Abstract
Background:
The effects of chronic administration of efavirenz commonly used as part of highly active antiretroviral therapy (HAART) for the treatment of Human Immunodeficiency Virus (HIV) type-1 on the weight of the brain and inferior colliculus of adult Wistar rats was carefully studied.
Methods and Materials
The rats of both sexes (n = 24), with average weight of 200g were randomly assigned into two treatment (A & B) (n=16) and Control (c) (n=8) groups. The rats in the treatment group received 600mg/70kg bogy weight of efavirenz dissolved in distilled water daily for 30days through the orogastric tube. The control group received equal volume of distilled through the same route. All rats were fed with grower's mash and given water liberally. The rats were sacrificed by cervical dislocation method on the thirty-first day of the experiment and the lateral geniculate body was carefully dissected out and quickly fixed in 10% formal saline for histological study.
Results
The findings indicate that rats in the treated group showed some cellular degenerative changes like sparse cellular population, pyknotic nuclei with some microcystic changes and edema in the stroma of the lateral geniculate body as compared to the control group.
Conclusion
Chronic administration of efavirenz may have an adverse effect on the visual sensibilities by affecting the microanatomy of the lateral geniculate body of adult Wistar rats. It is recommended that further studies aimed at corroborating these observations be carried out.
Keywords: Efavirenz, histological effects, lateral geniculate body, visual sensibilities
Introduction
Efavirenz is an antiretroviral drug that belongs to the class of drugs called non-nucleoside reverse transcriptase inhibitor (NNRTI) used as part of highly active antiretroviral therapy (HAART) for the treatment of human immunodeficiency virus (HIV)[1]. Efavirenz has been found to be effective in many combination regimes for the treatment of HIV infection, both in previously untreated and in treated individuals. It is combined in regimens with other HAART agents[2–4].
Two-sanctuary of HIV has been conceptualized to depict the major impediment to successful HIV targeted therapy. According to the concept, the two sanctuaries are cellular and anatomical. The latter implicates the central nervous system. An understanding of the nature of HIV within these reservoirs is critical to devising strategies to hasten viral eradication[6–8]. The two-sanctuary concept identifies that most antiviral agents do not efficiently penetrate the blood brain barrier or are actively transported out of the central nervous system. Hence, even after antiviral treatment that successfully controls virus in the treatment compartments, the central nervous system may suffer continuing damage induced by HIV infection. Efavirenz is one of the HAART agents that can penetrate the central nervous system and spinal fluids with a capacity to strongly inhibit multi-drug resistant proteins[6–12]. Thus, efavirenz has emerged as cornerstone of regimens.
Some adverse effect in the central nervous system has been commonly associated with efavirenz. The most common central nervous system effects include confusion, insomnia, abnormal vivid dreams, dizziness and headache[3,13–15].
The superior colliculus and lateral geniculate body constitutes the intracranial visual relay centers. The lateral geniculate body in mammals is considered as part of the thalamic nuclei for processing visual information. In rats the lateral geniculate body receives input from the geniculate leaflet, which participates in the regulation of circadian function through its projection to the circadian pacemaker of the hypothalamus[16,17].
Since efavirenz crosses the blood brain barrier, it is relevant to investigate its effect on the lateral geniculate body. It is not unlikely that the adverse effects of efavirenz manifesting as dizziness and headache may be due to direct effect of efavirenz on the brain and lateral geniculate body in particular. The objective of this study is to investigate the effects of chronic administration of efavirenz on the histology of the lateral geniculate body of adult Wistar rats.
Materials and Methods
Animal care ethics
The School of Basic Medical Sciences, University of Benin grant approval before the work begins. The rats were obtained and maintained in the Animal Holdings of the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Edo State, Nigeria. The animals were fed with grower's mash obtained from Edo Feeds and Flour Mill Limited, Ewu, Edo State, Nigeria and given feeds liberally.
Drug administration
Efavirenz was obtained from the PEPFAR unit, University of Benin Teaching Hospital, Benin City, Edo State, Nigeria. Sixteen adult Wistar rats of both sexes with average weight of 200g were equally and randomly distributed into two groups, which comprised control (n=8) and treatment (n=8). The rats in the treatment group received 600mg/70kg body weight of efavirenz dissolved in distilled water for thirty days through orogastric tube administration while the control rats received equal volume of distilled water through the same route and for the same period. The rats were sacrificed by cervical dislocation on the thirty-first day of the experiment. The skulls were opened using bone forceps to expose the brain of the rats and the lateral geniculate body was quickly dissected out and fixed in 10% formal saline for routine histological techniques.
Histological study
The tissues were dehydrated in an ascending grade of alcohol (ethanol), cleared in xylene and embedded in paraffin wax. Serial sections of 7 microns thick were obtained using a rotatory microtome. The deparaffinized sections were stained routinely with Haematoxyline and Eosin. Photomicrographs of the results were obtained using research photographic microscope in the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Edo State, Nigeria
Results
The sections of the lateral geniculate body from the control animals showed normal histological features with the neurons appearing distinct and of various sizes. The neuron and glial cells appeared normal and no vacuolation in the stroma of the sections (Fig. 1).
Fig. 1.
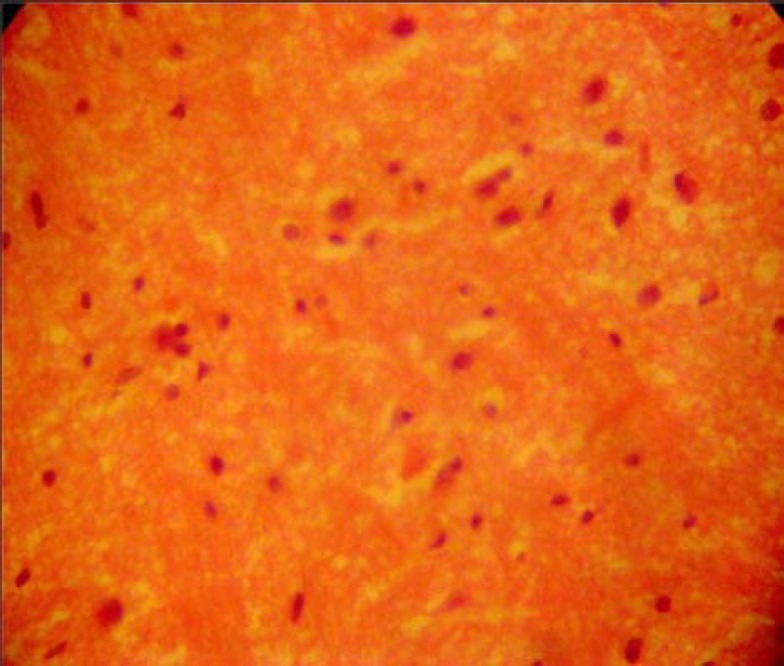
Control section of lateral geniculate body (H & E method ×400)
The lateral geniculate body of the treated groups revealed some cellular degenerative changes. Compared to the control group, sparse cellular population was observed as well as, pyknotic nuclei with some microcystic changes and edema in the stroma of the lateral geniculate body as (Fig. 2).
Fig. 2.
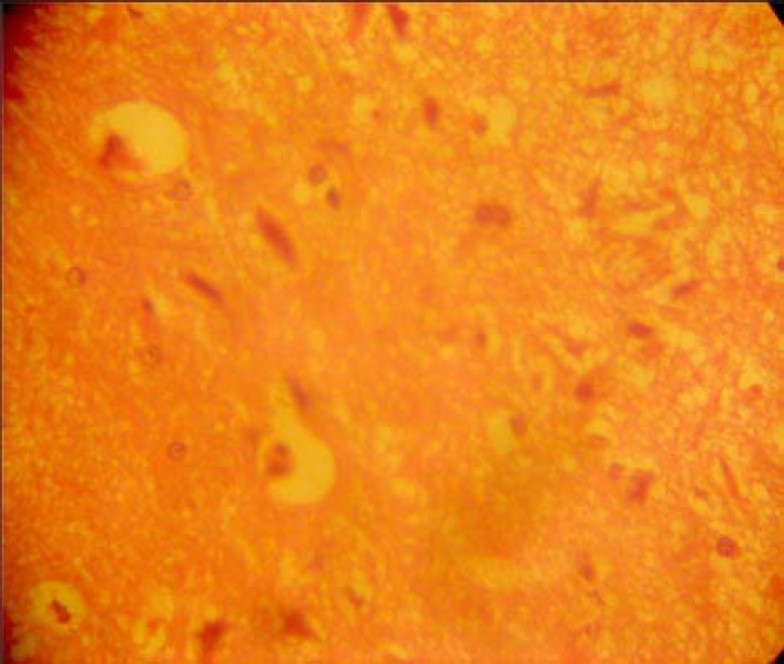
Efavirenz-treated section of lateral geniculate body (H & E ×400)
Discussion
The results of the haematoxyline and eosin (H&E) stain from this experiment revealed some cellular degenerative changes such as sparse cellular population, pyknotic nuclei with some microcystic changes and edema in the stroma of the lateral geniculate body (Fig. 2), when compared to the control group of the lateral geniculate body (Fig. 1).
Extensive cell death in the central nervous system is present in all neurodegenerative diseases. The type of nerve cell loss and the particular part of the brain affected dictate the symptoms associated with an individual disease[18]. In this study efavirenz may have acted as toxin to the cells of the lateral geniculate body, affecting their cellular integrity and causing defect in membrane permeability and cell volume homeostasis.
It is known that efavirenz has the potential to cause toxicity in the central nervous system, but this is yet to be elucidated[19]. In cellular necrosis, the rate of progression depends on the severity of the environmental insults. The prime candidates for inducing the massive cell destruction observed in neurodegeneration are neurotoxins. These may be substances present in small amounts in the environment, or even naturally occurring chemicals such as glutamate used by the brain as transmitter's substances. The latter when present at a critical level can be toxic to the brain cells in which they normally excite[20].
It could be inferred from this study that prolonged administration of efavirenz resulted in increased toxic effects on the lateral geniculate body. That is, the decrease in cellular population observed in this study may have been as a result of cell death caused by the toxic effect of efavirenz. In the same way, it has been reported that chronic administration of chloroquine resulted in cellular degenerative changes, sparse cellular population and vacuolation appearing in the stroma with some autophagic vacuoles in the inferior colliculus and medial geniculate body of adult Wistar rats[21,22].
The microcystic changes and edema observed in the stroma of the lateral geniculate body in this experiment may be due to efavirenz interference, since it is known to cross blood brain barrier and thus getting access to the cells of the brain. Given that the neurons of the central nervous system is affected by efavirenz, it is probable that the results obtain in this experiment may have been due to the neurotoxin effect of efavirenz on the neuronal cells of the lateral geniculate body of adult Wistar rats.
There is very limited, yet contrasting literature on the effects of antiretroviral therapy on visual sensibilities. While a case report has indicated that HAART could have significant resolution of vision problems[23], efaviren has been implicated to possess retinal toxicity[24]. How and/or whether the brain is involved has yet to be reported. Indeed, among the known side effects of HAART including efavirenz[3,13–15], visual defect is not listed. What this article contributes to literature is that the toxic effect of efaviren may be at the micro-anatomical level of the inferior colliculus vis-à-vis lateral geniculate body of the brain. It is probable that observable vision defect attributable to efavirenz would be dependent on the extent of effect on the micro-anatomical structure of the lateral geniculate body.
Conclusion
This study revealed that chronic administration of efavirenz can cause microanatomical changes in the lateral geniculate body of the adult Wistar rats. This histological effect may provoke cognitive dysfunction as well as affect the visual sensibility functions of the lateral geniculate body in the adult Wistar rats. A further study to corroborate this finding is warranted.
References
- 1.Maggiolo F. Efavirenz. a decade of clinical experience in the treatment of HIV. J Antimicrob Chemother. 2009;64:910–928. doi: 10.1093/jac/dkp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulick RM, Ribaudo HJ, Shikuma CM. Three- vs. four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA. 2006;296:769–781. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 3.Jena A, Sachdeva RK, Sharma A, Wanchu A. Adverse drug reactions to nonnucleoside reverse transcriptase inhibitor-based antiretroviral regimen: a 24-week prospective study. J Int Assoc Physicians AIDS Care (Chic Ill) 2009;8:318–322. doi: 10.1177/1545109709343967. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford GW, Sangani PR, Kennedy GE. Three- or four- versus two-drug antiretroviral maintenance regimens for HIV infection. Cochrane Database Syst Rev. 2003;4:CD002037. doi: 10.1002/14651858.CD002037. [DOI] [PubMed] [Google Scholar]
- 5.Staszewski S, Miller V, Sabin C, Schlecht C, Gute P, Stamm S, Leder T, Berger A, Weidemann E, Hill A, Phillips A. Determinants of sustainable CD4 lymphocyte count increases in response to antiretroviral therapy. AIDS. 1999;13:951–956. doi: 10.1097/00002030-199905280-00011. [DOI] [PubMed] [Google Scholar]
- 6.Schrager LK, D’Souza MP. Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. JAMA. 1998;280:67–71. doi: 10.1001/jama.280.1.67. [DOI] [PubMed] [Google Scholar]
- 7.Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82:A99–109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynn HE, Brundage RC, Fletcher CV. Clinical implications of CNS penetration of antiretroviral drugs. CNS Drugs. 2002;16:595–609. doi: 10.2165/00023210-200216090-00002. [DOI] [PubMed] [Google Scholar]
- 9.Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, Tarwater P, Clements J, Barber S. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA. 2005;293:2003–2011. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]
- 10.Fox HS, Weed MR, Huitron-Resendiz S, Baig J, Horn TF, Dailey PJ, Bischofberger N, Henriksen SJ. Antiviral treatment normalizes neurophysiological but not movement abnormalities in simian immunodeficiency virus-infected monkeys. J Clin Invest. 2000;106:37–45. doi: 10.1172/JCI9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puzantian T. Central nervous system adverse effect with efavirenz: case report and review. Pharmacotherapy. 2002;22:930–933. doi: 10.1592/phco.22.11.930.33624. [DOI] [PubMed] [Google Scholar]
- 12.Thomas SA. Anti-HIV drug distribution to the central nervous system. Curr Pharm Des. 2004;10:1313–1324. doi: 10.2174/1381612043384835. [DOI] [PubMed] [Google Scholar]
- 13.Clifford DB, Evans S, Yang Y, Acosta EP, Goodkin K, Tashima K, Simpson D, Dorfman D, Ribaudo H, Gulick RM. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med. 2005;143:714–721. doi: 10.7326/0003-4819-143-10-200511150-00008. [DOI] [PubMed] [Google Scholar]
- 14.Kontorinis N, Dieterich DT. Toxicity of non-nucleoside analogue reverse transcriptase inhibitors. Semin Liver Dis. 2003;23:173–182. doi: 10.1055/s-2003-39948. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimoto E, Konishi M, Takahashi K, Murakawa K, Maeda K, Mikasa K, Yamashina Y. The first case of efavirenz-induced photosensitivity in a Japanese patient with HIV infection. Intern Med. 2004;43:630–631. doi: 10.2169/internalmedicine.43.630. [DOI] [PubMed] [Google Scholar]
- 16.Altman AS, Bayer CS. Time of origin of neurons of rat superior colliculus in relation to other components of the visual and visuomotor pathways. Exp Brain Res. 1981;42:424–434. doi: 10.1007/BF00237507. [DOI] [PubMed] [Google Scholar]
- 17.Moore RY, Card JP. Intergeniculate leaflet: an anatomically and functionally distinct subdivision of the lateral geniculate complex. J Comp Neurol. 1984;344:403–444. doi: 10.1002/cne.903440306. [DOI] [PubMed] [Google Scholar]
- 18.Eweka AO, Adjene JO. Histological studies of the effects of oral administration of artesunate on the medial geniculate body of adult Wistar rats. Rev Electron Biomed/Electron J Biomed. 2008;1:20–26. [Google Scholar]
- 19.Fortin C, Joly V. Efavirenz for HIV-1 infection in adults: an overview. Expert Rev Anti Infect Ther. 2004;2:671–684. doi: 10.1586/14789072.2.5.671. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell IJ, Lawson S, Moser B, Laidlaw SM, Cooper AJ, Walkinshaw G, Waters CM. Glutamate-induced apoptosis results in a loss of striatal neurons in the parkinsonian rat. Neuroscience. 1994;63:1–5. doi: 10.1016/0306-4522(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 21.Adjene JO, Adenowo TK. Histological studies of the effect of chronic administration of chloroquine on the inferior colliculus of adult wistar rat. UNIBEN JMBR. 2005;4(1):83–87. [Google Scholar]
- 22.Adjene JO, Caxton-Martins AE. Some histological effect of chronic Administration of Chloroquine on the medial geniculate body of Adult wistar rat. Afri J Med Sci. 2006;35:131–135. [PubMed] [Google Scholar]
- 23.Diller R, Thompson K. Visual loss secondary to acquired immunodeficiency virus-related progressive multifocal leukoencephalopathy demonstrating clinical improvement with highly active antiretroviral therapy. Optometry. 2007;78:63–70. doi: 10.1016/j.optm.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Lewis A, Mitchell S. Efavirenz and retinal toxicity. Eye. 2002;16:107. doi: 10.1038/sj.eye.6700038. [DOI] [PubMed] [Google Scholar]


