenesis and metastasis (58, 59).
The emerging data on embryonic pathways, such as Nodal and Notch signaling pathways that are reactivated in aggressive tumor cells, may provide valuable new therapeutic targets that exploit the convergence of embryonic and tumorigenic signaling. Suppression of these master plasticity pathways results in the inhibition of VM, tumorigenicity and the reversion of the stem cell-like phenotype to that of a differentiated cell type (16, 37). Going forward, it is noteworthy to mention that VM can be used as a reliable predictor of tumor cell aggressiveness and as a preclinical screen for vascular-disrupting agents (7, 49, 60). Adding this assay to the armamentarium of current predictive tests for clinical application could provide valuable insights regarding the effective targeting of tumor cell plasticity.
Conclusion
Tumor cell VM illustrates the functional plasticity of the aggressive cancer phenotype, and serves as a selective advantage for rapidly growing tumors in need of perfusion. VM can provide one of several sources for a tumor’s blood supply that can directly or indirectly interact with other vasculature. The underlying induction of VM appears to be related to hypoxia, which may also promote the plastic, transendothelial phenotype of tumor cells capable of VM. Since its introduction in 1999 as a novel paradigm for tumor perfusion, many studies have contributed new insights into the underlying molecular pathways supporting VM. Each of these pathways warrants serious scrutiny as potential therapeutic targets and diagnostic indicator(s) of plasticity, drug resistance and the aggressive metastatic phenotype.
Acknowledgments
Much of the work cited in this review was supported by National Institutes of Health grants R37CA59702, RO1CA121205, and U54CA143869 (MJH). Given the space limitations of the review, the authors sincerely apologize for their inability to cite everyone who has contributed to this field of inquiry.
Footnotes
Conflict of Interest: All authors declare no conflict of interest.
References
- 1.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LMG, Pe’er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am J Pathol. 1999;155:739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folberg R, Hendrix MJC, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–81. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix MJC, et al. Molecular classification of cutaneous malignant melanoma by gene expression: Shifting from a continuous spectrum to distinct biologic entries. Nature. 2000;406:536–40. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 5.Warren BA, Shubik P. The growth of the blood supply to melanoma transplants in the hamster cheek pouch. Lab Invest. 1966;15:464–78. [PubMed] [Google Scholar]
- 6.Paulis YWJ, Soetekouw PM, Verheul HMW, Tjan-Heijnen VCG, Griffioen AW. Signalling pathways in vasculogenic mimicry. Biochim Biophys Acta. 2010;1806:18–28. doi: 10.1016/j.bbcan.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Hendrix MJC, Seftor EA, Hess AR, Seftor REB. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–21. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 8.El Hallani S, Boisselier B, Peglion F, Rousseau A, Colin C, Ibdaih A, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133:973–82. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dome B, Hendrix MJC, Paku S, Tovari J, Timar J. Alternative vascularization mechanisms in cancer: Pathology and therapeutic implications. Am J Pathol. 2007;170:1–15. doi: 10.2353/ajpath.2007.060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–8. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 12.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Gever A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 13.Bissell MJ. Tumor plasticity allows vasculogenic mimicry, a novel form of angiogenic switch. Am J Pathol. 1999;155:675–79. doi: 10.1016/S0002-9440(10)65164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendrix MJC, Seftor EA, Hess AR, Seftor RE. Molecular plasticity of human melanoma cells. Oncogene. 2003;22:3070–75. doi: 10.1038/sj.onc.1206447. [DOI] [PubMed] [Google Scholar]
- 15.Demou Z, Hendrix MJC. Microgenomics profile of the endogenous angiogenic phenotype in subpopulations of aggressive melanoma. J Cell Biochem. 2008;105:562–73. doi: 10.1002/jcb.21855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy KM, Kirschmann DA, Seftor EA, Margaryan NV, Postovit LM, Strizzi L, et al. Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype. Cancer Res. 2010;70:10340–50. doi: 10.1158/0008-5472.CAN-10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruf W, Seftor EA, Petrovan RJ, Weiss RM, Gruman LM, Margaryan NV, et al. Differential role of tissue factor pathway inhibitors 1 and 2 (TFPI-1 and 2) in melanoma vasculogenic mimicry. Cancer Res. 2003;63:5381–9. [PubMed] [Google Scholar]
- 18.Hendrix MJC, Seftor REB, Seftor EA, Gruman LM, Lee LM, Nickoloff B, et al. Transendothelial function of human metastatic melanoma cells: Role of the microenvironment in cell-fate determination. Cancer Res. 2002;62:665–8. [PubMed] [Google Scholar]
- 19.Hendrix MJC, Seftor EA, Meltzer PS, Gardner LMG, Hess AR, Kirschmann DA, et al. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: Role in vasculogenic mimicry. Proc Natl Acad Sci USA. 2001;98:8018–23. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess AR, Margaryan NV, Seftor EA, Hendrix MJC. Deciphering the signaling events that promote melanoma tumor cell vasculogenic mimicry and their link to embryonic vasculogenesis: Role of the Eph receptors. Dev Dynam. 2007;236:3283–96. doi: 10.1002/dvdy.21190. [DOI] [PubMed] [Google Scholar]
- 21.Seftor REB, Seftor EA, Gardner LMG, Bilban M, Koshikawa N, Meltzer PS, et al. Cooperative interactions of Laminin5γ2, MMP-2 and MT1-MMP are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322–7. [PubMed] [Google Scholar]
- 22.Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW, Che N, et al. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology. 2010;51:545–56. doi: 10.1002/hep.23311. [DOI] [PubMed] [Google Scholar]
- 23.Vartanian A, Stepanova E, Grigorieva I, Solomko E, Baryshnokov A, Lichinitser M. VEGFR1 and PKCα signaling control melanoma vasculogenic mimicry in a VEGFR2 kinase-independent manner. Melanoma Res. 2011;21:91–8. doi: 10.1097/CMR.0b013e328343a237. [DOI] [PubMed] [Google Scholar]
- 24.Wang JY, Sun T, Zhao XL, Zhang SW, Zhang DF, Gu Q, et al. Functional significance of VEGF-A in human ovarian carcinoma: role in vasculogenic mimicry. Cancer Biol Ther. 2008;7:758–66. doi: 10.4161/cbt.7.5.5765. [DOI] [PubMed] [Google Scholar]
- 25.Cheng N, Brantley D, Fang WB, Hua L, Fanslow W, Cerretti DP, et al. Inhibition of VEGF-dependent multistage carcinogenesis by soluble EphA receptors. Neoplasia. 2003;5:445–56. doi: 10.1016/s1476-5586(03)80047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brantley-Sieders DM, Fang WB, Hwang Y, Hicks D, Chen J. Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res. 2006;66:10315–24. doi: 10.1158/0008-5472.CAN-06-1560. [DOI] [PubMed] [Google Scholar]
- 27.Luo H, Chen Z, Jin H, Zhuang M, Wang T, Su C, et al. Cyclooxygenase-2 up-regulates vascular endothelial growth factor via a protein kinase C pathway in non-small cell lung cancer. J Exp Clin Canc Res. 2011;30:6–10. doi: 10.1186/1756-9966-30-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu WKK, Sung JJY, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett. 2010;295:7–16. doi: 10.1016/j.canlet.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Basu GD, Liang WS, Stephan DA, Wegener LT, Conley CR, Pockaj BA, et al. A novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cells. Breast Cancer Res. 2006;8:R69. doi: 10.1186/bcr1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson FM, Simeone A-M, Lucci A, McMurray JS, Ghosh S, Cristofanilli M. Differential regulation of the aggressive phenotype of inflammatory breast cancer cells by prostonoid receptors EP3 and EP4. Cancer. 2010;116:2806–14. doi: 10.1002/cncr.25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welch L. Signal transduction by vascular endothelia growth factor receptors. Biochem J. 2011;437:169–83. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 32.Frank NY, Schatton T, Kim S, Zhan Q, Wilson BJ, Ma J, et al. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer Res. 2011;71:1474–85. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoshina D, Abe R, Shimizu H. The role of PEDF in tumor growth and metastasis. Curr Mol Med. 2010;10:292–5. doi: 10.2174/156652410791065327. [DOI] [PubMed] [Google Scholar]
- 34.Orgaz JL, Ladhani O, Hoek KS, Fernandez-Barral A, Mihic D, Aguilera O, et al. Loss of pigment epithelium-derived factor enables migration, invasion, and metastatic spread of human melanoma. Oncogene. 2009;28:4147–61. doi: 10.1038/onc.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schier AF, Shen MM. Nodal signaling in vertebrate development. Nature. 2000;403:385–9. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- 36.Brown S, Teo A, Pauklin S, Hannan N, Cho CH, Lim B, et al. Activin/Nodal signaling controls divergent transcriptional networks in human embryonic stem cells and in endoderm progenitors. Stem Cells. 2011;8:1176–85. doi: 10.1002/stem.666. [DOI] [PubMed] [Google Scholar]
- 37.Topczewska JM, Postovit L-M, Margaryan NV, Sam A, Hess AR, Wheaton WW, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–32. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 38.Strizzi L, Postovit L-M, Margaryan NV, Lipavsky A, Gardiot J, Blank C, et al. Nodal as a biomarker for melanoma progression and a new therapeutic target for clinical intervention. Expert Rev Dermatol. 2009;4:67–78. doi: 10.1586/17469872.4.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strizzi L, Hardy KM, Seftor EA, Costa FF, Kirschmann DA, Seftor REB, et al. Development and cancer: At the crossroads of nodal and notch signaling. Cancer Res. 2009;69:7131–4. doi: 10.1158/0008-5472.CAN-09-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocrine Rev. 2007;28:339–63. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 41.De Bock K, Mazzone M, Carmeliet P. Antiantiogenic therapy, hypoxia, and metastasis: risky liansons, or not? Nat Rev Clin Oncol. 2011;8:393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- 42.Ma JL, Han SX, Zhu Q, Zhao J, Zhang D, Wang L, et al. Role of Twist in vasculogenic mimicry formation in hypoxic hepatocellular carcinoma cells in vitro. Biochem Biophys Res Commun. 2011;408:686–91. doi: 10.1016/j.bbrc.2011.04.089. [DOI] [PubMed] [Google Scholar]
- 43.van der Schaft DWJ, Hillen F, Pauwels P, Kirschmann DA, Castermans K, oude Egbrink MGA, et al. Tumor cell plasticity in Ewing sarcoma: an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005;65:11520–28. doi: 10.1158/0008-5472.CAN-05-2468. [DOI] [PubMed] [Google Scholar]
- 44.Zhang S, Li M, Zhang D, Xu S, Wang X, Liu Z, et al. Hypoxia influences linearly patterned programmed cell necrosis and tumor blood supply patterns formation in melanoma. Lab Invest. 2009;89:575–86. doi: 10.1038/labinvest.2009.20. [DOI] [PubMed] [Google Scholar]
- 45.Helcynska K, Kronblad A, Jogi A, Nilsson E, Beckman S, Landberg G, et al. Hypoxia promotes a dedifferentiated phenotype in ductal breast carcinoma in situ. Cancer Res. 2003;63:1441–4. [PubMed] [Google Scholar]
- 46.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–28. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 47.Quail DF, Taylor MJ, Walsh LA, Dieters-Castator D, Das P, Jewer M, et al. Low oxygen levels induce the expression of the embryonic morphogen Nodal. Mol Biol Cell. 2011;22:4809–21. doi: 10.1091/mbc.E11-03-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steeg PS. Angiogenesis inhibitors: motivators of metastasis? Nat Med. 2003;9:822–3. doi: 10.1038/nm0703-822. [DOI] [PubMed] [Google Scholar]
- 49.van der Schaft DWJ, Seftor RE, Seftor EA, Hess AR, Gruman LM, Kirschmann DA, et al. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J Natl Cancer Inst. 2004;96:1473–7. doi: 10.1093/jnci/djh267. [DOI] [PubMed] [Google Scholar]
- 50.Conley SJ, Gheordunescu E, Kakarala P, Newman B, Korkaya H, Heath AN, et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cong R, Sun Q, Yang L, Gu H, Zeng Y, Wang B. Effect of Genistein on vasculogenic mimicry formation by human uveal melanoma cells. J Exp Clin Cancer Res. 2009;28:124–30. doi: 10.1186/1756-9966-28-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L-X, He Y-J, Zhao S-Z, Wu J-G, Wang J-T, Zhu L-M, et al. Inhibition of tumor growth and vasculogenic mimicry by curcumin through downregultaion of the EphA2/PI3K/MMP pathway in a murine choroidal melanoma model. Cancer Biol Ther. 2011;11:229–35. doi: 10.4161/cbt.11.2.13842. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Li M, Gu Y, Liu Z, Xu S, Cui Y, et al. Thalidomide influence growth and vasculogenic mimicry channel formation in melanoma. J Exp Clin Cancer Res. 2008;27:60–9. doi: 10.1186/1756-9966-27-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su M, Feng Y-J, Yao L-Q, Cheng M-J, Xu C-J, Huang Y, et al. Plasticity of ovarian cancer cell SKOV3ip and vasculogenic mimicry in vivo. Int J Gynecol Cancer. 2008;18:476–86. doi: 10.1111/j.1525-1438.2007.01034.x. [DOI] [PubMed] [Google Scholar]
- 55.Soda Y, Marumoto T, Friedmann-Morvinski D, Soda M, Liu F, Michiue H, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci USA. 2011;108:4274–80. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J-L, Sainson RCA, Oon CE, Turley H, Leek R, Sheldon H, et al. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res. 2011;71:6073–83. doi: 10.1158/0008-5472.CAN-11-1704. [DOI] [PubMed] [Google Scholar]
- 57.Ridgway J, Zhang G, Wu Y, Stawicki S, Lian W-C, Chanthery Y, et al. Inhibition of DII4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–7. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 58.Smalley KSM, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–44. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 59.Ebos JML, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–21. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao L, Marshall ES, Kelland LR, Baguley BC. Evidence for the involvement of p38 MAP kinase in the action of the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) Invest New Drugs. 2007;25:271–6. doi: 10.1007/s10637-006-9029-0. [DOI] [PubMed] [Google Scholar]