Abstract
Carbon monoxide (CO) is an endogenously produced gas resulting from the degradation of heme by heme oxygense or from fatty acid oxidation. Heme oxygenase (HO) enzymes are constitutively expressed in the kidney (HO-2) and HO-1 is induced in the kidney in response to several physiological and pathological stimuli. While the beneficial actions of HO in the kidney have been recognized for some time, the important role of CO in mediating these effects has not been fully examined. Recent studies using CO inhalation therapy and carbon monoxide releasing molecules (CORMs) are demonstrating that increases in CO alone can be beneficial to the kidney in several forms of acute renal injury by limiting oxidative injury, decreasing cell apoptosis, and promoting cell survival pathways. Renal CO is also emerging as a major regulator of renal vascular and tubular function acting to protect the renal vasculature against excessive vasoconstriction and to promote natriuresis by limiting sodium reabsorption in tubule cells. Within this review, recent studies on the physiological actions of CO in the kidney will be explored as well as the potential therapeutic avenues that are being developed targeting CO in the kidney which may be beneficial in diseases such as acute renal failure and hypertension.
Keywords: Heme oxygenase, renal failure, blood pressure, bilirubin, acute renal injury
Introduction
Throughout human history, carbon monoxide (CO) has been considered a toxic environmental gas. Inhalation of as little of 800 parts per million (ppm) can be fatal due to its binding to hemoglobin which renders it incapable of carrying oxygen. While the toxicity of environmental CO is widely known, we have only recently begun to appreciate the important role for endogenously formed CO in regulating physiological processes in organs such as the kidney. CO is formed in the body from the catabolism of heme by heme oxygenase (HO) enzymes as well as the oxidation of lipids. It is estimated that the production rate of CO is 384 μmoles/day in the body with an average CO concentration in the tissues in the nanomolar range [1]. In the kidney, the primary HO enzymes responsible for the generation of CO are the constitutively expressed HO-2 isoform and the inducible HO-1 isoform. It is difficult to discuss CO and the kidney without mentioning these important enzymes as manipulation of the levels of the HO proteins has been a major strategy in altering the levels of CO in the kidney. Although HO enzymes have been greatly studied in the kidney under normal and pathological conditions, the role of CO in mediating the response to experimental manipulations of HO protein is not clear. The purpose of this review is to outline the important role of CO in the regulation of renal function and in protecting the kidney from injury as well as to highlight areas that may pose new opportunities for the development of novel therapeutic targets for the treatment of kidney disease.
CO Signaling in the Kidney
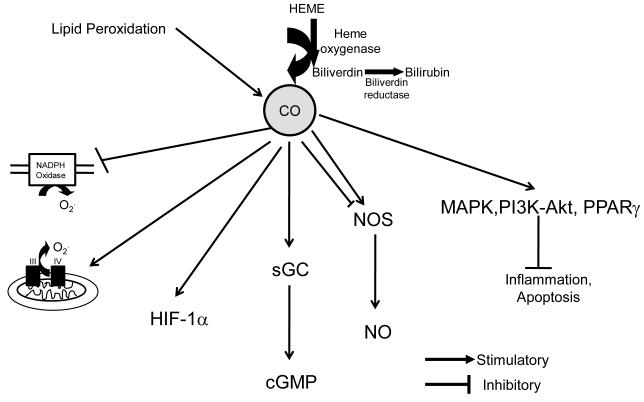
Endogenously produced CO can signal in the cell through multiple pathways as outlined in Figure 1. One important signaling pathway is the direct binding of CO to metal containing proteins such as soluble guanylate cyclase (sGC), cytochrome P450 proteins, cytochrome c oxidase, NADPH oxidase, and nitric oxide synthase [2]. The binding of CO to these proteins is able to confer a conformational change which can alter their biological activity [3]. Perhaps the most significant signaling pathway for CO in the kidney is the activation of sGC resulting in the increase in cGMP levels in the kidney. Several studies have demonstrated an important role for cGMP in the regulation of both vascular and tubular function in the kidney [4,5,6,7]. A second important signaling pathway for CO is through p38 mitogen-activated protein kinase (MAPK) pathway which is thought to mediate the cytoprotective, anti-inflammatory, and anti-apoptotic effects of CO [8,9,10]. Another emerging signaling pathway for CO is modulation of reactive oxygen species (ROS) formation. CO can either decrease or increase ROS formation depending on cell compartment where it is acting. For instance, CO in the cytosol inhibits the activity of NADPH oxidase which lowers superoxide production in the cell [11,12,13,14,15]. This decrease in superoxide can have several effects in the kidney including: decreasing sodium reabsorption in the tubules as well as increasing the bioavailability of nitric oxide to promote vasodilatation of the renal vasculature [16,17]. In contrast, CO in the mitochondria can promote superoxide [11,18]. The increase in mitochondrial superoxide production results from CO binding to complex IV proteins which allows for electrons to accumulate in complex III which drives their reaction with oxygen forming superoxide [19]. This increase in superoxide release from the mitochondria can induce superoxide dismutases which convert the superoxide to hydrogen peroxide (H2O2) which can drive further cell signaling [20]. Finally, CO can also signal through other signaling cascades such as PI3K-Akt, peroxisome proliferator-activated receptor γ (PPAR-γ), and hypoxia-inducible factor 1 α (HIF-1α) [10,21,22,23].
Figure 1.
Carbon monoxide (CO) signaling in the kidney. Carbon monoxide is mainly generated through metabolism of heme by heme oxygenase as well as a by-product of lipid peroxidation. CO mainly signals through activation of soluble guanylate cyclase (sGC) which increase cellular cGMP levels. CO can also inhibit cystolic NADPH oxidase to limit superoxide formation (02.) while promoting superoxide formation in mitochondria. CO has both stimulatory as well as inhibitory actions on nitric oxide (NO) and inhibits inflammation and apoptosis via activation of mitogen activated protein kinase (MAPK), Phosphoinositide 3-kinase (PI3K)/Atk and peroxisome proliferator-activated receptor γ (PPAR-γ).
CO and Kidney Injury
Cisplatin Nephrotoxicity
Cisplatin (CP) is a very effective anti-cancer drug used in the treatment of cancers of the testis, ovary, head and neck, bladder, lung, cervix and endometrium [24,25,26]. However, its use is frequently complicated by acute renal injury that often develops as a result of its use in humans. CP accumulates throughout the nephron with highest concentrations observed in the S3 segment of the proximal tubule and the thick ascending limb of Henle (TALH). These two regions of the nephron are also the most susceptible to CP-induced nephrotoxicity [27]. Despite various hydration protocols devised to minimize nephrotoxicity, 25-35% of patients experience a significant decline in renal function following CP treatment.
HO-1 induction has previously been demonstrated to limit the extent of CP-induced tubular cell damage in both in vitro and in vivo models [28,29,30]. Whether the protective effect of HO-1 induction is due to the actions of CO or bilirubin, is not known. One way to determine if increases in CO alone can protect the kidney against CP induced nephrotoxicity is to increase renal CO levels using carbon monoxide releasing molecules (CORMs). CORMs are newly developed compounds which have the ability to release CO in vivo [31,32,33,34]. The first generation of CORMs were designed with a metal containing carbonyl group to which the CO was bound [31,32,33]. The first generation CORMs have several caveats which one needs to take into account before their use including: 1) the fast release time of CO at physiological pH which occurs with a half-life between 1-5 minutes, 2) the metal group of these compounds can result in induction of HO-1 which can lead to further increases in CO and bilirubin independent of the actions of the CORMs [35], 3) some of these CORMs are not water soluble and must be delivered in an organic vehicle such as DMSO. However, a second generation of CORMs has been created in which the CO is bound to a boron atom instead of a transition metal. These CORMS offer several advantages of the first generation compounds including 1) slower release of CO with a half-life of 20 min, 2) since these compounds lack a metal groups they do not induce HO-1 in vivo, 3) they are easily soluble in water. The ability of an intraperitoneal (ip) injection of CORM-A1 to increase blood carboxyhemoglobin (COHb) levels in mice is summarized in Table 1. CORM-A1 administration at 7.5 mg/kg resulted in a significant increase in blood COHb levels for up to 45 min post administration; whereas, administration at 5 mg/kg resulted in a slight increase in blood COHb levels at 30 minutes following administration.
Table 1. Blood COHb levels following interpertional administration of CORM-A1 in mice.
Blood COHb levels were measured by absorbance at 420 and 432nm. Percentage COHb was calculated from the A420/A432 ratio using previously published molar absorptivities of mouse hemoglobin, F1=1.301, F2=0.464, F3=2.053 [79]. CORM-A1 when administered at a concentration of 7.5 mg/kg resulted in a significant increase in blood COHb levels for up to 45 min post administration, n=6/group. * = P <0.05 as compared to baseline, iCORM-A1=inactive CORM-A1.
| CORM-A1 Concentration |
Blood COHb (Percentage of baseline) |
||||
|---|---|---|---|---|---|
| Time Post Administration (min) | |||||
| 15 | 30 | 45 | 60 | 120 | |
| 7.5mg/kg | 147±10* | 134±1* | 118±1* | 108±2 | 105±8 |
| 5mg/kg | 105±11 | 112±8 | 94±5 | 90±4 | 94±5 |
| 3mg/kg | 107±3 | 105±8 | 88±6 | 93±15 | 89±4 |
| 1.5mg/kg | 105±12 | 102±7 | 100±10 | 100±3 | 100±2 |
| iCORM-A1 (7.5 mg/kg) | 101±10 | 93±7 | 93±9 | 90±2 | 100±8 |
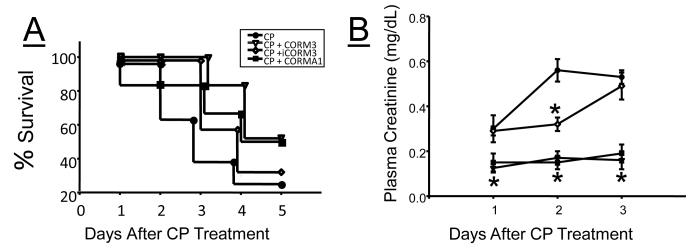
Our group tested whether increasing CO via treatment with CORMs protects against cisplatin nephrotoxicity. Mice were treated with either CORM-3, a first generation CORM (40 mg/kg), or CORM-A1 (5 mg/kg) a second generation CORM prior to exposure to a single dose of cisplatin (20 mg/kg) and daily for 5 days. Survival rates of 60%, after 3 days and 25% after 5 days were observed in vehicle treated mice (Figure 2A). Treatment with CORM-3 or CORM-A1 resulted in an improvement in survival with a 100% and 83% survival rate after 3 days and a 50% survival rate 5 days after cisplatin (CP) treatment in each group respectively (Figure 2A). Treatment of mice with the inactive CORMs, iCORM-3 was associated with a 100% survival rate at 3 days; however, the survival rate after 5 days was only 29 % (Figure 2A). The increased survival rate in CORM-3 and CORM-A1 treated mice was associated with a significantly attenuated increase in plasma creatinine as compared to vehicle and iCORM-3 treated mice (Figure 2B). Our results with CORM-3 were similar to results of CP nephrotoxicity in the rat [36]. In this study, treatment of rats with CORM-3 prior to the administration of CP prevented the increase in plasma creatinine and completely protected the kidney against CP induced tubular apoptosis, necrosis, cellular desquamation and vacuolization [36]. The anti-apoptotic effects of CO in CP nephrotoxicity were believed to be mediated via increases in cGMP since blockade with a sGC inhibitor prevented CORM-3 from attenuating caspase-3 activation in cultured renal tubular cells [36]. The results of these studies highlight the potential use of CO donors or CO inhalation therapy to protect the kidney against CP induced nephrotoxicity by limiting renal tubule cell apoptosis.
Figure 2.
Effect of carbon monoxide releasing molecules (CORMs), CORM-3, and CORM-A1 on mortality and plasma creatinine in C57BL/6J mice treated with cisplatin. A) Kaplan–Meier survival curve of mice after injection of cisplatin (CP) and subsequent treatment with vehicle, CORM-3, inactive CORM-3 (iCORM-3), and CORM-A1 (n=8). Treatment with CORM-3 and CORM-A1 increased the survival rate as compared to untrated (CP) and iCORM-3 treated mice. B) Plasma creatinine levels in mice following CP treatment. Treatment with CORM-3 and CORM-A1 significantly blunted the increase in plasma creatinine as compared to CP treated mice, n=8. *= statistically significant P< 0.05 as compared to CP treated mice.
Renal Transplantation & Ischemic Renal Failure
There are many factors that contribute to renal allograft rejection including: ischemia-reperfusion injury and immune rejection of the transplanted kidney. Several studies have demonstrated a pivotal role for CO in protecting transplanted kidneys from both of these potential complications. The first line of evidence suggesting the CO may be beneficial to the kidney during ischemia-reperfusion arose from transplantation studies utilizing CO inhalation therapy [37,38,39]. In these studies, CO was administered via inhalation for a little as one hour at a concentration of 250 ppm or continuously at a concentration of 20 ppm for a period of 30 days following transplantation [37,39]. CO inhalation therapy was associated with improvements in renal cortical blood flow, creatinine clearance, preservation of glomerular vascular structures and podocyte viability, decreased tubular cell apoptosis, and significant lengthening of survival by 2.5 fold as compared to animals breathing standard room air [39,40]. The protective role for CO in ischemia-reperfusion injury has also been examined using CORMs as a means of delivering CO. Pretreatment of mice with CORM-3 one hour before the onset of 40 minutes of renal ischemia-reperfusion resulted in a significant reduction in the rise in plasma creatinine measured 24 hours after recovery [35]. Interestingly, administration of CORM-3 after the ischemic period did not have the same effect. CORM-3 administration was also associated with a decrease in renal tubule cell injury and excretion of neutrophil gelatinase–associated lipocalin (NGAL), a marker of renal tubule cell injury [35]. Although CORM-3 can induce HO-1, blockade of HO activity with a specific HO inhibitor did not significantly alter the ability of CORM-3 to lower plasma creatinine and limit renal injury in this model [35]. All of the protective actions of CORM-3 on renal function and structure occurred without any significant increase in blood COHb levels which can occur during CO inhalation therapy especially at higher levels of CO [37].
CO has also been demonstrated to modulate the immune response following ischemia-reperfusion. The hallmarks of the inflammatory response following ischemia-reperfusion injury are activation of the innate immune system leading to the activation of the adaptive alloimmune response as well as macrophage infiltration. In a rat transplantation model, CO inhalation limits both the activation of CD4+ T-cells and alloreactive T-cells and to also decrease ED1(+) macrophage infiltration [37,41]. CO has also been demonstrated to reduce the level of inflammatory cytokines such as interferon-γ, interleukin (IL)-6, and tumor necrosis factor (TNF)-α [37,40]. In addition to its anti-inflammatory properties, CO also has significant anti-fibrotic actions through inhibition of the extracellular regulated kinase (ERK)-MAPK and TGF-β pathways [40].
Another area in which CO has been demonstrated to be beneficial is in protection against cold storage induced injury of the kidney. Cold storage-induced organ injury is a major determinant of post transplant organ dysfunction. Clinical data has demonstrated that kidney biopsies of cold stored allografts postreperfusion exhibited greater levels of apoptosis as compared to allografts obtained from living-related donors suggesting that reperfusion of cold stored organs may be associated with increased levels of apoptotic cell death [42]. First, studies in cultured proximal tubule cells demonstrated that treatment with the CO donor, CORM-3, decreased the amount of apoptosis and lactate dehydrogenase (LDH) release in cells pretreated before 48 hours of cold storage at 4°C followed by 18 hours of rewarming at 37°C [43]. Similar results were observed when whole kidneys were perfused with a Celsior solution supplemented with CORM-3 prior to cold storage at 4°C [44]. Kidneys treated with CORM-3 prior to cold storage displayed higher perfusion flow rate (PFR), glomerular filtration rate, and reabsorption rates of sodium and glucose as compared to control kidneys flushed with Celsior solution alone after transplantation [44]. In a separate study, administration of CORM-3 improved renal blood flow and creatinine clearance when administered immediately prior to renal implantation after 18 hours of cold storage [45]. While the protective mechanism of CO in cold induced renal failure is not known, experimental evidence suggest that the protective actions of CO may be mediated through stimulation of guanylate cyclase, HIF-1 and vascular endothelial growth factor (VEGF) [23,44].
The experimental evidence demonstrating the protective effects of both CO inhalation therapy and CORMs in renal ischemia-reperfusion injury associated with transplantation is strong raising the possibility that CO therapy could be used to protect against ischemia-reperfusion injury and consequently delayed graft function; however, this data has not resulted in the widespread adaptation of CO therapy to protect against ischemia reperfusion injury following transplantation. A recent case report described the successful transplantation outcome despite greater than 24 hours of ischemia in a kidney from donor who succumbed to CO poisoning [46]. Although the blood COHb levels in CO poisoning victims are clearly too high for clinical use, the potential benefit of CO therapy in renal transplantation merits further investigation to determine a safe level of CO inhalation can be achieved in potential organ donors to limit ischemia-reperfusion injury of transplanted organs.
CO, the Kidney and Blood Pressure Regulation
CO and the Renal Vasculature
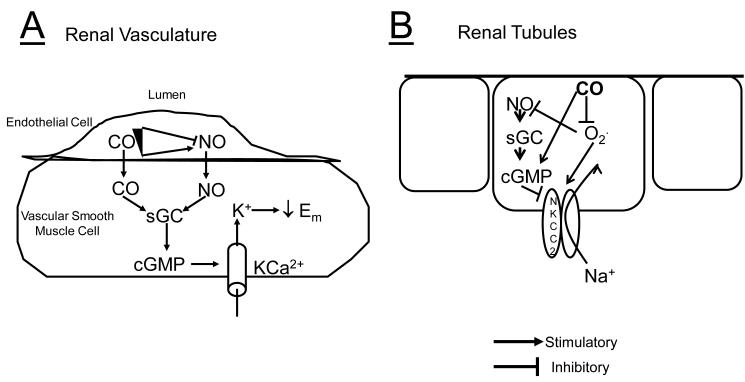
The role of CO in the regulation of renal vascular tone is controversial. Several studies using CO gas, CORMs, HO inducers or HO inhibitors have demonstrated that CO is a vasodilator of the renal circulation [4,5,47,48]. While the effects of CO in the renal vasculature under basal conditions may be considered modest, increases in CO are important to protect the renal vasculature from excessive vasoconstriction due to agents such as angiotensin II, phenylephrine, and 20-Hydroxyeicosatetrenoic acid (20-HETE) [5,47]. CO has also been demonstrated to be the major dilatory agent of the renal microcirculation in response to blockade of nitric oxide (NO) production [49]. CO is believed to result in vasodilatation ultimately through activation of large conductance calcium-activated potassium (KCa) channels either directly or as a result of stimulation of sGC resulting in the increase levels of cGMP (Figure 3A) [4,50,51,52,53]. Renal blood flow remains constant to the kidney despite fluctuations in blood pressure through autoregulation of renal blood flow. The role of CO in the renal autoregulatory response is an emerging area. Recent studies have suggested that induction of HO-1 in the kidney reduces renal autoregulation (decreases constriction of the afferent arteriole) and that this effect is reversed by inhibition of HO-1 [54]. Interestingly, the attenuation of renal autogulation can be restored by CO but not biliverdin following HO inhibition suggesting that CO derived from renal tubular sources may be an important modulator of afferent arteriolar vasoconstriction in vivo [54,55]. However, because autoregulation occurs via two distinct mechanisms, myogenic constriction and tubuloglomerular feedback (TGF), the effect of CO on autoregulation may be due to effects on TGF via CO acting on renal tubular cells.
Figure 3.
Schematic diagram summarizing the effect of CO on A) the renal vasculature and B) renal tubules. In the vasculature, CO can cause vasodilatation through increases in cGMP via stimulation of soluble guanylate cyclase (sGC). The increase in cGMP levels results in activation of the large conductance calcium activated potassium channel (KCa). Both CO and NO can also directly activate KCa channels. Low levels of CO also stimulate NO which leads to activation of KCa via increases in cGMP. However, high levels of CO can inhibit NO production in the vasculature and cause vasoconstriction. In renal tubules, CO inhibits superoxide (02.) production to decrease the activity of the sodium-potassium-2 chloride transporter (NKCC2) in the thick ascending loop of Henle (TALH). Superoxide also decreases NO levels. NO is an endogenous inhibitor of NKCC2 via cGMP mediated decreases in apical insertion of the transporter. CO may also have a direct effect on the NKCC2 transporter to decreases its activity resulting in a decrease in sodium reabsorption in the TALH.
Although CO can be an important dilator of the renal circulation, it has also been reported to act as a vasoconstrictor as well. The vasoconstrictor actions of CO result primarily from the unusual relationship that it has with NO in the vasculature. Low levels of CO have been reported to enhance NO production and NO has been reported to induce HO-1 [56,57]. On the other hand, inhibition of NO stimulates CO production and an increase in CO via overexpression of HO-1 in vascular smooth muscle cells decreases NO production [58,59]. Recently, it was demonstrated that treatment of rat interlobular arteries with the CO donor, CORM-3, resulted in vasoconstriction due to increases in cellular superoxide production [60]. Additional studies in angiotensin II hypertensive mice demonstrated that although induction of HO-1 lowered blood pressure, it did not normalize and actually resulted in further impairment of acetylcholine induced NO relaxation [61]. Therefore, it appears that CO can be both a constrictor and dilator in the renal vasculature depending on the balance between the levels of HO/CO and NO.
CO and Renal Tubule Function
The first reports of the effect of CO on thick ascending loop of Henle (TALH) function were performed in isolated, perfused tubules and TALH cells obtained from rats fed a high potassium diet [62,63]. In these studies, CO was found to stimulate the apical 70-pS K+ channel by patch clamp analysis in isolated TALH cells. Further studies in isolated perfused TALH tubules demonstrated that incubation with chromium mesoporphyrin (CrMP), an inhibitor of HO, resulted in a decrease in sodium and water reabsorption [63]. These studies would suggest that increases in CO would result in increases in sodium and water reabsorption in the TALH promoting fluid retention and increases in blood pressure. However, studies in whole animals have not supported a pro-hypertensive role for HO/CO in the kidney. First, intravenous administration of the HO inducer, heme, which raised urinary CO levels, resulted in increase sodium and water excretion [64]. Second, chronic intrarenal medullary interstitial infusion of CrMP, which decrease renal CO levels as measured by in vivo microdialysis–oxyhemoglobin CO trapping, resulted in decreased sodium excretion and increased blood pressure in rats fed normal and high salt diets [65]. Third, direct induction of HO-1 in the renal medulla with cobalt protoporphyrin (CoPP) significantly attenuated the development of angiotensin II-dependent hypertension in mice [66]. Last, low level of CO inhalation 60 ppm for 2 hours a day, was demonstrated to reduce blood pressure in angiotensin II infused low-density lipoprotein (LDL) receptor knockout mice [67]. The reason for the discrepancy between the results of the initial in vitro studies and the later in vivo studies is not known but may be due to the differences in approaches (in vitro vs. in vivo) or the different diets of the animals studies (high potassium versus normal and high salt).
The major signaling pathway for CO is believed to be mediated by stimulation of sGC resulting in increased levels of cGMP in the kidney. Both CO production as well as cGMP levels are increased by elevations in renal perfusion pressure [6,65,68]. Recent studies have also determined the important role of each of these molecules in mediating increases in sodium excretion in response to increases in renal perfusion pressure (pressure-natriuresis); however, the direct link between increased levels of CO and cGMP in the kidney has not been established. Studies with acute administration of CORMs and chronic induction of renal HO-1 with hemin in deoxycorticosterone acetate (DOCA)-salt treated rats have documented increases in urinary and tissue cGMP levels but the effects of chronic increases in CO alone on cGMP levels in the kidney have not been examined [48,69]. One major limitation in delineating the relationship between renal cGMP and CO is the ability to specifically increase renal CO levels chronically without inducing HO-1. CORMs are a potential solution to the problem; however, they exhibit a very short half-life which makes chronic infusion problematic [34].
One emerging aspect of CO signaling which may be important to the regulation of sodium reabsorption in the kidney is its affect on the generation of reactive oxygen species via cytosolic and mitochondrial sources (Figure 3B) [18]. Studies in immortalized, cultured, thick ascending loop of Henle (TALH) cells have demonstrated the ability of CO to directly attenuate angiotensin II-mediated superoxide production [15]. Angiotensin II is known to increase cellular superoxide production via stimulation of NAD(P)H oxidase [70]. NAD(P)H oxidase mediated increases in superoxide production have also been reported to increase sodium reabsorption in the thick ascending loop of Henle directly and through decreases in the bioavailability of NO [71,72]. The effects of CO on angiotensin II-mediated superoxide production in cultured TALH cells are similar to those observed in primary cultures of rat TALH cells where overexpression of HO-1 was demonstrated to limit angiotensin II induced oxidative injury [73]. The decrease in NAD(P)H oxidase mediated superoxide production in the thick ascending loop of Henle by CO may mediate the anti-hypertensive actions of HO induction in the kidney in models that exhibit increased levels of cytosolic superoxide production such as angiotensin II dependent and deoxycorticosterone acetate (DOCA)-salt hypertension [66,74].
CO may also regulate sodium reabsorption in the kidney via actions on membrane insertion of transport proteins. For example, recent studies have highlighted an important role for cGMP in the regulation of apical levels of the Na/K/2Cl co-transporter (NKCC2) in the TALH [75,76]. It is hypothesized that increased levels of cGMP result in the increased turnover of cAMP via activation of cGMP stimulated phosphodiesterase 2 (PDE2) which in turn decreases the insertion of the NKCC2 channel into the apical membrane, a process that is regulate by cAMP [77]. Whether CO can sufficiently increase cGMP levels in the TALH to activate PDE2 and decrease apical NKCC2 levels is not known. In the proximal tubule, cGMP has also been demonstrated to alter the activity of the Na/Pi cotransporter by decreasing insertion in the brush-border membrane [78]; however, the ability of CO to mimic this effect has not been investigated.
CO and the Kidney: Where do we go from here?
As the evidence for the beneficial effects of CO in the kidney accumulates, we are faced with the daunting task of translating basic experimental findings into clinical application. Clearly this is a significant challenge as we move into the next decade of research. There are currently several significant limitations that must be overcome in order to convert what we have learned about the beneficial actions of CO in the kidney into routine clinical practice. The first is a paradigm shift from considering CO as a toxic gas when present at high levels to a potential therapeutic gas when delivered at lower levels. The most intriguing approach for immediate clinical application would be CO inhalation therapy. One advantage of CO inhalation therapy is that it could be easily implemented in both clinical and non-clinical settings. One could imagine patients being treated chronically at home using accurate, dose-limited inhalation systems. However, much work regarding the appropriate concentration of CO as well as duration of treatment is needed. The second potential therapeutic avenue of CO rests in the development of CORMs that could be developed into practical drugs. Currently, CORMS are limited in the ability to deliver them chronically at therapeutic doses as well as the ability to have them become specifically activated in vivo. Both of these areas represent significant hindrances to the development of CORMs as pharmacological tools to deliver CO to patients in a non-clinical setting.
Much of what we currently know about the affects of CO on the kidney is derived from studies in which the levels of HO-1 have been altered. Thus, alterations of renal HO levels offer another potential therapeutic approach to exploiting the therapeutic actions of CO. However, since HO enzymes also generate metabolites such as free iron and bilirubin, targeting HO could have additional benefits or create unwanted side-effects depending on the nature of the specific disease to be treated. CO signaling in the kidney is another area that can be utilized for the development of potential therapeutics in kidney disease. Activation of sGC, stimulation of NO production, inhibition of cytostolic superoxide production, stimulation of PI3K-Akt, are all examples of the pathways that could be targeted to mimic the action of CO in the kidney to protect the kidney. However, more research is needed to specifically identify which pathway(s) activated by CO would provide the most benefit to the kidney in diseases such as acute renal failure, diabetic nephropathy, and hypertension.
Acknowledgements
This work was supported in part by an Institutional Research Award from the University of Mississippi Medical Center and by a grants from the National Heart, Lung, and Blood Institute HL088421, and HL088421-S1 (D.E.S.), and PO1HL-5197.
References
- [1].Coburn RF. Endogenous carbon monoxide production and body CO stores. Acta Med. Scand. Suppl. 1967;472:269–282. doi: 10.1111/j.0954-6820.1967.tb12633.x. [DOI] [PubMed] [Google Scholar]
- [2].Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu. Rev. Pharmacol. Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- [3].Roberts GP, Youn H, Kerby RL. CO-sensing mechanisms. Microbiol. Mol. Biol. Rev. 2004;68:453–473. doi: 10.1128/MMBR.68.3.453-473.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ryan MJ, Jernigan NL, Drummond HA, McLemore GR, Jr., Rimoldi JM, Poreddy SR, Gadepalli RS, Stec DE. Renal vascular responses to CORM-A1 in the mouse. Pharmacol. Res. 2006;54:24–29. doi: 10.1016/j.phrs.2006.01.012. [DOI] [PubMed] [Google Scholar]
- [5].Kaide JI, Zhang F, Wei Y, Jiang H, Yu C, Wang WH, Balazy M, Abraham NG, Nasjletti A. Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. J. Clin. Invest. 2001;107:1163–1171. doi: 10.1172/JCI11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jin XH, Siragy HM, Carey RM. Renal interstitial cGMP mediates natriuresis by direct tubule mechanism. Hypertension. 2001;38:309–316. doi: 10.1161/01.hyp.38.3.309. [DOI] [PubMed] [Google Scholar]
- [7].Jin XH, McGrath HE, Gildea JJ, Siragy HM, Felder RA, Carey RM. Renal interstitial guanosine cyclic 3′, 5′-monophosphate mediates pressure-natriuresis via protein kinase G. Hypertension. 2004;43:1133–1139. doi: 10.1161/01.HYP.0000123574.60586.7d. [DOI] [PubMed] [Google Scholar]
- [8].Kim HP, Wang X, Zhang J, Suh GY, Benjamin IJ, Ryter SW, Choi AM. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: involvement of p38 beta MAPK and heat shock factor-1. J. Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- [9].Otterbein LE, Bach FH, Alam J, Soares M, Tao LH, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- [10].Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J. Biol. Chem. 2003;278:1248–1258. doi: 10.1074/jbc.M208419200. [DOI] [PubMed] [Google Scholar]
- [11].Taille C, El Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J. Biol. Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- [12].Matsumoto H, Ishikawa K, Itabe H, Maruyama Y. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction. Mol. Cell Biochem. 2006;291:21–28. doi: 10.1007/s11010-006-9190-y. [DOI] [PubMed] [Google Scholar]
- [13].Srisook K, Han SS, Choi HS, Li MH, Ueda H, Kim C, Cha YN. CO from enhanced HO activity or from CORM-2 inhibits both O2- and NO production and downregulates HO-1 expression in LPS-stimulated macrophages. Biochem. Pharmacol. 2006;71:307–318. doi: 10.1016/j.bcp.2005.10.042. [DOI] [PubMed] [Google Scholar]
- [14].Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J. Biol. Chem. 2007;282:1718–1726. doi: 10.1074/jbc.M607610200. [DOI] [PubMed] [Google Scholar]
- [15].Kelsen S, Patel BJ, Parker LB, Vera T, Rimoldi JM, Gadepalli RS, Drummond HA, Stec DE. Heme oxygenase attenuates angiotensin II-mediated superoxide production in cultured mouse thick ascending loop of Henle cells. Am. J. Physiol Renal Physiol. 2008;295:F1158–F1165. doi: 10.1152/ajprenal.00057.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am. J. Physiol Renal Physiol. 2005;288:F982–F987. doi: 10.1152/ajprenal.00348.2004. [DOI] [PubMed] [Google Scholar]
- [17].Mori T, Cowley AW., Jr. Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension. 2003;42:588–593. doi: 10.1161/01.HYP.0000091821.39824.09. [DOI] [PubMed] [Google Scholar]
- [18].Bilban M, Haschemi A, Wegiel B, Chin BY, Wagner O, Otterbein LE. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J. Mol. Med. 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- [19].Sandouka A, Balogun E, Foresti R, Mann BE, Johnson TR, Tayem Y, Green CJ, Fuller B, Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) modulate respiration in isolated mitochondria. Cell Mol. Biol. (Noisy. -le-grand) 2005;51:425–432. [PubMed] [Google Scholar]
- [20].Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol. Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- [21].Hoetzel A, Dolinay T, Vallbracht S, Zhang Y, Kim HP, Ifedigbo E, Alber S, Kaynar AM, Schmidt R, Ryter SW, Choi AM. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am. J. Respir. Crit Care Med. 2008;177:1223–1232. doi: 10.1164/rccm.200708-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tsoyi K, Ha YM, Kim YM, Lee YS, Kim HJ, Kim HJ, Seo HG, Lee JH, Chang KC. Activation of PPAR-gamma by Carbon Monoxide from CORM-2 Leads to the Inhibition of iNOS but not COX-2 Expression in LPS-Stimulated Macrophages. Inflammation. 2009;32:364–371. doi: 10.1007/s10753-009-9144-0. [DOI] [PubMed] [Google Scholar]
- [23].Faleo G, Neto JS, Kohmoto J, Tomiyama K, Shimizu H, Takahashi T, Wang Y, Sugimoto R, Choi AM, Stolz DB, Carrieri G, McCurry KR, Murase N, Nakao A. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation. 2008;85:1833–1840. doi: 10.1097/TP.0b013e31817c6f63. [DOI] [PubMed] [Google Scholar]
- [24].Alberts DS, Garcia D, Mason-Liddil N. Cisplatin in advanced cancer of the cervix: an update. Semin. Oncol. 1991;18:11–24. [PubMed] [Google Scholar]
- [25].Raghavan D. Management of advanced bladder cancer in the elderly. Urol. Clin. North Am. 1992;19:797–806. [PubMed] [Google Scholar]
- [26].Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur. J. Cancer. 1998;34:1522–1534. doi: 10.1016/s0959-8049(98)00224-x. [DOI] [PubMed] [Google Scholar]
- [27].Taguchi T, Nazneen A, Abid MR, Razzaque MS. Cisplatin-associated nephrotoxicity and pathological events. Contrib. Nephrol. 2005;148:107–121. doi: 10.1159/000086055. [DOI] [PubMed] [Google Scholar]
- [28].Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, Nick HS, Agarwal A. Heme oxygenase-1 gene ablation or expression modulates cisplatininduced renal tubular apoptosis. Am. J. Physiol Renal Physiol. 2000;278:F726–F736. doi: 10.1152/ajprenal.2000.278.5.F726. [DOI] [PubMed] [Google Scholar]
- [29].Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995;48:1298–1307. doi: 10.1038/ki.1995.414. [DOI] [PubMed] [Google Scholar]
- [30].Schaaf GJ, Maas RF, de Groene EM, Fink-Gremmels J. Management of oxidative stress by heme oxygenase-1 in cisplatin-induced toxicity in renal tubular cells. Free Radic. Res. 2002;36:835–843. doi: 10.1080/1071576021000005267. [DOI] [PubMed] [Google Scholar]
- [31].Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ. Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- [32].Motterlini R, Mann BE, Johnson TR, Clark JE, Foresti R, Green CJ. Bioactivity and pharmacological actions of carbon monoxide-releasing molecules. Curr. Pharm. Des. 2003;9:2525–2539. doi: 10.2174/1381612033453785. [DOI] [PubMed] [Google Scholar]
- [33].Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ. Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- [34].Motterlini R, Sawle P, Hammad J, Bains S, Alberto R, Foresti R, Green CJ. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 2005;19:284–286. doi: 10.1096/fj.04-2169fje. [DOI] [PubMed] [Google Scholar]
- [35].Vera T, Henegar JR, Drummond HA, Rimoldi JM, Stec DE. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J. Am. Soc. Nephrol. 2005;16:950–958. doi: 10.1681/ASN.2004090736. [DOI] [PubMed] [Google Scholar]
- [36].Tayem Y, Johnson TR, Mann BE, Green CJ, Motterlini R. Protection against cisplatin-induced nephrotoxicity by a carbon monoxide-releasing molecule. Am. J. Physiol Renal Physiol. 2006;290:F789–F794. doi: 10.1152/ajprenal.00363.2005. [DOI] [PubMed] [Google Scholar]
- [37].Neto JS, Nakao A, Kimizuka K, Romanosky AJ, Stolz DB, Uchiyama T, Nalesnik MA, Otterbein LE, Murase N. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am. J. Physiol Renal Physiol. 2004;287:F979–F989. doi: 10.1152/ajprenal.00158.2004. [DOI] [PubMed] [Google Scholar]
- [38].Nakao A, Neto JS, Kanno S, Stolz DB, Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE, Murase N. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am. J. Transplant. 2005;5:282–291. doi: 10.1111/j.1600-6143.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- [39].Neto JS, Nakao A, Toyokawa H, Nalesnik MA, Romanosky AJ, Kimizuka K, Kaizu T, Hashimoto N, Azhipa O, Stolz DB, Choi AM, Murase N. Low-dose carbon monoxide inhalation prevents development of chronic allograft nephropathy. Am. J. Physiol Renal Physiol. 2006;290:F324–F334. doi: 10.1152/ajprenal.00026.2005. [DOI] [PubMed] [Google Scholar]
- [40].Nakao A, Faleo G, Nalesnik MA, Seda-Neto J, Kohmoto J, Murase N. Lowdose carbon monoxide inhibits progressive chronic allograft nephropathy and restores renal allograft function. Am. J. Physiol Renal Physiol. 2009;297:F19–F26. doi: 10.1152/ajprenal.90728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Martins PN, Reutzel-Selke A, Jurisch A, Denecke C, Attrot K, Pascher A, Kotsch K, Pratschke J, Neuhaus P, Volk HD, Tullius SG. Induction of carbon monoxide in donor animals prior to organ procurement reduces graft immunogenicity and inhibits chronic allograft dysfunction. Transplantation. 2006;82:938–944. doi: 10.1097/01.tp.0000232716.91887.c5. [DOI] [PubMed] [Google Scholar]
- [42].Burns AT, Davies DR, McLaren AJ, Cerundolo L, Morris PJ, Fuggle SV. Apoptosis in ischemia/reperfusion injury of human renal allografts. Transplantation. 1998;66:872–876. doi: 10.1097/00007890-199810150-00010. [DOI] [PubMed] [Google Scholar]
- [43].Stec DE, Bishop C, Rimoldi JM, Poreddy SR, Vera T, Salahudeen AK. Carbon monoxide (CO) protects renal tubular epithelial cells against cold-rewarm apoptosis. Ren Fail. 2007;29:543–548. doi: 10.1080/08860220701391878. [DOI] [PubMed] [Google Scholar]
- [44].Sandouka A, Fuller BJ, Mann BE, Green CJ, Foresti R, Motterlini R. Treatment with CO-RMs during cold storage improves renal function at reperfusion. Kidney Int. 2006;69:239–247. doi: 10.1038/sj.ki.5000016. [DOI] [PubMed] [Google Scholar]
- [45].Bagul A, Hosgood SA, Kaushik M, Nicholson ML. Carbon monoxide protects against ischemia-reperfusion injury in an experimental model of controlled nonheartbeating donor kidney. Transplantation. 2008;85:576–581. doi: 10.1097/TP.0b013e318160516a. [DOI] [PubMed] [Google Scholar]
- [46].Bojakowski K, Gaciong Z, Grochowiecki T, Szmidt J. Carbon monoxide may reduce ischemia reperfusion injury: a case report of complicated kidney transplantation from a carbon monoxide poisoned donor. Transplant. Proc. 2007;39:2928–2929. doi: 10.1016/j.transproceed.2007.08.063. [DOI] [PubMed] [Google Scholar]
- [47].Kaide J, Zhang F, Wei Y, Wang W, Gopal VR, Falck JR, Laniado-Schwartzman M, Nasjletti A. Vascular CO counterbalances the sensitizing influence of 20-HETE on agonist-induced vasoconstriction. Hypertension. 2004;44:210–216. doi: 10.1161/01.HYP.0000135658.57547.bb. [DOI] [PubMed] [Google Scholar]
- [48].Arregui B, Lopez B, Garcia SM, Valero F, Navarro C, Fenoy FJ. Acute renal hemodynamic effects of dimanganese decacarbonyl and cobalt protoporphyrin. Kidney Int. 2004;65:564–574. doi: 10.1111/j.1523-1755.2004.00409.x. [DOI] [PubMed] [Google Scholar]
- [49].Botros FT, Navar LG. Interaction between endogenously produced carbon monoxide and nitric oxide in regulation of renal afferent arterioles. Am. J. Physiol Heart Circ. Physiol. 2006;291:H2772–H2778. doi: 10.1152/ajpheart.00528.2006. [DOI] [PubMed] [Google Scholar]
- [50].Wang R, Wang Z, Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br. J. Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang R, Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J. Biol. Chem. 1997;272:8222–8226. doi: 10.1074/jbc.272.13.8222. [DOI] [PubMed] [Google Scholar]
- [52].Wang R, Wu L, Wang Z. The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch. 1997;434:285–291. doi: 10.1007/s004240050398. [DOI] [PubMed] [Google Scholar]
- [53].Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ. Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Botros FT, Prieto-Carrasquero MC, Martin VL, Navar LG. Heme oxygenase induction attenuates afferent arteriolar autoregulatory responses. Am. J. Physiol Renal Physiol. 2008;295:F904–F911. doi: 10.1152/ajprenal.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ren Y, D’Ambrosio MA, Wang H, Liu R, Garvin JL, Carretero OA. Heme oxygenase metabolites inhibit tubuloglomerular feedback (TGF) Am. J. Physiol Renal Physiol. 2008;295:F1207–F1212. doi: 10.1152/ajprenal.90243.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am. J. Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- [57].Motterlini R, Green CJ, Foresti R. Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antioxid. Redox. Signal. 2002;4:615–624. doi: 10.1089/15230860260220111. [DOI] [PubMed] [Google Scholar]
- [58].Rodriguez F, Lamon BD, Gong W, Kemp R, Nasjletti A. Nitric oxide synthesis inhibition promotes renal production of carbon monoxide. Hypertension. 2004;43:347–351. doi: 10.1161/01.HYP.0000111721.97169.97. [DOI] [PubMed] [Google Scholar]
- [59].Imai T, Morita T, Shindo T, Nagai R, Yazaki Y, Kurihara H, Suematsu M, Katayama S. Vascular smooth muscle cell-directed overexpression of heme oxygenase-1 elevates blood pressure through attenuation of nitric oxide-induced vasodilation in mice. Circ. Res. 2001;89:55–62. doi: 10.1161/hh1301.092679. [DOI] [PubMed] [Google Scholar]
- [60].Lamon BD, Zhang FF, Puri N, Brodsky SV, Goligorsky MS, Nasjletti A. Dual pathways of carbon monoxide-mediated vasoregulation: modulation by redox mechanisms. Circ. Res. 2009;105:775–783. doi: 10.1161/CIRCRESAHA.109.197434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Stec DE, Vera T, McLemore GR, Jr, Kelsen S, Rimoldi JM, Gadepalli RS, Ryan MJ. Heme Oxygenase-1 Induction Does Not Improve Vascular Relaxation in Angiotensin II Hypertensive Mice. Am. J. Hypertens. 2008;21:189–193. doi: 10.1038/ajh.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Liu H, Mount DB, Nasjletti A, Wang W. Carbon monoxide stimulates the apical 70-pS K+ channel of the rat thick ascending limb. J. Clin. Invest. 1999;103:963–970. doi: 10.1172/JCI5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang T, Sterling H, Shao WA, Yan Q, Bailey MA, Giebisch G, Wang WH. Inhibition of heme oxygenase decreases sodium and fluid absorption in the loop of Henle. Am. J. Physiol Renal Physiol. 2003;285:F484–F490. doi: 10.1152/ajprenal.00135.2003. [DOI] [PubMed] [Google Scholar]
- [64].Rodriguez F, Kemp R, Balazy M, Nasjletti A. Effects of exogenous heme on renal function: role of heme oxygenase and cyclooxygenase. Hypertension. 2003;42:680–684. doi: 10.1161/01.HYP.0000085785.40581.1A. [DOI] [PubMed] [Google Scholar]
- [65].Li N, Yi F, Dos Santos EA, Donley DK, Li PL. Role of renal medullary heme oxygenase in the regulation of pressure natriuresis and arterial blood pressure. Hypertension. 2007;49:148–154. doi: 10.1161/01.HYP.0000250086.06137.fb. [DOI] [PubMed] [Google Scholar]
- [66].Vera T, Kelsen S, Stec DE. Kidney-specific induction of heme oxygenase-1 prevents angiotensin II hypertension. Hypertension. 2008;52:660–665. doi: 10.1161/HYPERTENSIONAHA.108.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kobayashi A, Ishikawa K, Matsumoto H, Kimura S, Kamiyama Y, Maruyama Y. Synergetic Antioxidant and Vasodilatory Action of Carbon Monoxide in Angiotensin II Induced Cardiac Hypertrophy. Hypertension. 2007;50:1040–1048. doi: 10.1161/HYPERTENSIONAHA.107.097006. [DOI] [PubMed] [Google Scholar]
- [68].Lieb DC, Kemp BA, Howell NL, Gildea JJ, Carey RM. Reinforcing feedback loop of renal cyclic guanosine 3′ 5′ -monophosphate and interstitial hydrostatic pressure in pressure-natriuresis. Hypertension. 2009;54:1278–1283. doi: 10.1161/HYPERTENSIONAHA.109.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jadhav A, Torlakovic E, Ndisang JF. Hemin therapy attenuates kidney injury in deoxycorticosterone acetate-salt hypertensive rats. Am. J. Physiol Renal Physiol. 2009;296:F521–F534. doi: 10.1152/ajprenal.00510.2007. [DOI] [PubMed] [Google Scholar]
- [70].Touyz RM, Schiffrin EL. Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension. 1999;34:976–982. doi: 10.1161/01.hyp.34.4.976. [DOI] [PubMed] [Google Scholar]
- [71].Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am. J. Physiol Renal Physiol. 2002;283:F957–F962. doi: 10.1152/ajprenal.00102.2002. [DOI] [PubMed] [Google Scholar]
- [72].Ortiz PA, Garvin JL. Interaction of O(2)(-) and NO in the thick ascending limb. Hypertension. 2002;39:591–596. doi: 10.1161/hy0202.103287. [DOI] [PubMed] [Google Scholar]
- [73].Quan S, Yang L, Shnouda S, Schwartzman ML, Nasjletti A, Goodman AI, Abraham NG. Expression of human heme oxygenase-1 in the thick ascending limb attenuates angiotensin II-mediated increase in oxidative injury. Kidney Int. 2004;65:1628–1639. doi: 10.1111/j.1523-1755.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- [74].Nath KA, d’Uscio LV, Juncos JP, Croatt AJ, Manriquez MC, Pittock ST, Katusic ZS. An Analysis of the DOCA-salt Model of Hypertension in HO-1-/- Mice and the Gunn Rat. Am. J. Physiol Heart Circ. Physiol. 2007 doi: 10.1152/ajpheart.00870.2006. [DOI] [PubMed] [Google Scholar]
- [75].Herrera M, Ortiz PA, Garvin JL. Regulation of thick ascending limb transport: role of nitric oxide. Am. J. Physiol Renal Physiol. 2006;290:F1279–F1284. doi: 10.1152/ajprenal.00465.2005. [DOI] [PubMed] [Google Scholar]
- [76].Ares GR, Caceres P, Alvarez-Leefmans FJ, Ortiz PA. cGMP decreases surface NKCC2 levels in the thick ascending limb: role of phosphodiesterase 2 (PDE2) Am. J. Physiol Renal Physiol. 2008;295:F877–F887. doi: 10.1152/ajprenal.00449.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ortiz PA. cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am. J. Physiol Renal Physiol. 2006;290:F608–F616. doi: 10.1152/ajprenal.00248.2005. [DOI] [PubMed] [Google Scholar]
- [78].Bacic D, Hernando N, Traebert M, Lederer E, Volkl H, Biber J, Kaissling B, Murer H. Regulation of the renal type IIa Na/Pi cotransporter by cGMP. Pflugers Arch. 2001;443:306–313. doi: 10.1007/s004240100695. [DOI] [PubMed] [Google Scholar]
- [79].Rodkey FL, Hill TA, Pitts LL, Robertson RF. Spectrophotometric measurement of carboxyhemoglobin and methemoglobin in blood. Clin. Chem. 1979;25:1388–1393. [PubMed] [Google Scholar]