Abstract
A case of Stevens-Johnson syndrome following treatment with sodium valproate is presented here. A 20-year-old male was put on sodium valproate monotherapy for the migraine, with generalized epilepsy. He developed vesicles and bullae in the oral and nasal mucosa with conjunctivitis, after 10 days of treatment. The lesions resolved after treating with systemic steroids. This case has been presented because Stevens-Johnson syndrome with sodium valproate monotherapy has been very rarely reported.
Keywords: Side effect, Stevens-Johnson syndrome, valproate
INTRODUCTION
Sodium valproate is a broad-spectrum antiepileptic that has proven efficacy against all seizure types, which makes it a useful antiepileptic when the exact seizure classification is unknown or multiple seizure-types exists.[1] The aromatic antiepileptics, namely, phenytoin, carbamazepine, phenobarbitone, and primidone can cause hypersensitivity reactions.[2] For example, carbamazepine can cause pruritic and erythematous rashes, urticaria, photosensitivity reactions, exfoliative dermatitis, alopecia, diaphoresis, erythema multiforme, Stevens-Johnson syndrome (SJS), and Toxic epidermal necrolysis (TEN).[3] In such a case carbamazepine is discontinued and nonaromatic antiepileptics like sodium valproate or a benzodiazepine is usually recommended as an alternative therapy.[1] The common side effects of valproate include anorexia, nausea, vomiting, sedation, ataxia, tremor, alopecia, stimulation of appetite, elevation of hepatic transaminases, and rarely fulminant hepatitis.[4] Stevens-Johnson syndrome is rarely reported with it.[5] In most of the reported cases of SJS due to valproate, it has been found when it is given with other drugs.[5–7] SJS due to valproate monotherapy is rarely reported.
We present a case of Stevens-Johnson syndrome due to valproate monotherapy.
CASE REPORT
A 20-old-medical student had a past medical history of migraine and generalized seizures, with postictal visual and auditory hallucinations, for which sodium valproate 900 mg was prescribed. His baseline investigations, such as, liver function and complete hemogram were within normal limits. After seven days he developed fever with myalgia, followed by lesions in the oral cavity two days later. He also had difficulty in swallowing. On examination there were erosions in the oral and nasal mucosa [Figure 1]. Conjunctivitis was present. Tzank smear was negative. There were hemorrhagic crusts on his lips [Figure 2]. There were no skin lesions. No genital or anal involvement. No arthritic features. His complete hemogram, liver function tests, and chest X-ray, were within normal limits. His HIV and HbsAg status were negative. Sodium valproate was withdrawn and he was started on systemic steroids and antihistaminics. The lesions resolved within a few days.
Figure 1.
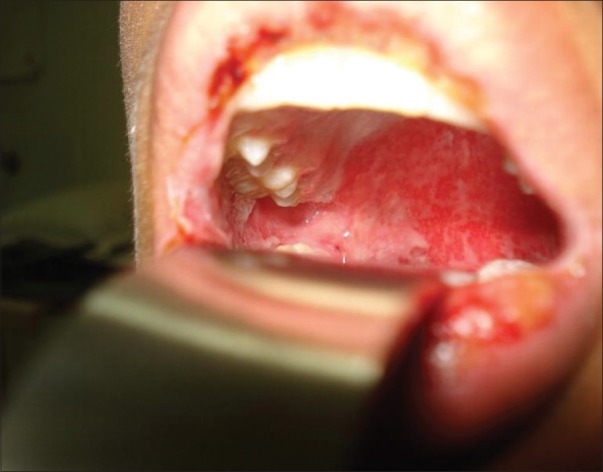
Mucosal erosions
Figure 2.
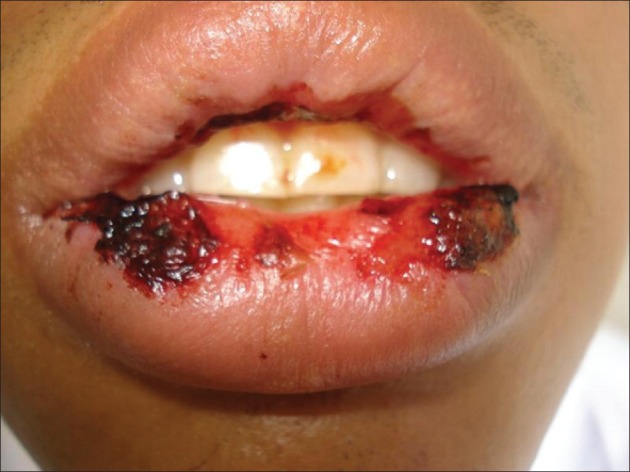
Hemorrhagic crusts on lips
DISCUSSION
The Stevens-Johnson syndrome and Toxic epidermal necrolysis are rarely reported side effects of valproate.[5–8] Rzany et al, in a case control study on the risk of SJS and TEN with various anti-epileptic agents, reported the univariate relative risk as, 120 for carbamazepine, 91 for phenytoin, 57 for Phenobarbital, 25 for lamotrigine, and 24 for valproate within the first eight weeks of therapy. The association of SJS with valproate was confounded by concomitant short-term therapy with other casual drugs in many studies.[9] Roujeau et al, conducted a case control study to quantify the risks associated with the use of specific drugs. Drug use before the onset of disease was compared in 245 people who were hospitalized because of TEN or SJS and 1147 patients hospitalized for other reasons. The crude relative risk for SJS or TEN was as follows: carbamazepine, 90; phenytoin, 53; Phenobarbital, 45, and valproic acid, 25.[10]
Suresh Kumar PN et al, reported a case of SJS after he was put on sodium valproate 600 mg per day, haloperidol 10 mg per day, and trihexiphenidyl 4 mg per day, which resolved after withdrawing sodium valproate and putting him on systemic steroids.[5]
In most of the studies, SJS was caused by valproate, when it was used in combination with other drugs.[5–7] In our case SJS was found with valproate monotherapy and was limited only to the mucosa. There was no skin involvement. This case is presented because valproate monotherapy-induced SJS is rarely reported.
CONCLUSION
As sodium valproate may also cause Stevens-Johnson syndrome, one should be careful while initiating therapy with valproate.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chogtu B, Chawla S, Gupta U, Reddy BS. Carbamazepine and sodium valproate cross reactivity. Indian J Pharmacol. 2005;37:194–5. [Google Scholar]
- 2.Schlienger RG, Shear NH. Antiepileptic drug hypersensitivity syndrome. Epilepsia. 1998;39(Suppl 7):S3–7. doi: 10.1111/j.1528-1157.1998.tb01678.x. [DOI] [PubMed] [Google Scholar]
- 3.Cada DJ. Anticonvulsants. In: Kastrup EK, editor. Drug facts and comparisons. 57th ed. St. Louis: A Wolters Kluwer Company; 2003. pp. 1106–56. [Google Scholar]
- 4.James O, McNamara . Pharmacotherapy of the epilepsies. In: Brunton LL, Laza JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw Hills; 2006. p. 515. [Google Scholar]
- 5.Kumar PN, Kumar SK. Stevens-Johnson syndrome induced by Sodium Valproate. Indian J Psychiatry. 2004;46:269–70. [PMC free article] [PubMed] [Google Scholar]
- 6.Platin P, Cartier H, Li Bihan G, Clouard P, Lellouche F, Leroy JP. Drug Hypersensitivity syndrome during treatment with valproic acid. Presse Med. 1995;24:1624. [PubMed] [Google Scholar]
- 7.Mishra BN, Mohapatra PK, Roy D. Stevens - Johnson Syndrome during treatment with lithium and valproate in mood disorder in a bipolar patient. Indian J Psychiatry. 2002;44:301–2. [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai SJ, Chen YS. Valproic acid induced Stevens-Johnson syndrome. J Clin Psychopharmacol. 1998;18:420. doi: 10.1097/00004714-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Rzany B, Corriea O, Kelly JP. Risk of Stevens - Johnson Syndrome and Toxic epidermal necrolysis during the first week antiepileptic therapy: Study group of the international case control study on the severe cutaneous adverse reactions. Lancet. 1999;353:2190–4. doi: 10.1016/s0140-6736(98)05418-x. [DOI] [PubMed] [Google Scholar]
- 10.Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk Stevens - Johnson Syndrome and toxic epidermal necrolysis. N Engl J Med. 1995;333:1600–7. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]


