Abstract
Myositis ossificans (MO) is an inflammatory pseudotumor of the muscle that may be mistaken clinically and even histologically for a malignant soft tissue tumor. The aim of this article is to report the imaging characteristics of MO, the emphasis being on the early diagnostic clues. USG can be used at an early stage to reveal the ‘zone phenomenon,’ which is highly suggestive of MO. A short course of nonsteroidal anti-inflammatory drug therapy may be an efficient treatment for early MO.
Keywords: Myositis ossificans, ossification, tumor, ultrasonography, zone phenomenon
Introduction
In this article, we examine the performance of various imaging methods for the early diagnosis of myositis ossificans (MO) and optimal patient management.
MO is a pseudo-inflammatory tumor that originates from skeletal muscle and corresponds to a heterotopic, metaplastic, nonmalignant bone tumor.[1,2] The etiology and potential predisposing factors of MO remain unclear. MO might develop secondary to a muscular trauma. However, in most cases no causative factor can be identified.[3] MO has a rich cortege of clinical symptoms,[1,4] but one common presentation is as a very inflammatory, rapidly-growing, and painful muscular mass.[1,4] In a typical case the dramatic onset and the symptom intensity strongly suggest the diagnosis of MO.
Two weeks from onset, MO displays specific histological characteristics that allow a definitive diagnosis to be made, provided that the biopsy has been performed adequately.[5] Conversely, in its early phase MO can be mistaken for a malignant soft tissue sarcoma.[5] Ossifications are usually observed in MO but should be distinguished from soft tissue ossifications of other causes, e.g., periarticular ossifications (paraosteoarthropathies) that usually occur in a context of central neurologic pathologies.[2,6]
Pathology
As MO develops it passes through three characteristic phases, leading to the so-called ‘zone phenomenon’. The ossifications in MO are peripheral and centripetal, while they are central and centrifugal in osteosarcomas[5] [Figures 1–3].
Figure 1.

Diagram showing the different stages of MO. USG is the most sensitive technique for early demonstration of the zone phenomenon. If biopsy is done it must not be performed during the early phases of MO, as there are high false positive rates for the misdiagnosis of sarcomatous tumor.
Figure 3.

Histological section of extraskeletal osteosarcoma (H and E, ×100). The section shows an ill-defined lesion composed of a linear bony framework (large arrows) irregularly interspersed between tumor cells (stars). Adipocytes can also be observed (cross)
Figure 2.
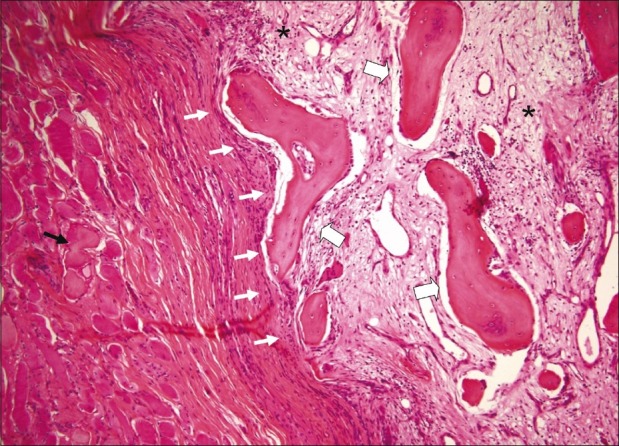
Histological section of a mature lesion of MO (H and E, ×100). The section shows a central myxoid matrix with fibroblasts (pseudo-fibrosarcomatous zone) (stars) surrounded by mature bone at the periphery of the lesion (large arrows). The lesion is well-circumscribed (white arrows). Muscle fibers are displayed around the lesion (black arrow)
The acute phase lasts one week. Histologically, the proliferation is composed of mesenchymal cells secreting a myxoid matrix as well as fibroblasts exhibiting numerous mitoses, which gives it a pseudo-fibrosarcomatous appearance.[5] This is followed by the subacute phase, which continues for about 10 days. The fibroblasts differentiate into osteoblasts and secrete an osteoid matrix at the periphery of the initial myxoid zone, giving it a pseudo-osteosarcomatous appearance.[5] The late phase, also called the maturation phase, usually starts between the second and fifth weeks of the evolution of MO. Bone production can be observed at the periphery of the lesion. At this stage, biopsy will reveal the three characteristic zones of MO and thus allow the correct final diagnosis to be made.[5] Later on in the evolution of MO, a fatty metaplastic evolution can also appear at the lesion center.[5]
On one hand, premature biopsy done at the early stage of MO may lead to a wrong diagnosis of sarcoma and, on the other hand, there is a risk that if biopsy is delayed a true sarcoma may be missed and result in tumor dissemination. It is therefore critical to identify the zone phenomenon of MO as early as possible using imaging. Another important feature that helps differentiate between MO and osteosarcoma is the well-circumscribed appearance of the former on histopathology.
Imaging
Standard radiographs do not disclose any anomaly in the early stages of MO.[7] Radiographs repeated later on in the evolution of the disease may show de novo pathognomonic ossification surrounding a clear central area [Figure 4]; typically, these ossifications are distant from adjacent bony structures. However, the ossifications are often missed on radiographs when these are performed 2 to 3 weeks after MO onset and sometimes even later.[7]
Figure 4 (A-G).

Painful left anterolateral thigh mass in a 9-year-old boy. There was no history of trauma. Initial standard radiographs were normal. Anteroposterior radiograph (A) of the femur at day 8 shows unilamellar periosteal reaction (arrow). USG at day 15 (B) shows an intramuscular mass with a hypoechoic center (star) and hyperechoic periphery (arrow). Anteroposterior radiographs at day 15 (C) and day 23 (D) show de novo ossifications arranged along the muscle fiber axis (large arrows) and located around a central noncalcified area. At day 23, coronal T1W (E) and T2W (F) MRI images show the lesion with a hypointense rim (arrows). The T2W MRI also shows a central hyperintensity area and soft tissue edema surrounding the lesion (star). Axial contrast-enhanced T1W MRI (G) shows rim enhancement (arrowhead), indicating a zone phenomenon
CT scan examination is more sensitive than radiography for detecting ossification and may also show a central fatty metaplastic area[8] [Figures 5–7]. MRI may show the so-called zone phenomenon before ossification appears. On MRI scans, an iso- or slight hyperintensity can be observed within the intramuscular mass on T1W and T2W images respectively[9] [Figures 4 and 6]. MRI can also depict an inflammatory edema extending beyond the MO [Figure 6]. On gadolinium-enhanced T1W images, a hyperintense rim is suggestive of the zone phenomenon and may correspond to active hypervascularized osteoid matrix[10] [Figures 4 and 8]. This annular enhancement is distinct from the heterogeneous enhancement seen in sarcomatous tumors.[10] Although rim enhancement is common in the acute phase of MO, diffuse enhancement may also be seen[11] [Figure 5]. Starting in the subacute phase of MO, the rim may be hypointense on all MRI sequences, indicating mineralization[9] [Figure 4]. Other important imaging characteristics of MO include the lack of invasion of adjacent tissues and the presence of viable muscle fibers within the lesion, which are often involved in case of tumor.[7]
Figure 5 (A-E).

De novo inflammatory mass on the posterior aspect of the left arm in a 57-year-old man. There was no history of trauma. Axial CT scan at day 16 (A), shows the presence of a slightly hypointense lesion with peripheral ossifications (arrow). USG at day 16 (B), shows a central hypoechoic area (star) encircled by a peripheral hyperechoic area, which corresponds to the calcified area (arrowheads). The third zone is the most peripheral area and is hypoechoic (large arrow). Surrounding hyperemia can be seen on Doppler (black arrows). Axial contrast enhanced fat-suppressed T1W MRI (C) shows global homogeneous enhancement of the lesion (arrow). Two months later, axial contrast enhanced fat-suppressed T1W MRI (D) shows dramatic reduction in the size and intensity of contrast enhancement (arrow). CT scan (E) demonstrates the disappearance of most of the ossifications (arrow)
Figure 7.

A 15-year-old girl with a history of chronic lumbar pain. Axial CT scan shows a mass typical of MO in the right paraspinal muscle, with peripheral ossifications (arrow head) and a low-density central area, indicating fatty metaplastic transformation (arrow)
Figure 6 (A-D).

Rapidly-growing painful mass in the left arm in a 15-year-old girl. There was no history of trauma. Axial T1W MRI (A) shows moderate hyperintensity of the lesion (black arrows). Axial contrast enhanced fat-suppressed T1W MRI (B) shows rim enhancement of the lesion (large arrow). Sagittal fat-suppressed T2W MRI (C) shows hypointense areas at the periphery of the lesion (arrows) and edema in the surrounding soft tissues (arrowhead). Sagittal CT scan (D), performed on the same day as the MRI shows the characteristic rim ossifications (white arrows)
Figure 8 (A–F).

Rapidly-increasing (within a single day) claudication and pain of the left hip in a 15-year-old girl. There was no history of trauma. Nonsteroidal anti-inflammatory drug therapy was initiated early. Initial anteroposterior radiographs of the left hip (A) are normal. Doppler USG at day 3 (B) shows a lesion with a central hypoechoic area (star), a hyperechoic rim (arrow), and a third hypoechoic area (large arrow). Pulsed Doppler (C) shows perilesional vascularization with arterial spectrum. Axial T2W (D) and coronal T1W contrast enhanced MRI (E) at day 5 show an enhancing rim (large arrows), suggesting a zone phenomenon. Axial contrast enhanced CT scan at day 20 (F) shows plain homogenous enhancement of the lesion (arrow). Unenhanced CT images did not reveal any ossification. No ossification was observed during follow-up and the lesion eventually disappeared
USG may be the most sensitive imaging modality to early depict the zone phenomenon in MO, as it can demonstrate this characteristic finding even before de novo ossifications can be reported on other imaging modalities[12] [Figures 4–6]. Thomas et al. have described three concentric zones, corresponding to the above-described characteristic MO zones. The first zone, the most peripheral, is hypoechoic and encircles the lesion; contiguous hyperemia can be observed on Doppler usG0 [Figure 8]. The second, thinner, zone is hyperechoic and corresponds to the ossifications. The third, central, zone is hypoechoic and corresponds to the central stromal fibroblastic component.[12]
Diagnosis
The diagnosis of MO is usually based on the patient's history (of trauma), clinical symptoms and on imaging findings when the zone phenomenon can be depicted. The main differential diagnosis is extraskeletal osteosarcoma, which in fact has ‘mirror’ characteristics both on imaging and pathology [Figure 9] The ossification phenomenon becomes first visible at two weeks. At that time, nonsteroidal anti-inflammatory drug (NSAID) therapy could be initiated leading to further clinical improvement and concomitant decrease of the soft tissue swelling and the echoic abnormality, thus preventing further useless invasive biopsy. When USG and diagnosis are delayed and the ossification is still visible, NSIA therapy could be administered. The final diagnosis should eventually be considered as the ossification progressively vanishes. If this is not the case, histologic confirmation would be necessary and biopsy should be performed by using USG and include the full length of the target lesion (complete sampling of the lesion).[5]
Figure 9 (A-C).

A 56-year-old male with intramuscular osteosarcoma of the right arm. Frontal radiographs of the right elbow (A) show irregular ossifications within the soft tissues (arrow). Axial CT scan (B) and sagittal contrast enhanced T1W MRI (C) show an intramuscular mass (arrowheads in B). In contrast to MO, the ossifications are seen at the lesion's heart (star) as a hyperdense mass (arrowhead in B) on CT scan and as a hypointense lesion on MRI (star in C). The MRI shows a large irregular lesion with global heterogeneous enhancement (arrowhead) and central areas of hypointensity, corresponding to the ossifications (star)
Treatment
Treatment is difficult owing to the chronological multistep process of MO. MO may heal and disappear spontaneously [Figure 5]. Surgical resection is sometimes suggested if the MO remains persistent; however, invasive surgical resection of the calcified tumor-like mass may compromise local function and lead to local relapse.[7]
Paraosteoarthropathies and MO share similar features: in both cases, the clinical onset includes inflammatory signs and evolution of disease is characterized by heterotopic bone production within soft tissues.[13] It is well recognized in the literature that nonsteroidal anti-inflammatory drugs (NSAID) may stop the evolutionary process of paraosteoarthropathies.[13] As mentioned previously, we suggest that NSAID might be used early to stop the evolutionary process of MO[14] [Figure 8]. Indeed, since efficacious treatment must be started early, it is necessary to diagnose MO as early as possible.
Conclusion
We have described herein the utility of MRI and USG for the diagnosis of MO. USG is probably the most sensitive modality and might enable a very early diagnosis provided the radiologist bears in mind the patient's history (trauma, clinical symptoms) and the historadiological correlations of the zonation process evolution. Early NSAID treatment may prevent the appearance of ossification in MO and thus help prevent unnecessary biopsy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kransdorf MJ, Meis JM. From the archives of the AFIP.Extraskeletal osseous and cartilaginous tumors of the extremities. Radiographics. 1993;13:853–84. doi: 10.1148/radiographics.13.4.8356273. [DOI] [PubMed] [Google Scholar]
- 2.Olsen KM, Chew FS. Tumoral calcinosis: Pearls, polemics, and alternative possibilities. Radiographics. 2006;26:871–85. doi: 10.1148/rg.263055099. [DOI] [PubMed] [Google Scholar]
- 3.Nuovo MA, Norman A, Chumas J, Ackerman LV. Myositis ossificans with atypical clinical, radiographic, or pathologic findings: A review of 23 cases. Skeletal Radiol. 1992;21:87–101. doi: 10.1007/BF00241831. [DOI] [PubMed] [Google Scholar]
- 4.Spencer JD, Missen GA. Pseudomalignant heterotopic ossification (‘myositis ossificans’).Recurrence after excision with subsequent resorption. J Bone Joint Surg Br. 1989;71:317–9. doi: 10.1302/0301-620X.71B2.2925755. [DOI] [PubMed] [Google Scholar]
- 5.Mirra JM. Osseous soft tumors. In: Mirra JM, Picci P, Gold RH, editors. Bone tumors: Clinical, radiologic and pathologic correlations. London: Lea and Febiger; 1989. pp. 1549–86. [Google Scholar]
- 6.Naraghi FF, DeCoster TA, Moneim MS, Miller RA, Rivero D. Heterotopic ossification. Orthopedics. 1996;19:145–51. doi: 10.3928/0147-7447-19960201-10. [DOI] [PubMed] [Google Scholar]
- 7.Goldman AB. Myositis ossificans circumscripta: A benign lesion with a malignant differential diagnosis. Am J Roentgenol. 1976;126:32–40. doi: 10.2214/ajr.126.1.32. [DOI] [PubMed] [Google Scholar]
- 8.Amendola MA, Glazer GM, Agha FP, Francis IR, Weatherbee L, Martel W. Myositis ossificans circumscripta: Computed tomographic diagnosis. Radiology. 1983;149:775–9. doi: 10.1148/radiology.149.3.6647854. [DOI] [PubMed] [Google Scholar]
- 9.De Smet AA, Norris MA, Fisher DR. Magnetic resonance imaging of myositis ossificans: Analysis of seven cases. Skeletal Radiol. 1992;21:503–7. doi: 10.1007/BF00195231. [DOI] [PubMed] [Google Scholar]
- 10.Shirkhoda A, Armin AR, Bis KG, Makris J, Irwin RB, Shetty AN. MR imaging of myositis ossificans: Variable patterns at different stages. J Magn Reson Imaging. 1995;5:287–92. doi: 10.1002/jmri.1880050312. [DOI] [PubMed] [Google Scholar]
- 11.Parikh J, Hyare H, Saifuddin A. The imaging features of post-traumatic myositis ossificans with emphasis on MRI. Clin Radiol. 2002;57:1058–66. doi: 10.1053/crad.2002.1120. [DOI] [PubMed] [Google Scholar]
- 12.Thomas EA, Cassar-Pullicino VN, McCall IW. The role of ultrasound in the early diagnosis and management of heterotopic bone formation. Clin Radiol. 1991;43:190–6. doi: 10.1016/s0009-9260(05)80478-7. [DOI] [PubMed] [Google Scholar]
- 13.Carlier RY, Mompoint D, Denys P, Denormandie P, Feydy A, Chevallier P, et al. L’échographie dans l’exploration des paraostéo-arthropathies neurogènes (POA) In: Pelissier J, Minaire P, Chantraine A, editors. Les para-ostéo-arthropathies neurogènes. Paris: Masson; 1997. pp. 78–84. [Google Scholar]
- 14.Mann D, McCormack B. Commentary: Myositis ossificans. CJEM. 1999;1:199. doi: 10.1017/s148180350000419x. [DOI] [PubMed] [Google Scholar]


