Summary
The pharyngeal endoderm is hypothesized as the source of local signals that specify the identity of neural crest-derived mesenchyme in the arches. Sox9 is induced and maintained in pre-chondrogenic cells during condensation formation and endochondral ossification. Using explant culture we determined that pharyngeal endoderm was sufficient, but not necessary for specifying pre-chondrogenic identity, as surrounding tissues including the otic vesicle can compensate for signals from the pharyngeal endoderm. Multiple Fgf genes are expressed specifically in the pharyngeal endoderm subjacent to the neural crest-derived mesenchyme. FGF signaling is both sufficient and required for specification of Sox9 expression and specification of pre-chondrogenic identity, as demonstrated by the addition of recombinant FGF protein or the FGF receptor inhibitor (SU5402) to explanted tissue, respectively. However, FGF signaling cannot maintain Sox9 expression or initiate the chondrogenic program as indicated by the absence of Col2a1 transcripts. BMP4 signaling can induce and maintain Sox9 expression in isolated mesenchyme, but only in combination with FGF signaling induce Col2a1 expression, and thus, chondrogenesis. Given the spatio-temporal expression patterns of FGFs and BMPs in the pharyngeal arches, we suggest that this may represent a general mechanism of local signals specifying pre-chondrogenic identity and initiation of the chondrogenic program.
Keywords: BMP4, Col2a1, columella, condensation, chondrogenesis, development, endochondral, FGF, pharyngeal arch, Sox9, SU5402
Introduction
The mechanism involved in induction and patterning of neural crest (NC)-derived Hox-positive mesenchyme in the pharyngeal arches is an area of intense interest (Creuzet et al., 2005). Mesenchyme of the 2nd pharyngeal arch gives rise to several skeletal elements, including the retroarticular process, columella, posterior basihyoid and ceratobranchial elements of the tongue skeleton (Ruhin et al., 2003; Kulesa et al., 2004). NC migrates from the chick midbrain region at Hamburger and Hamilton (HH)9 (Hamburger and Hamilton, 1951) in an anterior to posterior wave, with the hyoid stream beginning its migrating from rhombomere 4 (r4) at approximately HH11 (Couly et al., 1992). Small r3 and r5 contributions, which do not undergo apoptosis, add to the anterior and posterior hyoid stream, respectively (Kulesa and Fraser, 2000; Graham et al., 2004; Kulesa et al., 2004). Cranial NC migration is completed by HH14 with proximal and distal populations forming medial to more lateral hyoid elements (Kontges and Lumsden, 1996).
Avian development of the hyoid derived cartilage depends on signals that arise from pharyngeal endoderm (Ruhin et al., 2003; Creuzet et al., 2004a). Removal of anterior to progressively more posterior stripes of medial, or lateral foregut endoderm at premigratory stages, demonstrates that the pharyngeal endoderm is required for patterning of specific skeletal elements. At the level of the 2nd arch, these include elements such as the basihyoid and ceratobranchial (Ruhin et al., 2003). Turning mesenchyme into cartilage condensations necessitates that epithelial-mesenchymal interactions perform several functions. Migrating skeletogenic mesenchyme is first localized, with local epithelial-mesenchymal interactions influencing the size of the progenitor population for each cranial skeletal element, providing condensation initiation signals, and then permitting differentiation of chondrocytes and, finally, ossification (Hall, 2008). Previous studies looked at the presence or absence of cartilage elements, but did not examine the intervening steps to determine when the failure took place (Creuzet et al., 2005). Mesenchyme localization, specification of pre-chondrogenic identity or the chondrogenic program might each be affected.
The columella arises from NC mesenchyme that migrates to the proximal 2nd arch, apposing the pharyngeal endoderm (Kontges and Lumsden, 1996). The single chick middle ear bone, the columella, is a composite structure consisting of persistent and replacement cartilage elements. The proximal bony columella inserts into the oval window of the cochlear duct and the more distal extracolumella cartilage inserts into the tympanic membrane, spanning the width of the middle ear cavity (Jaskoll and Maderson, 1978; Wood et al., 2010). The effect of signals from the subjacent pharyngeal endoderm on the proximally localized mesenchyme of the columella was not analyzed in previous studies (B. Ruhin and N. le Douarin, personal communication). We wanted to know if the pharyngeal endoderm provided signals sufficient and necessary for specifying the putative columella mesenchyme to a chondrogenic fate. The positioning of the columella mesenchyme and endoderm make an ideal tissue to investigate the intervening time points between migration and chondrogenesis.
We hypothesize that signals required for specifying pre-chondrogenic identity and the onset of the chondrogenic program in this mesenchymal population are localized to the subjacent pharyngeal endoderm. To examine this question extirpated tissues from the middle ear region were grown in collagen gel culture. Sox9, a member of the high mobility group (HMG) domain containing transcription factors, is implicated in NC specification, with Sox9 expression in mesenchyme a marker of pre-chondrogenic identity (Cheung and Briscoe, 2003; Wood et al., 2010). The onset of Col2a1 expression indicates the initiation of the chondrogenic program at E7.5 in chick (Zhao et al., 1997; Eames et al., 2004; Betancur et al., 2010). Through our tissue recombination experiments we show that the pharyngeal endoderm adjacent to the post-migratory mesenchyme, giving rise to the putative columella condensation, is sufficient, but not required to induce Sox9 expression and, thus, pre-chondrogenic identity.
Endodermal signals arising in the caudal part of the foregut influence the Hox-positive mesenchyme in the 2nd and more posterior arches, inducing pre-chondrogenic identity (Ruhin et al., 2003). Reciprocal signaling between the Fgf8 expressing endoderm and NC cells in the facial region is required for condensation formation (Creuzet et al., 2004b). Numerous FGF genes with spatially restricted expression patterns are present in the region (Ohyama et al., 2007; Schimmang, 2007), potentially mediating these epithelial-mesenchymal interactions (Shigetani et al., 2000). Therefore, we surveyed known FGF family members to identify Fgf genes expressed in the pharyngeal endoderm. Embryos were analyzed at the migratory and post-NC migratory stages HH14 and HH18/19 when mesenchymal Sox9 expression is first detected.
Multistep crosstalk is required between the endoderm and NC-derived mesenchyme, leading to cartilage formation (Creuzet et al., 2005). Our functional studies demonstrate that FGF signaling is both sufficient and necessary for induction of pre-chondrogenic identity, but cannot maintain Sox9expression to initiate the chondrogenic program. Expression of the chondrocyte marker Col2a1 is, however, elicited with pharyngeal endoderm present, indicating the requirement for a second signal. BMP4 signaling alone is able to induce and maintain Sox9 expression, but it is the combination of BMP and FGF signaling that is required for induction of the chondrogenic program. We show that BMP4 in combination with FGF8 is sufficient to initiate the chondrogenic program and suggest that this may be a general patterning mechanism within the more posterior arches, which all express combinations of these secreted signaling molecules.
Results
Pharyngeal endoderm is sufficient to induce Sox9 expression
Ablation of caudal endoderm results in loss of ceratobranchial and epibranchial cartilage elements (Ruhin et al., 2003); however, the timing of the mechanism involved has not been closely examined. We hypothesize that signals from pharyngeal endoderm are sufficient to specify pre-chondrogenic identity, which is required for condensation and chondrogenesis to occur. Pharyngeal endoderm and the proximal mesenchyme of the 2nd arch are directly apposed (Fig. 1). It is likely that local signals, specifying the NC-derived mesenchyme pre-chondrogenic fate, arise from the endoderm. Neural crest cells located in the dorsal neural tube have Sox9 expression at premigratory stages HH9-12, which is down regulated at the onset of migration (Cheung and Briscoe, 2003). The NC migrates to the second arch and is located proximally at the entrance to the arch and distally within the arch pouch by HH14. The putative skeletogenic mesenchyme does not regain Sox9 expression until HH18 (Cheung and Briscoe, 2003)(our unpublished results).
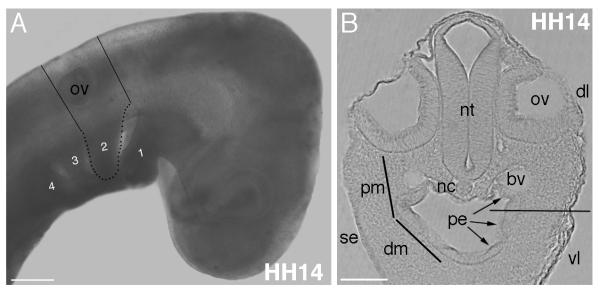
Figure 1. Location of tissues and anatomical landmarks for the explant cultures.
(A) Lines on HH14 embryo show the level of cuts to remove a transverse slice of the head and 2nd arch region. Pharyngeal arches numbered 1-4, with 2nd arch outlined in black dots. (B) A section through the level of the otic vesicle and 2nd arch region showing the tissues present. Proximal to distal mesenchyme continuum indicated by lines on the left side. Pharyngeal endoderm lines the oral cavity (arrowed). Horizontal line through arch on right side indicates approximate level of cut to exclude ventral arch mesenchyme from the explants. Abbreviations: bv, blood vessel; dl, dorsolateral level; dm, distal mesenchyme; nc, notochord; nt, neural tube; ov, otic vesicle; pe, pharyngeal endoderm; pm, proximal mesenchyme; se, surface ectoderm; vl, ventrolateral level. Scale bars: A, 200 μm, B, 100 μm.
To test the sufficiency of pharyngeal endoderm to induce Sox9 expression in mesenchyme we used an explant culture system. Mesenchyme from the proximal 2nd arch region, including cells fated to form the columella (the single middle ear bone of the chick) (Wood et al., 2010; Chapman, 2011) were explanted with or without the subjacent endoderm from HH14, HH19 and HH24 embryos into collagen gel culture (Exp. 1-3, Table 1). The more distal mesenchyme within the 2nd arch pouch was excluded (Fig. 1, see Experimental Procedures for a detailed description).
Table 1.
Sox9 expression at E6 in explanted tissues.
| Exp # |
Explants | Explant stage |
Embryonic Day |
# with expression |
% with expression |
Marker | Stage harvested |
|---|---|---|---|---|---|---|---|
| 1 | Mesenchyme + PE | HH14 | E2 | 36/36 | 100% | Sox9 | E6 |
| Mesenchyme alone | HH14 | E2 | 4/26 | 15.4% | Sox9 | E6 | |
| 2 | Mesenchyme + PE | HH19 | E3 | 3/4 | 75% | Sox9 | E6 |
| Mesenchyme alone | HH19 | E3 | 3/3 | 100% | Sox9 | E3 | |
| 3 | Mesenchyme + PE | HH24 | E4 | 8/8 | 100% | Sox9 | E6 |
| Mesenchyme alone | HH24 | E4 | 8/8 | 100% | Sox9 | E6 | |
| 4 | Arch 2 slice - PE | HH14 | E2 | 10/10 | 100% | Sox9 | E6 |
| Arch 2 slice + PE | HH14 | E2 | 10/10 | 100% | Sox9 | E6 | |
| 5 | Mesenchyme + otic vesicle |
HH14 | E2 | 13/13 | 100% | PNA | E6 |
| 6 | Mesenchyme + otic vesicle |
HH14 | E2 | 16/16 | 100% | Sox9 | E6 |
| 7 | Mesenchyme + surface ectoderm |
HH14 | E2 | 5/20 | 25% | Sox9 | E6 |
Abbreviations: PE, pharyngeal endoderm.
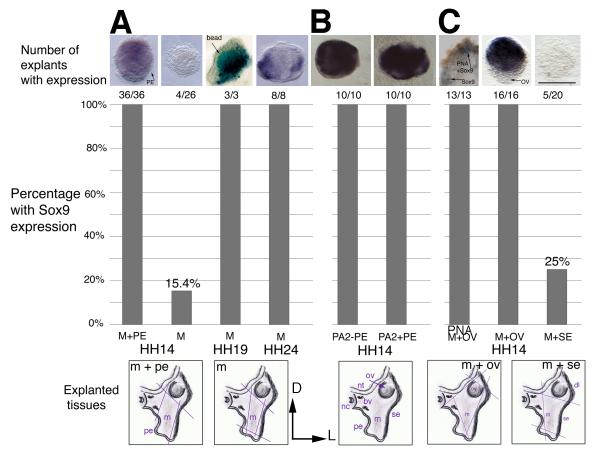
Mesenchymal tissue explanted at HH14 was grown to embryonic day (E)6 and analyzed for the presence of Sox9 transcripts (Exp. 1, Fig. 2A). Mesenchyme with endoderm present expressed Sox9 in all cases (n=36/36, Fig. 2), whereas explants of mesenchyme alone did not (n=4/26). Tissue sections of zero hour explants show mesenchyme with endoderm or mesenchyme alone as expected (not shown). We also confirmed that transplanted mesenchyme does not express Sox9 at HH14 (zero hour explants, not shown). The small number of explants with Sox9 expression likely included remnants of pharyngeal endoderm. Cleanly separating the thin endodermal layer is challenging without the use of enzymes. To avoid disrupting secreted signaling factors we avoided the use of enzymes to separate these tissues. This result indicates that signals from pharyngeal endoderm are sufficient for induction and maintenance of Sox9 expression.
Figure 2. Sox9 expression in explants from the proximal 2nd arch region.
The schematics below the graph illustrate the tissues in the region. Orientation is dorsal to the top and lateral to the right. The colored lines indicate the cuts made to extirpate the tissue. The graph shows the number and percentage of explants that tested positive for Sox9. The tissues included in each graph are listed on the horizontal axis. (A) Explants were removed from chick embryos at HH14, HH19 or HH24 and grown in collagen gel culture to E6. Mesenchyme cultured with pharyngeal endoderm from HH14 tissues expresses Sox9 at E6, whereas mesenchyme alone does not. At HH19 and HH24, Sox9 is expressed in the mesenchyme at E6 in the absence of pharyngeal endoderm. (B) Transverse sections of the head at the level of the 2nd arch with or without pharyngeal endoderm present express Sox9 in all cases. The schematic below is labeled with the tissues found in this region at the time of culture. (C) Extirpation of the otic vesicle with mesenchyme results in PNA and Sox9 expression. Mesenchyme explanted with surface ectoderm expresses Sox9 in a quarter of cases. Abbreviations: bv, blood vessel; D, dorsal; dl, dorsolateral; L, lateral; m/M, mesenchyme; nc, notochord; nt, neural tube; ov/OV, otic vesicle; PA2, 2nd pharyngeal arch level tissue; pe/PE, pharyngeal endoderm; PNA, Peanut Agglutinin Lectin; se/SE, surface ectoderm. Scale bar: 100 μm.
From HH18, mesenchyme fated to form skeletal condensations expresses Sox9 (not shown). We wanted to know if the expression of Sox9 in this tissue indicated that it was specified to a pre-chondrogenic identity and would thus maintain Sox9 expression when cultured in isolation. Mesenchyme was explanted from HH19 and HH24 embryos and grown to E6 with or without pharyngeal endoderm present (Exp. 2 and 3, Fig. 2). When cultured with pharyngeal endoderm, HH19 and HH24 explants maintained Sox9 expression at E6 (n=3/4 and 8/8, respectively, Table 1). When cultured alone, in the absence of pharyngeal endoderm, mesenchyme also maintained Sox9 expression (n=3/3 and 8/8, respectively, Fig. 2). This experiment indicates that mesenchyme was able to maintained Sox9 expression in the absence of any signals from the pharyngeal endoderm and the cells were thus specified as pre-chondrogenic by HH19.
These results support our hypothesis that signals from the pharyngeal endoderm at post-migratory stages (HH14 onward) are sufficient to induce Sox9, specifying the NC-derived mesenchyme to a pre-chondrogenic fate, and that by HH19 these signals are no longer required to maintain Sox9 expression. Moreover, this experiment confirms that the NC requires local epithelial derived signals and is not pre-patterned to a pre-chondrogenic fate. Furthermore, based on these results, the surface ectoderm subjacent to which these cells migrate does not appear to be required for the onset of Sox9 expression.
Pharyngeal endoderm is not required for Sox9 expression
Although sufficient, it is not clear if pharyngeal endoderm is required for Sox9 expression. Ablation of subjacent pharyngeal endoderm in ovo results in the loss of the hyoid skeletal elements: the ceratobranchial and epibranchial cartilages. In these experiments, whole mount embryos were manipulated at HH9 were incubated until E8 and then stained for the presence of cartilage (Ruhin et al., 2003). In this context, pharyngeal endoderm is required for skeletogenesis. However, the requirement for signals from the pharyngeal endoderm in inducing and maintaining Sox9 expression, and thus pre-chondrogenic identity was not addressed.
A transverse slice of the head at the level of the 2nd arch (Fig. 1A) was explanted from HH14 embryos and cultured to E6 (Exp. 4, Fig. 2B). The slice included all the tissues at this level; neural tube, notochord, otic vesicle, mesenchyme, surface ectoderm, pharyngeal endoderm and blood vessels. The slice was hemisected into left and right sides. The intact left side slice acted as a controlwith all tissues left intact, whereas the pharyngeal endoderm was removed from the right hand slice, leaving all other tissues undisturbed (Fig. 2B). In both control and experimental tissues Sox9 was expressed in all cases (n=10/10), demonstrating that although sufficient, pharyngeal endoderm is not required for Sox9 expression and the specification of pre-chondrogenic identity. Signals from surrounding tissues must, therefore, compensate for the loss of the pharyngeal endoderm.
Signals from other tissues are sufficient to induce Sox9 expression
It must be noted that our explant experiments likely include mesenchymal cells fated to become other skeletogenic structures, not just the proximal 2nd arch NC mesenchyme fated to become the columella. Some of the lateral otic capsule population is likely included when we extirpate the mesenchyme. The origin of otic capsule mesenchyme is more complex than for the proximally situated columella, with contributions from the first somite, cephalic mesoderm and NC (Couly et al., 1993), our unpublished data). For the otic capsule, endogenous inductive epithelial-mesenchymal signals originate from the placodal ectoderm of the otic epithelium (Hall, 2008). Sox9 is a common marker of pre-chondrogenic cells and, thus, will label both otic capsule and columella populations. The only marker to distinguish between these populations is Peanut Agglutinin Lectin (PNA). Otic capsule mesenchyme does not label with PNA unless pre-treated with neuraminidase to remove the sialic acid residues present in this population, allowing us to distinguish 2nd arch mesenchyme from otic capsule (Wood et al., 2010). Thus, we could distinguish which pre-cartilaginous populations were present in our explants (Exp. 5, n=13/13, Fig. 2C). Explants with Sox9 positive cells were incubated with PNA-HRP. Some Sox9 cells were labeled with PNA, whereas others were not labeled. This result leads us to conclude that both NC-derived mesenchyme and otic capsule cell populations were included in our explants.
Next we determined which other tissues in the region have signals capable of inducing Sox9 (Exp. 6). When otic vesicle was included with mesenchyme, Sox9 was expressed in all cases (n=16/16, Fig. 2). As the otic epithelium is adjacent to the otic capsule mesenchyme, signals from the otic epithelium would be expected to normally induce and maintain Sox9 expression in the otic capsule mesenchyme {Hall, 2008 #8}. Signaling molecules diffuse away from their source into the mesenchyme. It is likely that inducer molecules from the otic epithelium are able to substitute for signals from the pharyngeal endoderm and induce Sox9 in the NC-derived mesenchyme. Of interest, removal of the otic placode does not affect development of the columella, indicating that although sufficient, signals from the otic epithelium are not required for columella development (Reagan, 1917). We also found that neural tube and notochord are sufficient to induce Sox9 expression, although the endothelium of blood vessels was not (not shown).
A long-standing question is whether the surface ectoderm at these stages in the chick has the potential to specify the mesenchyme (Exp. 7). Our explants of pharyngeal endoderm and mesenchyme demonstrate that the surface ectoderm, past which NC migrates, is not required to induce Sox9 expression (Exp. 1). However, ectoderm at the level of the pharyngeal groove, when combined with the adjacent post-migratory mesenchyme, resulted in 5/20 (25%) cases positive for Sox9 expression. There are two possible explanations for this result. Firstly, the explant was contaminated with pharyngeal endoderm, giving a similar result to the baseline results for mesenchyme alone. Or secondly, that the surface ectoderm of the 2nd arch expresses an inducer, whereas the more dorsolateral surface ectoderm encountered during migration does not (Fig. 1B). Pharyngeal endoderm and surface ectoderm at this level both express Fgf8, a known inducer of Sox9 (Monsoro-Burq et al., 2003). Thus, in some of our surface ectoderm and mesenchyme explants we may have inadvertently included the Fgf8 expressing ventral ectoderm, leading to induction of Sox9 expression. These results lead us to survey the spatio-temporal expression patterns of Fgf genes in the surrounding tissues.
Spatiotemporal analysis of Fgf expression in the ear-forming region
Neural crest migrates into a proximal position in the 2nd pharyngeal arch, undergoes condensation and by endochondral ossification forms the single middle ear bone of the chick, the columella (Wood et al., 2010). We hypothesize that FGF signals are required for induction of Sox9 and specifying pre-chondrogenic identity. Ectopic hyoid (tongue) cartilage is evident when additional endoderm or FGF8 beads are grafted into the 2nd arch region (Ruhin et al., 2003; Creuzet et al., 2004a). However, the effect on the columella was not analyzed (B. Ruhin and N. le Douarin, personal communication). We analyzed Fgf expression patterns to identify the spatio-temporal expression of FGF family members in the region. Cranial NC migration begins at HH9 in the midbrain and progresses in an anterior to posterior direction, migrating at HH11 from rhombomere 4 toward the 2nd arch. We, therefore, chose to analyze Fgf expression using in situ hybridization at HH9 (pre-migration), HH14 (post-migration) and at HH19 when PNA labeling and Sox9 expression indicates the first signs of NC differentiation into pre-chondrogenic mesenchyme (Wood et al., 2010). Sox9 expression is maintained in chondrogenic cells of the columella up to E8, when it is down regulated in anticipation of ossification, as marked by the onset of Ihh expression at E10 (Wood et al., 2010).
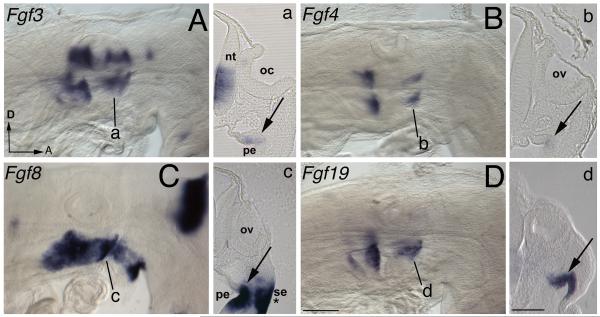
At pre-migration stages Fgf3, 4, 8 and 19 are expressed in the endoderm fold adjacent to where NC-derived mesenchyme will migrate (Karabagli et al., 2002; Paxton et al., 2010). Following completion of NC migration at HH14, expression of Fgf3, 4, 8 and 19 are maintained in the fold of the pharyngeal endoderm (Fig. 2A-D). Fgf3 and Fgf4 are weakly expressed (Fig. 2a, b), whereas Fgf8 and Fgf19 have strong expression (Fig. 2c, d), with Fgf8 also being expressed in the surface ectoderm at the groove of the arch (asterisk).
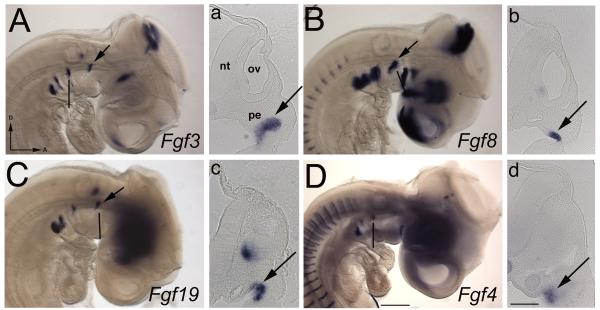
At HH19, Fgf3 is expressed in the pharyngeal endoderm between the first and second arch (Fig. 3A). Fgf8 and Fgf19 are expressed in the pharyngeal endoderm at the anterior edge of the 2nd arch (Fig. 3B, C). Fgf8 is no longer expressed in the surface ectoderm. Fgf4 expression has strengthened, but is limited to the posterior of the 2nd arch (Fig. 3D). We find no other Fgfs expressed in the pharyngeal endoderm.
Figure 3. Fgf expression at the completion of NC migration.
(A-D) Whole mount HH14 stage embryos with anterior facing to the right and dorsal to the top of the page. (a-d) Corresponding 50 μm gelatin sections are indicated by a black line on the whole mount images. (A, B) Weak Fgf3 and Fgf4 expression is detected in the pharyngeal endoderm lip (arrowed a, b), whereas strong Fgf8 and Fgf19 (C, D) endoderm expression is observed (arrowed, c, d), with Fgf8 also expressed in the adjacent surface ectoderm at the groove of the pharyngeal arch (asterisk). Abbreviations: A, anterior; D, dorsal; nt, neural tube; pe, pharyngeal endoderm; oc, otic cup; ov, otic vesicle; se, surface ectoderm. Scale bars: (A-D) 100 μm, (a-d) 100 μm.
These expression patterns indicate that Fgf8 and Fgf19 are consistently expressed in the pharyngeal endoderm pre and post migration stages, with weaker early Fgf3 and Fgf4 expression (HH14). Fgf4 expression may play a role in induction, but not maintenance, as it is down regulated by HH19 in the endoderm at the anterior of the arch where the columella will form.
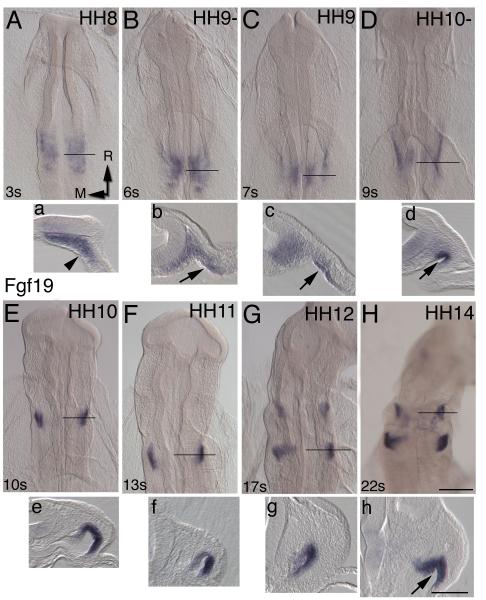
Although FGF19 has a known role in inner ear induction (Ladher et al., 2005), as candidate inducer of Sox9, a more detailed analysis of Fgf19’s spatio-temporal expression pattern was needed. We analyzed expression in an extended stage series (HH8-14) by in situ hybridization. Expression is initially detected in the mesenchyme and weakly in the ventral hindbrain at the level of the ear-forming region (Fig. 4A). By the six somite stage pharyngeal endoderm begins to express Fgf19 (Fig. 4B, C). Folding of the anterior intestinal portal forms the pharyngeal endoderm lip (Fig. 4D, arrowed). This coincides with the onset of NC migration. Low levels of Fgf19 expression in hindbrain continue until around HH10 with pharyngeal endoderm showing increased numbers of transcripts (Fig.4E). At HH12, expression is visible at the level of 2nd arch and a new domain of expression appears in the endoderm of the 4th arch (Fig. 4G, g). By HH14, Fgf19 expression in pharyngeal endoderm is directly subjacent to the NC-derived columella mesenchyme (Fig. 4H). Given the spatio-temporal Fgf expression patterns, we next tested if FGF signaling was sufficient and necessary for Sox9 expression.
Figure 4. Fgf expression in during specification of pre-chondrogenic identity.
(A-D) HH18/19 stage embryos, with the head to the right and dorsal towards the top of the page. The black line indicates the level of the corresponding transverse 50 μm gelatin sections (a-d). (A) Fgf3 is expressed in the pharyngeal endoderm in the 1st pouch (arrowed). (a) The section is through the endoderm at the anterior of the 3rd arch. (B, b) Fgf8 and (C, c) Fgf19 are expressed in the proximal anterior region of the 2nd arch (arrowed) and the vestibuloacoustic ganglion. (D, d) Fgf4 expression is limited to the posterior 2nd arch endoderm (arrowed). Scale bars: (A-D) 100 μm, (a-d) 100 μm.
FGF signaling is required and sufficient for induction of Sox9 expression
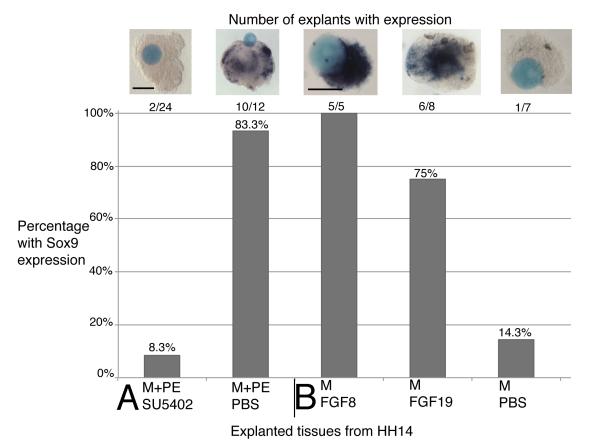
To test if FGF signaling was required to induce Sox9 expression we turned again to our culture system (Exp. 8, Table 2). A bead soaked in 10 mM SU5402 Fgf-receptor inhibitor or PBS (control) was cultured with a HH14 explant of mesenchyme with pharyngeal endoderm. Explants cultured for 24 hours with the FGF-receptor inhibitor failed to express Sox9 (n=2/24, Fig. 6A), while explanted tissue cultured with control PBS soaked beads did induce expression of Sox9 (n=10/12). These results indicate that FGF signaling is required to induce Sox9 expression and thus, specification of NC mesenchyme to a pre-chondrogenic fate.
Table 2.
Induction of Sox9 in explanted tissues.
| Exp # |
Explants | Explant stage |
Embryonic day |
# with expression |
% with expression |
Marker | Stage harvested |
|---|---|---|---|---|---|---|---|
| 8 | Mesenchyme + PE + SU5402 bead |
HH14 | E2 | 2/24 | 8.3% | Sox9 | + 24 hrs |
| Mesenchyme + PE + PBS bead |
HH14 | E2 | 10/12 | 83.3% | Sox9 | + 24 hrs | |
| 9 | Mesenchyme + FGF8 bead |
HH14 | E2 | 5/5 | 100% | Sox9 | + 24 hrs |
| Mesenchyme + FGF19 bead |
HH14 | E2 | 6/8 | 75% | Sox9 | + 24 hrs | |
| Mesenchyme + PBS bead |
HH14 | E2 | 1/7 | 14.3% | Sox9 | + 24 hrs |
Abbreviations: PBS, phosphate buffered saline; PE, pharyngeal endoderm.
Figure 6. The role of FGF signaling in inducing Sox9 in mesenchyme.
The graph shows the number and percentage of explants that tested positive for Sox9. The tissues included in each graph are listed on the horizontal axis. (A) Mesenchyme and pharyngeal endoderm cultured for 24 hours with SU5402 soaked beads failed to express Sox9, whereas Sox9 expression was unaffected by addition of PBS control beads. (B) In the converse experiment adding FGF8 or FGF19 soaked beads to mesenchyme alone, induced Sox9 expression. PBS beads in control explants did not have Sox9 expression. Scale bars: (A) 100 μm, (B) 100 μm.
In the converse experiment, we tested if addition of recombinant FGF protein was sufficient to induce Sox9 expression (Exp. 9). We added a bead soaked in recombinant FGF8 protein to HH14 explants with only mesenchyme present, resulting in Sox9 expression in all cases (n=5/5) after 24 hours in culture (Fig. 6B). Similarly, FGF19 soaked beads induced Sox9 when added to mesenchyme explants (n=6/8). In controls, mesenchyme only explants placed together with PBS soaked control beads showed no induction of Sox9 expression (n=1/7).
FGF signaling can induce, but not maintain Sox9 expression
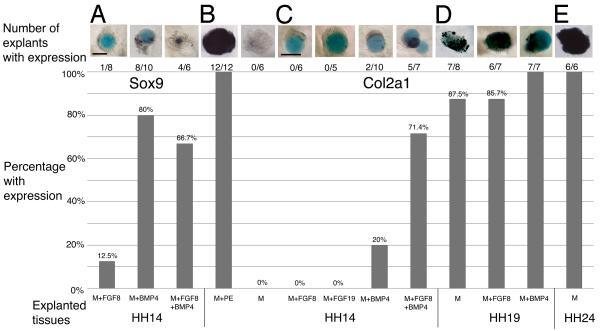
FGF signals are able to induce Sox9 expression during a 24 hour culture period, but we wanted to know if this one signal was sufficient to maintain Sox9 over time (Exp. 10, Table 3). Mesenchyme explants cultured with an FGF8 soaked bead to E6 could not maintain Sox9 expression (n=1/8, Fig. 7A). Neither could FGF19 soaked beads (Table 3). However, we have shown that signals from the pharyngeal endoderm are able to maintain Sox9, suggesting that a second secreted signaling molecule is required in addition to FGF.
Table 3.
Initiation of the chondrogenic program in explants.
| Exp # |
Explants | Explant stage |
Embryonic day |
# with expression |
% with expression |
Marker | Stage harvested |
|---|---|---|---|---|---|---|---|
| 10 | Mesenchyme + FGF8 bead |
HH14 | E2 | 1/8 | 12.5% | Sox9 | E6 |
| Mesenchyme + BMP4 bead |
HH14 | E2 | 8/10 | 80% | Sox9 | E6 | |
| Mesenchyme + FGF8 bead +BMP4 bead |
HH14 | E2 | 4/6 | 66.7% | Sox9 | E6 | |
| 11 | Mesenchyme + PE | HH14 | E2 | 12/12 | 100% | Col2a1 | E8 |
| Mesenchyme alone | HH14 | E2 | 0/6 | 0% | Col2a1 | E8 | |
| 12 | Mesenchyme + FGF8 bead |
HH14 | E2 | 0/6 | 0% | Col2a1 | E8 |
| Mesenchyme + FGF19 bead |
HH14 | E2 | 0/5 | 0% | Col2a1 | E8 | |
| 13 | Mesenchyme + BMP4 bead |
HH14 | E2 | 2/10 | 20% | Col2a1 | E8 |
| Mesenchyme + FGF8 bead +BMP4 bead |
HH14 | E2 | 5/7 | 71.4% | Col2a1 | E8 | |
| 14 | Mesenchyme alone | HH19 | E3 | 7/8 | 87.5% | Col2a1 | E8 |
| Mesenchyme + FGF8 bead |
HH19 | E3 | 6/7 | 85.7% | Col2a1 | E8 | |
| Mesenchyme + BMP4 bead |
HH19 | E3 | 7/7 | 100% | Col2a1 | E8 | |
| 15 | Mesenchyme alone | HH24 | E4 | 6/6 | 100% | Col2a1 | E8 |
Abbreviations: BMP, Bone Morphogenetic Protein; FGF, Fibroblast Growth Factor; PBS, phosphate buffered saline; PE, pharyngeal endoderm.
Figure 7. FGF and BMP signaling in initiation of the chondrogenic program.
The graph shows the number and percentage of explants that tested positive for Sox9 and Col2a1. The tissues included in each graph are listed on the horizontal axis. (A) Cultured HH14 mesenchyme expresses Sox9 when exposed to BMP4 alone, and in combination with FGF8 soaked beads, but not FGF8 beads alone at E6. (B) Mesenchyme in the presence of pharyngeal endoderm, but not alone, strongly expresses Col2a1 at E8. (C) Neither FGF8 or FGF19 protein soaked beads is sufficient for Col2a1 expression. BMP4 is a weak inducer of Col2a1. Only when BMP4 and FGF8 are combined does mesenchyme tissue express Col2a1at E8. (D) By HH19, mesenchyme explanted in isolation expresses Col2a1 and is unaffected by the presence of FGF8 or BMP4. (E) Col2a1 is expressed throughout the mesenchyme in tissue isolated at HH24. Scale bars: (A, B) 100 μm, (C-E) 100 μm.
Bmp2, 4 and 7 are expressed in restricted temporospatial patterns in the pharyngeal arch epithelia and mesenchyme (Shigetani et al., 2000; Bell et al., 2004; Darnell et al., 2007; Wood et al., 2010). We confirmed that Bmp4 is expressed in both the pharyngeal endoderm and mesenchyme of HH14 explants (not shown). When we applied BMP4 soaked beads alone (n=8/10), or together with FGF8 soaked beads (n=4/6), explants were able to induce and maintain Sox9 expression to E6(Fig. 7A).
Together these data demonstrate that FGF signaling is sufficient and required for induction of Sox9 expression and, thus, specification of pre-chondrogenic identity in mesenchyme tissue. However, FGF signals are unable to maintain this identity. Somewhat unexpectedly, even in the absence of FGF signaling, recombinant BMP protein was able to not only induce, but also maintain Sox9 expression in long-term cultures. We know from experiment 8 that FGF signals are required for induction of Sox9, when mesenchyme and pharyngeal endoderm are the only tissues present. It will be interesting to determine if BMP signaling could induce Sox9 in mesenchyme alone when FGF signals were being blocked by SU5402, indicating an important interaction between the two signaling pathways.
Next we examined the role of pharyngeal endoderm and secreted signaling factors in initiating the chondrogenic program, leading to over differentiation and cartilage formation.
Pharyngeal endoderm signals sets mesenchymal cells on a pathway to chondrogenesis
Col2a1 expression is normally detected in mesenchyme cells from E7.5 with the onset of overt chondrogenesis (Wood et al., 2010). To determine if mesenchyme was able to express the chondrogenic marker Col2a1, HH14 explants were grown to E8 (Exp. 11, Table 3). Mesenchyme in combination with pharyngeal endoderm strongly expressed Col2a1 (n=12/12, Fig. 7B), with the endoderm remaining negative for Col2a1 transcripts. HH14 mesenchyme cultured alone did not express Col2a1 (n=0/6), indicating that pharyngeal endoderm contains all the signals necessary for chondrogenesis to proceed. We next examined to role of FGF8 and BMP4 in this process.
Both FGF and BMP signaling are required to initiate the chondrogenic program
When HH14 mesenchyme was cultured with either an FGF8 (n=0/6) or FGF19 (n=0/5) soaked bead (Exp. 12), none of the explants expressed Col2a1 at E8. FGF signaling induces, but cannot maintain Sox9 expression and is also insufficient for induction of the chondrogenic program as indicated by the absence of Col2a1 transcripts at E8. It is possible that this was because FGF signals that are required to induce Col2a1 expression must be sustained for an interval that cannot be achieved using the bead culture system. We repeated the experiment, replacing the media each day with recombinant FGF8 protein included in the media (1 mg/ml) to supply the tissue constantly over the incubation period, but were unsuccessful in inducing Col2a1 expression (n=0/8, not shown). Taken together, these results suggest that another signaling pathway is required to facilitate induction of the chondrogenic program.
BMP4 recombinant protein soaked beads were added to mesenchyme explants (Exp. 13), with or without addition of FGF8 beads (Fig. 7C). Explants were analyzed for Col2a1 expression at E8. BMP4 alone was only able to induce Col2a1 expression in the mesenchyme in 20% of cases (n=2/10). However, FGF8 together with BMP4 soaked beads, induced Col2a1 expression in the majority of cases (n=5/7). This result suggests that the pharyngeal endoderm uses the combination of BMP and FGF signals to induce pre-chondrogenic identity and initiate the chondrogenic program.
To test if FGF and BMP signals were required following specification of pre-chondrogenic identity, Sox9 expressing mesenchyme explanted from HH19 embryos was incubated to E8 (Exp. 14). Zero hour explants all expressed Sox9 (not shown). Isolated mesenchyme explants cultured to E8 all strongly expressed Col2a1 (n=7/8, Fig. 7D). Combining explants with either FGF8 (n=6/7) or BMP4 (n=7/7) soaked beads had did not change the Col2a1 expression. These results suggest that the chondrogenic program is able to proceed autonomously in skeletogenic mesenchyme from HH19, without further input following specification of pre-chondrogenic identity. Mesenchyme explants extirpated at HH24 all showed Col2a1 expression at E8 (n=6/8).
In conclusion, these results demonstrate that FGF signaling induced, but could not maintain Sox9 expression, whereas BMP4 signaling was sufficient to both induce and maintain Sox9 expression. Together with our FGF receptor inhibitor experiment demonstrating that FGF signaling is required for Sox9 expression we conclude that FGF and BMP signaling together specify pre-chondrogenic identity. Moreover, combined FGF8 and BMP4 signaling was needed to initiate the chondrogenic program as indicated by Col2a1 expression.
Discussion
A refined model of cartilage formation in the neural crest-derived mesenchyme
We present a model in which pharyngeal endoderm is sufficient, but not required, to specify pre-chondrogenic identity and initiate the chondrogenic program in skeletogenic mesenchyme from HH14 embryos, as defined by the presence of Sox9 and Col2a1 transcripts respectively. Specification of pre-chondrogenic identity and initiation of the autonomous chondrogenic program is complete by HH19 in the presence of signals from the pharyngeal endoderm. Mesenchyme isolated at HH19 and HH24 expresses Sox9 and Col2a1 during long-term culture.
Furthermore, our gain- and loss-of-function studies using HH14 explants of isolated mesenchyme demonstrate that FGF signaling is both sufficient and required for induction of Sox9, but cannot maintain this expression long term. BMP4 recombinant protein can, however, both induce and maintain Sox9 expression, although by itself it is a weak initiator of the chondrogenic program. FGF and BMP signaling together are required for overt differentiation and expression of the chondrogenic marker, Col2a1.
As mesenchyme shares a common ground pattern (Minoux et al., 2009), with multiple FGF and BMP signaling molecules temporospatially restricted to the pharyngeal endoderm of all the arches {Creuzet, 2004 #13}, this is likely a general patterning mechanism, although this remains to be tested.
Pharyngeal endoderm is sufficient to induce and maintain Sox9 expression, but not required
Ablating pharyngeal endoderm, leaving all other tissues in the region intact elicits Sox9 expression. As demonstrated by our recombination experiments, signals from the otic epithelium act as an inducer of Sox9 in the NC-derived mesenchyme. Similarly, neural tube, notochord and 2nd arch Fgf8 expressing surface ectoderm can acts as inducers, but endothelial vessels, such as the dorsal aorta, cannot. Although other tissues are sufficient, given the distance from the putative columella in vivo, it is likely that the pharyngeal endoderm acts as the endogenous inducer.
In order to chondrify, premigratory cranial NC cells in mammals must interact with the cranial ectoderm in an inducer/responder relationship. In chick, signals from the surface ectoderm of the face are required to pattern the beak at later stages, but not other skeletal elements (Helms and Schneider, 2003; Schneider and Helms, 2003). In lower vertebrates, such as amphibians, post-migratory NC interacts with the branchial endoderm (Hall, 2008). Our results demonstrate that in chick, the non-FGF expressing dorsolateral surface ectoderm is unable to specify pre-chondrogenic identity. However, Fgf8-expressing surface ectoderm at the level of the pharyngeal groove is sufficient in this regard. We note that Fgf8 expression in this tissue is short-lived, appearing and then disappearing between HH8 and HH15. Thus, ectodermal expression might attract or prime NC cells as they migrate past the groove to reach the distal portion of each pouch.
FGF signaling is sufficient and required to induce, but cannot maintain, Sox9 expression
Specifying cells that follow the skeletogenic pathway requires exposure to FGF signaling, with FGF8 signaling being a potent inducer of pre-chondrogenic identity (Ruhin et al., 2003; Creuzet et al., 2004a; Minoux et al., 2009). The role of FGF8 in specification of skeletogenic mesenchyme is undisputed, but interestingly we show that FGF8 is unable to maintain Sox9 expression in isolated mesenchyme.
There are other Fgf genes in the region, with FGF8 a known regulator of Fgf19 expression (Ladher et al., 2005; Gimeno and Martinez, 2007). In our explants the effect of FGF19 on mesenchymal cells appears less robust than FGF8, with a smaller number of cells expressing Sox9. Although we did not quantify this, it is tempting to speculate that the endogenous role of FGF19 may be specification or patterning of only a subset of mesenchymal cells. Given the proximity of the columella cells to pharyngeal endoderm the columella mesenchyme may specifically require FGF19, although this remains to be tested. Fgf19 expression is detected only in distal portions of the 2nd and 4th arches, lending credence to this idea. The weaker, spatio-temporally restricted expression of Fgf3 and Fgf4 in 2nd arch endoderm will also be the subject of future examination. Zebrafish fgf3 endoderm expression is required for formation of arches 1-4 (David et al., 2002). Interestingly, these authors also suggest that signals in addition to FGFs are required in endoderm to pattern the skeletal elements arising from the arches.
Pharyngeal endoderm is required for induction of the chondrogenic program
Explants from HH14 embryos cultured with pharyngeal endoderm were able to express Col2a1 at E8. Once the mesenchyme population is specified, by HH19, chondrogenesis can proceed even in the absence of pharyngeal endoderm. Thus, pharyngeal endoderm is not only sufficient to specify pre-chondrogenic identity, but must be the source of a multiple signaling molecules, acting to initiate chondrogenesis (Creuzet et al., 2005). We show that BMP4 is sufficient in this regard.
BMP signaling has a role in promoting compaction of pre-chondrogenic cells leading to formation of the cartilage anlagen (the pre-chondrogenic condensation) and is an obvious candidate as an additional signal. BMP4 is expressed in a suitable temporospatial pattern (Wood et al., 2010). Loss of BMP signaling leads to failure of condensation formation and failure to differentiate into chondrocytes, even in condensed cells (Duprez et al., 1996; Yoon et al., 2005; Bandyopadhyay et al., 2006; Barna and Niswander, 2007; Karamboulas et al., 2010). Furthermore, BMP4 is a known inducer of Sox9, and our results support this finding, showing that BMP4 also maintains Sox9 expression over the longer term. BMP4 can also act as a direct transcriptional activator of Col2a1 expression (Semba et al., 2000). In our mesenchyme explants, FGF8 or BMP4 signaling alone are able to induce, but only BMP4 signaling can maintain Sox9 expression. Neither signal alone leads to Col2a1 expression, it is only when FGF and BMP signaling is combine that Col2a1 transcripts are detected at E8.
Reconciling models of hyoid skeletal formation
The results presented here add new insight into our understanding of the tissue and signaling mechanism required to induce and pattern NC-derived mesenchyme. NC-derived pharyngeal arch cells appear to share a common ground pattern (Minoux et al., 2009), with earlier transplantation experiments demonstrating the sufficiency and requirement for pharyngeal endoderm in imparting regional identity (Ruhin et al., 2003; Creuzet et al., 2004a). Supporting this, ablation of the adjacent pharyngeal endoderm in ovo resulted in the loss of the basihyoid and ceratobranchial skeletal elements (Ruhin et al., 2003). However, the mechanism resulting in failure was not evaluated in these studies. There are several possible stages at which skeletal elements that might be affected: NC migration, specification of pre-chondrogenic identity, condensation formation or chondrogenesis.
FGF signals act as an attractant for migrating NC cells (Creuzet et al., 2005). Genetic ablation of endoderm, in zebrafish van gogh, cas and bon mutants results in failure to form cartilage (Piotrowski and Nusslein-Volhard, 2000; David et al., 2002; Piotrowski et al., 2003). In the cas and bon mutants particularly, the lack of endoderm formation leads to failure of branchial arch formation. The NC cells migrate, but fail to enter the branchial arches, clustering instead on the surface of the yolk in disorganized masses, losing the markers of pre-chondrogenic mesenchyme. We suggest that a similar mechanism acts in chick, where removal of endoderm at the 2nd arch level during pre-migratory stages (Ruhin et al., 2003; Creuzet et al., 2004a), results in failure of the NC to become correctly localized and/or specified as pre-chondrogenic (David et al., 2002). Addition of an FGF8 source in place of the ablated endoderm rescues this phenotype by attracting the migrating cells and specifying pre-chondrogenic identity. Furthermore, our results show that FGF signals from other inducer tissues in the region can act to induce pre-chondrogenic identity, but would not localize the NC to the correct position. Thus, our results indicate that pharyngeal endoderm is sufficient, but not required for imparting pre-chondrogenic identity.
Due to the experimental paradigm used in earlier studies this distinction was not apparent. Failure of the NC cells to migrate to the correct location and thus be induced and maintain pre-chondrogenic identity would account for loss of specific skeletal elements. Thus, our experimental paradigm benefits from using NC cells that are post-migratory, avoiding the complications of localization. Thus, it appears to be the case that FGF signals arising from the pharyngeal endoderm are required to localize the migrating NC. Both FGF and BMP signals can induce pre-chondrogenic identity, although only BMP can maintain this state. In our longer-term cultures neither FGF, nor BMP signals alone are sufficient to induce the chondrogenic program. In conclusion, it is only when both signaling factors are combined that the chondrogenic program induced, as marked by the onset of Col2a1 expression.
Experimental Procedures
Embryos
Fertilized Bovan Brown x Rhode Island Red chicken eggs (Morgan Poultry Center, Clemson University) were incubated at 38.5°C in a rocking incubator to the desired stage. Whole mount in situ hybridization was performed as previously described (Chapman et al., 2002). Peanut Agglutinin Lectin (PNA) labeling was performed according to our standard protocol (Wood et al., 2010). For section analysis embryos were mounted in 20% gelatin, fixed in 4% PFA (paraformaldehyde) and sectioned at 50 μm using a Leica VT1000S vibratome, or embedded in 30% Sucrose/PBS (phosphate buffered saline) and cryosectioned at 10 μm using a Leica CM3050.
Tissue explants
Morphology of the head at HH14 and a section of the 2nd arch region are shown in figure 1. To preserve the integrity of cell surface receptors no enzymatic treatments were used to separate tissues. Embryos were harvested, washed in saline and transferred to L15 media during the fine manipulation before being transferred to collagen gel culture. Whole mount embryos were harvested in saline, with the head transected at the level of the 1/2 pharyngeal arch boundary anteriorly and the 2/3 arch boundary posteriorly. The remaining tissue formed a slice at the level of the 2nd arch (Fig. 1A). The tissues needed for explants at HH14 (E2), HH19 (E3) or HH24 (E4) were dissected from the equivalent region at each stage. The same basic morphology is present at each of the stages used. The slice was laid flat on the base of the petri dish and a flame sharpened tungsten needle (0.125 mm, WPI, Sarasota, FL) was used to remove tissues not required. For example, mesenchyme alone required the removal of the surface ectoderm, pharyngeal endoderm, distal pharyngeal arch, otic vesicle, neural tube, notochord and blood vessels, leaving only the proximal mesenchyme (Fig. 1B and Fig. 2 schematics). Using this methodology ensured that the proximal mesenchyme was the only tissue explanted to collagen gel culture, whereas the more distal mesenchyme within the pharyngeal arch was excluded. The older the tissue, the easier the tissue was to extirpate, as the size of the embryo had increased and thus the mesenchyme was considerably larger. To ensure that we explanted only the desired tissues, explants at zero hours were embedded and sectioned to determine the tissues present.
Collagen gel culture
Isolates were cultured in 3.3 mg/ml rat tail collagen (Roche Diagnostics, Indianapolis, IN) re-suspended in sterile 0.2% acetic acid. Collagen was prepared on ice using 440 μl collagen, 80 μl DEPC-H2O, 60 μl 10X DMEM and 20 μl 7.5% bicarbonate solution. Following collagen embedding carbonated Neurobasal medium supplemented with Glutamax and Penicillin/Streptomycin was added. Cultures were grown at 37°C in a 5% CO2 incubator, with daily changes in media until the desired stage was reached. Following fixation, tissue was processed for in situ hybridization or immunocytochemistry.
Bead implantation
Affi-Gel Blue Beads (100-200 μm, Bio-Rad, Hercules, CA) were washed in PBS for 1 hour. Beads were then soaked in 10 mM SU5402 (Pfizer), or 1 mg/ml human recombinant FGF8 (423-F8/CF, R&D systems), FGF19 (100-32, Peprotech), or 100 ng/ml mouse recombinant BMP4 (314-BP/CF, R&D systems) for 1 hour. SU5402 is dissolved in DMSO and beads were washed three times in PBS before implanting. Control beads were similarly treated with DMSO and washed in PBS before implanting. For the recombinant protein experiment the FGF and BMP proteins were re-suspended in PBS and, thus, no further washing of beads was necessary. PBS washed control beads were used in these experiments. Beads were kept on ice and as each tissue sample was prepared a bead was inserted in the collagen gel adjacent to the tissue. For experiment requiring both FGF and BMP, two beads were added adjacent to the tissue.
Figure 5. Fgf19 expression in the ear region.
(A-H) HH8-14 embryos with rostral towards the top of the page and (a-h) corresponding transverse 50 μm gelatin sections. The black line on the whole mount indicates the level of the section. (A) Initial Fgf19 expression is in the mesoderm and is weakly expressed in the ventral neural folds, but not the pharyngeal endoderm (arrowhead). (B, C) At HH9, expression is detectable in the future pouch endoderm. (D-F) Expression is particularly noticeable in the fold of the pharyngeal endoderm (arrowed). (G, g) As NC cells migrate into the 2nd arch the cells are exposed to Fgf19 signaling in the endoderm. A second region of Fgf19 expression is detected at level of the 4th arch from HH12. (H) At the conclusion of NC migration Fgf19 expression is directly subjacent to the putative columella mesenchyme population. Scale bars: (A-D) 100 μm, (a-d) 100 μm.
Acknowledgements
This study was supported by and NIH/NIDCD grant DC009236 and ARRA supplemental funding. Technical Contribution No. 5893 of the Clemson University Experiment Station. We thank Gary Schoenwolf (University of Utah) for cDNA clones of Fgf13 and 14 and Sean Kitch and Kiesha Staley for technical assistance.
Grant Sponsor: NIH Grant number: DC009236 and ARRA supplement
References
- Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS genetics. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna M, Niswander L. Visualization of cartilage formation: insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Developmental cell. 2007;12:931–941. doi: 10.1016/j.devcel.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Bell GW, Yatskievych TA, Antin PB. GEISHA, a whole-mount in situ hybridization gene expression screen in chicken embryos. Developmental dynamics : an official publication of the American Association of Anatomists. 2004;229:677–687. doi: 10.1002/dvdy.10503. [DOI] [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, Sauka-Spengler T. Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial NC. Proc Natl Acad Sci U S A. 2010;107:3570–3575. doi: 10.1073/pnas.0906596107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SC. Can you hear me now? Understanding vertebrate middle ear development. Front Biosci. 2011;16:1675–1692. doi: 10.2741/3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SC, Schubert FR, Schoenwolf GC, Lumsden A. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev Biol. 2002;245:187–199. doi: 10.1006/dbio.2002.0641. [DOI] [PubMed] [Google Scholar]
- Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Couly G, Le Douarin NM. Patterning the NC derivatives during development of the vertebrate head: insights from avian studies. Journal of anatomy. 2005;207:447–459. doi: 10.1111/j.1469-7580.2005.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet S, Schuler B, Couly G, Le Douarin NM. Reciprocal relationships between Fgf8 and NC cells in facial and forebrain development. Proc Natl Acad Sci U S A. 2004a;101:4843–4847. doi: 10.1073/pnas.0400869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet S, Schuler B, Couly G, Le Douarin NM. Reciprocal relationships between Fgf8 and NC cells in facial and forebrain development. Proceedings of the National Academy of Sciences of the United States of America. 2004b;101:4843–4847. doi: 10.1073/pnas.0400869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell DK, Kaur S, Stanislaw S, Davey S, Konieczka JH, Yatskievych TA, Antin PB. GEISHA: an in situ hybridization gene expression resource for the chicken embryo. Cytogenetic and genome research. 2007;117:30–35. doi: 10.1159/000103162. [DOI] [PubMed] [Google Scholar]
- David NB, Saint-Etienne L, Tsang M, Schilling TF, Rosa FM. Requirement for endoderm and FGF3 in ventral head skeleton formation. Development. 2002;129:4457–4468. doi: 10.1242/dev.129.19.4457. [DOI] [PubMed] [Google Scholar]
- Duprez D, Bell EJ, Richardson MK, Archer CW, Wolpert L, Brickell PM, Francis-West PH. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mechanisms of development. 1996;57:145–157. doi: 10.1016/0925-4773(96)00540-0. [DOI] [PubMed] [Google Scholar]
- Eames BF, Sharpe PT, Helms JA. Hierarchy revealed in the specification of three skeletal fates by Sox9 and Runx2. Dev Biol. 2004;274:188–200. doi: 10.1016/j.ydbio.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Gimeno L, Martinez S. Expression of chick Fgf19 and mouse Fgf15 orthologs is regulated in the developing brain by Fgf8 and Shh. Dev Dyn. 2007;236:2285–2297. doi: 10.1002/dvdy.21237. [DOI] [PubMed] [Google Scholar]
- Graham A, Begbie J, McGonnell I. Significance of the cranial NC. Dev Dyn. 2004;229:5–13. doi: 10.1002/dvdy.10442. [DOI] [PubMed] [Google Scholar]
- Hall B. The NC and NC cells in vertebrate development and evolution. Springer; New York: 2008. [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Helms JA, Schneider RA. Cranial skeletal biology. Nature. 2003;423:326–331. doi: 10.1038/nature01656. [DOI] [PubMed] [Google Scholar]
- Jaskoll TF, Maderson PF. A histological study of the development of the avian middle ear and tympanum. Anat Rec. 1978;190:177–199. doi: 10.1002/ar.1091900203. [DOI] [PubMed] [Google Scholar]
- Karabagli H, Karabagli P, Ladher RK, Schoenwolf GC. Comparison of the expression patterns of several fibroblast growth factors during chick gastrulation and neurulation. Anat Embryol (Berl) 2002;205:365–370. doi: 10.1007/s00429-002-0264-7. [DOI] [PubMed] [Google Scholar]
- Karamboulas K, Dranse HJ, Underhill TM. Regulation of BMP-dependent chondrogenesis in early limb mesenchyme by TGFbeta signals. Journal of cell science. 2010;123:2068–2076. doi: 10.1242/jcs.062901. [DOI] [PubMed] [Google Scholar]
- Kontges G, Lumsden A. Rhombencephalic NC segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Kulesa P, Ellies DL, Trainor PA. Comparative analysis of NC cell death, migration, and function during vertebrate embryogenesis. Dev Dyn. 2004;229:14–29. doi: 10.1002/dvdy.10485. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Fraser SE. In ovo time-lapse analysis of chick hindbrain NC cell migration shows cell interactions during migration to the branchial arches. Development. 2000;127:1161–1172. doi: 10.1242/dev.127.6.1161. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005;19:603–613. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoux M, Antonarakis GS, Kmita M, Duboule D, Rijli FM. Rostral and caudal pharyngeal arches share a common NC ground pattern. Development. 2009;136:637–645. doi: 10.1242/dev.028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development. 2003;130:3111–3124. doi: 10.1242/dev.00531. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Groves AK, Martin K. The first steps towards hearing: mechanisms of otic placode induction. Int J Dev Biol. 2007;51:463–472. doi: 10.1387/ijdb.072320to. [DOI] [PubMed] [Google Scholar]
- Paxton CN, Bleyl SB, Chapman SC, Schoenwolf GC. Identification of differentially expressed genes in early inner ear development. Gene Expr Patterns. 2010;10:31–43. doi: 10.1016/j.gep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski T, Ahn DG, Schilling TF, Nair S, Ruvinsky I, Geisler R, Rauch GJ, Haffter P, Zon LI, Zhou Y, Foott H, Dawid IB, Ho RK. The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development. 2003;130:5043–5052. doi: 10.1242/dev.00704. [DOI] [PubMed] [Google Scholar]
- Piotrowski T, Nusslein-Volhard C. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio) Dev Biol. 2000;225:339–356. doi: 10.1006/dbio.2000.9842. [DOI] [PubMed] [Google Scholar]
- Reagan FP. The role of the auditory sensory epithelium in the formation of the stapedial plate. J. Exp. Zool. 1917;23:85–108. [Google Scholar]
- Ruhin B, Creuzet S, Vincent C, Benouaiche L, Le Douarin NM, Couly G. Patterning of the hyoid cartilage depends upon signals arising from the ventral foregut endoderm. Dev Dyn. 2003;228:239–246. doi: 10.1002/dvdy.10380. [DOI] [PubMed] [Google Scholar]
- Schimmang T. Expression and functions of FGF ligands during early otic development. Int J Dev Biol. 2007;51:473–481. doi: 10.1387/ijdb.072334ts. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Semba I, Nonaka K, Takahashi I, Takahashi K, Dashner R, Shum L, Nuckolls GH, Slavkin HC. Positionally-dependent chondrogenesis induced by BMP4 is co-regulated by Sox9 and Msx2. Developmental dynamics : an official publication of the American Association of Anatomists. 2000;217:401–414. doi: 10.1002/(SICI)1097-0177(200004)217:4<401::AID-DVDY7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Shigetani Y, Nobusada Y, Kuratani S. Ectodermally derived FGF8 defines the maxillomandibular region in the early chick embryo: epithelial-mesenchymal interactions in the specification of the craniofacial ectomesenchyme. Dev Biol. 2000;228:73–85. doi: 10.1006/dbio.2000.9932. [DOI] [PubMed] [Google Scholar]
- Wood JL, Hughes AJ, Mercer KJ, Chapman SC. Analysis of chick (Gallus gallus) middle ear columella formation. BMC Dev Biol. 2010;10:16. doi: 10.1186/1471-213X-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Eberspaecher H, Lefebvre V, De Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]