Abstract
Wnt1 inducible signaling pathway protein 1 (WISP1/CCN4) is a CCN family member more broadly identified with development and tumorigenesis. However, recent studies have shed new light and enthusiasm on WISP1 as a novel target directed against toxic cell degeneration. Here we show WISP1 prevents apoptotic degeneration in primary neurons during oxidant stress through the activation of protein kinase B (Akt1), the post-translational maintenance of β-catenin integrity that is consistent with inhibition of glycogen synthase kinase-3β (GSK-3β), and the subcellular trafficking of β-catenin to foster its translocation to the nucleus. Interestingly, WISP1 autoregulates its expression through the promotion of β-catenin activity and may employ β-catenin to have a limited control over autophagy, but neuronal injury during oxidant stress as a result of autophagy appears portioned to a small population of neurons without significant impact upon overall cell survival. New strategies that target WISP1, its autoregulation, and the pathways responsible for neuronal cell injury may bring forth new insight for the treatment of neurodegenerative disorders.
Keywords: Akt1, apoptosis, autophagy, β-catenin, Beclin 1, CCN4, glycogen synthase kinase-3β, LC3, neurons, oxidative stress, p62, WISP, Wnt1
INTRODUCTION
Wnt1 inducible signaling pathway protein 1 (WISP1) was initially identified as a gene in a mouse mammary epithelial cell line [1], later determined to modulate gastric tumor growth [2], and is regulated by Wnt1 [1], a cysteine-rich glycosylated protein that controls cell growth, development, and survival in multiple systems of the body [3–11]. WISP1, also known as CCN4, is part of the CCN family of proteins that is defined by the first three members of the family that include Cysteine-rich protein 61, Connective tissue growth factor, and Nephroblastoma overexpressed gene [12]. The CCN family is characterized by four cysteine-rich modular domains that include insulin-like growth factor-binding domain, von Willebrand factor type C module, thrombospondin domain, and C-terminal cysteine knot-like domain [13].
WISP1 has been associated with neoplastic growth [14, 15] and early studies have demonstrated that WISP1 can block p53 mediated DNA damage and apoptosis [16]. However, subsequent work has shown that WISP1 may be beneficial as a proliferative agent. WISP1 may be involved with cardiac remodeling following myocardial infarction [17], with lung tissue repair and re-growth [18], with the stimulation of vascular smooth muscle growth [19], and with the induction of cardiomyocyte proliferation [20]. In addition, WISP1 is cytoprotective against cardiac toxicity [21], may foster bone formation and fracture repair [22, 23], and can limit neuronal cell injury during oxidative stress [24].
WISP1 utilizes several signal transduction pathways to yield cellular proliferation, survival, and repair that include the modulation of downstream apoptotic signaling involving mitochondrial stress, cytochrome c release, and caspase activation [16, 21, 24, 25]. Further upstream from these pathways, WISP1 can prevent cell injury through the activation of phosphoinositide 3-kinase (PI 3-K) and Akt1, primary mediators of cellular growth and survival [3, 26–31]. WISP1 phosphorylates Akt under conditions of cardiac tissue injury, oxidative stress, neuronal degeneration, and vascular smooth muscle proliferation [16, 19, 21, 24]. With the phosphorylation of Akt, WISP1 also leads to the inhibitory phosphorylation of glycogen synthase kinase-3β (GSK-3β) [21, 24] and the nuclear expression of β-catenin in cardiomyocytes [21]. Interestingly, β-catenin through Wnt signaling promotes the expression of WISP1 [32]. During the inhibition of GSK-3β, β-catenin is not phosphorylated, ubiquinated, or degraded and therefore can translocate to the nucleus to initiate “antiapoptotic” pathways and prevent cellular apoptosis [3, 33–39]. Furthermore, β-catenin also may function to limit cell injury through the blockade of autophagy [40, 41].
WISP1 and its signaling pathways with β-catenin represent a novel target that has the potential to promote tissue proliferation, repair, and regeneration in multiple cell systems, especially the central nervous system. We show in primary hippocampal neurons that oxygen-glucose deprivation (OGD) primarily leads to apoptotic cell death and that autophagy represents a smaller subset of the neuronal cell death observed. In primary neurons, WISP1 prevents apoptotic neuronal cell death, phosphorylates Akt1, limits β-catenin phosphorylation through the suggested inhibition of GSK-3β, and fosters the subcellular trafficking of β-catenin from the cytoplasm to the cell nucleus. β-catenin is necessary for WISP1 to prevent neuronal apoptotic degeneration and to autoregulate its expression, but β-catenin may play a lesser role in OGD mediated neuronal autophagy since WISP1 through β-catenin has limited effects upon the lipidation of light chain 3 -I (LC3-I) to LC3-II, the expression of Beclin 1, and the expression of p62 during the induction of autophagy with OGD.
MATERIALS AND METHODS
Hippocampal Neuronal Cultures
Per our prior protocols [4, 24, 42–44], hippocampi were obtained from E-19 Sprague-Dawley rat pups and incubated in Hanks’ balanced salt solution (HBBS) supplemented with 1 mM sodium pyruvate and 10 mM HEPES buffer solution (Invitrogen, Carlsbad, CA). The neurons were isolated by trituration for 10 times, centrifuged for 2 min at 200 g and then dissociated in growth medium (Leibovitz's L-15 medium, Invitrogen, Carlsbad, CA) containing 6% sterile rat serum (ICN, Aurora, OH), 150 mM NaHCO3, 2.25 mg/ml of transferrin, 2.5 µg/ml of insulin, 10 nM progesterone, 90 µM putrescine, 15 nM selenium, 35 mM glucose, 1 mM L-glutamine, penicillin and streptomycin (50 µg/ml), and vitamins. Cells were then plated at a density of ~1.5 ×103cells/mm2 in 35 mm polylysine/laminin-coated plates (Falcon Labware, Lincoln Park, NJ). Neurons were maintained in growth medium at 37 °C in a humidified atmosphere of 5% CO2 and 95% room air for 10–14 days.
Experimental Treatments
Oxygen-glucose deprivation (OGD) in microglia was performed by replacing the media with glucose-free HBSS containing 116 mmol/l NaCl, 5.4 mmol/l KCl, 0.8 mmol/l MgSO4, 1 mmol/l NaH2PO4, 0.9 mmol/l CaCl2, and 10 mg/l phenol red (pH 7.4) and cultures were maintained in an anoxic environment (95% N2 and 5% CO2) at 37 °C per our prior experimental protocols [29, 45, 46). For treatments applied prior to OGD, human recombinant WISP1 protein (R&D Systems, Minneapolis, MN) was continuous. The phosphatidylinositol-3-kinase (PI3-K) inhibitors wortmannin (0.5 µM, Calbiochem, La Jolla, CA) and LY294002 (10 µM, Sigma, St Louis, MO), the autophagy inhibitor 3-methyladenine) 3MA))10 mM, Sigma, St Louis, MO), the β-catenin agonist 2-amino-4-)3,4-)methylenedioxy) benzyl-amino)-6-)3-methoxyphenyl)pyrimidine (AMBP, 1–20 µM, EMD, Billerica, MA), and the β-catenin antagonist 2-phenoxybenzoic acid-(5-methyl-2-furanyl) methylene (hydrazide) PNU74654, PNU, 1–10 µM, R&D Systems, Minneapolis, MN) were each administered directly to the cultures 1 hour prior to OGD and treatments were continuous.
Assessment of Cell Survival
Neuronal cell injury was determined by bright field microscopy using a 0.4% trypan blue dye exclusion method 24 hours following treatment with OGD per our previous protocols [27, 46, 47]. The mean survival was determined by counting eight randomly selected non-overlapping fields with each containing approximately 10–20 cells (viable + non-viable). Each experiment was replicated 4–6 times with different cultures
Assessment of DNA Fragmentation
Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay [48–50]. Briefly, neuronal cells were fixed in 4% paraformaldehyde/0.2% picric acid/0.05% glutaraldehyde and the 3’-hydroxy ends of cut DNA were labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Promega, Madison, WI) followed by streptavidin-peroxidase and visualized with 3,3’-diaminobenzidine (Vector Laboratories, Burlingame, CA).
Expression of Phosphorylated Akt1, Beclin 1, p62, β-Catenin, WISP1, and LC3
Cell protein extracts were subjected to SDS-PAGE (7.5%, Akt1, Beclin 1, p62, and β-catenin; 12.5%, WISP1 and LC3) separation. After blocking for 1 hour at room temperature with 5% skim milk, the membranes were incubated overnight at 4 °C with a rabbit polyclonal antibody against WISP1 (1:200, Santa Cruz Biotechnologies, Santa Cruz, CA), a rabbit polyclonal antibody against phospho-Akt1 (p-Akt1, Ser473, 1:1000, Cell Signaling, Beverly, MA), a rabbit monoclonal antibody against phospho-Beclin 1 (1:1000), a rabbit polyclonal antibody against phospho-β-catenin (p-β-catenin, Ser33,37, 1:1000), a rabbit polyclonal antibody against p62 (1:1000), and a rabbit polyclonal antibody against LC3 (1:1000) (Cell Signaling, Beverly, MA). After incubation of the membranes with horseradish peroxidase conjugated secondary antibody (goat anti-mouse IgG, 1:5000) (Pierce, Rockford, IL), the antibody-reactive bands were revealed by enhanced chemiluminescence detection on Hyperfilm (Amersham Pharmacia Biotech, Piscataway, NJ).
Western blot analysis for β-Catenin in the cytoplasm and the nucleus: Cells were homogenized, the cytoplasmic and nuclear proteins were prepared by using NE-PER nuclear and cytoplasmic extraction reagents according to manufacture’s instructions (Pierce, Rockford, IL). The expression of β-catenin in nucleus and cytoplasm was determined by Western blot. Each sample (50 µg/lane) was subjected to 7.5% SDS-polyacrylamide gel electrophoresis and Western blot was performed as description as above.
Immunocytochemistry for β-Catenin
For staining of β-catenin, neurons were fixed with 4% paraformaldehyde and were permeabilized using 0.2% Triton X-100. The cells were then incubated with rabbit anti-β-catenin 1 (1:100, Cell Signaling Technology, Beverly, MA) over night at 4°C and then with biotinylated anti-rabbit IgG (1:50, Vector laboratories) for 2 hours followed by Texas Red streptavidin (1:50, Vector laboratories, Burlingame, CA) for 1 hour. Cells are washed in PBS and then stained with DAPI (Sigma, St. Louis, MO) for nuclear identification. Fluorescence imaging used the wavelengths of 565 nm (red) and 400 nm (DAPI).
Statistical Analysis
For each experiment, the mean and standard error were determined. Statistical differences between groups were assessed by means of analysis of variance (ANOVA) from 6 replicate experiments with the post-hoc Dunnett's test. Statistical significance was considered at P<0.05.
RESULTS
WISP1 Reduces Neuronal Apoptosis during Oxygen Glucose Deprivation (OGD)
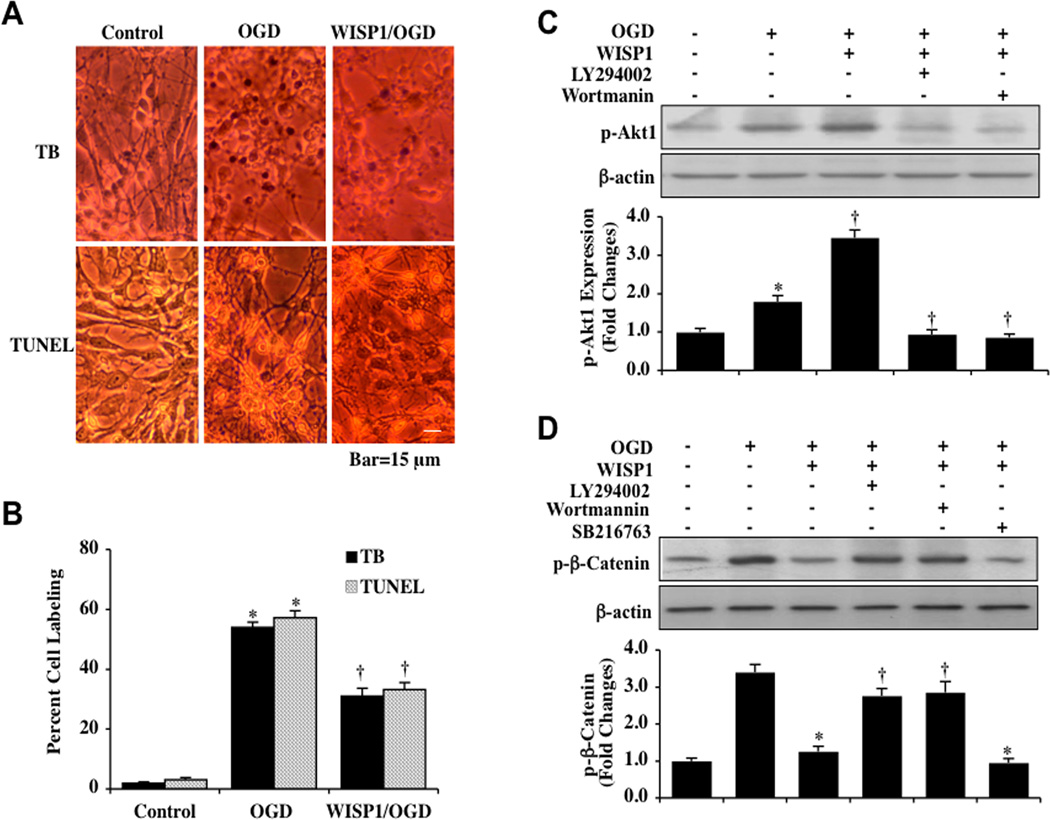
Recombinant human WISP1 protein (10 ng/ml) was administered to neuronal cultures 1 hour prior to a 3 hour period of OGD and cell injury was determined 24 hours later through trypan blue dye exclusion method and TUNEL assay. This concentration of WISP1 was chosen since it previously was shown to provide significant cytoprotection in neuronal cells and to significantly lead to the phosphorylation of Akt1 (Ser473) [24]. In Fig. (1A), representative images demonstrate that untreated neurons were not significantly stained with trypan blue and TUNEL, but exposure to OGD resulted in significant trypan blue staining and nuclear DNA damage 24 hours following OGD exposure in neurons. In contrast, WISP1 (10 ng/ml) significantly reduced trypan blue staining and nuclear DNA degradation. Quantitative results illustrate indicate that percent trypan blue staining and DNA fragmentation were significantly increased in neurons 24 hours following OGD (Fig. 1B). In contrast, WISP1 (10 ng/ml) treatment significantly reduced percent trypan blue staining and DNA fragmentation.
Fig. (1). WISP1 prevents neuronal cell apoptosis and controls the phosphorylation of β-catenin through the PI 3-K/Akt1 pathway during OGD.
[A] Primary hippocampal neurons were exposed to OGD for 3 hours and cell injury was determined by trypan blue [TB] dye exclusion and TUNEL assay 24 hours after OGD. Representative images illustrate that OGD led to neuronal cell injury with increased trypan blue and TUNEL staining. WISP1 protein [10 ng/ml] applied to the neuronal cultures 1 hour prior to OGD significantly reduced trypan blue and TUNEL staining in neurons 24 hours following OGD. [B] Quantitative analysis shows that percent cell trypan blue staining and apoptotic DNA fragmentation was significantly increased 24 hours following OGD [*P<0.01 vs. control]. In contrast, WISP1 significantly decreased percent trypan blue staining and DNA fragmentation [†P < 0.01 vs. OGD]. Each data point represents the mean and SEM from 6 experiments. [C] Equal amounts of neuronal protein extracts [50 µg/lane] were immunoblotted at 3 hours after a 3 hour period of OGD with anti–phospho-Akt1 [p-Akt1, Ser 473] antibody. Administration of WISP1 [10 ng/ml] 1 hour prior to OGD significantly enhanced p-Akt1 expression. Yet, application of the specific PI 3-K inhibitors wortmannin [0.5 µM] or LY294002 [10 µM] 1 hour prior to a 3 hour period of OGD blocked expression of p-Akt1 [*P<0.01 vs. Control; †P <0.01 vs. OGD]. Quantitative analysis of the western blots for the data from 3 experiments was performed using the public domain NIH Image program [available on the Internet at http://rsb.info.nih.gov/nih-image/]. [D] Equal amounts of neuronal protein extracts [50 µg/lane] were immunoblotted with p-β-catenin [Ser33/37] antibody at 3 hours following a 3 hour period of OGD. Expression of p-β-catenin was increased at 3 hours following OGD exposure and was significantly decreased by WISP1 [10 ng/ml] administration 1 hour prior to OGD. Yet, combined treatment with specific PI 3-K inhibitors wortmannin [0.5 µM] or LY294002 [LY, 10 µM] abrogated the ability of WISP1 to reduce the expression of p-β-catenin. Application of the GSK-3β inhibitor SB21673 [5 µM] 1 hour prior to OGD significantly reduced the expression of p-β-catenin 3 hours following OGD [*P <0.01 vs. OGD; †P <0.01 vs. WISP1/OGD]. Each data point represents the mean and SEM from 3 experiments.
WISP1 Regulates Phosphorylation of β-Catenin through the PI 3-K/Akt1 Pathway during OGD
To assess the ability of WISP1 to phosphorylate Akt1, western blot assay was performed for phosphorylated Akt1 (p-Akt1) (Ser473) (activated form of Akt1) at 3 hours after administration of WISP1 (10 ng/ml) to neurons. As shown in Fig. 1C, WISP1 significantly increased p-Akt1 expression at 3 hours following OGD exposure. However, this increase in the expression of p-Akt1 was abrogated by the PI 3-K inhibitors wortmannin (0.5 µM) and LY294002 (10 µM) during treatment with WISP1 (10 ng/ml) (Fig. 1C). Wortmannin (0.5 µM) forms a covalent link with the lysine residue of PI 3-K [51] and LY294002 (10 µM) reversibly competes for ATP binding [52].
Since β-catenin is the downstream target of Wnt/GSK-3β and Akt signaling pathways [53, 54], we next examined the effects of WISP1 on β-catenin phosphorylation that can lead to β-catenin ubiquination and degradation. In Fig. (1D), expression of p-β-catenin (Ser33/37) was significantly increased 3 hours following OGD exposure. In contrast, WISP1 (10 ng/ml) applied 1 hour prior to a 3 hour period of OGD significantly prevented the expression of p-β-catenin 3 hours following OGD exposure (Fig. 1D). The ability of WISP1 to block phosphorylation of β-catenin was prevented during treatment with the PI 3-K inhibitors wortmannin (0.5 µM) and LY294002 (10 µM), illustrating that the PI 3-K pathway is utilized by WISP1 to control β-catenin phosphorylation. In addition, the phosphorylation of β-catenin was prevented during the administration of WISP1 (10 ng/ml) with the GSK-3β inhibitor SB21673 (5 µM) to a similar degree as observed with application of WISP1 alone during OGD, suggesting that blockade of β-catenin phosphorylation during OGD exposure was also mediated by GSK-3β inhibition.
WISP1 through the PI 3-K Pathway Promotes the Nuclear Translocation of β-Catenin in Neurons during OGD
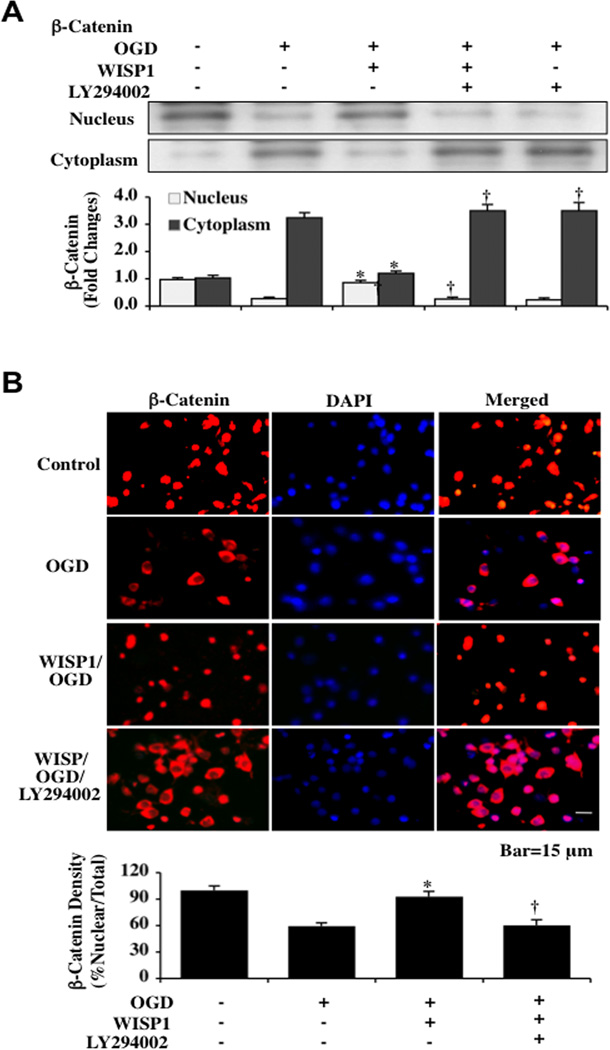
β-catenin that is not phosphorylated can translocate to the nucleus and foster protection against apoptosis [3, 35, 45, 55–58] and autophagy [40, 41, 59]. We therefore investigated the ability of WISP1 to regulate the translocation of β-catenin in neurons during OGD exposure. WISP1 (10 ng/ml) was applied to neuronal cultures 1 hour prior to a 3 hour period of OGD and western blot analysis for β-catenin in the extracts from both nucleus and cytoplasm was performed. As shown in Fig. (2A), OGD resulted in an increase in the expression of β-catenin in the cytoplasm and a decrease in the expression of β-catenin in the nucleus. In contrast, WISP1 treatment significantly increased the expression of β-catenin in the nuclei of neurons. Application of the PI 3-K inhibitor LY294002 (10 µM) prevented the nuclear translocation of β-catenin during WISP1 administration, suggesting that the PI 3-K pathway was necessary for WISP1 to traffic β-catenin. Immunofluorescence staining for β-catenin (Texas-red) was performed at 3 hours following OGD and nuclei of neurons were counterstained with DAPI (Fig. 2B). In merged images, cells with OGD alone demonstrate neuronal nuclei with minimal β-catenin staining (blue/white) and neuronal cytoplasms with significant β-catenin staining (red). This is in contrast to neurons treated with WISP1 that demonstrate significant increase in nuclear staining of β-catenin. Similar to the western analysis studies, administration of the PI 3-K inhibitor LY294002 (10 µM) prevented the nuclear translocation of β-catenin during WISP1 exposure. The immunofluorescence studies with β-catenin parallel the western blot studies for β-catenin expression in the cytoplasm and the nucleus (Fig. 2A).
Fig. (2). WISP1 through the PI 3-K pathway fosters nuclear translocation of β-catenin during OGD.
[A] Equal amounts of cytoplasmic [cytoplasm] or nuclear [nucleus] protein extracts were immunoblotted with anti-total β-catenin antibody at 3 hours following a 3 hour period of OGD. WISP1 [10 ng/ml] treatment significantly increased the expression of β-catenin in the nuclei of neurons. Application of the PI 3-K inhibitor LY294002 [10 µM] prevented the nuclear translocation of β-catenin during WISP1 administration and OGD exposure [*P<0.01 vs. OGD; †P< 0.01 vs. WISP1/OGD]. Quantitative analysis of the western blots for the data was performed using the public domain NIH Image program [available on the Internet at http://rsb.info.nih.gov/nih-image/]. [B] WISP1 [10 ng/ml] was administered 1 hour prior to a 3 hour period of OGD and immunofluorescence staining for β-catenin [Texas-red] was performed 3 hours after OGD. Nuclei of neurons were counterstained with DAPI. In merged images, cells with OGD alone show neuronal nuclei with minimal β-catenin staining [blue/white] with significant β-catenin staining [red] in the cytoplasm. In contrast to neurons treated with WISP1 [10 ng/ml] and OGD exposure, β-catenin is predominantly in the nuclei of neurons [nuclei primarily red]. Treatment with the PI 3-K inhibitor LY294002 [10 µM] combined with WISP1 resulted in the lost of nuclear staining of β-catenin [*P<0.01 vs. OGD; †P< 0.01 vs. WISP1/OGD]. Intensity of β-catenin nuclear staining was performed using the public domain NIH Image program [available on the Internet at http://rsb.info.nih.gov/nih-image/] and control = untreated neurons.
WISP1 Protects Neurons through β-Catenin Activation during OGD Exposure
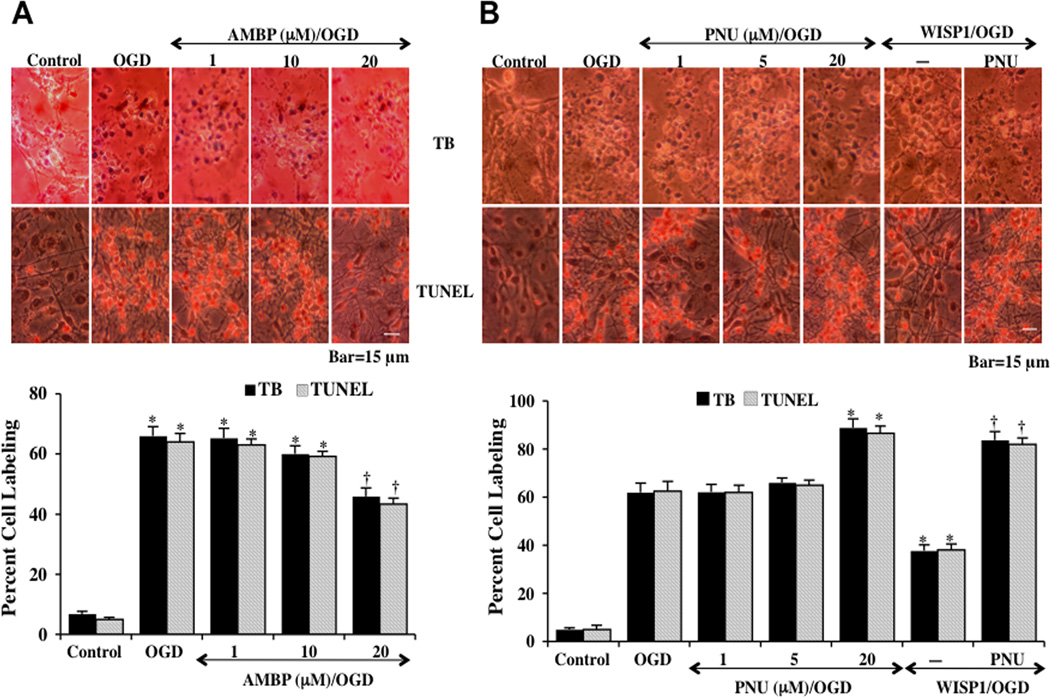
The β-catenin agonist AMBP (1, 10, and 20 µM) was applied to neuronal cultures 1 hour prior to a 3 hour period of OGD and cell injury was determined 24 hours following OGD exposure through trypan blue (TB) dye exclusion and apoptotic nuclear DNA fragmentation (TUNEL). AMBP (20 µM) significantly reduced trypan blue uptake and DNA fragmentation in neuronal cells (Fig. 3A) and the β-catenin inhibitor PNU (20 µM) significantly increased neuronal cell injury (Fig. 3B). Quantitative results demonstrate that the percent cell labeling of typan blue and TUNEL was significantly decreased by the treatment with the β-catenin agonist. In contrast, application of β-catenin inhibitor PNU (20 µM) increased trypan blue staining and DNA fragmentation during OGD exposure when compared with neurons treated with OGD alone (Figs. 3A and 3B). In addition, application of PNU (20 µM) with WISP1 significantly reduced the ability of WISP1 to protect cells during OGD exposure, suggesting that WISP1 employs β-catenin to foster neuronal protection (Fig. 3B).
Fig. (3). WISP1 protects neurons against OGD through β-catenin activation.
[A] The β-catenin agonist [AMBP, 1, 10, and 20 µM] was applied 1 hour prior to a 3 hour period of OGD. Cell injury was determined by trypan blue dye exclusion method and TUNEL staining 24 hours following OGD. Representative images demonstrate that OGD led to a significant increase in trypan blue staining and DNA fragmentation in neuronal cells 24 hours after OGD compared to untreated control cultures. AMBP [20 µM] application 1 hour prior to OGD significantly reduced neuronal cell injury. Quantitative results illustrate that AMBP [20 µM] application significantly decreased percent trypan blue uptake and DNA fragmentation 24 hours after OGD when compared to OGD exposure alone [*P <0.01 vs. untreated control; †P <0.01 vs. OGD]. [B] The β-catenin inhibitor [PNU, 1, 5, and 20 µM] was administered 1 hour prior to a 3 hour period of OGD and cell injury was determined by trypan blue dye exclusion and TUNEL staining 24 hours following OGD. Representative images demonstrate that PUN [20 µM] increased trypan blue staining and DNA fragmentation in neuronal cells 24 hours after OGD. Combined application of the β-catenin inhibitor PNU [20 µM] with WISP1 [10 ng/ml] 1 hour prior to a 3 hour period of OGD attenuated the efficacy of WISP1 to prevent cell injury [trypan blue] and apoptosis [TUNEL] 24 hours following OGD [*P <0.01 vs. OGD; †P <0.01 vs. WISP1/OGD]. In all cases, each data point represents the mean and SEM from 6 experiments.
WISP1 Autoregulates its Expression through β-Catenin During OGD
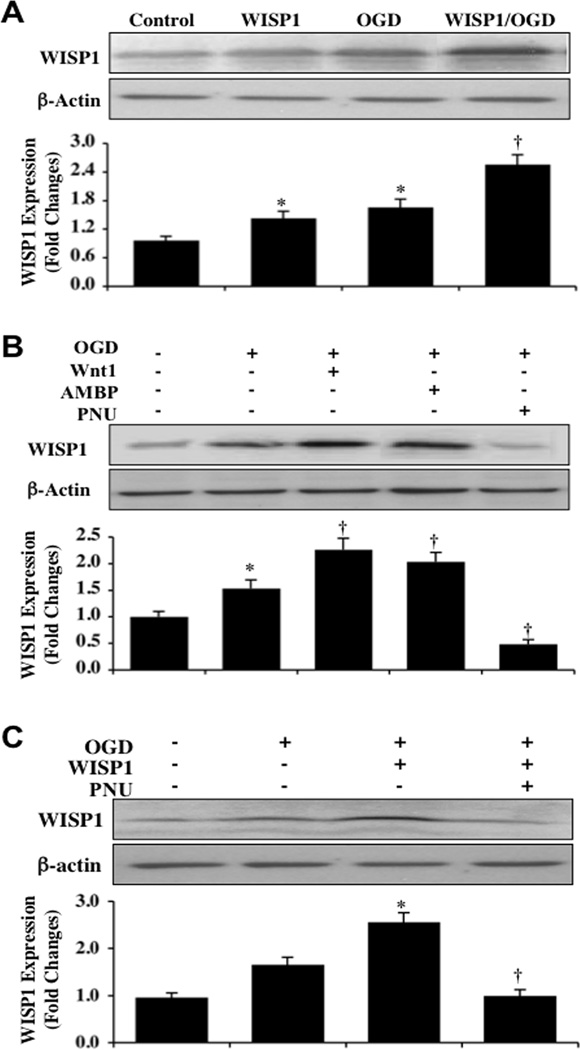
Increased activity of β-catenin can promote WISP1 expression in cells exclusive of the nervous system [9, 21, 60]. Given that WISP1 can prevent the phosphorylation of β-catenin and translocate this protein to the cell nucleus in primary neurons, we examined whether β-catenin could then regulate the expression of WISP1 as a feedback mechanism. Hippocampal neuronal protein extracts (50 µg/lane) were immunoblotted with anti-WISP1 antibody at 24 hours following a 3 hour period of OGD. As shown in Fig. (4A), WISP1 can lead to the induction of its own expression. WISP1 expression was increased in control untreated neurons and in neurons exposed to OGD 24 hours following OGD exposure. Application of WISP1 (10 µM) 1 hour prior to OGD significantly increased the expression of WISP1 in neurons 24 hours following OGD exposure.
Fig. (4). WISP1 increases its expression and autoregulates its expression through β-catenin during OGD exposure.
[A] Hippocampal neuronal protein extracts [50 µg/lane] were immunoblotted with anti-WISP1 antibody 24 hours following WISP1 [10 ng/ml] administration alone or following a 3 hour period of OGD with 1 hour pretreatment with WISP1 [10 ng/ml]. WISP1 treatment significantly increased WISP1 expression 24 hours both in untreated control cultures and in OGD exposed neurons [*P <0.01 vs. Control; †P <0.01 vs. OGD]. [B] Wnt1 [100 ng/ml], β-catenin agonist [AMBP, 20 µM], or β-catenin inhibitor [PNU, 20 µM] was applied to neuronal cultures 1 hour prior to a 3 hour period of OGD and the expression of WISP1 was determined 24 hours following OGD. Both Wnt1 and AMBP treatment significantly increased the expression of WISP1 following OGD. In contrast, PUN significantly decreased WISP1 expression following OGD [*P <0.01 vs. Control; †P <0.01 vs. OGD]. Control=untreated cultures. [C] Application of PNU [20 µM] with WISP1 [10 ng/ml] 1 hour prior to a 3 hour period of OGD blocked the ability of WISP1 to increase its expression 24 hours following OGD [*P <0.01 vs. OGD; †P <0.01 vs. WISP1/OGD]. Quantitative analysis of the western blots for the data from 3 experiments was performed using the public domain NIH Image program [available on the Internet at http://rsb.info.nih.gov/nih-image/].
We next assessed whether WISP1 can regulate its expression through β-catenin. Wnt1 protein (100 ng/ml), the β-catenin agonist AMBP (20 µM), or the β-catenin inhibitor PNU (20 µM) were applied to neuronal cultures 1 hour prior to a 3 hour period of OGD (Figs. 4B and 4C). The cysteine-rich glycosylated protein Wnt1, known to lead to β-catenin activation [54, 61], was also used in these studies, since it has previously been shown that Wnt1 can increase WISP1 expression in primary neurons [24]. Western blot analysis was performed for WISP1 24 hours following OGD. Both Wnt1 and the β-catenin agonist AMBP significantly increased WISP1 expression during OGD exposure. In contrast, WISP1 expression was significantly reduced during application of the β-catenin inhibitor PNU (20 µM) alone or during WISP1 administration, illustrating that β-catenin activity is necessary for WISP1 expression.
Autophagy has a Limited Role for Neuronal Survival during OGD Exposure
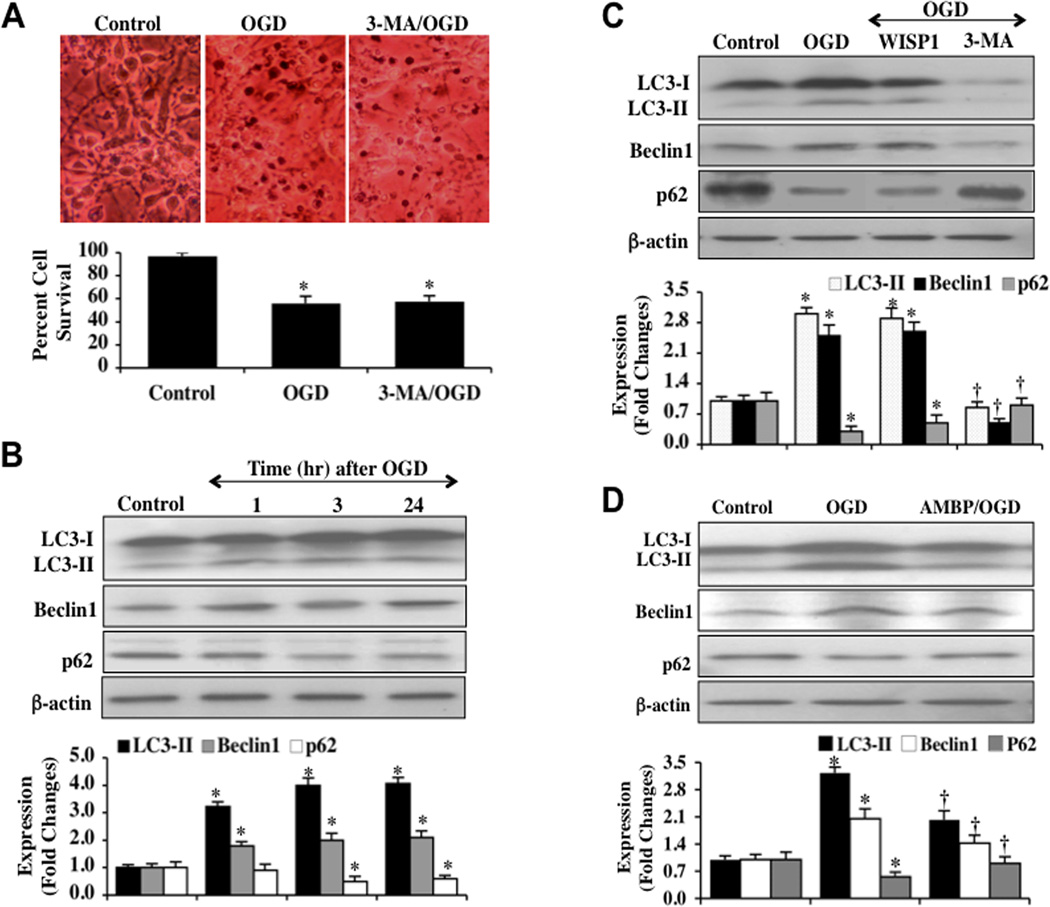
To investigate the regulatory effects of autophagy on neuronal survival during OGD, we applied the autophagy inhibitor 3MA (10 mM) to neuronal cultures 1 hour prior to a 3 hour period of OGD. Neuronal cell injury was determined 24 hours following OGD by using the trypan blue dye exclusion method. In Fig. (5A), representative images demonstrate that untreated neurons were without evidence of trypan blue uptake, but exposure to OGD resulted in significant trypan blue staining 24 hours following OGD in neurons. Application of the autophagy inhibitor 3MA did not significantly affect neuronal cell injury during OGD exposure. Quantitative results show that percent trypan blue staining (Fig. 5A) were not significantly affected by 3MA treatment in neurons 24 hours following OGD, suggesting that autophagy has a minimal role for cell survival in possibly only a subset of primary neurons during OGD exposure.
Fig. (5). Autophagy has a limited role in cell injury and WISP1 cytoprotection involving β-catenin.
[A] Autophagy inhibitor 3- methyladenine [3MA, 10 mM] was applied to neuronal cultures 1 hour prior to a 3 hour period of OGD and cell survival was determined by trypan blue dye exclusion method. Representative images and quantitative results demonstrate that OGD led to a significant increase in trypan blue staining in neuronal cells at 24 hours after OGD. Inhibition of autophagy by 3MA did not improve neuronal cell survival significantly when compared with OGD treated alone neurons [*P <0.01 vs. untreated Control]. Each data point represents the mean and SEM from 6 experiments. [B] Equal amounts of neuronal protein extracts [25–50 µg/lane] were immunoblotted at 1, 3 and 24 hours following a 3 hour period of OGD with anti–LC3-II, p62, and Beclin-1 antibodies. OGD resulted in a significant increase in the expression of LC3-II and Beclin-1 and a decrease in the expression of p62 [*P<0.01 vs. Control]. [C] WISP1 [10 ng/ml] was applied to neuronal cultures 1 hour prior to a 3 hour period of OGD and the expressions of LC3-II, p62, and Beclin-1 were determined by western blot analysis 24 hours following OGD. WISP1 treatment did not significantly change the expression of LC3-II and Beclin-1, but mildly increased p62 expression following OGD. In contrast, the autophagy inhibitor 3MA [10 mM] significantly decreased the expression of LC3-II and Beclin-1 and increased the expression of p62 at 24 hours following OGD [*P<0.01 vs. Control; †P <0.01 vs. OGD]. [D] Application of the β-catenin agonist AMBP [20 µM] 1 hour prior to a 3 hour period of OGD decreased the expression of LC3-II and Beclin-1 and increased the expression of p62 24 hours following OGD [*P<0.01 vs. Control; †P <0.01 vs. OGD].]. Each data point represents the mean and SEM from 3 experiments. In B, C, and D, quantitative analysis of the western blots for the data from 3 experiments was performed using the public domain NIH Image program [available on the Internet at http://rsb.info.nih.gov/nih-image/].
WISP1 through β-Catenin has a Minimal Effect upon Autophagy Parameters during OGD Exposure
For our analysis of autophagic flux, we assessed LC3-II, Beclin-1, and p62 expression, since the presence of LC3-II alone may not always correlate with autophagy activity [62]. Hippocampal neuronal protein extracts were immunoblotted with anti- microtubule-associated protein 1 light chain 3 (LC3), p62, and Beclin-1 antibodies at 1, 3, and 24 hours following a 3 hour period of OGD. OGD exposure resulted in increased expression of LC3-II (autophagosome-specific) and Beclin-1, but an expected decrease in p62 expression, consistent with the induction of autophagy in primary neurons (Fig. 5B). Administration of WISP1 (10 ng/ml) to neuronal cultures 1 hour prior to OGD resulted in a minor increase in p62 expression without significant changes in LC3-II and Beclin-1 expressions. Application of 3MA as a control during OGD significantly prevented increased expression of LC3-II and Beclin-1 and blocked reduction in p62 expression, consistent with the prevention of autophagy (Fig. 5C). The β-catenin agonist AMBP (20µM) was applied to neuronal cultures 1 hour prior to OGD and the expressions of LC3-II, Beclin-1, and p62 were determined 24 hours following a 3 hour period of OGD. Activation of β-catenin with AMBP decreased the expressions of LC3-II and Beclin-1 while increasing p62 expression to a greater degree that WISP1 alone 24 hours following OGD exposure, suggesting that the limited response of WISP1 against autophagy may be mediated in part through β-catenin activation.
DISCUSSION
WISP1 has a broad role in multiple systems of the body that include bone formation and repair [22, 23], tumorigenesis and aggressive cancer progression in the gastric system [2, 15], and cellular hypertrophy [20]. WISP1 also can enhance cell survival during cardiomyocyte injury [21, 63], potentially limit vascular cell death during crush injury in human saphenous veins [64], and block primary neuronal early and late apoptotic injury during oxidative stress [24]. We show that exogenous application of WISP1 prevents neuronal cell injury and blocks apoptotic DNA degradation during OGD exposure consistent with prior studies illustrating that WISP1 can prevent cardiomyocyte death against tumor necrosis factor [63], increase survival in human lung carcinoma cells during UV irradiation and etoposide treatment [16], and block neuronal degeneration during oxidant stress [24].
WISP1 provides neuronal cellular protection against oxidative stress during OGD exposure through the phosphorylation and activation of Akt1 and the prevention of β-catenin phosphorylation that can occur through GSK-3β and lead to the degradation of β-catenin. During oxidative stress, Akt1 activation can protect and promote the proliferation of endothelial cells [3, 27–29, 31, 47, 65–70], lead to enhanced survival and activity of glial cells [10, 11, 30, 35, 45, 71–73], foster cardiac tissue protection [74, 75], and prevent the degeneration of neurons [4, 24, 76–82]. We show that WISP1 phosphorylates Akt1 and subsequently prevents the phosphorylation of β-catenin through a PI 3-K mediated pathway. In addition, GSK-3β inhibition with WISP1 application did not synergistically decrease the phosphorylation of β-catenin, suggesting that prevention of β-catenin phosphorylation by WISP1 during OGD exposure was also mediated by GSK-3β inhibition. Inhibition of GSK-3β is known to prevent β-catenin phosphorylation [3, 33–36, 83–85].
WISP1 also controls the subcellular trafficking of β-catenin. In osteoclasts [60], vascular cells [9], and cardiomyocytes [21], WISP1 has been associated with increased nuclear expression of β-catenin. We show that WISP1 through a PI 3-K mediated pathway in primary neurons fosters the translocation of β-catenin from the cytoplasm of neurons to the nucleus that can allow for the transcription and eventual translation of pathways that can limit apoptosis [3, 35, 45, 55–58] and autophagy [40, 41, 59]. In addition, WISP1 requires β-catenin to limit neuronal cell injury during OGD exposure, since inhibition of β-catenin activity during OGD increases cell injury and blocks neuroprotection against apoptosis by WISP1.
The ability of WISP1 to control the phosphorylation and cellular trafficking of β-catenin appears to be necessary for WISP1 to autoregulate its expression. Increased activity of β-catenin can promote WISP1 expression in osteoclasts and vascular cells [9, 60] and administration of exogenous WISP1 can increase the expression of endogenous WISP1 in cardiomyocytes [21]. In primary hippocampal neurons, we demonstrate that exogenous application of WISP1 not only increases WISP1 in untreated control neurons, but also significantly increases WISP1 expression during oxidative stress exposure. As a result, WISP1 can lead to the induction of its own expression. Furthermore, we show that loss of β-catenin activity leads to depressed WISP1 expression while increased β-catenin activity promotes WISP1 expression, suggesting that WISP1 expression is governed by β-catenin activity and that WISP1 regulates its own expression through the ability of WISP1 to control β-catenin phosphorylation and nuclear translocation.
However, the ability of WISP1 to control β-catenin activity does not appear to alter autophagy progression in primary neurons during OGD exposure. Our studies demonstrate that inhibition of autophagy with 3MA does not significantly protect neurons during OGD, suggesting that autophagy may play a small role in acute neuronal injury during oxidative stress. Furthermore, during OGD exposure, soluble cytosolic LC3-I is lipidated to the autophagosome bound LC3-II per western analysis that indicates activation of the autophagy pathways. Since the presence of LC3-II may not always correlate with autophagy activity [62], we also assessed Beclin-1 expression [86, 87] and p62 expression [88] to evaluate autophagic flux. We show that Beclin-1 expression is increased during OGD exposure over time, but that p62 expression is decreased, suggesting that autophagy occurs in at least a small subset of neurons during OGD exposure. Yet, WISP1 does not significantly alter LC3-II, Beclin-1, or p62 expression when compared to neurons exposed only to OGD, illustrating that WISP1 with the concentration that we employed does not significantly modulate autophagy during OGD exposure in primary neurons. Administration of the β-catenin agonist AMPB did more prominently alter autophagy in neurons, suggesting that in this model of neuronal oxidative stress β-catenin appears to have some influence to limit autophagy. When examining the three parameters used to assess autophagy, WISP1 was able to increase p62 expression without significant alterations to LC3-II and Beclin-1. β-catenin activation had a more effective response in decreasing the expressions of LC3-II and Beclin-1 and increasing p62 expression when compared to neurons exposed to OGD alone, suggesting that WISP1 may, in part, slightly limit autophagy through β-catenin activation.
As a member of the Wnt signaling pathway, WISP1 may play an important role in both development and neoplastic growth [8, 12]. However, the capacity of WISP1 to promote cell survival during toxic environments is generating a new line of excitement for this cellular target. Although WISP1 may rely upon PI 3-K, mitochondrial, and cytokine pathways to elicit increased cell survival [16, 21, 24, 63], WISP1 also has been associated with β-catenin activity [9, 60]. Our studies highlight the vital roles Akt1 and β-catenin hold for WISP1 not only to autoregulate the expression of WISP1, but also for WISP1 to prevent neuronal cell injury during oxidant stress. Protection by WISP1 in neuronal cells is primarily directed against apoptotic cell death with minimal influence against autophagic cell injury, suggesting that the Akt1 pathway may be central for WISP1 to control multiple cell survival pathways that include mitochondria and β-catenin. Novel therapies that can focus upon WISP1 (CCN4), its autoregulation, and the cytoprotective pathways controlled by this CCN family member may offer unique advantages for the treatment of degenerative disorders in the nervous system.
ACKNOWLEDGEMENTS
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, LEARN Foundation Award, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
REFERENCES
- 1.Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, et al. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A. 1998 Dec 8;95(25):14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies SR, Davies ML, Sanders A, Parr C, Torkington J, Jiang WG. Differential expression of the CCN family member WISP-1, WISP-2 and WISP-3 in human colorectal cancer and the prognostic implications. Int J Oncol. 2010 May;36(5):1129–1136. doi: 10.3892/ijo_00000595. [DOI] [PubMed] [Google Scholar]
- 3.Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3beta, and beta-Catenin to Foster Vascular Integrity During Experimental Diabetes. Curr Neurovasc Res. 2011 May 1;8(2):103–120. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010 Mar-Apr;3(2):153–165. doi: 10.4161/oxim.3.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ. Differential expression of wnts after spinal cord contusion injury in adult rats. PLoS ONE. 2011;6(11):e27000. doi: 10.1371/journal.pone.0027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, et al. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005 Apr 21;24(18):3054–3058. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- 7.L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Cossetti C, et al. Reactive astrocytes and Wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Neurobiol Dis. 2011 Feb;41(2):508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008 Apr;118(1):58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchand A, Atassi F, Gaaya A, Leprince P, Le Feuvre C, Soubrier F, et al. The Wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell. 2011 Apr;10(2):220–232. doi: 10.1111/j.1474-9726.2010.00661.x. [DOI] [PubMed] [Google Scholar]
- 10.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res. 2011 Oct 19;8(4):270–285. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012 Mar 3;4(3):187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berschneider B, Konigshoff M. WNT1 inducible signaling pathway protein 1 [WISP1]: a novel mediator linking development and disease. Int J Biochem Cell Biol. 2010 Mar;43(3):306–309. doi: 10.1016/j.biocel.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Yeger H, Perbal B. The CCN family of genes: a perspective on CCN biology and therapeutic potential. J Cell Commun Signal. 2007 Dec;1(3–4):159–164. doi: 10.1007/s12079-008-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou CH, Chiang YC, Fong YC, Tang CH. WISP-1 increases MMP-2 expression and cell motility in human chondrosarcoma cells. Biochem Pharmacol. 2011 Jun 1;81(11):1286–1295. doi: 10.1016/j.bcp.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Nagai Y, Watanabe M, Ishikawa S, Karashima R, Kurashige J, Iwagami S, et al. Clinical significance of Wnt-induced secreted protein-1 [WISP-1/CCN4] in esophageal squamous cell carcinoma. Anticancer Res. 2011 Mar;31(3):991–997. [PubMed] [Google Scholar]
- 16.Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002 Jan 1;16(1):46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colston JT, de la Rosa SD, Koehler M, Gonzales K, Mestril R, Freeman GL, et al. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1839–H1846. doi: 10.1152/ajpheart.00428.2007. [DOI] [PubMed] [Google Scholar]
- 18.Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem. 2011 May 20;286(20):17435–17444. doi: 10.1074/jbc.M110.137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy VS, Valente AJ, Delafontaine P, Chandrasekar B. Interleukin-18/WNT1-inducible signaling pathway protein-1 signaling mediates human saphenous vein smooth muscle cell proliferation. J Cell Physiol. 2011 Dec;226(12):3303–3315. doi: 10.1002/jcp.22676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanmugam P, Valente AJ, Prabhu SD, Venkatesan B, Yoshida T, Delafontaine P, et al. Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011 Jun;50(6):928–938. doi: 10.1016/j.yjmcc.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010 May;22(5):809–820. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French DM, Kaul RJ, D'Souza AL, Crowley CW, Bao M, Frantz GD, et al. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol. 2004 Sep;165(3):855–867. doi: 10.1016/S0002-9440(10)63348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohara H, Tabata Y. Enhancement of ectopic osteoid formation following the dual release of bone morphogenetic protein 2 and Wnt1 inducible signaling pathway protein 1 from gelatin sponges. Biomaterials. 2011 Aug;32(24):5726–5732. doi: 10.1016/j.biomaterials.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Chong ZZ, Shang YC, Maiese K. Wnt1 inducible signaling pathway protein 1 [WISP1] blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res. 2012 Feb;9(1):20–31. doi: 10.2174/156720212799297137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157(3):429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Sweidi S, Sanchez MG, Bourque M, Morissette M, Dluzen D, Di Paolo T. Oestrogen receptors and signalling pathways: implications for neuroprotective effects of sex steroids in Parkinson's disease. J Neuroendocrinol. 2012 Jan;24(1):48–61. doi: 10.1111/j.1365-2826.2011.02193.x. [DOI] [PubMed] [Google Scholar]
- 27.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002 Dec 3;106(23):2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 28.Hou J, Chong ZZ, Shang YC, Maiese K. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010 Mar 4;321(2):194–206. doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity During Oxidant Stress. Curr Neurovasc Res. 2011 Aug 1;8(3):220–235. doi: 10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pineda D, Ampurdanes C, Medina MG, Serratosa J, Tusell JM, Saura J, et al. Tissue plasminogen activator induces microglial inflammation via a noncatalytic molecular mechanism involving activation of mitogen-activated protein kinases and Akt signaling pathways and AnnexinA2 and Galectin-1 receptors. Glia. 2012 Apr;60(4):526–540. doi: 10.1002/glia.22284. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F, Ding T, Yu L, Zhong Y, Dai H, Yan M. Dexmedetomidine protects against oxygen-glucose deprivation-induced injury through the I2 imidazoline receptor-PI3K/AKT pathway in rat C6 glioma cells. J Pharm Pharmacol. 2012 Jan;64(1):120–127. doi: 10.1111/j.2042-7158.2011.01382.x. [DOI] [PubMed] [Google Scholar]
- 32.Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ. WISP-1 is a Wnt-1- and beta-catenin-responsive oncogene. Genes Dev. 2000 Mar 1;14(5):585–595. [PMC free article] [PubMed] [Google Scholar]
- 33.Baryawno N, Sveinbjornsson B, Eksborg S, Chen CS, Kogner P, Johnsen JI. Small-molecule inhibitors of phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma growth. Cancer Res. 2010 Jan 1;70(1):266–276. doi: 10.1158/0008-5472.CAN-09-0578. [DOI] [PubMed] [Google Scholar]
- 34.Chong ZZ, Li F, Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005 Jan;20(1):299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007 Jun;19(6):1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Deleidi M, et al. Plasticity of Subventricular Zone Neuroprogenitors in MPTP [1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine] Mouse Model of Parkinson's Disease Involves Cross Talk between Inflammatory and Wnt/beta-Catenin Signaling Pathways: Functional Consequences for Neuroprotection and Repair. J Neurosci. 2012 Feb 8;32(6):2062–2085. doi: 10.1523/JNEUROSCI.5259-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006 Jan;21(1):103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Wang Y, Pan Q, Su Y, Zhang Z, Han J, et al. Wnt/beta-catenin pathway forms a negative feedback loop during TGF-beta1 induced human normal skin fibroblast-to-myofibroblast transition. J Dermatol Sci. 2012 Jan;65(1):38–49. doi: 10.1016/j.jdermsci.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Toku AE, Tekir SD, Ozbayraktar FB, Ulgen KO. Reconstruction and crosstalk of protein-protein interaction networks of Wnt and Hedgehog signaling in Drosophila melanogaster. Comput Biol Chem. 2011 Oct 12;35(5):282–292. doi: 10.1016/j.compbiolchem.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen TM, Subramanian IV, Xiao X, Ghosh G, Nguyen P, Kelekar A, et al. Endostatin induces autophagy in endothelial cells by modulating Beclin 1 and beta-catenin levels. J Cell Mol Med. 2009 Sep;13(9B):3687–3698. doi: 10.1111/j.1582-4934.2009.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Ding L, Wang X, Zhang J, Han W, Feng L, et al. Pterostilbene simultaneously induces apoptosis, cell cycle arrest and cyto-protective autophagy in breast cancer cells. Am J Transl Res. 2012;4(1):44–51. [PMC free article] [PubMed] [Google Scholar]
- 42.Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005 Dec;2(5):387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chong ZZ, Lin SH, Kang JQ, Maiese K. The tyrosine phosphatase SHP2 modulates MAP kinase p38 and caspase 1 and 3 to foster neuronal survival. Cell Mol Neurobiol. 2003 Oct;23(4–5):561–578. doi: 10.1023/A:1025158314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J Cereb Blood Flow Metab. 2000;20(9):1380–1391. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006 Aug;3(3):187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shang YC, Chong ZZ, Hou J, Maiese K. FoxO3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis. Curr Neurovasc Res. 2009 Nov;6(4):223–238. doi: 10.2174/156720209789630302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007 Apr;150(7):839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008 Oct 10;283(41):27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome, c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003 Mar;23(3):320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- 50.Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009 Feb;6(1):20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, et al. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996 Apr;16(4):1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-[4-morpholinyl]-8-phenyl-4H-1-benzopyran-4-one [LY294002] J Biol Chem. 1994 Feb 18;269(7):5241–5248. [PubMed] [Google Scholar]
- 53.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14(16):1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maiese K, Chong ZZ, Shang YC, Hou J. A "FOXO" in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009 May;29(3):395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim, and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006 May;3(2):107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawamoto EM, Gleichmann M, Yshii LM, Lima Lde S, Mattson MP, Scavone C. Effect of activation of canonical Wnt signaling by the Wnt-3a protein on the susceptibility of PC12 cells to oxidative and apoptotic insults. Braz J Med Biol Res. 2012 Jan;45(1):58–67. doi: 10.1590/S0100-879X2011007500157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu YL, Yang HP, Zhou XD, Gong L, Tang CL, Wang HJ. The Hypomethylation Agent Bisdemethoxycurcumin Acts on the WIF-1 Promoter, Inhibits the Canonical Wnt Pathway and Induces Apoptosis in Human Non-Small-Cell Lung Cancer. Curr Cancer Drug Targets. 2011 Sep 20; doi: 10.2174/156800911798073041. [DOI] [PubMed] [Google Scholar]
- 58.Wang XH, Sun X, Meng XW, Lv ZW, Du YJ, Zhu Y, et al. beta-catenin siRNA regulation of apoptosis- and angiogenesis-related gene expression in hepatocellular carcinoma cells: potential uses for gene therapy. Oncology reports. 2010 Oct;24(4):1093–1099. [PubMed] [Google Scholar]
- 59.Ghanevati M, Miller CA. Phospho-beta-catenin accumulation in Alzheimer's disease and in aggresomes attributable to proteasome dysfunction. J Mol Neurosci. 2005;25(1):79–94. doi: 10.1385/JMN:25:1:079. [DOI] [PubMed] [Google Scholar]
- 60.Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008 Oct 24;283(43):29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011;52(4):1173–1185. [PMC free article] [PubMed] [Google Scholar]
- 62.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009 Apr;20(7):1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venkatachalam K, Venkatesan B, Valente AJ, Melby PC, Nandish S, Reusch JE, et al. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-alpha [TNF-alpha]-stimulated cardiac fibroblast proliferation but inhibits TNF-alpha-induced cardiomyocyte death. J Biol Chem. 2009 May 22;284(21):14414–14427. doi: 10.1074/jbc.M809757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Price RM, Tulsyan N, Dermody JJ, Schwalb M, Soteropoulos P, Castronuovo JJ., Jr Gene expression after crush injury of human saphenous vein: using microarrays to define the transcriptional profile. J Am Coll Surg. 2004 Sep;199(3):411–418. doi: 10.1016/j.jamcollsurg.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 65.Chong ZZ, Kang JQ, Maiese K. AKT1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-xL and caspase 1, 3, and 9. Exp Cell Res. 2004 Jun 10;296(2):196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 66.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, mitochondrial caspase activation. Curr Neurovasc Res. 2010 May;7(2):95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koh SH, Noh MY, Cho GW, Kim KS, Kim SH. Erythropoietin increases the motility of human bone marrow-multipotent stromal cells [hBM-MSCs] and enhances the production of neurotrophic factors from hBM-MSCs. Stem Cells Dev. 2009 Apr;18(3):411–421. doi: 10.1089/scd.2008.0040. [DOI] [PubMed] [Google Scholar]
- 68.Mannell HK, Pircher J, Chaudhry DI, Alig SK, Koch EG, Mettler R, et al. ARNO regulates VEGF-dependent tissue responses by stabilizing endothelial VEGFR-2 surface expression. Cardiovasc Res. 2012 Jan 1;93(1):111–119. doi: 10.1093/cvr/cvr265. [DOI] [PubMed] [Google Scholar]
- 69.Su KH, Shyue SK, Kou YR, Ching LC, Chiang AN, Yu YB, et al. beta Common receptor integrates the erythropoietin signaling in activation of endothelial nitric oxide synthase. J Cell Physiol. 2011 Dec;226(12):3330–3339. doi: 10.1002/jcp.22678. [DOI] [PubMed] [Google Scholar]
- 70.Toba H, Sawai N, Morishita M, Murata S, Yoshida M, Nakashima K, et al. Chronic treatment with recombinant human erythropoietin exerts renoprotective effects beyond hematopoiesis in streptozotocin-induced diabetic rat. Eur J Pharmacol. 2009 Jun 10;612(1–3):106–114. doi: 10.1016/j.ejphar.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 71.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007 Feb;19(2):263–272. [PMC free article] [PubMed] [Google Scholar]
- 72.Chugh P, Fan S, Planelles V, Maggirwar SB, Dewhurst S, Kim B. Infection of human immunodeficiency virus and intracellular viral Tat protein exert a pro-survival effect in a human microglial cell line. J Mol Biol. 2007 Feb 9;366(1):67–81. doi: 10.1016/j.jmb.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou X, Wang L, Wang M, Xu L, Yu L, Fang T, et al. Emodin-induced microglial apoptosis is associated with TRB3 induction. Immunopharmacol Immunotoxicol. 2011 Dec;33(4):594–602. doi: 10.3109/08923973.2010.549135. [DOI] [PubMed] [Google Scholar]
- 74.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets. 2012 Jan 10;16(2):167–178. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005 Jan 5;293(1):90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chattopadhyay M, Walter C, Mata M, Fink DJ. Neuroprotective effect of herpes simplex virus-mediated gene transfer of erythropoietin in hyperglycemic dorsal root ganglion neurons. Brain. 2009 Apr;132(Pt 4):879–888. doi: 10.1093/brain/awp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chong ZZ, Kang J, Li F, Maiese K. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr Neurovasc Res. 2005 Jul;2(3):197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003 Mar;138(6):1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004 Jul;24(7):728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- 80.Komandirov MA, Knyazeva EA, Fedorenko YP, Rudkovskii MV, Stetsurin DA, Uzdensky AB. On the role of phosphatidylinositol 3-kinase, protein kinase b/akt, and glycogen synthase kinase-3beta in photodynamic injury of crayfish neurons and glial cells. J Mol Neurosci. 2011 Oct;45(2):229–235. doi: 10.1007/s12031-011-9499-1. [DOI] [PubMed] [Google Scholar]
- 81.Shen J, Wu Y, Xu JY, Zhang J, Sinclair SH, Yanoff M, et al. ERK-and Akt-dependent neuroprotection by erythropoietin [EPO] against glyoxal-AGEs via modulation of Bcl-xL. Bax, and BAD. Invest Ophthalmol Vis Sci. 2010 Jan;51(1):35–46. doi: 10.1167/iovs.09-3544. [DOI] [PubMed] [Google Scholar]
- 82.Zeng KW, Wang XM, Ko H, Kwon HC, Cha JW, Yang HO. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid beta-protein via the PI3K/Akt/Bad/Bcl[XL]-regulated mitochondrial apoptotic pathway. Eur J Pharmacol. 2011 Dec 15;672(1–3):45–55. doi: 10.1016/j.ejphar.2011.09.177. [DOI] [PubMed] [Google Scholar]
- 83.Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, et al. beta-Catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007 Nov;25(11):2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 84.Lee HN, Jeon GS, Kim DW, Cho IH, Cho SS. Expression of adenomatous polyposis coli protein in reactive astrocytes in hippocampus of kainic Acid-induced rat. Neurochem Res. 2010 Jan;35(1):114–121. doi: 10.1007/s11064-009-0036-3. [DOI] [PubMed] [Google Scholar]
- 85.Shahjee HM, Koch KR, Guo L, Zhang CO, Keay SK. Antiproliferative factor decreases Akt phosphorylation and alters gene expression via CKAP4 in T24 bladder carcinoma cells. J Exp Clin Cancer Res. 2010;29:160. doi: 10.1186/1756-9966-29-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qin AP, Liu CF, Qin YY, Hong LZ, Xu M, Yang L, et al. Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy. 2010 Aug 16;6(6):738–753. doi: 10.4161/auto.6.6.12573. [DOI] [PubMed] [Google Scholar]
- 87.Xin XY, Pan J, Wang XQ, Ma JF, Ding JQ, Yang GY, et al. 2-methoxyestradiol attenuates autophagy activation after global ischemia. Can J Neurol Sci. 2011 Jul;38(4):631–638. doi: 10.1017/s031716710001218x. [DOI] [PubMed] [Google Scholar]
- 88.Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol. 2004 Sep;24(18):8055–8068. doi: 10.1128/MCB.24.18.8055-8068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]