Abstract
Administration of the exogenous nitric oxide (NO) donor dipropylenetriamine-NONOate (DPTA-NO) to mice during Plasmodium berghei ANKA (PbA) infection largely prevents development of experimental cerebral malaria (ECM). However, a high dose (1mg/mouse twice a day) is necessary and causes potent side effects such as marked hypotension. In the present study we evaluated whether an alternative, physiologically relevant NO donor, S-nitrosoglutathione (GSNO), was able to prevent ECM at lower doses with minimal side effects. Prophylactic treatment with high (3.5mg), intermediate (0.35mg) or low (0.035mg) doses of GSNO decreased incidence of ECM in PbA-infected mice, decreasing also edema, leukocyte accumulation and hemorrhage incidence in the brain. The high dose inhibited parasite growth and also induced transient hypotension. Low and intermediate doses had no or only mild effects on parasitemia, blood pressure, and heart rate compared to saline-treated mice. PbA infection decreased brain total and reduced (GSH) glutathione levels. Brain levels of oxidized (GSSG) glutathione and the GSH/GSSG ratio were positively correlated with temperature and motor behavior. Low and intermediate doses of GSNO failed to restore the depleted brain total glutathione and GSH levels, suggesting that ECM prevention by GSNO was probably related to other effects such as inhibition of inflammation and vascular protection. These results indicate that ECM is associated with depletion of the brain glutathione pool and that GSNO is able to prevent ECM development in a wide range of doses, decreasing brain inflammation and inducing milder cardiovascular side effects.
Keywords: cerebral malaria, nitric oxide, S-nitrosoglutathione, Plasmodium berghei
Introduction
Malaria still imposes a heavy burden to a large fraction of the World population. Estimates by the World Health Organization points to the occurrence of 225 million cases of malaria worldwide in 2010, with 709,000 deaths, 85% of which occurring in children under 5 years of age (WHO 2010). Cerebral malaria (CM) is one of the most lethal complications of malaria by Plasmodium falciparum and despite effort in this area there are no currently available adjunctive therapies (John et al. 2010). Experimental (murine) cerebral malaria (ECM) caused by Plasmodium berghei ANKA (PbA) is characterized by brain microcirculatory complications leading to vasoconstriction, marked decreases in cerebral blood flow and eventually vascular collapse (Cabrales et al. 2010). These complications are associated with a state of low nitric oxide (NO) bioavailability likely caused by the NO-scavenging action of gram amounts of cell-free hemoglobin in the plasma consequent to red blood cell (RBC) lysis during the parasite cycle, which can be reverted by the administration of high doses of the potent NO-donor dipropylenetriamine-NONOate (DPTA-NO) (Gramaglia et al. 2006). Treatment with DPTA-NO during PbA infection reduces ECM incidence, improves cerebral blood flow and venous return, prevents blood brain barrier leakage, and decreases hemorrhage incidence, leukocyte and platelet adherence, and expression of endothelial cell adhesion molecules including ICAM-1 and P-selectin in the brain (Cabrales et al. 2011; Zanini et al. 2011). Protection is also associated with decreased levels of inflammatory markers in the plasma and restoration of cyclic guanosine monophosphate levels in the brain (Gramaglia et al. 2006).
Although DPTA-NO has been valuable in providing proof of concept for the role of NO in ECM, its further use to explore potential therapeutic applications of this concept is hindered by a number of limitations. The high dose of DPTA-NO effective in preventing ECM (1mg/mouse), combined with a fast and short-lived release of NO by DPTA-NO in aqueous solutions, results in the generation of a huge amount of NO in a short period of time, which leads to the occurrence of acute side effects such as marked hypotension (Gramaglia et al. 2006). We have been researching alternative strategies to increase NO bioavailability during PbA infection, such as arginine and/or tetrahydrobiopterin supplementation, with or without arginase inhibition with nor-NOHA, but such compounds were not effective in preventing ECM, at least in the doses and schemes used. Inhibition of phosphodiesterase 5 (PDE-5) by sildenafil was not effective by itself, however sildenafil greatly lowered the amount of DPTA-NO needed to prevent ECM, and resulted in milder side effects, probably due to prolongation of the downstream effects of NO by maintenance of cyclic guanosine monophosphate (cGMP) levels (Martins YC, Zanini GM, Frangos JA, Carvalho LJ. Efficacy of different nitric oxide-based strategies in preventing experimental cerebral malaria by Plasmodium berghei ANKA, manuscript submitted). These data show that strategies to decrease the amount of exogenous NO needed to prevent ECM and its consequent side effects are feasible. Based on these results, we anticipated that slower-releasing NO-donors might also prove to be effective in preventing ECM by restoring the endogenous NO pools and improving endothelial and vascular function while generating mild side effects.
In the present study, we evaluated the efficacy of the nitrosothiol S-nitrosoglutathione (GSNO) in preventing ECM in PbA-infected mice. GSNO has been shown to be a potent neuroprotective agent against stroke in rats, whereas fast-release NO-donors such as S-nitroso-N-acetyl-penicillamine, sodium nitroprusside, methylamine-hexamethylene-methylamine-NONOate, propylamine-propylamine-NONOate, and 3-morpholinosydnonimine (SIN-1) had limited or no protective effect (Khan et al. 2006). In addition to being a widely used NO-donor in a number of experimental studies with therapeutic purposes in brain inflammatory conditions (Khan et al. 2005; Khan et al. 2006; Haq et al. 2007; Nath et al. 2010), GSNO has the advantage of being a naturally-occurring, physiologically-relevant molecule. GSNO is an important endogenous reservoir and transporter of NO, contributing to the regulation of NO physiology (Zeng et al. 2001; Zhang and Hogg 2002). In fact, GSNO as well as L-glutathione reduced (GSH) and oxidized (GSSG) are potent endogenous anti-oxidant agents which were considered candidates for the prevention of ECM, a disease characterized by a state of oxidative stress (Wiese et al. 2006). We tested different concentrations of GSNO to evaluate its effect on survival and on critical parameters such as brain edema and inflammation, blood pressure and heart rate.
Methods
Animals, parasite and infection
Animal handling and care followed the NIH Guide for Care and Use of Laboratory Animals. All protocols were approved by the La Jolla Bioengineering Institutional Animal Care and Use Committee. Six to eight-week old C57Bl/6 (Jackson Laboratories, ME) were inoculated intraperitoneally (IP) with 1×106 Plasmodium berghei ANKA (PbA) parasites expressing the green fluorescent protein (a donation from the Malaria Research and Reference Reagent Resource Center – MR4, Manassas, VA; deposited by CJ Janse and AP Waters; MR4 number: MRA-865). Motor behavior and rectal temperature were checked daily from day 4 as previously described (Clemmer et al. 2011). Briefly, a set of six behavioral tests (transfer arousal, locomotor activity, tail elevation, wire maneuver, contact righting reflex and righting in arena) adapted from the SHIRPA protocol (Lackner et al. 2006; Martins et al. 2010) was used to provide a better estimate of the overall clinical status of the mice during infection. The performance in each test was assessed and a composite score was built ranging from 0 to 23, where 23 indicates maximum performance and 0 indicates complete impairment – usually coma. Body temperature was monitored using an Accorn Series Thermocouple thermometer with a mouse rectal probe (Oakton Instruments, Vernon Hills, IL). ECM was defined as the presentation of one or more of the following clinical signs of neurological involvement: ataxia, limb paralysis, , seizures, roll-over, coma. Parasitemia was determined by flow cytometry by detecting the number of fluorescent GFP-expressing pRBCs in relation to 10,000 RBCs.
Drugs and Treatments
GSNO and DPTA-NO were purchased from Cayman Chemical (Ann Arbor, MI). PbA-infected mice were treated twice a day starting on day 0 of infection until day 8 with freshly prepared GSNO or saline. Groups of mice received doses of GSNO of 3.5, 0.35 or 0.035mg per mouse, in saline, via IP, 100µL per mouse. These doses are equivalent in NO generation to the dose of DPTA-NO (1mg) shown to be effective in preventing ECM, as well as 10 times and 100 times lower, respectively. Treatment in all groups was discontinued after day 8 and survivor mice were followed up to day 12 of infection.
Exhaled NO, plasma nitrite levels and hematocrit
For determination of exhaled NO levels, one hour after the morning treatment on day 5 post infection mice were held for 1 minute in a plastic chamber connected to a Sievers 280i NO-analyzer (Sievers, Boulder, CO). Air accumulated for one minute in the chamber was released by opening the ports and the NO content of an exhaled air sample was measured in parts per billion by the NO-analyzer. On day 6 of infection, 30µL of blood was collected from the tail vein in a heparinized micro-hematocrit capillary tube (Chase, Rockwood, TN) and centrifuged. Hematocrit was measured and the plasma was separated and frozen at −20°C. Later, plasma was thawed, mixed with an equal amount of methanol to precipitate proteins and centrifuged at 10,000g for 10 min. Supernatant was collected and frozen (−80°C) until reading. Nitrite plasma content was measured using an ENO-20 NOx Analyzer (Eicom, San Diego, CA) according to the manufacturer’s instructions.
Measurements of brain glutathione (GSH-GSSG) levels
Total glutathione, GSH, GSSG concentrations in brain tissue were measured using enzyme-linked assay kits (Cayman Chemical, Ann Arbor, MI) in accordance with the manufacturer’s instructions. Mice were anesthetized (ketamine 150 mg/kg; xylazine 10 mg/kg), the thoracic cavity was opened, and perfusion was made with 5 ml of saline using a gravity perfusion setup. The brain was carefully removed, weighed and one of the hemispheres was processed for the measurement of total glutathione (GSH+GSSG) and GSSG. The brain sample was homogenized in 5 ml of suggested cold buffer per gram of tissue using a T25 basic electric homogenizer (IKA Works, Wilmington, NC). The precipitate was removed by centrifugation and supernatant was collected and frozen (−80°C) until assaying.
Histology
Infected animals treated with saline or GSNO were euthanized at day 6 post-infection and their brains and spleens collected and processed for histology. The brain was carefully collected in the same way described for glutathione and cGMP measurement, weighed, and stored in 10% paraformaldehyde during 48 hours for fixation. Brains were cut in four coronal slices of 2–3mm using a mouse brain blocker (David Kopf Instruments, Tujunga, CA) and each slice was embedded in paraffin. Sections of 5µm were obtained at approximate 400µm intervals (four sections per slice), mounted in glass slides, and stained with hematoxylin-eosin (HE). The number of leukocytes in each meningeal vessel, the number of tamponated vessels in parenchyma, and the number and area of hemorrhages of each section was quantified using an ocular grid calibrated with a 100× magnification (field dimensions: 200 × 180µm) in a Nikon microscope (Eclipse E200). The whole area of each section was similarly quantified with the grid calibrated at 40× magnification. Quantification was performed by an experienced investigator in a blinded fashion. Pictures were taken with a SPOT camera (Diagnostic Instruments Inc. USA; 1014 × 721 pixels).
Cardiovascular parameters
To evaluate the effects of GSNO on cardiovascular physiology, remote measurement of heart rate and blood pressures (systolic, diastolic and mean arterial pressures) was made with Data System International telemetry devices (DSI, St. Paul, MN) on individually housed mice at room temperature as described before (Astrand et al. 2004). Briefly, following anesthesia an incision was made in the neck exposing the left carotid artery and the catheter tip of a TA11PA-C20 unit was inserted into the vessel and secured with 5-0 suture. The transducer device was then placed subcutaneously in the animal’s back and the incision was stapled. The animals received analgesics and were allowed to recover for a minimum of 5 days. Before drug administration, animals were allowed to acclimate on the telemetry platform for 30 minutes. Each animal then received an injection of saline or GSNO at different concentrations and were immediately returned to the telemetry platform. Hemodynamic parameters were continuously recorded starting 30 minutes before to 60 minutes after injection. During the baseline and experimental periods mice were left alone in the procedure room providing a noise free environment.
Statistical analyses
Results were expressed as mean and standard error of the mean unless otherwise stated. The log-rank test was used to compare different survival curves followed by post hoc log-rank tests for comparisons between different treatment groups. Two-way ANOVA with Bonferroni posttests was used to analyze parasitemia. Curve analyses were made only at days 4–6 post-infection because mice started to die from cerebral malaria after this period, introducing bias in the sample. Temperature, motor behavior, hematocrit, exhaled NO, plasma nitrite, body and brain weight, and brain histopathology parameters were initially analyzed using one-way ANOVA or Kruskal-Wallis test if the variances between groups differed significantly (Bartlett`s test for equal variances). Body and brain weight, temperature, behavior, exhaled NO, and plasma nitrite data were further analyzed by planned post hoc t-tests with Welch`s correction for comparisons between different treatment groups. Due to the high number of comparisons, post-tests p-values were corrected using Šidák-Holm’s p-value adjustment, to reduce the number of false positive tests. Tukey's multiple comparison test was used to check for differences in hematocrit and brain histopathology parameters between experimental groups. When multiple concentrations of GSNO were tested, post-tests to check for linear trend following log-rank, one-way ANOVA, and Kruskal-Wallis tests were also performed. Spearman correlation coefficients and two-tailed p-values were calculated to estimate the degree and significance of association between two parameters. A p-value <0.05 was considered significant. Heart rate, systolic, diastolic, and pulse pressures data were averaged as 10 seconds bins for each animal and the average for each bin from the same time point in each group was determined. Means from bins representing the first 30 minutes before injection were then averaged to calculate a baseline for each group. Data from each bin was converted and plotted as percentage of baseline. Lowess curves were then calculated to show the trend of the data in each group. Normal ranges for each parameter were calculated based in values obtained from four saline treated animals. The range of values falling within the mean plus and minus two standard deviations (SD) was considered normal and treatments that decrease or increase the parameter values outside this range were considered to affect the parameter. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, San Diego, CA) and Stata 9.0 (StataCorp LP, College Station, TX).
Results
GSNO protects against ECM
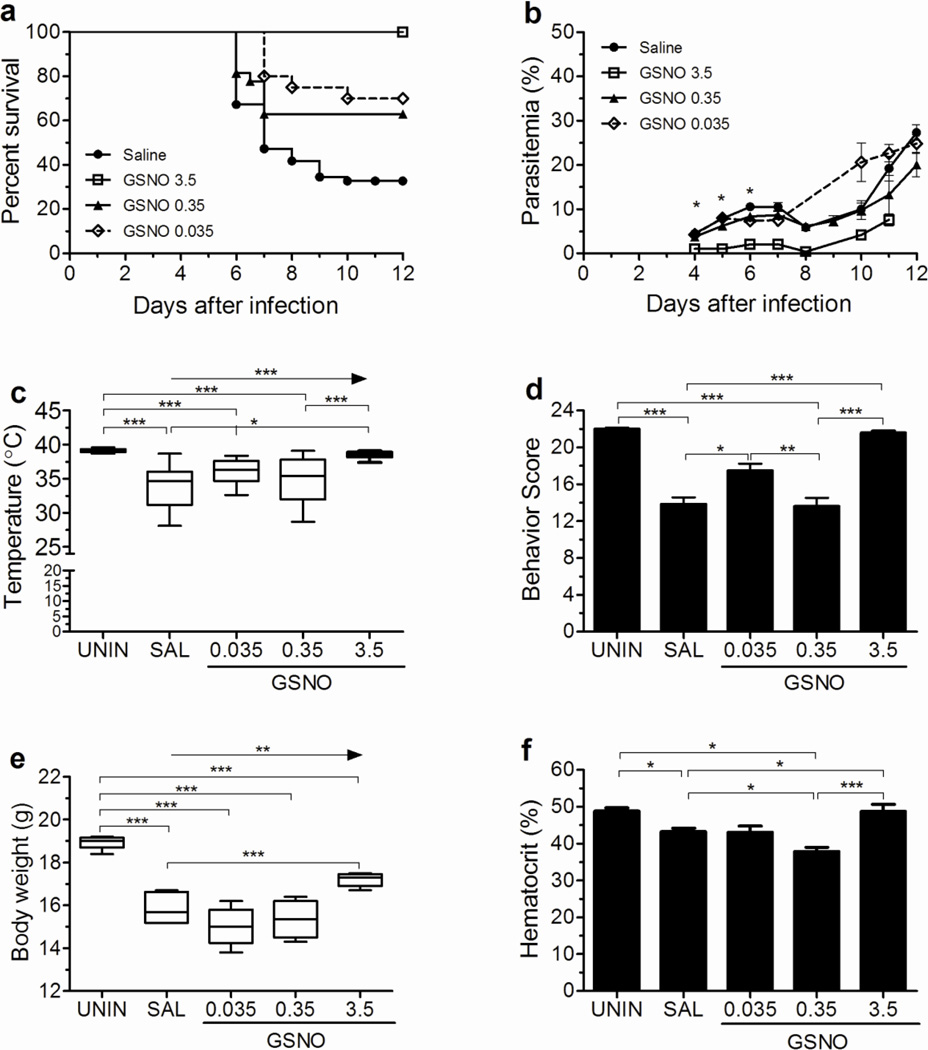
Treatment with GSNO resulted in significant protection against the development of ECM with the three doses used (3.5mg, 0.35mg and 0.035mg) (Fig. 1a). The higher dose (3.5mg/mouse) was chosen to be equivalent in NO molar terms to the dose of DPTA-NO (1mg/mouse) shown to afford protection against ECM. GSNO at doses 10 times and 100 times lower was also able to afford significant protection against ECM (Fig. 1a). Survival curves of mice supplemented with 0.035 or 0.35 mg were not significantly different, indicating that both doses were equally efficient to prevent ECM (Fig. 1a). Treatment with 3.5mg per mouse caused inhibition of parasite growth whereas the low and intermediate doses of GSNO had no effect on the course of parasitemia compared to saline-treated mice (Fig. 1b). The decrease in parasite growth caused by the high dose of GSNO also decreased disease morbidity as shown by the maintenance of temperature and behavior score at similar levels to uninfected mice and the mild decrease in body weight by day 6 of infection (Fig. 1c–e). Supplementation with 0.035 and 0.35 mg per mouse showed a trend to improve temperature and body weight in a dose response manner (Fig. 1c and 1e). However, a similar pattern was not observed for the behavior score with mice treated with the intermediate dose not showing improvement in this parameter at day 6 of infection (Fig. 1d). Treatments with the different doses of GSNO had opposite effects on hematocrit. The group of mice receiving the higher dose presented hematocrit levels similar to uninfected control animals (Fig. 1f). This effect on preventing RBC destruction can be ascribed to the inhibitory effect on parasite growth induced by this dose. On the other hand, the group receiving the intermediate dose of GSNO had hematocrit levels significantly lower compared to the group receiving saline (Fig. 1f), despite similar levels of parasitemia. The lower dose of GSNO had no effect on hematocrit compared to saline.
Figure 1. GSNO prevents ECM development.
Cumulative survival (A), course of parasitemia (B), rectal temperature (C), and motor behavior score (D) of PbA-infected mice treated with saline (n=55) or GSNO at 0.035 (n=18), 0.35 (n=28), and 3.5 (n=20) mg/mouse. Body weight (E, n=4–6 per group) and hematocrit (F, n=cumulative survival) were also evaluated. Rectal temperature, motor behavior score, body weight and hematocrit were measured on day 6 of infection. *p<0.05, **p<0.01, ***p<0.001, arrows indicate the presence of a linear trend.
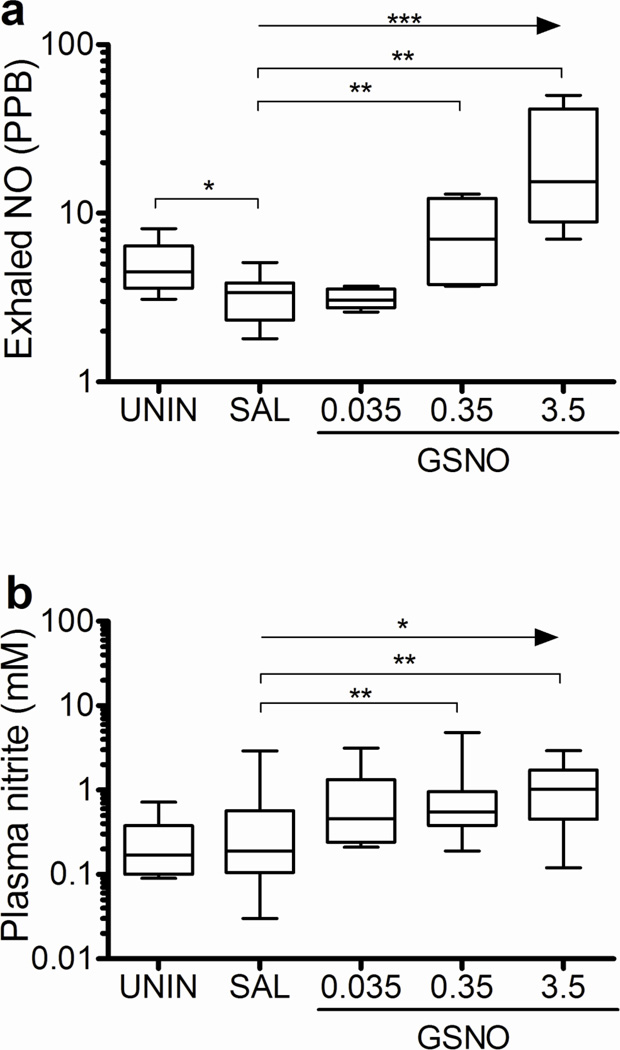
Effects of GSNO on exhaled NO and plasma nitrite levels
Treatment with GSNO resulted in a trend for increased levels of exhaled NO and plasma nitrite (Fig. 2a,b). However, the increase in exhaled NO and plasma nitrite in relation to the saline group was statistically significant only for the intermediate and higher dose GSNO-treated mice (Fig. 2a,b). As expected mice receiving saline presented low levels of exhaled NO when compared to uninfected mice (Fig. 2a). These results indicate that GSNO supplementation of PbA-infected mice resulted in increased NO bioavailability in a dose response manner.
Figure 2. GSNO increases NO bioavailability in PbA infected mice.
Exhaled NO (A) of uninfected and PbA-infected mice treated with saline or GSNO at 0.035, 0.35, and 3.5 mg/mouse was measured 1 hour after the morning treatment on day 5 of infection (n≥5 per group). Plasma nitrite (F) of uninfected and PbA-infected mice treated with saline or GSNO at 0.035, 0.35, and 3.5 mg/mouse was measured on samples collected prior to the morning dosing on day 6 of infection (n≥7 per group). *p<0.05, **p<0.01, ***p<0.001, arrows indicate the presence of a linear trend.
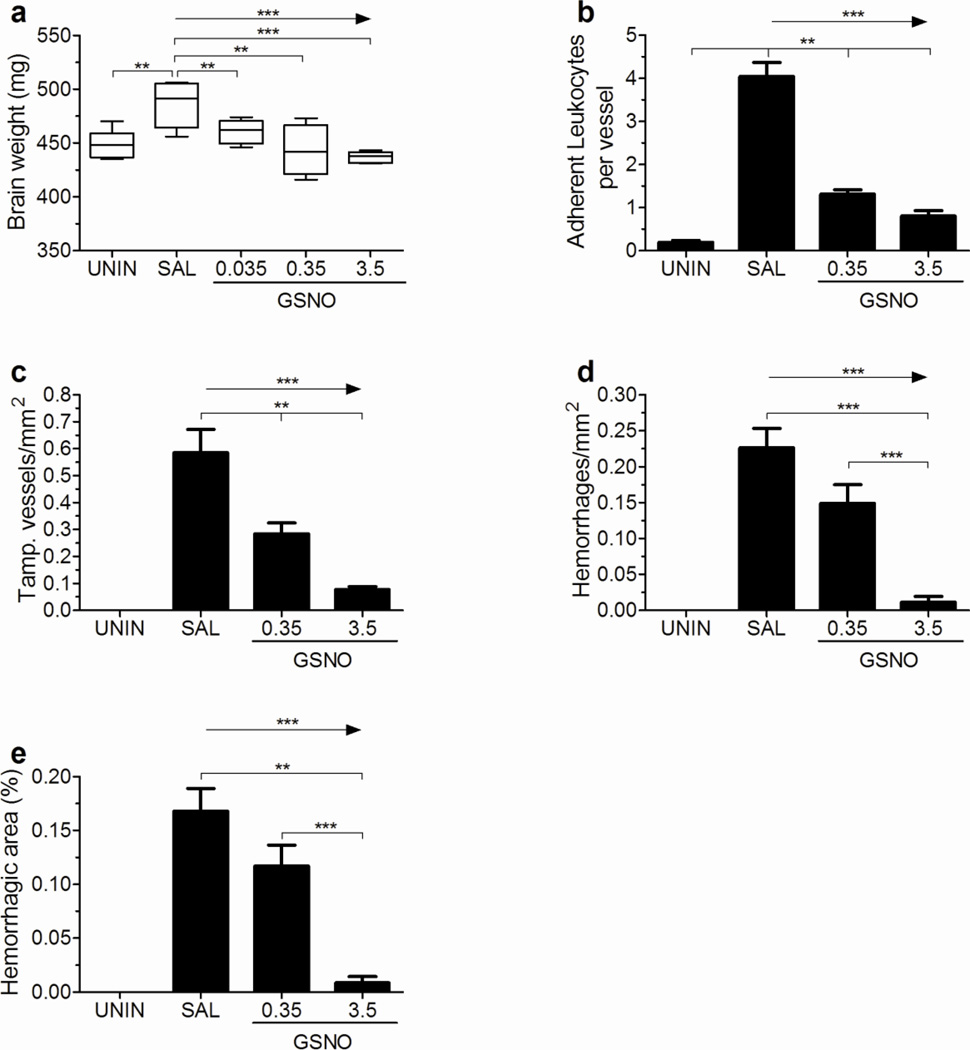
GSNO supplementation prevents brain edema and decreases brain inflammation and hemorrhages
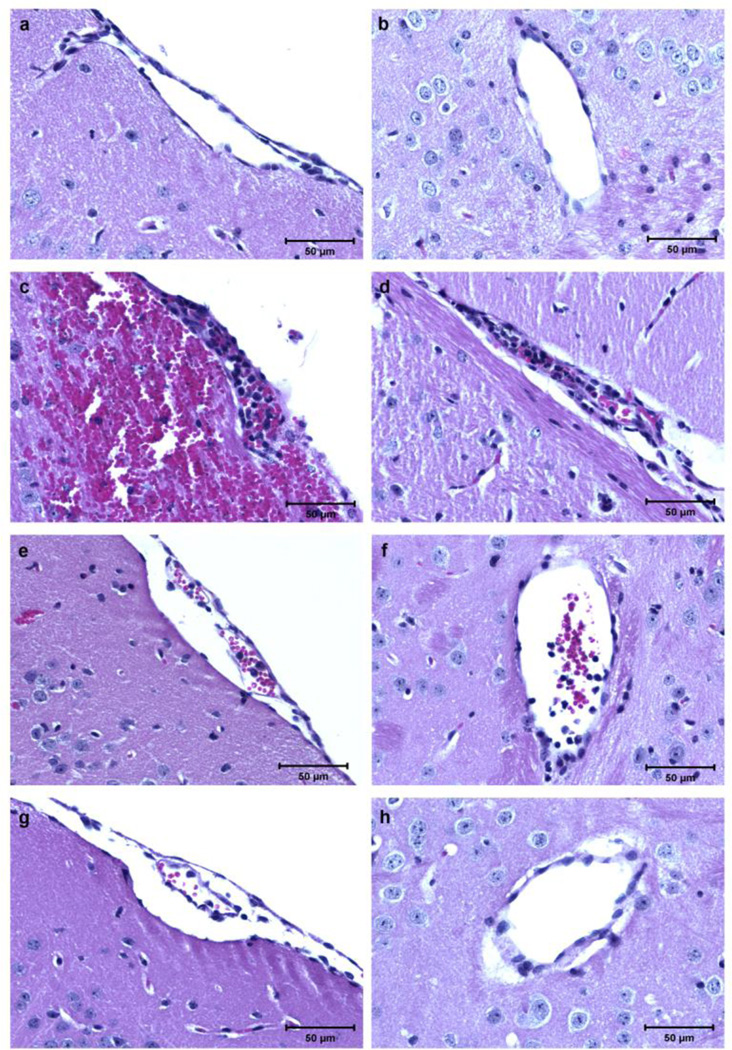
PbA-infected mice showed brain edema on day 6 of infection, which was prevented by any of the three doses of GSNO, with a trend for less edema with increasing GSNO doses (Fig. 3a). GSNO treatment also prevented or decreased the accumulation of leukocytes in brain vessels (Fig. 3b,c) and the occurrence of brain hemorrhages (Fig. 3d,e), again with a trend for more robust effects with increasing GSNO doses. Representative brain histology images from uninfected mice and infected mice given saline or GSNO (0.35 and 3.5 mg/mouse) are shown in Figure 4a–h. Interestingly, the few adherent leukocytes in mice receiving 3.5mg of GSNO were observed mostly in meningeal vessels, being the vast majority of brain parenchymal vessels in this group totally clean (Figures 4g,h).
Figure 3. GSNO decreases brain edema and inflammation in PbA infected mice.
Mice receiving GSNO at 0.35 and 3.5 mg/mouse did not show an increase in total brain weight (A) upon infection. Treated mice also presented a smaller number of adherent leukocytes per meningeal vessel (B), a lesser number of tamponated vessel in brain parenchyma (C), and a small hemorrhagic burden as reflected by a decreased number of hemorrhages/mm2 (D) and hemorrhagic area (E, defined as the area occupied by hemorrhages in relation to the total area analyzed) of brain parenchyma in a dose response manner on day 6 of infection. n= 4–5 per group, *p<0.05, **p<0.01, ***p<0.001, arrows indicate the presence of a linear trend.
Figure 4. Inhibition of inflammation in the brain of PbA-infected mice treated with GSNO.
(A and B) Pial (A) and parenchymal (B) vessels of uninfected control mice, showing no accumulation or adhesion of leukocytes; (C and D) pial (C) and parenchymal (D) vessels of mice receiving saline with ECM, plugged with leukocytes (black arrows); an extensive subarachnoid hemorrhage is seen in panel C (white arrows); (E and F) pial (C) and parenchymal (F) vessels of mice receiving GSNO at 0.35 mg/mouse showing a less intense leukocyte adhesion (black arrows); (G and H) pial (G) and parenchymal (H) vessels of mice receiving GSNO at 3.5 mg/mouse, a small number of adherent leukocytes is shown in panel G (black arrows), and a clean parenchymal vessel is shown in panel H. Magnitude of leukocyte accumulation and adherence was heterogeneous among vessels in mice receiving saline and GSNO at 0.35 and 3.5 mg/mouse. All sections were stained with H&E (magnification, ×400).
Effect of PbA infection on brain total glutathione, GSH, and GSSG levels
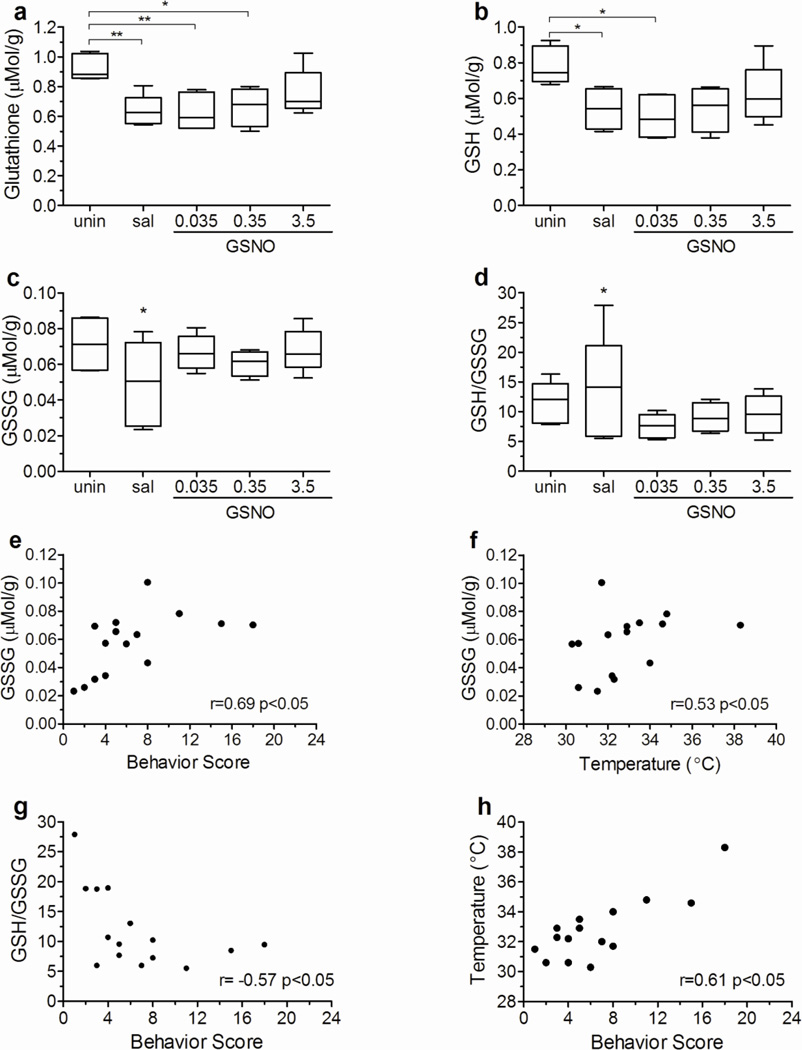
PbA-infected mice receiving saline showed a marked decrease in brain total glutathione and GSH levels on day 6 of infection (Fig. 5a,b). Treatment with the lower and intermediate doses of GSNO did not affect total glutathione and GSH levels in the brain of infected animals, but mice receiving the higher dose showed an improvement in brain GSH levels (Fig. 5a,b). Mean GSSG levels and GSH/GSSG ratio did not differ among the groups on day 6 of infection (Fig. 5c,d). However, the variance in these parameters in mice receiving saline was significantly greater than in uninfected and GSNO-supplemented mice (p<0.05) indicating that the former population was not homogeneous. A correlation matrix was built to test if brain GSH and GSSG levels in saline-treated mice on day 6 of infection could be related to body and brain weight, behavior score, temperature or parasitemia. Brain GSSG levels were positively correlated with behavior score and temperature (Fig. 5e,f), GSH/GSSG ratio was negatively correlated with behavior score (Fig. 5g), and, as expected, temperature was positively correlated with behavior score (Fig. 5h). Interestingly GSH levels were not correlated with behavior score or temperature. These data suggest that the brain of mice with ECM is seriously affected by a loss of its anti-oxidant defense mechanisms, with a marked decrease in total glutathione and GSH levels, and GSSG levels appear to be particularly an indicator of disease severity.
Figure 5. PbA infection decreases brain total glutathione and GSH levels, but GSSG levels and GSH/GSSG ratio are correlated with ECM development.
Brain levels of total glutathione (A), GSH (B), and GSSG (C) and GSH/GSSG ratio of uninfected and PbA infected mice receiving saline or GSNO at 0.035, 0.35, and 3.5 mg/mouse. Brain GSSG levels were positively correlated with motor behavior score (E) and temperature (F), and negatively correlated with the GSH/GSSG ratio (G) in PbA infected mice. Temperature was also positively correlated with motor behavior score (H). All measurements were made on day 6 of infection. *p<0.05, **p<0.01, r= spearman correlation coefficient.
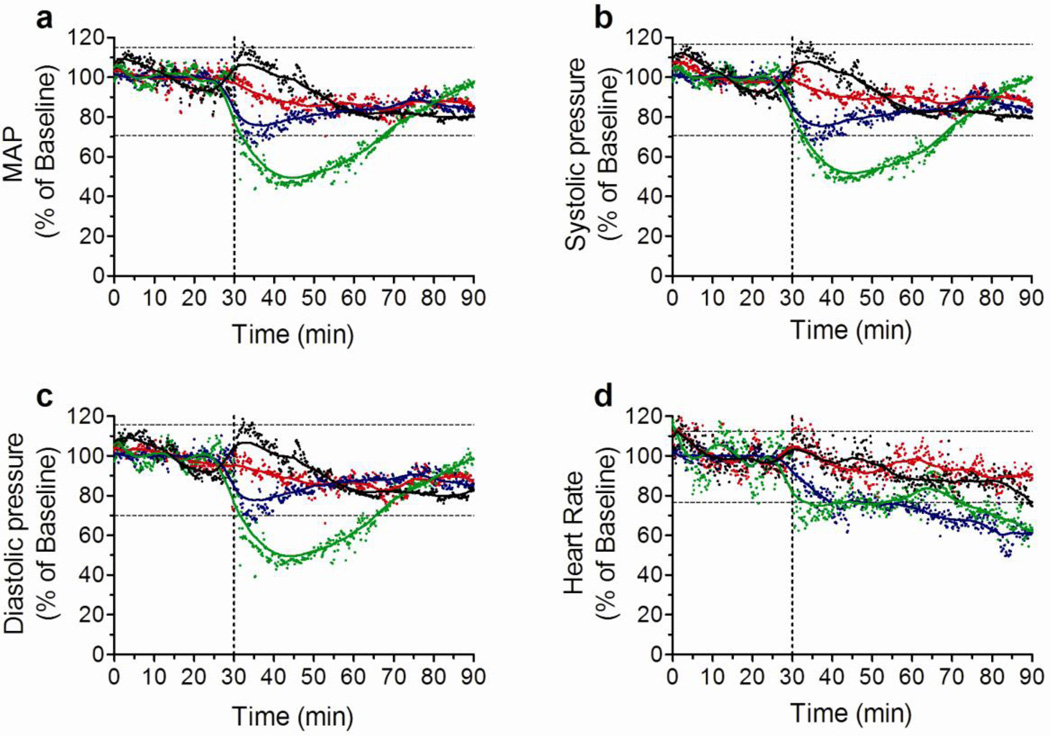
Low and intermediate doses of GSNO do not induce hypotension
Injection of the higher (3.5mg) dose of GSNO induced a sharp but transient decrease in mean arterial pressure (MAP) (Fig. 6a). By 60 minutes MAP returned to normal. A similar pattern was observed for the systolic and diastolic pressures (Fig. 6b,c), whereas heart rate presented a less abrupt but longer lasting decrease (Fig. 6d). The intermediate dose induced no significant changes in MAP or systolic and diastolic pressures (Fig. 6a–c), however changes in heart rate and pulse pressure were similar to those induced by the higher dose. Saline injection as well as the lower dose of GSNO caused no changes in any of the parameters (Fig. 6a–d).
Figure 6. GSNO effects on cardiovascular parameters.
Changes in mean arterial pressure (MAP, A), systolic pressure (B), diastolic pressure (C), and heart rate (D) following one IP injection of saline (black dots and lines), and GSNO at 0.035 (red dots and lines), 0.35 (blue dots and lines), and 3.5 (green dots and lines). Vertical doted lines represent the time when the IP injection was given and separate the baseline period from the experimental period. Results are expressed as the percentage change in relation to the mean of the baseline period for each group. Horizontal doted lines represent the range of values falling within the mean plus and minus two standard deviations (SD) of the baseline value calculated for saline treated group. N = 4 per group.
Discussion
Both human and murine CM have been shown to be associated with endothelial dysfunction and brain microcirculatory complications including vascular occlusion, reduced blood flow, vascular collapses or ghost vessels, vasoconstriction and inflammation (Beare et al. 2009; Cabrales et al. 2010). In ECM, exogenous NO has been shown to be beneficial in preventing the syndrome (Gramaglia et al. 2006; Cabrales et al. 2011). Nevertheless, the NO donor used in the murine studies, DPTA-NO, presents a number of limitations including rapid clearance and the induction of potent side effects including a major drop in arterial pressure. While it remains a useful tool to increase NO bioavailability in mechanistic studies, the adverse effects limit its clinical potential. We considered whether GSNO could be a better alternative NO-donor based on the fact that GSNO is an important endogenous reservoir and carrier of NO, contributing to regulate its physiological roles (Chiueh and Rauhala 1999).
Physiologically, GSNO is stable and will release NO only under certain conditions, and its auto-oxidation is slow (Singh et al. 1996; Zhang and Hogg 2002). Much of the NO in GSNO can actually be used to transnitrosylate protein thiols which can act either as an endogenous reservoir for NO extending its bioavailability or have physiological roles on their own (Radomski et al. 1987; Foster et al. 2009).
Here we showed that GSNO was indeed superior to DPTA-NO, as it was able to decrease ECM incidence and increase survival of PbA-infected mice when administered at doses 10–100 times lower than the comparable effective dose of DPTA-NO. While the higher dose of GSNO (3.5mg per mouse) induced transient hypotension as does DPTA-NO, the lower and intermediate doses had no or only mild effects on blood pressure or heart rate. These results make GSNO a more promising candidate to be used with the purpose of restoring NO bioavailability. It should be stressed, however, that the present study was not designed to verify its potential efficacy as an adjunctive therapy in ECM, and additional studies are necessary to address this issue.
GSNO has several properties that may be relevant for its protective effect on ECM, which may be linked to its NO moiety but may also be modulated by, or independent of, its glutathione backbone. ECM is associated with endothelial dysfunction, vascular obstruction, decreased cerebral blood flow and a vasospasm-like phenomenon develops in the pial vessels (Cabrales et al. 2010). Diffuse microhemorrhages and eventually larger sub-arachnoid hemorrhages are observed (Carvalho et al. 2000; Cabrales et al. 2011). These features can be ameliorated by exogenous NO (DPTA-NO) and therefore GSNO may act similarly. These ECM features also resemble stroke, and GSNO has been shown to be beneficial in experimental stroke by reducing oxidative stress and inhibiting the activation of both endothelial cells and macrophages in the brain (Khan et al. 2005). In addition, GSNO treatment increased cerebral blood flow, decreased mRNA expression of E-selectin, and inhibited the expression of inducible NOS, TNF-α, IL-1β, ICAM-1, and LFA-1 in a rat model of stroke (Khan et al. 2006).
ECM is also characterized by brain inflammation, with increased expression of cell adhesion molecules and leukocyte and platelet migration/adherence to brain vessels (Carvalho et al. 2000; Bauer et al. 2002; Cabrales et al. 2010, 2011). We showed that GSNO supplementation decreased brain leukocyte accumulation and edema during PbA infection, indicating that some of these anti-inflammatory mechanisms could be claimed to explain the phenomenon. GSNO has indeed been shown to attenuate central nervous system inflammatory syndromes such as experimental autoimmune encephalomyelitis (Nath et al. 2010) as well as experimental autoimmune uveitis (Haq et al. 2007). Furthermore, GSNO is also an inhibitor of platelet aggregation (Radomski et al. 1992).
Oxidative stress, particularly affecting brain endothelial cells, plays an important role in ECM (Wiese et al. 2006). Here we show that PbA-infected mice presented marked decreases in the brain levels of GSH, indicating that indeed infection depleted one of the major antioxidant and redox buffer systems in the brain (Aoyama et al. 2008), which likely contributes significantly to ECM pathogenesis. GSNO is a potent antioxidant against peroxynitrite and protects neurons against oxidative stress (Rauhala et al. 1998). However, our data suggest that the protective effect of GSNO was not derived directly from the restoration of GSH pools in the brain since animals treated with the lower and intermediate doses still presented glutathione depletion. Indeed, it has been shown that GSNO is not able to cross the blood brain barrier when administered i.p. in healthy mice (Peyrot et al. 2005). Accordingly, only 0.5% of radiolabeled GSH administered by intra-carotid injection was detectable in brain extracts (Cornford et al. 1978). Taken together these data suggest that the resistance to ECM development conferred by GSNO supplementation is likely related to effects for instance on the endothelium, an interpretation supported by the decreased level of inflammation observed in GSNO-treated mice. In addition, 8-oxoguanine staining of the brain revealed that oxidative stress during ECM affects mainly the endothelial cells (Wiese et al. 2006). The effect of the higher GSNO dose in preventing glutathione depletion was likely secondary to the anti-malarial activity of this dose.
Alternatively, GSNO promotes the formation of S-nitroso-(bCys93) hemoglobin (SNO-Hb) which right shifts the oxygen dissociation curve of hemoglobin favoring the oxygen offload and providing an alternative, vasorelaxation-independent mechanism for improving oxygen delivery (Patel et al. 1999). For example, SNO-Hb can mediate oxygen tension (pO2)-dependent vasodilatory activity (Singel and Stamler 2005). The fact that GSNO or nitrosylated proteins store NO making it available upon demand may help to explain why only the intermediate and high doses of GSNO were able to generate detectable increased levels of exhaled NO and plasma nitrite. In addition, although GSNO is considered an NO-donor, in vitro NO generation accounts for only a part of the decomposition products of GSNO by endothelial cells (Zeng et al. 2001) and nitrosothiols may exert its biological effects through NO-independent mechanisms.
The higher dose of GSNO also showed a strong anti-parasite effect, keeping parasitemia low compared to saline-treated animals. It has been previously shown that GSNO as well as other NO donors can have anti-plasmodial effects in vitro (Rockett et al. 1991; Totino et al. 2008). Interestingly, treatment with DPTA-NO, which generates similar amounts of NO, does not inhibit PbA growth in vivo (Gramaglia et al. 2006; Cabrales et al. 2011). The reasons for the differential anti-parasite effect of these two donors are unclear. Malaria parasites grow readily in saturated NO solutions but are highly sensitive to S-nitrosothiols (Rockett et al. 1991). In any case, the fact that GSNO presents anti-plasmodial activity also adds to its potential for anti-malarial therapies.
In summary, the present study shows that GSNO in a wide range of doses can prevent the development of CM in PbA-infected mice. At a higher dose it can also inhibit parasite growth but induces transient hypotension. Lower doses are also effective in preventing CM without inhibiting parasite growth and without changing blood pressure. These features reveal that GSNO plays a relevant role in ECM pathogenesis. While it is clear that the data shown here with GSNO used as a prophylactic intervention in ECM allow no inferences about its potential in a clinically relevant setting as an adjunctive therapy intervention, the characteristics above make GSNO a promising compound for further studies to determine its efficacy in CM-rescuing treatments with anti-malarial drugs.
Acknowledgements
This study was supported by NIH grants R01-HL087290 and R01-AI082610. GMZ was recipient of a CNPq (Brazil) post-doctoral fellowship. We thank Dr John Nolan (LJBI) for granting access to the flow cytometry facilities, Wisam Barkho for performing surgeries to implant the TA11PA-C20 units, and Diana Meays for animal care.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108(3):227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- Astrand A, Bohlooly YM, Larsdotter S, Mahlapuu M, Andersen H, Tornell J, Ohlsson C, Snaith M, Morgan DG. Mice lacking melanin-concentrating hormone receptor 1 demonstrate increased heart rate associated with altered autonomic activity. Am J Physiol Regul Integr Comp Physiol. 2004;287(4):R749–R758. doi: 10.1152/ajpregu.00134.2004. [DOI] [PubMed] [Google Scholar]
- Bauer PR, Van Der Heyde HC, Sun G, Specian RD, Granger DN. Regulation of endothelial cell adhesion molecule expression in an experimental model of cerebral malaria. Microcirculation. 2002;9(6):463–470. doi: 10.1038/sj.mn.7800159. [DOI] [PubMed] [Google Scholar]
- Beare NA, Harding SP, Taylor TE, Lewallen S, Molyneux ME. Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J Infect Dis. 2009;199(2):263–271. doi: 10.1086/595735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJ. Murine cerebral malaria is associated with a vasospasm-like microcirculatory dysfunction, and survival upon rescue treatment is markedly increased by nimodipine. Am J Pathol. 2010;176(3):1306–1315. doi: 10.2353/ajpath.2010.090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJ. Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. J Infect Dis. 2011;203(10):1454–1463. doi: 10.1093/infdis/jir058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LJ, Lenzi HL, Pelajo-Machado M, Oliveira DN, Daniel-Ribeiro CT, Ferreira-da-Cruz MF. Plasmodium berghei: cerebral malaria in CBA mice is not clearly related to plasma TNF levels or intensity of histopathological changes. Exp Parasitol. 2000;95(1):1–7. doi: 10.1006/expr.2000.4508. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Rauhala P. The redox pathway of S-nitrosoglutathione, glutathione and nitric oxide in cell to neuron communications. Free Radic Res. 1999;31(6):641–650. doi: 10.1080/10715769900301211. [DOI] [PubMed] [Google Scholar]
- Clemmer L, Martins YC, Zanini GM, Frangos JA, Carvalho LJ. Artemether and artesunate show the highest efficacies in rescuing mice with late-stage cerebral malaria and rapidly decrease leukocyte accumulation in the brain. Antimicrob Agents Chemother. 2011;55(4):1383–1390. doi: 10.1128/AAC.01277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford EM, Braun LD, Crane PD, Oldendorf WH. Blood-brain barrier restriction of peptides and the low uptake of enkephalins. Endocrinology. 1978;103(4):1297–1303. doi: 10.1210/endo-103-4-1297. [DOI] [PubMed] [Google Scholar]
- Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15(9):391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, Intaglietta M, van der Heyde HC. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med. 2006;12(12):1417–1422. doi: 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- Haq E, Rohrer B, Nath N, Crosson CE, Singh I. S-nitrosoglutathione prevents interphotoreceptor retinoid-binding protein (IRBP (161-180))-induced experimental autoimmune uveitis. J Ocul Pharmacol Ther. 2007;23(3):221–231. doi: 10.1089/jop.2007.0023. [DOI] [PubMed] [Google Scholar]
- John CC, Kutamba E, Mugarura K, Opoka RO. Adjunctive therapy for cerebral malaria and other severe forms of Plasmodium falciparum malaria. Expert Rev Anti Infect Ther. 2010;8(9):997–1008. doi: 10.1586/eri.10.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Jatana M, Elango C, Paintlia AS, Singh AK, Singh I. Cerebrovascular protection by various nitric oxide donors in rats after experimental stroke. Nitric Oxide. 2006;15(2):114–124. doi: 10.1016/j.niox.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Khan M, Sekhon B, Giri S, Jatana M, Gilg AG, Ayasolla K, Elango C, Singh AK, Singh I. S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J Cereb Blood Flow Metab. 2005;25(2):177–192. doi: 10.1038/sj.jcbfm.9600012. [DOI] [PubMed] [Google Scholar]
- Lackner P, Beer R, Heussler V, Goebel G, Rudzki D, Helbok R, Tannich E, Schmutzhard E. Behavioural and histopathological alterations in mice with cerebral malaria. Neuropathol Appl Neurobiol. 2006;32(2):177–188. doi: 10.1111/j.1365-2990.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- Martins YC, Werneck GL, Carvalho LJ, Silva BP, Andrade BG, Souza TM, Souza DO, Daniel-Ribeiro CT. Algorithms to predict cerebral malaria in murine models using the SHIRPA protocol. Malar J. 2010;9:85. doi: 10.1186/1475-2875-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath N, Morinaga O, Singh I. S-nitrosoglutathione a physiologic nitric oxide carrier attenuates experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5(2):240–251. doi: 10.1007/s11481-009-9187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RP, Hogg N, Spencer NY, Kalyanaraman B, Matalon S, Darley-Usmar VM. Biochemical characterization of human S-nitrosohemoglobin. Effects on oxygen binding and transnitrosation. J Biol Chem. 1999;274(22):15487–15492. doi: 10.1074/jbc.274.22.15487. [DOI] [PubMed] [Google Scholar]
- Peyrot F, Grillon C, Vergely C, Rochette L, Ducrocq C. Pharmacokinetics of 1-nitrosomelatonin and detection by EPR using iron dithiocarbamate complex in mice. Biochem J. 2005;387(Pt 2):473–478. doi: 10.1042/BJ20040828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski MW, Rees DD, Dutra A, Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol. 1992;107(3):745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhala P, Lin AM, Chiueh CC. Neuroprotection by S-nitrosoglutathione of brain dopamine neurons from oxidative stress. Faseb J. 1998;12(2):165–173. doi: 10.1096/fasebj.12.2.165. [DOI] [PubMed] [Google Scholar]
- Rockett KA, Awburn MM, Cowden WB, Clark IA. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991;59(9):3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- Singh SP, Wishnok JS, Keshive M, Deen WM, Tannenbaum SR. The chemistry of the S-nitrosoglutathione/glutathione system. Proc Natl Acad Sci U S A. 1996;93(25):14428–14433. doi: 10.1073/pnas.93.25.14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totino PR, Daniel-Ribeiro CT, Corte-Real S, de Fatima Ferreira-da-Cruz M. Plasmodium falciparum: erythrocytic stages die by autophagic-like cell death under drug pressure. Exp Parasitol. 2008;118(4):478–486. doi: 10.1016/j.exppara.2007.10.017. [DOI] [PubMed] [Google Scholar]
- WHO. World Malaria Report. Geneva: WHO Press; 2010. [Google Scholar]
- Wiese L, Kurtzhals JA, Penkowa M. Neuronal apoptosis, metallothionein expression and proinflammatory responses during cerebral malaria in mice. Exp Neurol. 2006;200(1):216–226. doi: 10.1016/j.expneurol.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Zanini GM, Cabrales P, Barkho W, Frangos JA, Carvalho LJ. Exogenous nitric oxide decreases brain vascular inflammation, leakage and venular resistance during Plasmodium berghei ANKA infection in mice. J Neuroinflammation. 2011;8:66. doi: 10.1186/1742-2094-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Spencer NY, Hogg N. Metabolism of S-nitrosoglutathione by endothelial cells. Am J Physiol Heart Circ Physiol. 2001;281281(1):H432–H439. doi: 10.1152/ajpheart.2001.281.1.H432. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hogg N. Mixing artifacts from the bolus addition of nitric oxide to oxymyoglobin: implications for S-nitrosothiol formation. Free Radic Biol Med. 2002;32(11):1212–1219. doi: 10.1016/s0891-5849(02)00829-8. [DOI] [PubMed] [Google Scholar]