Abstract
The indirect serotonin (5-HT) agonist 3,4-methylenedioxymethamphetamine (MDMA) produces a distinct behavioral profile in rats consisting of locomotor hyperactivity, thigmotaxis, and decreased exploration. The indirect 5-HT agonist α-ethyltryptamine (AET) produces a similar behavioral profile. Using the Behavioral Pattern Monitor (BPM), the present investigation examined whether the effects of MDMA and AET are dependent on the novelty of the testing environment. These experiments were conducted in Sprague-Dawley rats housed on a reversed light cycle and tested during the dark phase of the light/dark cycle. We found that racemic MDMA (RS-MDMA; 3 mg/kg, SC) increased locomotor activity in rats tested in novel BPM chambers, but had no effect on locomotor activity in rats habituated to the BPM chambers immediately prior to testing. Likewise, AET (5 mg/kg, SC) increased locomotor activity in non-habituated animals but not in animals habituated to the test chambers. These results were unexpected because previous reports indicate that MDMA has robust locomotor-activating effects in habituated animals. To further examine the influence of habituation on MDMA-induced locomotor activity, we conducted parametric studies with S-(+)-MDMA (the more active enantiomer) in habituated and non-habituated rats housed on a standard or reversed light cycle. Light cycle was included as a variable due to reported differences in sensitivity to serotonergic ligands during the dark and light phases. In confirmation of our initial studies, rats tested during the dark phase and habituated to the BPM did not show an S-(+)-MDMA (3 mg/kg, SC)-induced increase in locomotor activity, whereas non-habituated rats did. By contrast, in rats tested during the light phase, S-(+)-MDMA increased locomotor activity in both non-habituated and habituated rats, although the response in habituated animals was attenuated. The finding that habituation and light cycle interact to influence MDMA- and AET-induced hyperactivity demonstrates that there are previously unrecognized complexities associated with the behavioral effects of these drugs.
Keywords: 3,4-methylenedioxymethamphetamine; locomotor activity; habituation; novelty; circadian rhythm
3,4-Methylenedioxymethamphetamine (MDMA) is a derivative of amphetamine that increases the non-exocytotic release of serotonin (5-HT), dopamine (DA), and norepinephrine (Nichols et al., 1982, 1986; Baumann et al., 2008). MDMA, a popular drug of abuse for several decades, can induce stimulation, heightened mood, empathy, feelings of closeness to others, and minor perceptual alterations (Vollenweider et al., 2002). Although there are similarities between the effects of MDMA and psychostimulants such as amphetamine, qualitative differences exist as well (Gouzoulis-Mayfrank et al., 1999). It has been proposed that MDMA belongs to a novel class of psychoactive compounds known as entactogens (Nichols, 1986; Nichols et al., 1986; Nichols and Oberlender, 1990). In support of this distinction, it appears that the effects of MDMA are mediated primarily by increases in 5-HT release (Callaway et al., 1990; Liechti et al., 2000a,b), whereas amphetamine acts largely via effects on DA efflux (Kelly et al., 1975).
MDMA and related compounds produce a distinct profile of behavioral effects in rats, including locomotor hyperactivity, thigmotaxis, and a reduction in the frequency of investigatory rearings and holepokes (Gold et al., 1998; Callaway et al., 1990, 1991). MDMA-induced hyperactivity is markedly attenuated by 5-HT uptake inhibitors (Callaway et al., 1990, 1991), indicating that the effects of MDMA are dependent on carrier-mediated release of 5-HT. There is extensive evidence that the 5-HT1B receptor is responsible for mediating the behavioral effects of MDMA. For example, the 5-HT1A/1B agonist RU 24969 produces effects that are qualitatively similar to those of MDMA (Rempel et al., 1993), and RU 24969 and MDMA produce cross-tolerance (Callaway and Geyer, 1992). Furthermore, the locomotor-activating effects of MDMA are blocked by the selective 5-HT1B/1D antagonist GR127935 and attenuated in 5-HT1B knockout mice (McCreary et al., 1999; Scearce-Levie et al., 1999; Fletcher et al., 2002). There is also evidence that 5-HT2A receptors (Ball and Rebec, 2005; Kehne et al., 1996; Fletcher et al., 2002), D1 and D2 dopaminergic receptors (Bubar et al., 2004; Risbrough et al., 2006), and α1 adrenergic receptors (Selken and Nichols, 2007) contribute to the MDMA-induced locomotor response.
During the course of a series of pilot behavioral experiments with MDMA, we found evidence that the drug does not increase locomotor activity in animals tested in a familiar environment (data not shown). This finding was surprising because several other groups have reported that racemic MDMA and the more active enantiomer S-(+)-MDMA produced robust hyperactivity in rats habituated to the test chambers (Kehne et al., 1996; McCreary et al., 1999; Bankson and Cunningham, 2002; Fletcher et al., 2002). Given these discrepant observations, we conducted a series of experiments to determine whether habituation alters the behavioral response to MDMA. Parallel studies were conducted with α-ethyltryptamine (AET), a 5-HT releasing agent (Baker et al., 1980) that produces an MDMA-like behavioral profile in rats (Krebs and Geyer, 1993; Glennon, 1993). While most drugs with MDMA-like effects are based on the phenethylamine structure, AET is derived from tryptamine and thus more related structurally to 5-HT; hence, studies with AET were included to assess the generality of any findings with MDMA. In rodents, the baseline level of activity (Iuvone and Van Hartesveldt, 1977), as well as the behavioral response to serotonergic ligands (Singleton and Marsden, 1981; Lu and Nagayama, 1996; Darmani, 1998), varies over the diurnal light/dark cycle. Most investigators have tested MDMA in rats housed on a standard light cycle (i.e., behavioral testing occurred during the light phase), whereas our studies with MDMA were conducted in rats housed on a reversed light cycle. To determine whether the effects of MDMA are influenced by the phase of the light/dark cycle, we also conducted parametric studies with MDMA in habituated and non-habituated rats housed under a standard or a reversed light-dark cycle.
2. MATERIALS AND METHODS
2.1. Subjects
Male Sprague-Dawley rats (250–300 g; Harlan, San Diego, CA) were housed in pairs and maintained on either a standard or reversed 12-hour light/dark cycle (lights on or off at 0700 h, respectively) based on random group assignment. All animals were housed in compliance with AAALAC guidelines, and all procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of California San Diego. Food and water were provided ad libitum. Testing occurred between 0900 and 1600 h. Animals were handled upon arrival and allowed to acclimate for 1 week prior to testing.
2.2. Drugs and solutions
Racemic 3,4-methylenedioxymethamphetamine hydrochloride (RS-MDMA) and S-(+)-MDMA hydrochloride were generously donated by the National Institute on Drug Abuse (Rockville, MD, USA); α-ethyltryptamine acetate (AET) was obtained from Sigma-Aldrich (St. Louis, MO, USA). MDMA and AET were dissolved in 0.9% (w/v) sterile saline and injected subcutaneously at a volume of 1 ml/kg. MDMA and AET were injected 10 min prior to testing in the BPM.
2.3. Behavioral Pattern Monitor (BPM)
The BPM consisted of a 30.5 x 61 cm black Plexiglas chamber that provided quantitative and qualitative measures of exploratory and investigatory behavior, including sequential patterns of activity (see: Geyer et al., 1986 for more details). Each of the 8 chambers was enclosed for sound isolation, well ventilated, and illuminated with a red light bulb (7.5 W) for testing within a darkened room. A microcomputer recorded the animal’s successive holepokes, rearings, and position within the chamber. Holepokes were measured at 2.5-cm holes placed along the walls (3 along each long wall, 1 along the back short wall) and 3 along the floor of each chamber. Nose pokes in the holes were detected using infrared (IR) photobeams. The chamber contained a 4 x 8 array of IR photobeams located 2.5 cm above the floor, which detected the position of the animal in an X-Y plane. Rearing behavior along the walls was detected by a thin metal plate located 15.5 cm above the floor, on all four walls. A rearing was recorded when the animal’s forepaws touched the wall plate as the hind paws touched the floor. Activity within each chamber was monitored continuously by a computer (all the IR beams were sampled every 55 msec) and data were collected and stored for later analysis.
2.4. Testing procedure
One day before BPM studies, animals were taken to the testing room, weighed, handled briefly, placed in a clear Plexiglas box (24 × 46 cm) for approximately 30 s, and then returned to their cages in the vivarium. On the testing day, rats were brought to the testing room under dark cover and allowed to acclimate for 1 hour under red lights. In Experiment 3, the standard and reversed light cycle animals were handled in the same manner, but were tested on alternating days to avoid entry into multiple vivarium rooms on the same day.
2.5. Experimental design
Half of the animals tested in each experiment were habituated to the BPM chambers for 30 minutes prior to testing, and the other animals were naïve to the BPM environment. In the habituated groups, animals were placed in the BPM testing chambers 30 min prior to testing. Animals in the non-habituated groups were left in their transport cages during that time. At 10 min prior to testing, animals received injections of vehicle or the appropriate drug treatment, and were then returned to the same BPM chamber (habituated animals) or transport cage (non-habituated animals). After 10 min, the non-habituated animals were placed in the BPM chambers and then data collection commenced. All BPM test sessions lasted 60 min. Chambers were cleaned thoroughly between sessions and checked to ensure proper functioning. Details of the individual experiments are listed in Table 1.
Table 1.
Experimental Design
| Experiment | Group | N | Light/dark cyclea | Habituationb | Treatment |
|---|---|---|---|---|---|
| 1 | 1 | 8 | Reversed cycle | Non-Habituated | Vehicle |
| 2 | 9 | RS-MDMA 3 mg/kg | |||
| 3 | 8 | Habituated | Vehicle | ||
| 4 | 9 | RS-MDMA 3 mg/kg | |||
| 2 | 1 | 13 | Reversed cycle | Non-Habituated | Vehicle |
| 2 | 13 | AET 5 mg/kg | |||
| 3 | 13 | Habituated | Vehicle | ||
| 4 | 13 | AET 5 mg/kg | |||
| 3 | 1 | 8 | Reversed cycle | Non-Habituated | Vehicle |
| 2 | 8 | S-(+)-MDMA 3 mg/kg | |||
| 3 | 8 | Habituated | Vehicle | ||
| 4 | 7 | S-(+)-MDMA 3 mg/kg | |||
| 5 | 8 | Standard cycle | Non-Habituated | Vehicle | |
| 6 | 8 | S-(+)-MDMA 3 mg/kg | |||
| 7 | 8 | Habituated | Vehicle | ||
| 8 | 8 | S-(+)-MDMA 3 mg/kg | |||
Animals housed on a reversed 12-hour light/dark cycle (lights on at 1900 h) were tested during their dark phase; animals housed on a standard 12-hour light/dark cycle (lights on at 0700 h) were tested during their light phase.
Habituated animals were exposed to the BPM chambers for 30 min prior to data collection; non-habituated animals were naïve to the BPM chambers.
2.7. Data analysis
Locomotor activity was quantified by the number of crossings between eight equal 15.25 x 15.25 cm sectors within the BPM (see: Geyer et al., 1986). Investigatory behavior was quantified by the total number of rearings and holepokes. Locomotor activity was analyzed in 10-min blocks, and rearings and holepokes were analyzed in 30-min blocks. For Experiment 1 and Experiment 2, data were analyzed using three-way ANOVA with habituation and treatment as between-subject factors and time as a repeated measure. For Experiment 3, data were analyzed using four-way ANOVA with habituation, light cycle, and treatment as between-subject factors and time as a repeated measure. Three-way ANOVAs were also used in Experiment 3 to assess the two light cycle conditions individually. Specific post hoc comparisons were made using Tukey’s Studentized Range Method. The criterion for significance was set at p<0.05.
3. RESULTS
3.1. Experiment 1: Effect of habituation on the behavioral response to RS-MDMA
To determine whether the behavioral effects of MDMA are dependent on familiarity with the testing environment, we compared the effects in non-habituated and habituated rats. To induce habituation, rats were exposed to the BPM chambers for 30 min, which significantly reduced the baseline level of locomotor activity (F(1,29)=99.86, p<0.0001; Fig. 1). Figure 1 illustrates the effects of RS-MDMA on crossings, a measure of locomotor activity. In animals tested in a novel environment, RS-MDMA (3 mg/kg) increased locomotor activity (F(1,29)=15.44, p<0.0005), an effect that occurred primarily during the last 40 min of testing (MDMA × time: F(5,145)=7.43, p<0.0001). Interestingly, RS-MDMA had no effect on locomotor activity in rats habituated to the BPM chambers immediately prior to testing (MDMA × habituation: F(1,29)=4.94, p<0.04; MDMA × habituation × time: F(5,145)=11.61, p<0.0001). Habituation also reduced the baseline level of rearing (F(1,29)=14.56, p=0.0007) and holepoking behavior (F(1,29)=25.60, p<0.0001). Administration of RS-MDMA significantly reduced the number of rearings and holepokes in both non-habituated and habituated rats (see Table 2). Although there were significant interactions of drug treatment, time, and habituation for rearings (F(1,29)=35.23, p<0.0001) and holepokes (F(1,29)=19.02, p=0.0001), inspection of the data indicated that the interaction is likely a consequence of floor effects in the habituated animals, as opposed to changes in the effects of RS-MDMA.
Figure 1.
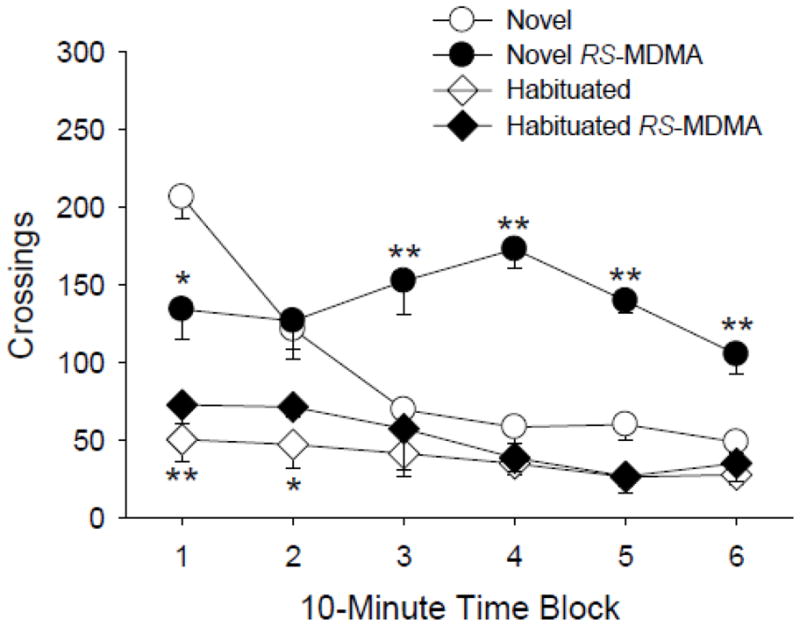
Effect of RS-MDMA (3 mg/kg) on locomotor activity. RS-MDMA increased crossings in rats tested in a novel environment but had no effect in rats tested in a familiar environment. The circles (○, ●) refer to non-habituated animals, and the diamonds (◇, ◆) refer to habituated animals. Data are reported as group means ± S.E.M. *p<0.05, **p<0.01 versus the non-habituated vehicle control group (Tukey’s test). ##p<0.01 versus the habituated vehicle control group (Tukey’s test).
Table 2.
Effects of RS-MDMA on investigatory behaviora
| Non-habituated | Habituated | |||
|---|---|---|---|---|
| Vehicle | RS-MDMA | Vehicle | RS-MDMA | |
| Rearings | ||||
| 0–30 Minutes | 105.3±13.3 | 6.4±2.8b | 37.5±6.5b | 3.9±1.9c |
| 30–60 Minutes | 24.0±6.5 | 40.4±12.8 | 26.6±5.9 | 8.0±5.6 |
| Holepokes | ||||
| 0–30 Minutes | 188.0±25.2 | 35.8±6.5b | 46.0±9.0b | 7.7±2.0 |
| 30–60 Minutes | 100.6±24.0 | 75.5±16.1 | 68.8±9.4 | 27.7±7.9 |
Data are reported as the mean number of events ± S.E.M.
p<0.05 versus the non-habituated vehicle control group (Tukey’s test).
p<0.05 versus the habituated vehicle control group (Tukey’s test).
3.2. Experiment 2: Effect of habituation on the behavioral response to AET
Previous BPM studies have shown that AET induces a MDMA-like behavioral profile in rats (Krebs and Geyer, 1993). Consistent with those findings, administration of 5 mg/kg AET significantly increased locomotor activity in non-habituated rats (AET effect: F(1,48)=6.61, p<0.02; Fig. 2). Importantly, as was found with MDMA in Experiment 1, AET did not increase locomotor activity in habituated rats (AET × habituation: F(1,48)=16.83, p<0.0002). AET reduced the total number of holepokes (F(1,48)=29.47, p<0.0001) and rearings (F(1,48)=47.49, p<0.0001) in both non-habituated and habituated rats (see Table 3). Habituation significantly reduced the baseline level of locomotor activity (F(1,48)=51.29, p<0.0001), holepokes (F(1,48)=16.23, p<0.0002), and rearing (F(1,48)=12.31, p<0.001).
Figure 2.
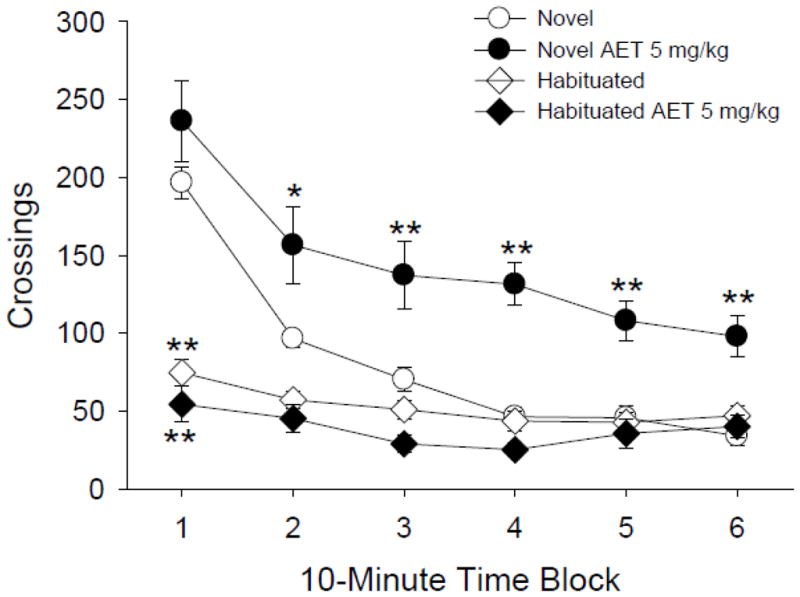
Effect of AET (5 mg/kg) on locomotor activity. AET increased crossings in rats tested in a novel environment but had no effect in rats tested in a familiar environment. The circles (○, ●) refer to non-habituated animals, and the diamonds (◇, ◆) refer to habituated animals. Data are reported as group means ± S.E.M. *p<0.05, **p<0.01 versus the non-habituated vehicle control group (Tukey’s test).
Table 3.
Effect of α-ethyltryptamine (AET) on investigatory behaviora
| Non-habituated | Habituated | |||
|---|---|---|---|---|
| Vehicle | AET | Vehicle | AET | |
| Rearings | ||||
| 0–30 Minutes | 113.2±12.2 | 19.2±5.7b | 54.2±7.3b | 16.4±4.8c |
| 30–60 Minutes | 39.7±9.3 | 26.9±5.7 | 35.5±5.9 | 10.8±2.0c |
| Holepokes | ||||
| 0–30 Minutes | 147.4±9.7 | 50.4±11.4b | 79.0±10.1b | 13.8±3.7c |
| 30–60 Minutes | 84.7±12.2 | 68.3±20.4 | 68.6±11.7 | 22.1±5.5 |
Data are reported as the mean number of events ± S.E.M.
p<0.05 versus the non-habituated vehicle control group (Tukey’s test).
p<0.05 versus the habituated vehicle control group (Tukey’s test).
3.3. Experiment 3: Effect of light cycle and habituation on the behavioral response to S-(+)-MDMA
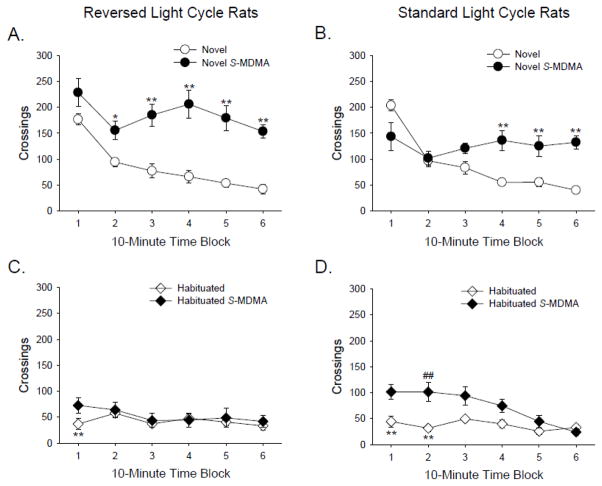
Light cycle had no effect on the baseline level of locomotor activity of vehicle-treated animals. Likewise, in non-habituated animals, there was no significant difference between the amount of locomotor hyperactivity induced by S-(+)-MDMA in reversed-cycle rats versus standard-cycle rats. There was, however, a significant three-way interaction between light cycle, habituation, and S-(+)-MDMA (F(1,56)=11.17, p<0.002); therefore, the results of Experiment 3 were analyzed separately for reversed-light cycle rats (i.e., rats tested during their dark phase) and standard-light cycle rats (i.e., rats tested during their light phase).
3.3.1. Reversed light-cycle rats
As we found in Experiment 1 with RS-MDMA, 3 mg/kg S-(+)-MDMA significantly increased locomotor activity in non-habituated rats (MDMA effect: F(1,28)=27.11, p<0.0001; Fig. 3A), but had no effect on locomotor activity in rats that were habituated to the BPM chambers (habituation × MDMA: F(1,28)=17.88, p<0.0002; Fig. 3C). There was a significant interaction of habituation and time (F(5,140)=8.16, p<0.0001), and habituation significantly reduced the locomotor activity of vehicle-treated rats during the first 10 min of testing (p<0.05, Tukey’s test). In both non-habituated and habituated rats, S-(+)-MDMA significantly decreased the number of holepokes (F(1,28)=22.98, p<0.0001) and rearings (F(1,28)=58.15, p<0.0001) compared to vehicle-treated animals (see Table 4).
Figure 3.
Effect of S-(+)-MDMA (3 mg/kg) on locomotor activity. S-(+)-MDMA was tested in rats housed on a reversed light cycle (A, C) or a standard light cycle (B, D). In animals housed on a reversed light cycle, S-(+)-MDMA increased locomotor activity in non-habituated animals (A) but had no effect in habituated animals (C). In animals housed on a standard light cycle, S-(+)-MDMA increased locomotor activity in non-habituated animals (B) and habituated animals (D). The circles (○, ●) refer to non-habituated animals, and the diamonds (◇, ◆) refer to habituated animals. Data are reported as group means ± S.E.M. *p<0.05, **p<0.01 versus the non-habituated vehicle control group housed on the same light cycle (Tukey’s test). ##p<0.01 versus the habituated vehicle control group housed on the same light cycle (Tukey’s test).
Table 4.
Effects of S-(+)-MDMA on investigatory behavior in reversed light cycle and standard light cycle ratsa
| Reversed Light Cycle Rats | Standard Light Cycle Rats | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-habituated | Habituated | Non-habituated | Habituated | |||||
| Vehicle | S-MDMA | Vehicle | S-MDMA | Vehicle | S-MDMA | Vehicle | S-MDMA | |
| Rearings | ||||||||
| 0–30 Minutes | 117.0±12.5 | 2.6±1.9b | 33.6±6.6b | 0.1±0.1c | 104.9±14.8 | 0.6±0.5b | 33.9±6.6b | 0.1±0.1c |
| 30–60 Minutes | 42.9±7.1 | 42.3±9.1 | 35.0±5.3 | 10.6±5.0 | 29.4±6.8 | 16.1±4.6 | 24.8±5.8 | 2.0±0.8c |
| Holepokes | ||||||||
| 0–30 Minutes | 145.4±19.9 | 34.1±7.0b | 69.0±16.4b | 23.4±7.0 | 130.3±12.3 | 33.0±6.6b | 54.8±8.4b | 11.0±2.3c |
| 30–60 Minutes | 54.1±19.6 | 16.0±4.6 | 13.4±5.9 | 3.0±1.2 | 26.8±7.5 | 17.0±3.5 | 13.0±6.4 | 1.8±0.8c |
Data are reported as the mean number of events ± S.E.M.
p<0.05 versus the non-habituated vehicle control group housed on the same light cycle (Tukey’s test).
p<0.05 versus the habituated vehicle control group housed on the same light cycle (Tukey’s test).
3.3.2. Standard light-cycle rats
For the animals housed on a standard light cycle, there was a main effect of S-(+)-MDMA (F(1,28)=22.28, p<0.0001) and an interaction between S-(+)-MDMA, habituation, and time (F(5,140)=13.04, p<0.0001). S-(+)-MDMA significantly increased locomotor activity in the non-habituated animals during the last 30 min of testing (p<0.01, Tukey’s test; Fig. 3B). Although S-(+)-MDMA also increased locomotor activity in habituated rats, the effect was attenuated compared with the non-habituated animals, and was only significant during the second 10-min block of testing (p<0.01, Tukey’s test; Fig. 3D). Examination of the data at a lower time resolution (i.e., over 30-min blocks) confirmed that habituation almost completely attenuated the effect of S-(+)-MDMA (data not shown). In the standard light-cycle rats, there was a significant interaction of habituation and time (F(5,140)=5.39, p<0.0001), and habituation significantly reduced the activity of vehicle-treated rats during the first 20 min of testing (p<0.01, Tukey’s test). As was found in the reversed light-cycle animals, S-(+)-MDMA reduced the number of holepokes (F(1,28)=44.87, p<0.0001) and rearings (F(1,28)=54.85, p<0.0001), regardless of habituation group (see Table 4).
3.3.3. Comparison of S-(+)-MDMA effects in rats housed on reversed and standard light-cycles
To directly compare the effects of S-(+)-MDMA over the different light-cycle and habituation conditions, S-(+)-MDMA-induced locomotor activity was normalized to baseline (saline injection) activity levels (Fig. 4). There was a significant difference between the normalized levels of locomotor activity induced by S-(+)-MDMA in the four groups of animals (F(3,28)=4.14, p<0.02). Compared to baseline activity levels, S-(+)-MDMA increased locomotor activity to a similar extent in non-habituated rats housed on a reversed light cycle (218.1% increase) and in habituated rats housed on a standard light cycle (199.4% increase). MDMA had less of an effect on locomotor activity in non-habituated rats housed on a standard light cycle (143.3% increase), and relatively little effect on locomotor activity in habituated rats housed on a reversed light cycle (124.5% increase). These data demonstrate that habituation markedly attenuates the ability of MDMA to increase locomotor activity during the active phase of the diurnal cycle, whereas habituation augments MDMA-induced locomotor hyperactivity during the sleep phase.
Figure 4.
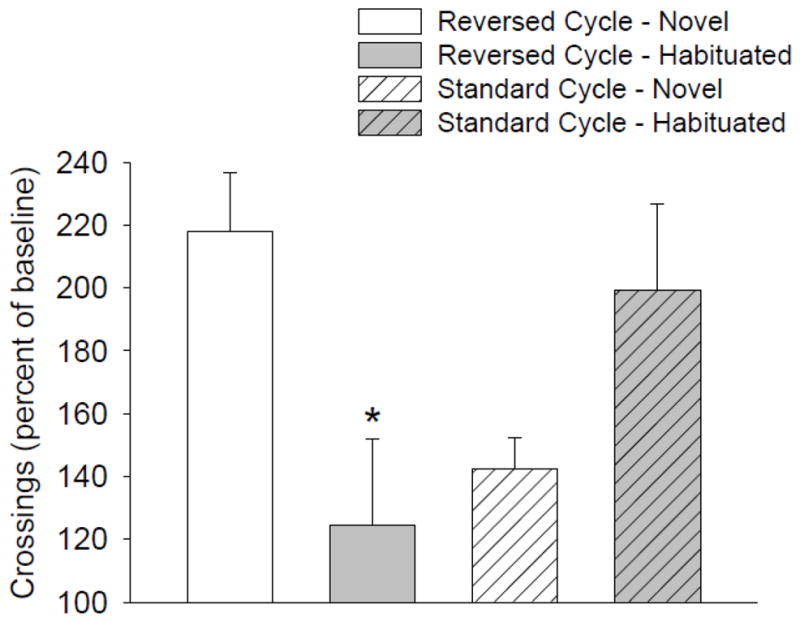
Effect of habituation and light cycle on the locomotor hyperactivity induced by S-(+)-MDMA (3 mg/kg). S-(+)-MDMA-induced locomotor activity (collapsed across the 60-min BPM session) was normalized to the mean activity of saline-treated rats. Data are reported as group means ± S.E.M. *p<0.05 versus S-(+)-MDMA in non-habituated rats housed on a reversed light cycle (Tukey’s test).
4. DISCUSSION
Despite their different chemical structures, the indirect 5-HT agonists MDMA and AET produce strikingly similar behavioral profiles in rats, including locomotor hyperactivity and reductions in the frequency of investigatory behaviors (Gold et al., 1998; Callaway et al., 1990; Krebs and Geyer, 1993). In this study, we investigated whether the effects of MDMA and AET are sensitive to the novelty of the testing environment and/or the time tested relative to the light/dark cycle. The present data demonstrate that a low dose of RS-MDMA increases locomotor activity in rats tested in novel BPM chambers during the dark phase of the light-dark cycle, but has no effect on locomotor activity in animals pre-exposed to the test environment. Similar to MDMA, the locomotor-activating effects of AET are dependent on the novelty of the test chambers. By contrast to the effect on locomotor activity, habituation did not alter the reduction of investigatory behavior induced by RS-MDMA or AET.
Although the present results indicate that RS-MDMA, S-(+)-MDMA, and AET induce locomotor hyperactivity in a novel environment but not in a familiar environment, we only tested single doses of those compounds, so we cannot eliminate the possibility that habituation is actually producing a rightward dose-response shift. Nonetheless, these studies clearly demonstrate that environmental factors exert a profound influence on the behavioral response to 5-HT releasing agents. Similar to our findings, it has been reported the 5-HT uptake inhibitor citalopram increases locomotor activity tested in a novel environment but not in a familiar environment, although these studies were done in mice (Brocco et al., 2002; Millan et al., 2003); therefore, it appears that sensitivity to environmental novelty may be a property common to multiple classes of indirect 5-HT agonists. Although the mechanism through which habituation influences the response to MDMA and AET is not certain, effects on serotonergic transmission are likely to be involved. The locomotor-activating effects of MDMA and AET are dependent on increases in carrier-mediated release of 5-HT (Callaway et al., 1990; Krebs and Geyer, 1993), which in turn activates 5-HT1B receptors (Callaway and Geyer, 1992; Rempel et al., 1993; McCreary et al., 1999; Fletcher et al., 2002). Therefore, it is possible that environmental familiarity could influence MDMA- and AET-induced hyperactivity by altering 5-HT release and/or the downstream 5-HT1B receptor-mediated response. Additional studies are necessary to determine the exact mechanism through which habituation alters the response to MDMA-like compounds.
While it was thought initially that the effects of MDMA and related substances were similar to those of psychostimulants and serotonergic hallucinogens such as LSD or DOM, drug discrimination studies (Glennon et al., 1988; Oberlender & Nichols 1988, 1990), BPM experiments (Paulus and Geyer, 1992; Krebs and Geyer, 1993), and controlled trials in humans (Hermle et al., 1993; Vollenweider et al., 1998; Gouzoulis-Mayfrank et al., 1999) have shown that these compounds cannot be characterized simply as stimulants or hallucinogens. Based on those findings, MDMA-like compounds are now recognized as being members of a novel pharmacological class. Interestingly, however, the results of the present studies demonstrate that there are at least some similarities between the effects of hallucinogens and MDMA-like compounds on locomotor activity. Like MDMA and AET, the effects of serotonergic hallucinogens on locomotor activity are sensitive to habituation and are attenuated in a familiar environment (Adams and Geyer 1985a,b; Wing et al., 1990; Halberstadt et al., 2008). Although hallucinogens typically reduce locomotor activity when tested in rats (Adams and Geyer 1985b; Wing et al., 1990), they can also increase locomotor activity under certain conditions (Mittman and Geyer, 1991; Halberstadt et al., 2008). Despite the fact that hallucinogens and MDMA-like compounds belong to distinct pharmacological classes, studies in rodents and humans demonstrate that there is some degree of overlap between their effects (Glennon et al., 1997; Liechti et al., 2000c); the present findings provide additional evidence that behavioral similarities exist between MDMA and hallucinogens.
In stark contrast to our finding that RS-MDMA has no effect on locomotor activity in a familiar environment, other groups have reported that MDMA induces robust hyperactivity in habituated animals (Kehne et al., 1996; McCreary et al., 1999; Bankson and Cunningham, 2002; Fletcher et al., 2002; Bubar et al., 2004; Herin et al., 2005). It is important to note, however, that in those previous studies, behavioral testing was conducted during the light phase of the light/dark cycle (i.e., the sleep period of rats, which are nocturnal). By contrast, our studies with RS-MDMA were conducted using rats housed on a reversed cycle, and testing occurred during the dark phase of the light/dark cycle. Previous reports have demonstrated that the behavioral effects of serotonergic agents can vary considerably depending on the phase of the light/dark cycle. One example is the head twitch response (HTR) in rodents, a 5-HT2A receptor-mediated behavior evoked by many agents that increase 5-HT outflow. p-Chloroamphetamine, a compound with MDMA-like effects on 5-HT release, induces a more robust HTR during the light phase compared with the dark phase (Singleton and Marsden, 1981). The selective 5-HT1A antagonist WAY-100635 induces the HTR by disinhibiting serotonergic neurons, but this effect occurs only during the light phase (Darmani, 1998). By contrast, behavioral and physiological sensitivity to the 5-HT1A agonist 8-OH-DPAT peaks during the dark phase (Lu and Nagayama, 1996, 1997). There is also significant circadian variation in the behavioral response to psychostimulants (Gaytan et al., 1997, 1998; Webb et al., 2009). Given those previous findings, it is possible that there may be fluctuations in the response to MDMA depending on the time of administration.
To determine whether differences in housing conditions (i.e., standard light cycle vs. reversed cycle) were responsible for the discrepant observations regarding the sensitivity of MDMA-induced locomotor-activating effects to habituation, we conducted a parametric experiment to compare the effects of habituation and light-cycle on the response to MDMA. As shown in Figure 3, S-(+)-MDMA failed to increase locomotor activity in habituated rats tested during the dark phase, but did produce an increase in locomotor activity in habituated rats tested during the light phase. These findings demonstrate that the locomotor-activating effects of MDMA are less dependent on novelty during the light phase compared with the dark phase; this difference may explain why previous studies detected MDMA-induced hyperactivity in habituated animals. It is not clear why there is circadian variability in the response to MDMA, but one possible explanation is that the ability of MDMA to provoke 5-HT release varies over the light/dark cycle. MDMA preferentially releases 5-HT from the newly synthesized cytoplasmic pool (Wichems et al., 1995), and the neurochemical and electrophysiological effects of MDMA are potentiated by administration of the 5-HT precursor L-tryptophan, which increases 5-HT synthesis (Bradberry et al., 1990; Sprouse et al., 1990; Evans et al., 2008). As the rate of 5-HT synthesis increases during the light phase (Hery et al., 1972), it is likely that the ability of MDMA to induce 5-HT release peaks during the light phase of the diurnal cycle. There is also evidence that the sensitivity of 5-HT1B receptors, as well as the density of 5-HT1 binding sites, increases during the light phase (Akiyoshi et al., 1989; Garabette et al., 2000). These diurnal fluctuations in serotonergic transmission could reduce the sensitivity of MDMA locomotor-activating effects to habituation. Other neurochemical alterations that occur over the circadian cycle, including changes in catecholaminergic transmission, intracellular signaling cascades, and membrane properties (Weiner et al., 1992), could also potentially alter the response to MDMA.
Electrophysiological investigations have established that the firing of serotonergic neurons in the midbrain raphe nuclei increases across the sleep-wake-arousal cycle and is positively correlated with the level of behavioral activation (Trulson and Jacobs, 1979; Sakai and Crochet, 2001). Therefore, the fluctuation we observed in the sensitivity to the locomotor-activating effects of MDMA over the diurnal cycle could potentially be a consequence of differences in serotonergic background activity. This interpretation, however, is difficult to reconcile with two findings. First, microdialysis studies in rats have shown that the increase in 5-HT efflux induced by 10 mg/kg MDMA is not altered by infusion of the Na+-channel blocker tetrodotoxin through the dialysis probe (Gudelsky and Nash, 1996), demonstrating that MDMA-induced 5-HT release is not dependent on impulse flow. Second, MDMA suppresses the firing of serotonergic neurons by indirectly activating 5-HT1A autoreceptors (Sprouse et al., 1989; Piercey et al., 1990; Gartside et al., 1997; Queree et al., 2009). Taken together, these findings suggest that the locomotor-activating effects of MDMA are unlikely to be influenced by fluctuations of serotonergic outflow over the diurnal cycle.
In summary, our experiments demonstrate that habituation reduces the locomotor-activating effects of MDMA, and show that the sensitivity to habituation is most prominent during the dark phase of the light/dark cycle. Although the exact mechanism for the observed effect of habituation is unclear, these findings indicate that the locomotor-activating effects of MDMA exhibit a complex dependence on the testing conditions. Although it is not clear whether the behavioral effects of MDMA in humans exhibit a similar dependence on environmental factors, there is some evidence that the human response to the drug can be affected by setting and expectancy (Parrott, 2007). Therefore, it is possible that the clinical effects of MDMA may be sensitive to the environment in which the drug is administered. Because clinical trials are now investigating MDMA as an adjunct for psychotherapy (Bouso et al., 2008; Mithoefer et al., 2011), it is important to consider the potential environmental influence on MDMA effects when human studies with the drug are conducted. The results of the present investigation highlight the fact that the behavioral response to serotonergic agents can vary depending on the testing conditions. The findings that the behavioral effects of MDMA-like compounds are influenced by the novelty of the testing environment and the time of administration should be taken into account when studies of these compounds are conducted. More generally, given the fact that the serotonergic system plays a role in mediating or modulating the effects of a variety of abused substances, the present findings raise the possibility that entactogens and serotonergic hallucinogens may not be the only drug classes that produce behavioral effects that are sensitive to environmental familiarity.
Highlights.
Studies examined whether the effects of MDMA are dependent on the novelty of the test environment
The ability of MDMA to induce locomotor hyperactivity was attenuated by habituation to the test environment
The effect of habituation on the locomotor response to MDMA varied over the light-cycle
Acknowledgments
Supported by National Institute on Drug Abuse Awards R01 DA002925 and F32 DA025412, and the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams LM, Geyer MA. A proposed animal model for hallucinogens based on LSD’s effects on patterns of exploration in rats. Behav Neurosci. 1985a;5:881–900. doi: 10.1037//0735-7044.99.5.881. [DOI] [PubMed] [Google Scholar]
- Adams L, Geyer MA. Effects of DOM and DMT in a proposed animal model of hallucinogenic activity. Prog Neuropsychopharmacol Biol Psychiatry. 1985b;9:121–32. doi: 10.1016/0278-5846(85)90074-0. [DOI] [PubMed] [Google Scholar]
- Akiyoshi J, Kuranaga H, Tsuchiyama K, Nagayama H. Circadian rhythm of serotonin receptor in rat brain. Pharmacol Biochem Behav. 1989;32:491–3. doi: 10.1016/0091-3057(89)90186-x. [DOI] [PubMed] [Google Scholar]
- Baker GB, Hiob LE, Dewhurst WG. Effects of monoamine oxidase inhibitors on release of dopamine and 5-hydroxytryptamine from rat striatum in vitro. Cell Mol Biol. 1980;26:183–6. [PubMed] [Google Scholar]
- Ball KT, Rebec GV. Role of 5-HT2A and 5-HT2C/B receptors in the acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on striatal single-unit activity and locomotion in freely moving rats. Psychopharmacology. 2005;81:676–87. doi: 10.1007/s00213-005-0038-z. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. Pharmacological studies of the acute effects of (+)-3,4- methylenedioxymethamphetamine on locomotor activity: role of 5-HT1B/1D and 5-HT2 receptors. Neuropsychopharmacology. 2002;26:40–52. doi: 10.1016/S0893-133X(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav. 2008;90:208–17. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Sprouse JS, Aghajanian GK, Roth RH. 3,4-Methylenedioxymethamphetamine (MDMA)-induced release of endogenous serotonin from the rat dorsal raphe nucleus in vitro: Effects of fluoxetine and tryptophan. Neurochem Int. 1990;17:509–13. doi: 10.1016/0197-0186(90)90037-t. [DOI] [PubMed] [Google Scholar]
- Brocco M, Dekeyne A, Veiga S, Girardon S, Millan MJ. Induction of hyperlocomotion in mice exposed to a novel environment by inhibition of serotonin reuptake. A pharmacological characterization of diverse classes of antidepressant agents. Pharmacol Biochem Behav. 2002;71:667–80. doi: 10.1016/s0091-3057(01)00701-8. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Pack KM, Frankel PS, Cunningham KA. Effects of dopamine D1- or D2-like receptor antagonists on the hypermotive and discriminative stimulus effects of (+)-MDMA. Psychopharmacology. 2004;173:326–36. doi: 10.1007/s00213-004-1790-1. [DOI] [PubMed] [Google Scholar]
- Bouso JC, Doblin R, Farré M, Alcázar MA, Gómez-Jarabo G. MDMA-assisted psychotherapy using low doses in a small sample of women with chronic posttraumatic stress disorder. J Psychoactive Drugs. 2008;40:225–36. doi: 10.1080/02791072.2008.10400637. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Geyer MA. Tolerance and cross-tolerance to the activating effects of 3,4-methylenedioxymethamphetamine and a 5-hydroxytryptamine1B agonist. J Pharmacol Exp Ther. 1992;263:318–26. [PubMed] [Google Scholar]
- Callaway CW, Johnson MP, Gold LH, Nichols DE, Geyer MA. Amphetamine derivatives induce locomotor hyperactivity by acting as indirect serotonin agonists. Psychopharmacology. 1991;104:293–301. doi: 10.1007/BF02246026. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Geyer MA. Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther. 1990;254:456–64. [PubMed] [Google Scholar]
- Darmani NA. The silent and selective 5-HT1A antagonist, WAY 100635, produces via an indirect mechanism, a 5-HT2A receptor-mediated behaviour in mice during the day but not at night. Short communication. J Neural Transm. 1998;105:635–43. doi: 10.1007/s007020050085. [DOI] [PubMed] [Google Scholar]
- Evans AK, Reinders N, Ashford KA, Christie IN, Wakerly JB, Lowry CA. Evidence for serotonin synthesis-dependent regulation of in vitro neuronal firing rats in the midbrain raphe complex. Eur J Pharmacol. 1008;590:136–149. doi: 10.1016/j.ejphar.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM, Robinson SR, Baker GB. Multiple 5-HT receptors are involved in the effects of acute MDMA treatment: studies on locomotor activity and responding for conditioned reinforcement. Psychopharmacology. 2002;162:282–91. doi: 10.1007/s00213-002-1104-4. [DOI] [PubMed] [Google Scholar]
- Garabette ML, Martin KF, Redfern PH. Circadian variation in the activity of the 5-HT1B autoreceptor in the region of the suprachiasmatic nucleus, measured by microdialysis in the conscious freely-moving rat. Br J Pharmacol. 2000;131:1569–76. doi: 10.1038/sj.bjp.0703753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside SE, McQuade R, Sharp T. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on 5-HT cell firing and release: comparison between dorsal and median raphe 5 -HT systems. Neuropharmacology. 1997;36:1697–703. doi: 10.1016/s0028-3908(97)00171-8. [DOI] [PubMed] [Google Scholar]
- Gaytan O, Ghelani D, Martin S, Swann A, Dafny N. Methylphenidate: diurnal effects on locomotor and stereotypic behavior in the rat. Brain Res. 1997;777:1–12. doi: 10.1016/s0006-8993(97)00880-9. [DOI] [PubMed] [Google Scholar]
- Gaytan O, Swann A, Dafny N. Diurnal differences in rat’s motor response to amphetamine. Eur J Pharmacol. 1998;345:119–28. doi: 10.1016/s0014-2999(97)01558-6. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–88. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Glennon RA. MDMA-like stimulus effects of alpha-ethyltryptamine and the alpha-ethyl homolog of DOM. Pharmacol Biochem Behav. 1993;46:459–62. doi: 10.1016/0091-3057(93)90379-8. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Dukat M, Cheng Y. Initial characterization of PMMA as a discriminative stimulus. Pharmacol Biochem Behav. 1997;57:151–8. doi: 10.1016/s0091-3057(96)00306-1. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Yousif M, Patrick G. Stimulus properties of 1-(3,4-methylenedioxyphenyl)-2-aminopropane (MDA) analogs. Pharmacol Biochem Behav. 1988;29:443–9. doi: 10.1016/0091-3057(88)90001-9. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. J Pharmacol Exp Ther. 1988;247:547–55. [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, Kunert HJ, Kovar KA, Lindenblatt H, Hermle L, Spitzer M, Sass H. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology. 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: implications for serotonin-dopamine interactions. J Neurochem. 1996;66:243–9. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology. 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herin DV, Liu S, Ullrich T, Rice KC, Cunningham KA. Role of the serotonin 5-HT2A receptor in the hyperlocomotive and hyperthermic effects of (+)-3,4-methylenedioxymethamphetamine. Psychopharmacology. 2005;178:505–13. doi: 10.1007/s00213-004-2030-4. [DOI] [PubMed] [Google Scholar]
- Hermle L, Spitzer M, Borchardt D, Kovar KA, Gouzoulis E. Psychological effects of MDE in normal subjects. Are entactogens a new class of psychoactive agents? Neuropsychopharmacology. 1993;8:171–6. doi: 10.1038/npp.1993.19. [DOI] [PubMed] [Google Scholar]
- Héry F, Rouer E, Glowinski J. Daily variations of serotonin metabolism in the rat brain. Brain Res. 1972;43:445–65. doi: 10.1016/0006-8993(72)90400-3. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Van Hartesveldt C. Diurnal locomotor activity in rats: effects of hippocampal ablation and adrenalectomy. Behav Biol. 1977;19:228–37. doi: 10.1016/s0091-6773(77)91518-8. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Ketteler HJ, McCloskey TC, Sullivan CK, Dudley MW, Schmidt CJ. Effects of the selective 5-HT2A receptor antagonist MDL 100,907 on MDMA-induced locomotor stimulation in rats. Neuropsychopharmacology. 1996;15:116–24. doi: 10.1016/0893-133X(95)00160-F. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–22. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Krebs KM, Geyer MA. Behavioral characterization of alpha-ethyltryptamine, a tryptamine derivative with MDMA-like properties in rats. Psychopharmacology. 1993;113:284–7. doi: 10.1007/BF02245712. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Baumann C, Gamma A, Vollenweider FX. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology. 2000a;22:513–21. doi: 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. The serotonin uptake inhibitor citalopram reduces acute cardiovascular and vegetative effects of 3,4-methylenedioxymethamphetamine (‘Ecstasy’) in healthy volunteers. J Psychopharmacol. 2000b;14:269–74. doi: 10.1177/026988110001400313. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA (“Ecstasy”) after pretreatment with the 5-HT2 antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000c;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Lu JQ, Nagayama H. Circadian rhythm in the response of central 5-HT1A receptors to 8-OH-DPAT in rats. Psychopharmacology. 1996;123:42–5. doi: 10.1007/BF02246279. [DOI] [PubMed] [Google Scholar]
- Lu JQ, Nagayama H. Circadian rhythm in the hypothermic response to serotonin1A receptor agonist 8-OH-DPAT in rats. Chronobiol Int. 1997;14:267–73. doi: 10.3109/07420529709001418. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Bankson MG, Cunningham KA. Pharmacological studies of the acute and chronic effects of (+)-3,4-methylenedioxymethamphetamine on locomotor activity: role of 5-hydroxytryptamine1A and 5-hydroxytryptamine1B/1D receptors. J Pharmacol Exp Ther. 1999;290:965–73. [PubMed] [Google Scholar]
- Millan MJ, Veiga S, Girardon S, Brocco M. Blockade of serotonin 5-HT1B and 5-HT2A receptors suppresses the induction of locomotor activity by 5-HT reuptake inhibitors, citalopram and fluvoxamine, in NMRI mice exposed to a novel environment: a comparison to other 5-HT receptor subtypes. Psychopharmacology. 2003;168:397–409. doi: 10.1007/s00213-003-1389-y. [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of {+/−}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol. 2011;25:439–52. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Dissociation of multiple effects of acute LSD on exploratory behavior in rats by ritanserin and propranolol. Psychopharmacology. 1991;105:69–76. doi: 10.1007/BF02316866. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: entactogens. J Psychoactive Drugs. 1986;18:305–13. doi: 10.1080/02791072.1986.10472362. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Hoffman AJ, Oberlender RA, Jacob P, 3rd, Shulgin AT. Derivatives of 1-(1,3-benzodioxol-5-yl)-2-butanamine: representatives of a novel therapeutic class. J Med Chem. 1986;29:2009–15. doi: 10.1021/jm00160a035. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Lloyd DH, Hoffman AJ, Nichols MB, Yim GKW. Effects of certain hallucinogenic amphetamine analogues on the release of [3H]serotonin from rat brain synaptosomes. J Med Chem. 1982;25:530–5. doi: 10.1021/jm00347a010. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Oberlender R. Structure-activity relationships of MDMA and related compounds: a new class of psychoactive drugs? Ann N Y Acad Sci. 1990;600:613–23. doi: 10.1007/978-1-4613-1485-1_7. [DOI] [PubMed] [Google Scholar]
- Oberlender R, Nichols DE. Drug discrimination studies with MDMA and amphetamine. Psychopharmacology. 1988;95:71–6. doi: 10.1007/BF00212770. [DOI] [PubMed] [Google Scholar]
- Oberlender R, Nichols DE. (+)-N-methyl-1-(1,3-benzodioxol-5-yl)-2-butanamine as a discriminative stimulus in studies of 3,4-methylenedioxy-methamphetamine-like behavioral activity. J Pharmacol Exp Ther. 1990;255:1098–106. [PubMed] [Google Scholar]
- Parrott AC. The psychotherapeutic potential of MDMA (3,4-methylenedioxyamphetamine): an evidence-based review. Psychopharmacology. 2007;191:181–93. doi: 10.1007/s00213-007-0703-5. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. The effects of MDMA and other methylenedioxy-substituted phenylalkylamines on the structure of rat locomotor activity. Neuropsychopharmacology. 1992;6:15–31. [PubMed] [Google Scholar]
- Piercey MF, Lum JT, Palmer JR. Effects of MDMA (‘ecstasy’) on firing rates of serotonergic, dopaminergic, and noradrenergic neurons in the rat. Brain Res 1990. 1990;526:203–6. doi: 10.1016/0006-8993(90)91222-3. [DOI] [PubMed] [Google Scholar]
- Quérée P, Peters S, Sharp T. Further pharmacological characterization of 5-HT2C receptor agonist-induced inhibition of 5-HT neuronal activity in the dorsal raphe nucleus in vivo. Br J Pharmacol. 2009;158:1477–85. doi: 10.1111/j.1476-5381.2009.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel NL, Callaway CW, Geyer MA. Serotonin1B receptor activation mimics behavioral effects of presynaptic serotonin release. Neuropsychopharmacology. 1993;8:201–11. doi: 10.1038/npp.1993.22. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–58. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Sakai K, Crochet S. Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and wake-sleep states. Neuroscience. 2001;104:1141–55. doi: 10.1016/s0306-4522(01)00103-8. [DOI] [PubMed] [Google Scholar]
- Scearce-Levie K, Viswanathan SS, Hen R. Locomotor response to MDMA is attenuated in knockout mice lacking the 5-HT1B receptor. Psychopharmacology. 1999;141:154–61. doi: 10.1007/s002130050819. [DOI] [PubMed] [Google Scholar]
- Selken J, Nichols DE. Alpha1-adrenergic receptors mediate the locomotor response to systemic administration of (+/−)-3,4-methylenedioxymethamphetamine (MDMA) in rats. Pharmacol Biochem Behav. 2007;86:622–30. doi: 10.1016/j.pbb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton C, Marsden CA. Circadian variation in the head twitch response produced by 5-methoxy-N1, N1-dimethyltryptamine and p-chloroamphetamine in the mouse. Psychopharmacology. 1981;74:173–6. doi: 10.1007/BF00432688. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Bradberry CW, Roth RH, Aghajanian GK. MDMA (3,4-methylenedioxymethamphetamine) inhibits the firing of dorsal raphe neurons in brain slices via release of serotonin. Eur J Pharmacol. 1989;167:375–83. doi: 10.1016/0014-2999(89)90446-9. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Bradberry CW, Roth RH, Aghajanian GK. 3,4-Methylenedioxymethamphetamine-induced release of serotonin and inhibition of dorsal raphe cell firing: potentiation by L-tryptophan. Eur J Pharmacol. 1990;178:313–20. doi: 10.1016/0014-2999(90)90110-r. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–50. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Gamma A, Liechti M, Huber T. Psychological and cardiovascular effects and short-term sequelae of MDMA (“ecstasy”) in MDMA-naïve healthy volunteers. Neuropsychopharmacology. 1998;19:241–51. doi: 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Liechti ME, Gamma A, Greer G, Geyer MA. Acute psychological and neurophysiological effects of MDMA in humans. J Psychoactive Drugs. 2002;34:171–84. doi: 10.1080/02791072.2002.10399951. [DOI] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms. 2009;24:465–76. doi: 10.1177/0748730409346657. [DOI] [PubMed] [Google Scholar]
- Weiner N, Clement HW, Gemsa D, Wesemann W. Circadian and seasonal rhythms of 5-HT receptor subtypes, membrane anisotropy and 5-HT release in hippocampus and cortex of the rat. Neurochem Int. 1992;21:7–14. doi: 10.1016/0197-0186(92)90062-v. [DOI] [PubMed] [Google Scholar]
- Wichems CH, Hollingsworth CK, Bennett BA. Release of serotonin induced by 3,4-methylenedioxymethamphetamine (MDMA) and other substituted amphetamines in cultured fetal raphe neurons: further evidence for calcium-independent mechanisms of release. Brain Res. 1995;695:10–8. doi: 10.1016/0006-8993(95)00774-k. [DOI] [PubMed] [Google Scholar]
- Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology. 1990;100:417–25. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]