Abstract
Objectives
Piperacillin is often used in preterm infants for intra-abdominal infections; however, dosing has been derived from small single-center studies excluding extremely preterm infants at highest risk for these infections. We evaluated the population pharmacokinetics (PK) of piperacillin using targeted sparse sampling and scavenged samples obtained from preterm infants ≤32 weeks gestational age at birth and <120 postnatal days.
Materials and Methods
A 5-center study was performed. A population PK model using nonlinear mixed effect modeling was developed. Covariate effects were evaluated based on estimated precision and clinical significance.
Results
Fifty-six preterm infants were evaluated and had a median (range) gestational age at birth of 25 (22–32) weeks, a postnatal age of 17 (1–77) days, a postmenstrual age of 29 (23–40) weeks, and a weight of 867 (400–2580) grams. The final PK data set contained 211 samples; 202/211 (96%) were scavenged from discarded clinical specimens. Piperacillin population PK was best described by a 1-compartment model. The population mean clearance (CL) was derived by the equation CL (liter/h)=0.479 x (weight)0.75 x 0.5/serum creatinine and using a volume of distribution (V) (liter) of 2.91 x (weight). The relative standard errors around parameter estimates ranged from 13.7–32.2%. A trend towards increased CL was observed with increasing gestational age at birth; infants with serum creatinine ≥1.2 mg/dL had a 60% reduction in piperacillin CL. The majority (>70%) of infants did not meet pre-defined pharmacodynamic efficacy targets.
Conclusions
Scavenged PK sampling is a minimal-risk approach that can provide meaningful information related to development of PK models but not dosing recommendations for piperacillin. The utility of scavenged sampling in providing definitive dosing recommendations may be drug-dependent and needs to be further explored.
Keywords: neonate, drug, pharmacokinetics, piperacillin
Piperacillin is a semisynthetic derivative of ampicillin with a ureido and piperazine side chain.1 The combination of piperacillin with tazobactam (beta-lactamase inhibitor) is approved by the U.S. Food and Drug Administration (FDA) for the treatment of children >2 months of age with appendicitis or peritonitis. The European Medicines Agency has approved the combination for the treatment of complicated intra-abdominal infections in children 2–12 years of age. Safety, efficacy, and dosing recommendations for infants <2 month of age have not been established. In adults and older children, more than 70% of piperacillin is primarily excreted unchanged in the urine. Total body clearance increases and elimination half-life decreases with increasing age.2,3 After intravenous administration, the elimination half-life of piperacillin in adults and children 2–12 years of age is short (0.5–1 hour); elimination half-life is prolonged in children and infants <23 months of age (0.9–1.4 hours).2,3
In infants <2 months of age, piperacillin-tazobactam is commonly used “off label” for the treatment of intra-abdominal infections, such as necrotizing enterocolitis, as well as severe infections due to bacterial resistance. Appropriate dosing recommendations for this population have been limited by single-center studies and exclusion of the most preterm (<28 weeks gestational age at birth) infants.4 Because the main clearance mechanism for piperacillin is the renal system, ontogenic pharmacokinetic (PK) changes in the developing infant are expected. Extrapolation of PK data from more mature infants is often difficult due to non-linear relationships between maturation and renal function.5 PK studies are needed in preterm infants to appropriately describe drug disposition in this population.
PK studies are rarely conducted in infants due to the research challenges posed by this population in the context of traditional study design. These challenges include limited blood volume for PK sampling, difficulty in obtaining PK samples due to the critical medical condition of the infants, and low parental informed consent rates. As such, investigators have explored novel minimal-risk methods to evaluate the PK of antimicrobials in this population. One approach is the use of scavenged samples, left over from the normal clinical care of infants. When combined with the collection of timed PK samples (collected specifically for study purposes), scavenged samples have allowed for characterization of the PK in preterm infants.6 Our research team has recently demonstrated the utility of this approach to describe the PK of metronidazole in premature infants.7 However, to our knowledge, the use of scavenged samples for PK analysis of other antimicrobials has not been evaluated. The present study was conducted to assess the population PK of intravenous piperacillin using scavenged samples collected from preterm infants <32 weeks gestational age at birth.
MATERIALS AND METHODS
Study Design
Pharmacokinetic samples for this analysis were obtained from the Antimicrobial PK in High-Risk Infants trial sponsored by the Pediatric Pharmacology Research Unit. This multi-center, prospective, open-label PK study of commonly used antimicrobial agents in the neonatal intensive care unit enrolled infants ≤32 weeks gestational age at birth who were <120 days old and who were receiving intravenous piperacillin or piperacillin-tazobactam per routine medical care. Piperacillin dosing was determined by the routine clinical practice in each unit, and no exclusion criteria were used. Infants were stratified by birth gestational age (BGA) at enrollment: <26 weeks, 26–29 weeks, and 30–32 weeks. The study was approved by the institutional review boards at each institution, and informed consent was obtained prior to enrollment.
The following information was collected for covariate analysis: BGA, postnatal age (PNA), postmenstrual age (PMA), weight, sex, race, serum creatinine, and ethnicity. Covariates that exhibited time-dependent changes (e.g., weight, PNA) were permitted to change with time, and the actual value in the data set reflects the observations made at each patient visit. Missing weights were imputed with the last recorded value carried forward for up to 7 days. Serum creatinine (SCR) was recorded when obtained for clinical care. Missing SCR values were imputed based on an exponential model of SCR and PMA derived from the data.
PK Sample Collection
A sparse sampling approach was followed in this study. Samples were divided into 2 types: scavenged and blood draw. Scavenged samples were defined as samples obtained without obtaining additional blood from the infant. These samples were collected from the clinical laboratory from discarded blood (heparinized or ethylenediaminetetraacetic acid [EDTA] tubes) obtained for routine clinical care. Blood draw samples were defined as samples obtained with collection of extra blood from the infant. Each blood draw was approximately 0.3 mL of blood collected in EDTA Microtainers. PK sample collection was planned at the following time points: immediately prior to piperacillin infusion, immediately after the completion of infusion (t=0), approximately 1 hour after completion of infusion (t=1 hour), approximately 2 hours after completion of infusion (t=2 hours), and immediately prior to infusion of the next dose. The duration of piperacillin infusion was performed according to routine clinical care at the site. Samples were refrigerated or placed on ice immediately after collection and then centrifuged at 1500 g and 4°C for 10 minutes. Plasma was removed and stored at −70° C. Samples from all sites were shipped on dry ice to Duke University Medical Center where they were stored at −70° C prior to analysis. Samples were stored for a maximum of 32 months prior to analysis.
Bioanalytical Assay
A liquid chromatography-tandem mass spectrometry (HPLC/MS/MS) assay for piperacillin detection in human plasma suitable for small plasma volumes was developed and validated. Sample analysis was performed on a triple quadrupole mass spectrometer API 4000 (Applied Biosystems–ABSciex, Foster City, CA, USA) operated with electrospray ionization (TurboV source using a TurboIonspray® probe). Instrument parameters were optimized for the piperacillin transition (518.2→143.2 m/z). Ionspray voltage and turbo heater temperature were kept at 2500 V and 500° C, respectively; a collision gas of 10 and a collision energy of 25 V were used. Piperacillin and dicloxacillin (internal standard [IS]) were purchased from the Sigma Chemical Company (St. Louis, MO, USA). HPLC separation was achieved using a reverse-phase C18 Aquasil column (Thermo Fisher, Waltham, MA, USA) with a flow-rate of 0.75 ml/min using a gradient mobile phase. Mobile phase A consisted of 0.1% formic acid in water, and mobile phase B consisted of 0.1% formic acid in methanol. Total run time was 6 minutes. Analytical data were acquired by Analyst Software 1.4.1 (Applied Biosystems–ABSciex, Foster City, CA, USA). The lower limit of quantitation of piperacillin in plasma was 0.3 mg/L. Intraday and interday coefficients of variation were <7% at concentrations ranging from 0.3–120 mg/L.
Population PK Analysis
PK data were analyzed with a nonlinear mixed effect modeling (NONMEM) approach using the computer program NONMEM (version 7) in conjunction with WINGS for NONMEM version 7.03 (Auckland, NZ). Output was summarized using STATA 10 (College Station, TX, USA). The first-order conditional estimation method with interaction was used for all model runs. One- and 2-compartment structural PK models were evaluated. Inter-individual (IIV) random effects were evaluated on clearance (CL) and volume of distribution (V). Covariance was described by a block Omega matrix. An exponential model for IIV variance for CL and V was used, and a proportional error model was deemed appropriate to describe residual variability. The potential impact of clinical covariates on PK parameters was explored if a relationship was suggested by visual inspection of scatter and box plots (continuous and categorical variables, respectively) of individual Bayesian estimates obtained from the base model and IIV (ETAs) against covariates. The following covariates were evaluated: weight (kg), BGA (weeks), PNA (days), PMA (defined as BGA plus PNA in weeks [PNA/7]), SCR, race, sex, and ethnicity. Once covariates were identified during the model-building process, covariate testing was performed via standard forward addition backward elimination methods. Potential covariates that reduced the objective function by more than 3.84 (P<~0.05) were included in the subsequent multivariable analysis. A forward inclusion with backwards elimination approach was used during the multivariable step, and a reduction of 6.63 (P<~0.01) was required for retention of a covariate in the final model. Continuous covariates were scaled to their median values. Empirical Bayesian estimates of individual infant PK parameters were generated from the final model using the post-hoc subroutine.
Model Evaluation
Models were evaluated based on successful minimization, goodness-of-fit plots, precision of parameter estimates, bootstrap procedures, and standardized visual predictive check. The precision of the final population PK model parameter estimates was evaluated using non-parametric bootstrapping (1000 replicates) to generate the 95% confidence intervals (CIs) for parameter estimates. For the standardized visual predictive check, the final model was used to generate 1000 Monte Carlo simulation replicates per time point of piperacillin exposure. Simulated results were compared at the subject level with those observed in the study by calculating and plotting the percentile of each observed concentration in relation to its 1000 simulated observations derived from the final model.8 The dosing and covariate values used to generate the predictions in the standardized visual predictive check were the same as those used in the study population. The number of observed concentrations outside of the 90% prediction interval for each time point was quantified.
Assessment of Dose-exposure Relationship
Piperacillin demonstrates time-dependent pharmacodynamics (PD). For target exposure, we chose the time above a minimum inhibitory concentration (MIC) of 16–64 mg/L at steady state. This MIC target is consistent with the Clinical and Laboratory Standards Institute-recommended MIC susceptibility breakpoint of piperacillin/tazobactam for enteric gram-negative organisms such Escherichia coli and Enterobacter sp. (16 mg/L) and Pseudomonas aeruginosa (64 mg/L).9 Monte Carlo simulations using the final population PK model were used to explore dose-exposure relationships using these defined targets. Commonly used and current piperacillin dosing recommendations listed in Neofax10 and The Harriet Lane Handbook11 were used in these simulated datasets to evaluate target attainment rates at steady state. When a dosing range was recommended, the highest end of the range was chosen for the simulations. Target attainment rates were calculated for infants who achieved target piperacillin concentrations for 50% or 75% of the dosing interval. Inclusion of the more stringent PD target criteria (75% of the dosing interval) was derived from the assumption of an immuno-compromised state of preterm infants and the need to achieve higher drug concentrations to achieve bacterial killing.
RESULTS
Study Population
A total of 77 subjects from 5 centers were evaluated for analysis. Subjects were excluded from the analysis if dosing, concentration, or sampling data were unreliable (i.e., unable to discern time when sample was collected relative to dose administered) (N=13) and if sampling was obtained during line flush or during drug infusion and no other samples were collected (N=8). The exclusion of these subjects and samples resulted in 56 subjects from 5 sites with 211 concentrations used in the modeling process. The overall median (range) BGA, PNA, PMA, weight, serum creatinine, and dose were 25 (22–32) weeks, 17 (1–77) days, 29 (23–40) weeks, 867 (400–2580) g, 0.8 (0.2–2.4) mg/dL, and 88 (39–147) mg/kg, respectively (Table 1). The majority of subjects were male (31/56, 56%) and white (29/56, 52%), and few were Hispanic (5/56, 9%).
TABLE 1.
Clinical Data by Gestational Age Group
| Characteristic | Gestational Age at Birth (Weeks)
|
||
|---|---|---|---|
| <26 | 26–29 | 30–32 | |
| N | 29 | 20 | 7 |
| Gestational age, weeks | 24 (22, 25) | 27 (26, 29) | 31 (28, 32) |
| Postnatal age, days | 17 (1, 77) | 15 (1, 75) | 24 (3, 65) |
| Postmenstrual age, weeks | 27 (23, 35) | 30 (27, 38) | 35 (31, 40) |
| Weight, g | 709 (400, 1400) | 1023 (555, 2580) | 1610 (1357, 1890) |
| Female sex | 14 (48) | 5 (25) | 6 (86) |
| Race | |||
| White | 15 (52) | 10 (50) | 4 (57) |
| Black | 12 (41) | 9 (45) | 2 (29) |
| Other | 2 (7) | 1 (5) | 1 (14) |
| Hispanic | 2 (7) | 1 (5) | 2 (29) |
| Serum creatinine (mg/dL) | 1 (0.3, 2.4) | 0.8 (0.2,2) | 0.5 (0.2,0.9) |
| Dose (mg/kg) | 80 (43,147) | 87 (44,116) | 100 (39,110) |
| Dosing frequency (h) | 8 (6,14) | 8 (6,13) | 8 (6,16) |
Data are median (range) for continuous data and n (%) for categorical data.
PK Specimens
A total of 41/261 (16%) outlier drug concentrations were removed from the analysis due to unreliability of sampling times related to time of infusion (N=18), time of flush (N=20), or sample contamination (N=3). Six of these samples did not have any measurable piperacillin concentrations. The median time of PK sampling was 4.7 (0–11.16) hours after the dose, and the median concentration was 25.6 (0.041–502) mg/L. An average of 3.7 samples per infant (range, 1–22) was collected, and the overwhelming majority of PK samples were scavenged from the clinical laboratory (202/211, 96%). Sixteen samples (16/211, 7.6%) had concentrations below the limit of quantitation; 6/211 (2.8%) had a reliable signal above background noise documented on the LC/MS/MS instrument, and this measurement was used in the PK analysis. A value of zero was assigned to the remaining samples (10/211 [4.7%]) as they were below the level of detection.
Population PK Model Building
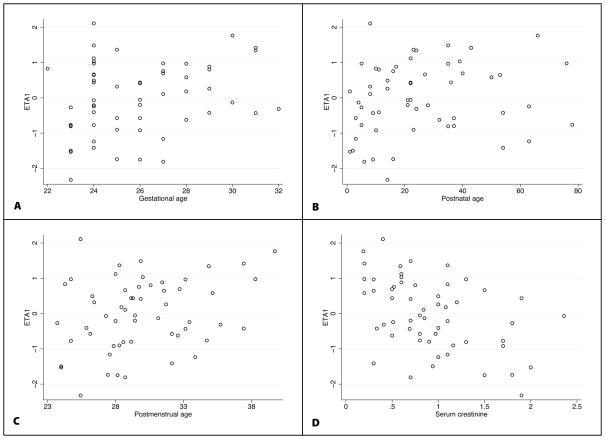
A 1-compartment model was the appropriate structural PK model for this data set (Figure 2). Because few samples were obtained within the first few hours post dose, it was not possible to estimate inter-compartmental clearance, and a 2-compartment model did not provide a better fit to the data. Weight was included in the base CL and V models (Table 2). Allometric scaling (Weight0.75) as well as estimation of the body size exponent (Weightθ) were explored as potential body size models for CL and V. The exponent estimates for CL and V were 0.62 and 0.86, respectively. However, given strong physiologic basis and no improvement in model fit, exponents for weight were fixed at 0.75 and 1 for CL and V, respectively. In the base model, scatter plots showed a correlation between IIV on CL (ETA1) and V (ETA2); a covariance term was added to the model. Age- and maturity-related covariates (BGA, PMA, PNA), as well as SCR, showed correlation with unexplained CL (ETA1) IIV (Figure 1). During the univariable evaluation (after inclusion of body weight), all age-related covariates resulted in a significant decrease in the objective function value (OFV); however, the largest drop in OFV occurred when SCR was added to the model (Table 2). A SCR power model with exponent estimation did not improve the goodness of fit. CL estimated by SCR was superior to age-related models, and the addition of a maturation covariate (PMA) in the multivariable analysis did not improve the model goodness of fit (Tables 2 and 3). No other covariates were evaluated in the V model due to lack of correlation observed between the covariate and unexplained V (ETA2) IIV. Due to the low number of blood draw samples, bias introduced by scavenged specimen collection compared with timed blood draws around the dose could not be assessed.
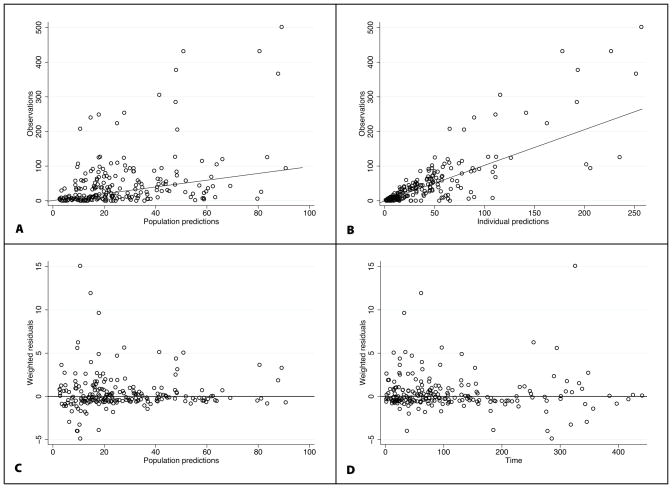
FIGURE 2.
Final population pharmacokinetic model diagnostic plots: observed vs. population prediction (A) and individual prediction (B), weighted residuals vs. population predictions (C) and time (D). For A and B, the line of identity is included as a reference. For weighted residuals, a solid line at y=0 is included as a reference.
TABLE 2.
Model-building Process
| Base Model and Univariable Analysis | Population Model | OFV | ΔOFV |
|---|---|---|---|
| V | V = θV X (wt)1 | 1653 | - |
| CL base model | CL = θCL X (wt)0.75 | 1653 | - |
| BGA | CL = θCL X (wt)0.75 X (BGA/25)θCL-BGA | 1647 | −6 |
| PMA | CL = θCL X (wt)0.75 X (PMA/29)θCL-PMA | 1643 | −10 |
| PNA | CL = θCL X (wt)0.75 X (PNA/17)θCL-PNA | 1645 | −8 |
| SCR | CL = θCL X (wt)0.75 X (0.5/SCR) | 1618 | −35 |
|
Multivariable Model | |||
| CL, PMA, and SCR | CL = θCL X (wt)0.75 X (0.5/SCR) X (PMA/29)θCL-PMA | 1618 | 0 |
V, volume of distribution; CL, clearance; BGA, gestational age at birth; PMA, postmenstrual age; PNA, postnatal age; SCR, serum creatinine (mg/dL); wt, weight; OFV, objective function value.
FIGURE 1.
Base model scatter plots of CL ETA1 estimates and the following: BGA (A), PNA (B), PMA (C), and SCR (D).
TABLE 3.
Final Population Pharmacokinetic Model Parameter Estimates
| Parameter | Symbol | Point Estimate | %RSE | Bootstrap CI
|
||
|---|---|---|---|---|---|---|
| 2.5% | Median | 97.5% | ||||
| CL (L/h/kg0.75) | θCL | 0.479 | 13.7 | 0.352 | 0.466 | 0.606 |
| V (L/kg) | θv | 2.91 | 30.8 | 1.640 | 2.935 | 5.615 |
| Inter-individual Variance | ||||||
| CL (CV%) | ω2CL | 91.54 | 19.5 | 83.86 | 94.88 | 103.87 |
| V (CV%) | ω2V | 119.16 | 32.2 | 88.39 | 108.54 | 124.14 |
| CL vs. V correlation | ω2CL-V | 101.5 | 21.5 | 94 | 98 | 100 |
| Residual Variance (CV%) | σ2 | 73.82 | 13.7 | 79.25 | 85.56 | 91.27 |
CL, clearance; V, volume of distribution; CV, coefficient of variation; CI, confidence interval; RSE, relative standard error.
Population PK Model Evaluation
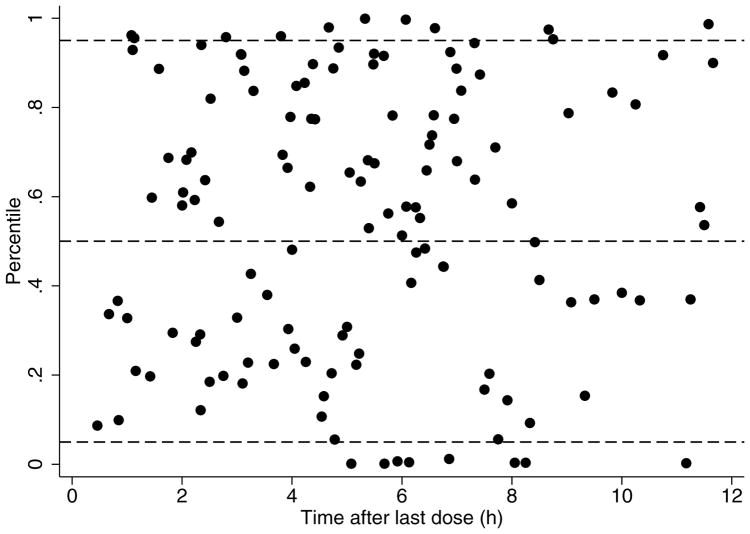
The final model had good precision as evidenced by relative standard errors around the parameter point estimates of 13.7–32.2% and by 95% confidence intervals generated by bootstrapping (N = 1000 simulated trials, 981 successful runs) (Table 3). Larger relative standard errors were observed for the point estimates of V. Goodness-of-fit diagnostic plots for the final model are shown in Figure 2. The standardized visual predictive check revealed a good fit between observed and predicted piperacillin concentrations as evidenced by the uniform distribution of calculated observation percentiles for each time point (Figure 3). In addition, only 8.5% (18/211) of observed concentrations were outside of the 90% prediction interval.
FIGURE 3.
Standardized visual predictive check of piperacillin observation percentiles versus time after last dose. Solid black circles represent calculated percentiles. Dashed lines represent the 5th, 50th, and 95th percentiles (bottom, middle, and top, respectively) of model predicted data.
Bayesian Estimates of CL, V, and Elimination Half-life
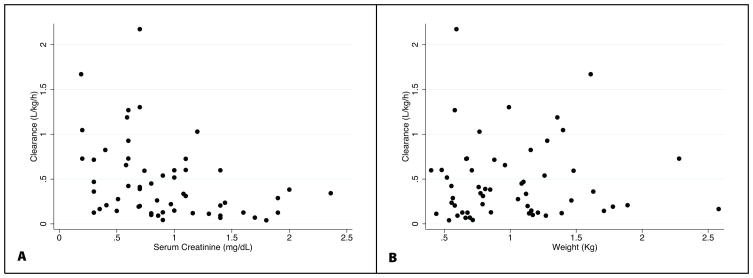
The median individual empirical Bayesian estimates for CL, V, and half-life were summarized by gestational age group (Table 4). There was a trend towards increasing median piperacillin weight-normalized CL with increasing BGA that was more apparent between infants <26 weeks BGA and infants 26–29 weeks BGA. Weight-adjusted piperacillin CL decreased with increasing SCR and, expectedly, did not change with increasing body weight (Figure 4). Infants with SCR ≥1.2 mg/dL had a 60% lower CL value. Half-life decreased with increasing BGA group.
TABLE 4.
Individual Empirical Bayesian Pharmacokinetic Parameter Estimates by Gestational Age Group
| Gestational Age (Weeks) | CL (L/h) | 95% CI | CL (L/h/kg) | 95% CI | V (L) | 95% CI | V (L/kg) | 95% CI | Half-life (h) | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| <26 | 0.239 | 0.048, 1.286 | 0.311 | 0.069, 1.303 | 2.15 | 0.32, 16.99 | 3.44 | 0.51, 20.91 | 8.8 | 2.7, 16.8 |
| 26–29 | 0.401 | 0.037, 1.306 | 0.371 | 0.055, 0.777 | 2.44 | 0.40, 10.09 | 2.83 | 0.39, 9.21 | 5.9 | 1.5, 17.4 |
| 30–32 | 0.393 | 0.248, 1.612 | 0.261 | 0.145, 1.671 | 3.38 | 1.47, 17.98 | 2.42 | 0.86, 13.25 | 4.5 | 1.6, 7.7 |
| Overall | 0.302 | 0.044, 1.612 | 0.338 | 0.067, 1.303 | 2.75 | 0.32, 16.99 | 2.72 | 0.48, 17.16 | 6.9 | 1.7, 16.7 |
CL, clearance, V, volume of distribution; CI, confidence interval.
FIGURE 4.
Weight-normalized piperacillin clearance versus serum creatinine (A) and body weight (B).
Dose-exposure Relationship
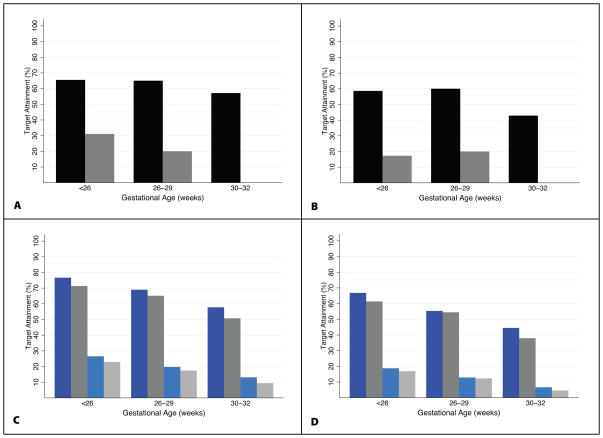
High failure rates were calculated when the surrogate PD targets of time above MIC of 16 and 64 mg/L for 50% and 75% of the dosing interval were evaluated. Only 60% of all infants achieved piperacillin concentrations >16 mg/L for 50% of the dosing interval. Only 30% of infants ≤29 weeks and none of those 30–32 weeks BGA achieved piperacillin concentrations >64 mg/L for 50% of the dosing interval (Figure 5). When the same targets were evaluated for 75% of the dosing interval, 50% of all infants achieved piperacillin concentrations >16 mg/L; 15% of infants ≤29 weeks and none of those 30–32 weeks BGA achieved piperacillin concentrations >64 mg/L for 75% of the dosing interval. Monte Carlo simulations using the final population PK model were used to explore dose-exposure relationships using current piperacillin dosing recommendations. Overall, dosing recommendations by Neofax (100 mg/kg every 8–12 hours) and The Harriet Lane Handbook (75 mg/kg every 8–12 hours) produced similar piperacillin exposures in simulated datasets; only 40–60% of infants across BGA achieved the PD target of concentrations above 16 mg/L for 50% of the dosing interval. Piperacillin concentrations >64 mg/L for 75% of the dosing interval were achieved in the minority of simulated patients (<25%); this finding was most pronounced among infants 30–32 weeks BGA (Figure 5).
FIGURE 5.
Pharmacodynamic target attainment rates by gestational age group. A: Proportion of patients who met PD target of desired concentrations for 50% of the dosing interval (black bar: 16 mg/L, gray bar: 64 mg/L); B: Proportion of patients who met PD target of desired concentrations for 75% of the dosing interval (black bar: 16 mg/L, gray bar: 64 mg/L); C: Proportion of simulated patients who met PD target of desired concentrations for 50% of the dosing interval (dark blue bar: Neofax 16 mg/L, light blue bar: Neofax 64 mg/L, dark gray bar: The Harriet Lane Handbook 16 mg/L, light gray bar: The Harriet Lane Handbook 64 mg/L); D: Proportion of simulated patients who met PD target of desired concentrations for 75% of the dosing interval (dark blue bar: Neofax 16 mg/L, light blue bar: Neofax 64 mg/L, dark gray bar: The Harriet Lane Handbook 16 mg/L, light gray bar: The Harriet Lane Handbook 64 mg/L).
DISCUSSION
Most antimicrobial products used in preterm infants lack some aspect of PK information specific to this population. Without appropriate studies specifically designed for preterm infants, clinicians are often forced to prescribe products “off-label,” exposing patients to potential adverse drug effects or less-than-optimal drug exposure without dosing evidence.12,13
The primary goal was to evaluate the PK of piperacillin in this population, evaluate potential covariates that would explain inter-individual variability in PK parameters, and assess the potential bias introduced by scavenged sampling compared with traditional sampling per study protocol. A population PK approach allowed for the use of sparse sampling, and stratified enrollment ensured a broad distribution of BGA and PNA.
In the present model, piperacillin CL increased with allometrically scaled body weight, and it decreased proportionally with increasing SCR. Allometric size adjustments in PK parameters have been well established as appropriate and physiologic-based models to describe CL changes among neonates and preterm infants.6,14 The association of CL with SCR is expected due to the high proportion (>70%) of piperacillin excreted in the urine.3 However, the clinical utility of SCR in preterm infants during the first few days of life is debated due to the confounding effect of maternal creatinine. Because only 3 (5%) of subjects in this study were <3 days of life, it is unlikely that bias was introduced by maternal creatinine contamination. The addition of maturational components (i.e., BGA, PNA, PMA) into the multivariable CL model did not change the goodness of fit nor did it explain remaining IIV. Due to ontogenic changes in renal function among preterm infants, SCR is strongly linked with maturational components such as BGA, PNA, and PMA. A correlation matrix between covariates included in this study revealed this association. This observation likely prevented the ability to discern the effect of each maturational component in CL IIV from SCR in the CL model-building process. Weight was the only covariate that explained V IIV, consistent with other studies in preterm infants.14
The population PK model developed performed well and showed good precision around parameter estimates. A high degree of IIV in CL (91.5% coefficient of variation [CV]), V (119 CV%), and residual variability (RV, 73.8 CV%) was observed. The large IIV in CL and V could have resulted from the diversity of study subjects included in this trial. However, large unexplained IIV in CL and V remained in the final model after inclusion of significant covariates. Because study efficiency was an important part of the study design, data collection (demographics and other clinically relevant covariates) was limited. The unexplained IIV could be attributed to other covariates not included in the study (i.e., concomitant medications). The large RV observed could be the result of several factors, such as documentation errors in sampling or dosing times, model misspecification, and drug degradation in scavenged samples. Ambient and refrigerator storage may affect piperacillin stability in plasma.15,16 Information regarding the duration and conditions under which the samples remained in the clinical laboratory before freezing were not collected, which limits our ability to assess the reliability of drug concentrations in scavenged samples. Because very few samples were drawn specifically for the study, the comparison between traditional and scavenged sampling schemes was not possible. Large RV was unlikely to be the result of drug concentration measurements given that the assay was validated according to FDA criteria.17
Because this study did not evaluate efficacy or clinical outcomes, surrogate piperacillin PD end points were used for the study population receiving piperacillin per routine clinical care and in simulated datasets including current dosing recommendations from commonly used pediatric resources.10,11 The proportion of patients achieving surrogate PD targets for efficacy was suboptimal. A substantial proportion of subjects (~40%) did not achieve piperacillin concentrations efficacious against common gram-negative enteric bacteria (i.e. Escherichia coli, enterobacter), and the vast majority (~70%) did not achieve concentrations above the susceptibility breakpoint for more resistant organisms such as Pseudomonas aeruginosa. A similar pattern was observed among simulated datasets using current dosing recommendation guidelines. Dosing by Neofax or The Harriet Lane Handbook resulted in similar target attainment rates, but similar to targets in the actual patient population, outcomes were suboptimal. These findings could suggest that current dosing per standard of care or as recommended in common pediatric resources is inadequate for this patient population. However, this should be interpreted with caution because lower (2–10 fold) than previously observed piperacillin concentrations2,4 were observed in this study, possibly as a result of scavenged sampling.
Our understanding of the PK of piperacillin in preterm infants is extremely limited. A single-center, single-dose PK study of piperacillin (75 mg/kg) conducted in 28 newborn infants with BGA of 29–40 weeks and PNA of 3–11 days demonstrated an increase in clearance with increasing gestational age.4 This study, however, excluded neonates <28 weeks estimated gestational age and infants beyond the second week of life. It is therefore difficult to compare our study results to this prior study, but overall the individual empirical Bayesian CL and V estimates of the present study were higher (2–10 fold) than previously reported after controlling for BGA and PMA. V estimates were particularly large when compared with other patient populations including older infants and adults.2,3 A large piperacillin V would be expected in preterm infants due to the high total water content relative to body mass18 and the hydrophilic nature of the drug. Alternatively, higher than expected CL and V estimates are likely the result of low piperacillin concentrations encountered in this trial. As mentioned, drug degradation in scavenged samples may have accounted for the low concentrations observed.
In summary, minimal-risk methods such as scavenged PK sampling can provide meaningful information related to development of structural PK models, as well as potential covariates that explain IIV in PK parameters. After allometric scaling, incorporating SCR as a CL model increased the model fit, and dose adjustments will likely be needed based on this parameter. The utility of scavenged sampling in providing dosing recommendations, however, is drug-dependent and likely not useful for unstable drugs such as piperacillin without stringent criteria regarding handling or documentation of handling to eliminate degraded samples from analysis. Future efforts evaluating this methodology should consider the physicochemical properties of the drug, more detailed documentation of sample collection and storage conditions, and simultaneous collection of traditional plasma samples to fully assess the extent of bias introduced by scavenged sampling. Results from a follow-up study (clinicaltrials.gov # NCT00873327) will further evaluate these findings.
Footnotes
Sources of funding and conflicts of interest: This study was sponsored by the National Institute of Health through the Pediatric Pharmacology Research Unit. Dr. Cohen-Wolkowiez receives support from NICHD 1K23HD064814-01 and the non-profit organization Thrasher Research Foundation for his work in pediatric clinical pharmacology and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. Benjamin receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C), the non-profit organization Thrasher Research Foundation for his work in neonatal candidiasis, and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. Ross has no conflicts to report. Dr. James receives research support from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK075936, 1R01DK081406, 1R01 G1-31616-99 [PI Hinson]); NIH/National Center for Research Resources (1UL1RR029884, PI Lowery); Center for Clinical and Translational Research (2289-2); NIH (1R01HD060543-01A1, PI van den Anker); and the Arkansas Children’s Hospital Research Institute (10469370, PI Stroud). Dr. Walsh has no conflicts to report. Dr. Sullivan received support from the U.S. government for her work in pediatric pharmacology (U10 HD045934 05) and from industry for neonatal and pediatric drug development. Ms. Zadell has no conflicts to report. Ms. Newman has no conflicts to report. Ms. White has no conflicts to report. Dr. Kashuba receives support from the U.S. government for work in clinical pharmacology (1U01AI095031, P30AI37260) and from industry for clinical pharmacology (ViiV, Abbott, Tibotec, and Merck). Dr. Ouellet is an employee of GSK and has no conflict of interest.
References
- 1.Michelow IC, McCracken GH., Jr . Antibacterial therapeutic agents. In: Feigin RD, editor. Textbook of Pediatric Infectious Diseases. Philadelphia, PA: Saunders; 2004. pp. 2990–2991. [Google Scholar]
- 2.Reed MD, Goldfarb J, Yamashita TS, et al. Single-dose pharmacokinetics of piperacillin and tazobactam in infants and children. Antimicrob Agents Chemother. 1994;38:2817–2826. doi: 10.1128/aac.38.12.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tjandramaga TB, Mullie A, Verbesselt R, et al. Piperacillin: human pharmacokinetics after intravenous and intramuscular administration. Antimicrob Agents Chemother. 1978;14:829–837. doi: 10.1128/aac.14.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kacet N, Roussel-Delvallez M, Gremillet C, et al. Pharmacokinetic study of piperacillin in newborns relating to gestational and postnatal age. Pediatr Infect Dis J. 1992;11:365–369. doi: 10.1097/00006454-199205000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Rhodin MM, Anderson BJ, Peters AM, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24:67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 6.Wade KC, Wu D, Kaufman DA, et al. National Institute of Child Health and Development Pediatric Pharmacology Research Unit Network. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother. 2008;52:4043–4049. doi: 10.1128/AAC.00569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Wolkowiez M, Ouellet D, Smith PB, et al. Population pharmacokinetics of metronidazole using scavenged samples from preterm infants. Antimicrob Agents Chemother. doi: 10.1128/AAC.06071-11. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang DD, Zhang S. Standardized visual predictive check versus visual predictive check for model evaluation. J Clin Pharmacol. 2012;52:39–54. doi: 10.1177/0091270010390040. [DOI] [PubMed] [Google Scholar]
- 9.Eagye KJ, Kuti JL, Sutherland CA, et al. In vitro activity and pharmacodynamics of commonly used antibiotics against adult systemic isolates of Escherichia coli and Pseudomonas aeruginosa at 40 U.S. hospitals. Clin Ther. 2009;31:2678–2688. doi: 10.1016/j.clinthera.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Thomson Reuters Clinical Editorial Staff . Neofax 2011. 24. Montvale, NJ: Thomson Reuters; 2011. [Google Scholar]
- 11.Tschudy M, Arcara K. The Harriet Lane Handbook: A Manual for Pediatric House Officers. 19. Philadelphia, PA: Mosby; 2011. p. 1035. [Google Scholar]
- 12.Choonara I. Unlicensed and off-label drug use in children: implications for safety. Expert Opin Drug Saf. 2004;3:81–83. doi: 10.1517/eods.3.2.81.27342. [DOI] [PubMed] [Google Scholar]
- 13.Roberts R, Rodriguez W, Murphy D, et al. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA. 2003;290:905–911. doi: 10.1001/jama.290.7.905. [DOI] [PubMed] [Google Scholar]
- 14.Suyagh M, Collier PS, Millership JS, et al. Metronidazole population pharmacokinetics in preterm neonates using dried blood-spot sampling. Pediatrics. 2011;127:e367–e374. doi: 10.1542/peds.2010-0807. [DOI] [PubMed] [Google Scholar]
- 15.Arzuaga A, Isla A, Gascón AR, et al. Quantitation and stability of piperacillin and tazobactam in plasma and ultrafiltrate from patients undergoing continuous venovenous hemofiltration by HPLC. Biomed Chromatogr. 2005;19:570–578. doi: 10.1002/bmc.482. [DOI] [PubMed] [Google Scholar]
- 16.Denooz R, Charlier C. Simultaneous determination of five beta-lactam antibiotics (cefepim, ceftazidim, cefuroxim, meropenem and piperacillin) in human plasma by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;864(1–2):161–167. doi: 10.1016/j.jchromb.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine. . Guidance for Industry: Bioanalytical Method Validation. Rockville, MD: Food and Drug Administration; 2001. [Google Scholar]
- 18.Fomon SJ, Haschke F, Ziegler EE, et al. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35(5 Suppl):1169–1175. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]