Abstract
Cardiovascular disease (CVD) is the driving force behind the discrepancy in life expectancy between indigenous and non-indigenous groups in many countries. Preceding CVD many indigenous groups exhibit a cluster of cardiometabolic risk factors, including overweight-obesity, diabetes, high cholesterol, and high blood pressure. In turn, modifiable lifestyle risk factors contribute to the development of this cluster of cardiometabolic conditions. Modifiable lifestyle risk factors include, but are not limited to, physical inactivity, poor nutrition, excessive alcohol consumption, and cigarette smoking. Notably, these metabolic and lifestyle risk factors are relatively simple to monitor and track. The current review will look at modifiable cardiometabolic (overweight-obesity, diabetes mellitus, high cholesterol, and high blood pressure) and lifestyle (physical inactivity, poor nutrition, risky alcohol behavior, and cigarette smoking) risk factors among indigenous populations from Australia (Aboriginal Australians and Torres Strait Islanders), New Zealand (Māori) and the United States (Native Americans). Discussion will focus on the causal relationship between modifiable lifestyle risk factors and cardiometabolic outcomes, as well as, simple measurements for tracking these risk factors.
Keywords: Heart disease, endothelial dysfunction, Maori, Aboriginal Australian, Native American
INTRODUCTION
There are more than 370 million indigenous people in 70 countries worldwide.[1] Indigenous people are not monolithic, there is a significant variation between and within these indigenous populations in terms of worldview, culture, political forces, education, socioeconomic status, living conditions, and familial factors. However, many indigenous groups do share a striking commonality: A discrepancy in life expectancy when compared to their non-indigenous kinsman — even in the so-called ‘wealthy’ countries, including Australia, New Zealand, and the United States. For example, the Aboriginal people of Australia have a life expectancy of 62 years, versus 81 years for non-indigenous Australians;[2] the Maori of New Zealand have a life expectancy of 73 years, versus 81 years for their white kinspeople;[3] and the Native Americans have a live expectancy of 75 years, versus 78 years for their white counterparts.[4]
Cardiovascular disease (CVD) is the driving force behind the discrepancy in life expectancy between indigenous and non-indigenous populations in many countries.[5] Preceding CVD, many indigenous groups exhibit a cluster of modifiable cardiometabolic risk factors, including overweight-obesity, diabetes mellitus, high cholesterol, and high blood pressure. In turn, modifiable lifestyle risk factors contribute to the development of this cluster of cardiometabolic conditions [Figure 1]. Modifiable lifestyle risk factors include, but are not limited to, physical inactivity, poor nutrition, risky alcohol behavior, and cigarette smoking. Notably, these metabolic and lifestyle risk factors are relatively simple to monitor and track [Table 1].
Figure 1.
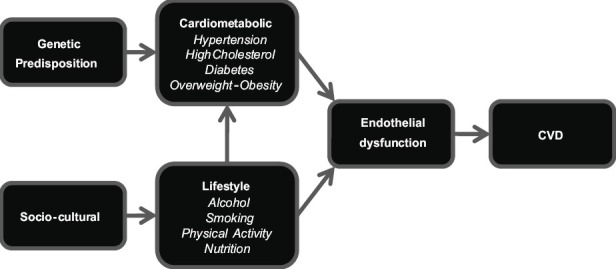
Causation pathway for cardiovascular disease
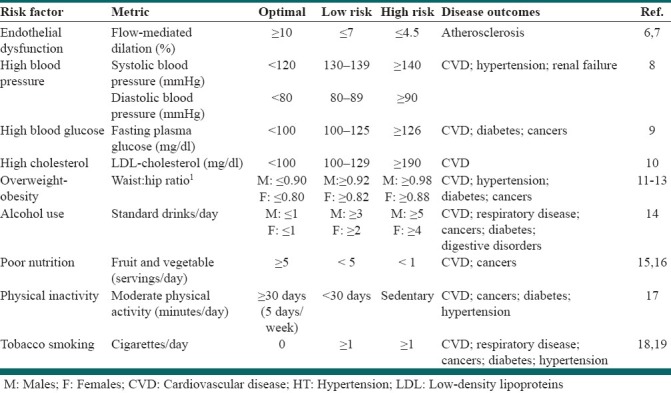
Table 1.
Lifestyle and metabolic risk factor analysis
The current review will look at modifiable cardiometabolic (overweight-obesity, diabetes mellitus, high cholesterol, and high blood pressure) and lifestyle (physical inactivity, poor nutrition, risky alcohol behavior, and cigarette smoking) risk factors among the indigenous populations from Australia (Aboriginal Australians and Torres Strait Islanders), New Zealand (Māori), and the United States (Native Americans). Discussion will focus on the causal relationship between modifiable lifestyle risk factors and cardiometabolic outcomes, as well as, simple measurements for tracking these risk factors.
MODIFIABLE CARDIOMETABOLIC METABOLIC RISK FACTORS
The following section will highlight the prevalence of modifiable cardiometabolic risk factors for CVD: Overweight-obesity, diabetes mellitus, high cholesterol, and high blood pressure. Each of these risk factors is relatively simple to monitor [Table 1].
OBESITY
Much higher rates of obesity have been found for the indigenous populations of Australia,[20] New Zealand,[21] and the US[15] Excess body fat increases the risk of developing a range of health problems, including high blood pressure, diabetes mellitus, and CVD. Population studies typically estimate the prevalence of overweight/obesity by calculating an individual's Body Mass Index (BMI) score.[22] Despite widespread use, BMI has been heavily criticized.[23–28] The BMI is calculated by dividing weight by height, and it is assumed that weight equates to body fat.
Romero-Corral et al.,[23] undertook a meta-analysis to determine the nature of the relationship between obesity and cardiovascular mortality in patients with CHD. Patients with severe obesity (BMI ≥35) had the greatest relative risk (RR) for cardiovascular mortality (RR=1.88), compared to people with a normal BMI (BMI 20 – 24.9). However, overweight patients (BMI 25 – 29.9) had the lowest risk (RR=0.88), and obese patients (BMI 30 – 35) had no increased risk (R=0.97). The authors suggested that these findings could be explained by the lack of discriminatory power of BMI to differentiate between body fat and lean mass.
Waist circumference, waist-to-height ratio, and waist-to-hip ratio (WHR) take into consideration body-fat distribution, especially abdominal obesity, and appear to be better predictors of CVD than BMI.[29] A recent study compared the predictive power of BMI, waist circumference, waist-to-height ratio, and WHR for diabetes mellitus, hypertension, and dyslipidemia in Australian Aboriginal and Torres Strait Islander adults.[28] The WHR was found to have the greatest predictive power. A number of studies, investigating a range of ethnic groups, have found WHR to better predict cardiometabolic and cardiovascular risk factors than BMI.[24–27] However, studies over the past two decades indicate that cutoffs for WHR differ by ethnic groups, therefore, the reference values provided in Table 1 should be used to provide a guideline,[13] not to ascertain the absolute risk.
DIABETES MELLITUS
The prevalence of diabetes mellitus is up to three times greater when comparing the indigenous populations of Australia,[20] New Zealand,[21] and the US[15] to their non-indigenous counterparts. Diabetes mellitus is a metabolic disease in which high blood glucose levels result from defective insulin secretion, insulin action or both.[30] Diabetes is a worldwide epidemic and a major risk factor for CVD.[15,31–35] The prevalence of diabetes for all age groups worldwide was estimated to be 2.8% in 2000 and is projected to be 4.4% in 2030.[31] CVD accounted for the primary cause of death for all patients with diabetes.[15] Several modifiable risk factors play a role in the onset of diabetes mellitus, including obesity, physical inactivity, and poor nutrition, as does genetic predisposition and aging.[36–40] Diabetes mellitus risk can be monitored by measuring fasting blood glucose. A fasting blood glucose of <100 mg/dl is considered optimal.[8]
HIGH CHOLESTEROL AND OTHER LIPIDS
The prevalence of high total cholesterol is greater for the indigenous population of the US[15] compared to the general population, but not for the indigenous populations in Australia[20] and New Zealand.[21] However, the lack of differences in cholesterol between the indigenous and non-indigenous populations in Australia and New Zealand, in these large surveys, may be misleading, as only total cholesterol was measured in the Australian survey, and in the New Zealand survey, the participants reported medicated high cholesterol levels, and thus, there were likely undiagnosed cases.
The two most common blood lipids are cholesterol and triglycerides. These two blood fats are carried on particles called lipoproteins, the most important of which are low density lipoprotein (LDL) and high density lipoprotein (HDL). Both carry cholesterol, but it is high LDL-cholesterol levels that have been shown to be pro-atherogenic,[41–45] whereas, low levels of HDL-cholesterol are associated with increased Coronary Heart Disease (CHD), morbidity, and mortality.[46–49] Conversely, high HDL-cholesterol levels convey reduced risk.[46–49] In population studies, serum total cholesterol is often used as a surrogate for LDL-cholesterol levels, however, measurement of LDL-cholesterol concentrations confer greater predictive value for cardiovascular events.[41–45] High cholesterol usually has no symptoms, and people may not be aware they have the condition unless they have had a blood test. Therefore, the best way to determine the true prevalence of high cholesterol in the community is through blood samples.[21] An LDL-cholesterol level <100 mg/dl is considered optimal.[50]
HYPERTENSION
The indigenous populations of Australia[20] and New Zealand,[21] but not the US,[15] have higher rates of hypertension compared to the general population — however, it should be noted that the prevalence of hypertension in the US among the total population is particularly high.[15] Hypertension is a major risk factor for CVD. For every 20 mmHg systolic or 10 mmHg diastolic increase in resting blood pressure, there is a two-fold increase in mortality from both ischemic heart disease and stroke.[51] Hypertension is associated with shorter overall life expectancy, shorter life expectancy free of CVD, and more years lived with CVD.[52] A systolic blood pressure <120 mmHg and a diastolic blood pressure <80 mmHG is considered optimal.[8] Blood pressure should be monitored using the ausculatory method, with a properly calibrated devise, following five minutes of quiet rest in a chair.[8]
MODIFIABLE LIFESTYLE RISK FACTORS
The following section will discuss the prevalence of poor nutrition, risky alcohol behavior, physical inactivity, and cigarette smoking. Although not exhaustive, these variables represent modifiable lifestyle risk factors, which have been proven to modulate the cardiometabolic factors discussed earlier in the text.
ALCOHOL
In Australia,[20] New Zealand,[53] and the US,[54] there is a lower prevalence of any alcohol consumption among the indigenous population compared to the general population. However, the indigenous people of each country are more likely to exhibit risky alcohol behavior (binge drinking: ≥5 standard drinks/day for males, ≥4 standard drinks/day for females). Although some scientific evidence indicates that light-to-moderate alcohol consumption may significantly reduce the risk of CVD and all-cause mortality, but excessive alcohol intake is toxic to both the heart and overall health.[14,55,56] In contrast, excessive alcohol intake is toxic to both the heart and overall health.[14,55,56] In particular, binge drinking, even among otherwise light drinkers, increases cardiovascular events and mortality.[14,55,56] The American Heart Association guidelines caution people not to start drinking if they do not already drink alcohol, because it is not possible to predict in which people alcohol abuse will become a problem.[57]
NUTRITION
Low fruit and vegetable consumption has been reported for the indigenous people of Australia,[20] New Zealand,[21] and the US[58] A diet high in fruits and vegetables can reduce the risk for many leading causes of death.[15,16,59–61] In a meta-analyses of prospective cohort studies, each daily serving of fruits or vegetables was associated with a 4% lower risk of CHD (RR: 0.96, 95% CI: 0.93 to 0.99) and a 5% lower risk of stroke (RR: 0.95, 95% CI 0.92 to 0.97).[60,61] Five or more daily servings of fruit and vegetables are considered optimal.[15,16] FFQs, including the freely available National Cancer Institute Diet History Questionnaire (http://riskfactor.cancer.gov/dhq2/),[62,63] allows for the assessment of the usual patterns of food intake over an extended period of time.[64,65] FFQs are inexpensive in both time and cost, in comparison to other measurement tools, which is an important consideration in studies involving large cohorts.[66]
PHYSICAL INACTIVITY
Higher rates of sedentary behavior have been reported for the indigenous populations of Australia[20] and the US,[67] but not New Zealand.[21] However, while similar physical activity levels have been reported for the Māori of New Zealand compared to the non-indigenous population, large scale studies are limited. It has been estimated that physical inactivity is responsible for 12% of the global burden of heart attacks.[68] Regular physical activity reduces CVD risk in its own right and also improves CVD risk factors such as overweight, high blood pressure, high cholesterol, and diabetes.[69–74] The American College of Sports Medicine (ACSM) recommends at least 30 minutes of moderate-intensity physical activity (e.g., walking briskly, mowing the lawn, dancing, swimming, bicycling) at least five days a week.[17]
A number of tools have been developed to measure physical activity, ranging from objective measures such as accelerometry to subjective questionnaires.[75] Physical activity questionnaires are prone to technical error, but are inexpensive, practical to use in population studies, and can provide information about the type of physical activity and context.[75] The International Physical Activity Questionnaire (IPAQ) (http://www.ipaq.ki.se/ipaq.htm) is a freely available, cross-national monitoring tool, which has been validated for use in adults[76–80] and children.[81–84]
CIGARETTE SMOKING
Much higher rates of cigarette smoking have been reported for the indigenous populations of Australia,[20] New Zealand,[21] and the US,[85] compared to their non-indigenous counterparts. Cigarette smoking increases the incidence of CVD in a dose-dependent manner,[86–92] with even occasional smoking increasing the risk of CVD.[93] The relationship between smoking and CVD lies in the multiple mechanisms that interact to contribute to atherosclerosis, vascular injury, vascular dysfunction, and thrombosis, although the precise mechanisms are largely unknown.[93–95]
MONITORING CARDIOVASCULAR DISEASE: ENDOTHELIAL FUNCTION
Upsetting the delicate balance of functions performed by the endothelium initiates a number of events that promote atherosclerosis, the precursor to CVD.[96–98] Although atherosclerosis is commonly described as the presence of plaques that obstruct the lumen of the conduit arteries, endothelial dysfunction precedes plaque formation.[99–101] Reduced endothelial responses can be observed early in the course of atherogenesis, preceding angiographic or ultrasonic evidence of the atherosclerotic plaque.[102] There is, therefore, widespread interest in the application of clinical tools, to assess the function and health of this essential monolayer.
Several biomarkers are available to identify the health of the endothelium, including total nitric oxide (NO), asymmetric dimethylarginine (ADMA), dimethylarginine (DDAH), and endothelin-1 (ET-1). ADMA is an endogenous inhibitor of NO synthases — NO is one of the most important molecules regulating endothelial function.[103,104] ADMA plasma concentration is elevated in numerous populations with vascular diseases or at high cardiovascular risk.[105–107] ADMA concentrations have also been found to significantly correlate with flow-mediated dilation (FMD), the gold standard for evaluating endothelial function.[108]
Endothelial function can also be evaluated non-invasively by using strain-gauge plethysmography,[109–112] pulse-wave analysis,[113–115] or flow-mediated dilation (FMD). The FMD test is the standard tool used to assess endothelial function.[116,117] Reduced FMD, an early marker of atherosclerosis[116], has been noted for its capacity to predict future CVD events,[6,118–120] and an impaired vascular response has also been demonstrated in children as young as seven years of age with familial hypercholesterolemia.[121] A recent meta-analysis by Inaba et al.[6] reported that the relative risk of cardiovascular events for a 1% absolute change in FMD is 0.87. This suggests that a 1% decrease in FMD is associated with a 13% (95% CI: 9 to 17%) increase in the risk of future cardiovascular events. A number of authors have developed standardized guidelines for conducting this test.[117,122–126]
DISCUSSION
Cardiovascular disease is the driving force behind the discrepancy in life expectancy between the indigenous and non-indigenous groups in many countries.[5] This trend extends to the indigenous populations of Australia, New Zealand, and the US. The historical underpinnings of this trend can be seen in the devastating effects of colonization on the health outcomes for indigenous people.[127,128] This review has provided a working model to outline the causal relationship between lifestyle choices, cardiometabolic risk factors (i.e., modifiable lifestyle-related diseases) and CVD. Guidelines have also been provided to monitor these risk factors.
Strategies for implementing change are beyond the scope of this article. There is a paucity of large-scale, methodologically rigorous interventions1 designed to improve indigenous health outcomes,[129] and as such, there is a limited evidence base to guide healthcare scientists and practitioners. Paul et al.,[129] recently identified 811 publications focusing on the major causes of death and illness for indigenous populations in Australia, New Zealand, the US, and Canada, and only 43 of these were identified as intervention studies. Of the 43 studies identified by Paul et al., only 19 were judged to be methodologically rigorous.[130,131] The preponderance of the literature on the health of indigenous populations is focused on describing or understanding problems, rather than on testing the effectiveness of the potential solutions. A small number of intervention studies do meet the standards for appropriate research methodology, but they do not provide a body of evidence in relation to any particular population, health topic, or intervention type. There remains a pressing need to develop culturally sensitive strategies geared toward improving the lifestyles of indigenous populations. The guidelines outlined in the current article will assist future research focusing on indigenous health outcomes.
CONCLUSION
Cardiovascular disease is the driving force behind the discrepancy in life expectancy between the indigenous and non-indigenous groups in many countries. Much of this risk can be offset through lifestyle changes. The current review provides a simple working model for managing and monitoring CVD risk among the indigenous populations.
Footnotes
The term ‘intervention’ is used in accordance with the scientific literature. The authors acknowledge that this term may be construed as culturally insensitive.
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.State of the world's indigenous populations. New York: Department of Economic and Social Affairs of the United Nations Secretariat; 2009. Department of Economic and Social Affairs of the United Nations Secretariat. [Google Scholar]
- 2.Australian Institute of Health and Welfare (AIHW) Life expectancy. 2011. [Last cited on 2011 Apr 14]. Available from: http://www.aihw.gov.au/life-expectancy/
- 3.Statistics New Zealand. New Zealand life tables: 2005-07. 2008. [Last cited on 2011 Apr 15]. Available from: http://www.stats.govt.nz/browse_for_stats/health/life_expectancy/nzlifetables_hotp05-07.aspx .
- 4.Facts on Indian health disparities. Rockville, MD: Indian Health Service; 2006. Indian Health Service. [Google Scholar]
- 5.Huffman MD, Galloway JM. Cardiovascular health in indigenous communities: successful programs. Heart Lung Circ. 2010;19:351–60. doi: 10.1016/j.hlc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int J Cardiovasc Imaging. 2010;26:631–40. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 7.Witte DR, Westerink J, de Koning EJ, van der Graaf Y, Grobbee DE, Bots ML. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J Am Coll Cardiol. 2005;45:1987–93. doi: 10.1016/j.jacc.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 9.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Cholesterol Education Program (NCEP) Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 11.Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist: hip ratio as predictors of cardiovascular risk–a review of the literature. Eur J Clin Nutr. 2010;64:16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 12.Qiao Q, Nyamdorj R. The optimal cutoff values and their performance of waist circumference and waist-to-hip ratio for diagnosing type II diabetes. Eur J Clin Nutr. 2010;64:23–9. doi: 10.1038/ejcn.2009.92. [DOI] [PubMed] [Google Scholar]
- 13.Lear SA, James PT, Ko GT, Kumanyika S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur J Clin Nutr. 2010;64:42–61. doi: 10.1038/ejcn.2009.70. [DOI] [PubMed] [Google Scholar]
- 14.O’Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: The razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50:1009–14. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics–2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC). State- Specific Trends in Fruit and Vegetable Consumption Among Adults — United States, 2000–2009. MMWR Morb Mortal Wkly Rep. 2010;59:1125–30. [PubMed] [Google Scholar]
- 17.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 18.Erhardt L. Cigarette smoking: An undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205:23–32. doi: 10.1016/j.atherosclerosis.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 19.White WB. Smoking-related morbidity and mortality in the cardiovascular setting. Prev Cardiol. 2007;10:1–4. doi: 10.1111/j.1520-037x.2007.06050.x. [DOI] [PubMed] [Google Scholar]
- 20.National Aboriginal and Torres Strait Islander health survey, 2004-05. Canberra: Australian Bureau of Statistics (ABS); 2006. Australian Bureau of Statistics (ABS) [Google Scholar]
- 21.A potrait of health: Key results of the 2006-07 New Zealand health survey. Wellington: Ministry of Health; 2008. Ministry of Health. [Google Scholar]
- 22.Organization WH. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. (1-253).World Health Organ Tech Rep Ser. 2000;894:i–xii. [PubMed] [Google Scholar]
- 23.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies. Lancet. 2006;368:666–78. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 24.Esmaillzadeh A, Mirmiran P, Azizi F. Waist-to-hip ratio is a better screening measure for cardiovascular risk factors than other anthropometric indicators in Tehranian adult men. Int J Obes Relat Metab Disord. 2004;28:1325–32. doi: 10.1038/sj.ijo.0802757. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Rowley K, Piers L, O’Dea K. Anthropometric indices and their relationship with diabetes, hypertension and dyslipidemia in Australian Aboriginal people and Torres Strait Islanders. Eur J Cardiovasc Prev Rehabil. 2007;14:172–8. doi: 10.1097/01.hjr.0000220580.34763.fb. [DOI] [PubMed] [Google Scholar]
- 26.Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr Res Rev. 2010;23:247–69. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- 27.Lear SA, Humphries KH, Frohlich JJ, Birmingham CL. Appropriateness of current thresholds for obesity-related measures among Aboriginal people. CMAJ. 2007;177:1499–505. doi: 10.1503/cmaj.070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, McDermott RA. Using anthropometric indices to predict cardiometabolic risk factors in Australian indigenous populations. Diabetes Res Clin Pract. 2010;87:401–6. doi: 10.1016/j.diabres.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Cepeda-Valery B, Pressman GS, Figueredo VM, Romero-Corral A. Impact of obesity on total and cardiovascular mortality–fat or fiction? Nat Rev Cardiol. 2011;8:233–7. doi: 10.1038/nrcardio.2010.209. [DOI] [PubMed] [Google Scholar]
- 30.Part 1: diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. [Google Scholar]
- 31.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 32.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: A systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–7. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 33.Mellbin LG, Anselmino M, Ryden L. Diabetes, prediabetes and cardiovascular risk. Eur J Cardiovasc Prev Rehabil. 2010;17(Suppl 1):S9–14. doi: 10.1097/01.hjr.0000368192.24732.2f. [DOI] [PubMed] [Google Scholar]
- 34.Fox CS. Cardiovascular disease risk factors, type 2 diabetes mellitus, and the Framingham Heart Study. Trends Cardiovasc Med. 2010;20:90–5. doi: 10.1016/j.tcm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preis SR, Pencina MJ, Hwang SJ, D’Agostino RB, Sr, Savage PJ, Levy D. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation. 2009;120:212–20. doi: 10.1161/CIRCULATIONAHA.108.846519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajpathak SN, Aggarwal V, Hu FB. Multifactorial intervention to reduce cardiovascular events in type 2 diabetes. Curr Diab Rep. 2010;10:16–23. doi: 10.1007/s11892-009-0084-8. [DOI] [PubMed] [Google Scholar]
- 37.Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: A meta-analysis. Diabetes Care. 2011;34:1228–37. doi: 10.2337/dc10-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar MR, Carbajal HA, Espeche WG, Dulbecco CA, Aizpurua M, Marillet AG, et al. Relationships among insulin resistance, obesity, diagnosis of the metabolic syndrome and cardiometabolic risk. Diab Vasc Dis Res. 2011;8:109–16. doi: 10.1177/1479164111403170. [DOI] [PubMed] [Google Scholar]
- 39.Reaven GM. Relationships among insulin resistance, type 2 diabetes, essential hypertension, and cardiovascular disease: Similarities and differences. J Clin Hypertens (Greenwich) 2011;13:238–43. doi: 10.1111/j.1751-7176.2011.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowden DW, Cox AJ, Freedman BI, Hugenschimdt CE, Wagenknecht LE, Herrington D, et al. Review of the Diabetes Heart Study (DHS) family of studies: A comprehensively examined sample for genetic and epidemiological studies of type 2 diabetes and its complications. Rev Diabet Stud. 2010;7:188–201. doi: 10.1900/RDS.2010.7.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones PH. Low-density lipoprotein cholesterol reduction and cardiovascular disease prevention: the search for superior treatment. Am J Med. 2004;116(Suppl 6A):17S–25S. doi: 10.1016/j.amjmed.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C. Metabolic syndrome, low-density lipoprotein cholesterol, and risk of cardiovascular disease: A population-based study. Atherosclerosis. 2006;189:369–74. doi: 10.1016/j.atherosclerosis.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Rosenson RS. Low high-density lipoprotein cholesterol and cardiovascular disease: Risk reduction with statin therapy. Am Heart J. 2006;151:556–63. doi: 10.1016/j.ahj.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 44.Karalis DG. Intensive lowering of low-density lipoprotein cholesterol levels for primary prevention of coronary artery disease. Mayo Clin Proc. 2009;84:345–52. doi: 10.1016/S0025-6196(11)60544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teramoto T, Nakaya N, Yokoyama S, Ohashi Y, Mizuno K, Nakamura H. Association between lowering low-density lipoprotein cholesterol with pravastatin and primary prevention of cardiovascular disease in mild to moderate hypercholesterolemic Japanese. J Atheroscler Thromb. 2010;17:879–87. doi: 10.5551/jat.4176. [DOI] [PubMed] [Google Scholar]
- 46.de Freitas EV, Brandao AA, Pozzan R, Magalhaes ME, Fonseca F, Pizzi O, et al. Importance of high-density lipoprotein-cholesterol (HDL-C) levels to the incidence of cardiovascular disease (CVD) in the elderly. Arch Gerontol Geriatr. 2011;52:217–22. doi: 10.1016/j.archger.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Windler E, Schoffauer M, Zyriax BC. The significance of low HDL-cholesterol levels in an ageing society at increased risk for cardiovascular disease. Diab Vasc Dis Res. 2007;4:136–42. doi: 10.3132/dvdr.2007.032. [DOI] [PubMed] [Google Scholar]
- 48.Davidson MH. Targeting high-density lipoprotein cholesterol in the management of cardiovascular disease. Am Heart Hosp J. 2007;5:210–6. doi: 10.1111/j.1541-9215.2007.07423.x. [DOI] [PubMed] [Google Scholar]
- 49.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: Adjustments and options. Am J Cardiol. 2005;96:53E–9E. doi: 10.1016/j.amjcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 52.Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: Life course analysis. Hypertension. 2005;46:280–6. doi: 10.1161/01.HYP.0000173433.67426.9b. [DOI] [PubMed] [Google Scholar]
- 53.Alcohol use in New Zealand: Key results of the 2007/08 New Zealand alcohol and drug use survey. Wellington: Ministry of Health; 2009. Ministry of Health. [Google Scholar]
- 54.Substance Abuse and Mental Health Services Administration (SAMHSA) 2007 National Survey on Drug Use and Health, detailed tables, dependence, abuse and treatment. 2008. [Last cited on 2011 Sept 26]. Available from: http://www.oas.samhsa.gov/NSDUH/2k7NSDUH/tabs/Sect7peTabs59to115.htm .
- 55.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: A meta-analysis. Addiction. 2000;95:1505–23. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- 57.Lucas DL, Brown RA, Wassef M, Giles TD. Alcohol and the cardiovascular system: Research challenges and opportunities. J Am Coll Cardiol. 2005;45:1916–24. doi: 10.1016/j.jacc.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 58.Holm JE, Vogeltanz-Holm N, Poltavski D, McDonald L. Assessing health status, behavioral risks, and health disparities in American Indians living on the northern plains of the US Public Health Rep. 2010;125:68–78. doi: 10.1177/003335491012500110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Meta-analysis of cohort studies. J Hum Hypertens. 2007;21:717–28. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- 60.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J Nutr. 2006;136:2588–93. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 61.Dauchet L, Amouyel P, Dallongeville J. Fruit and vegetable consumption and risk of stroke: a meta-analysis of cohort studies. Neurology. 2005;65:1193–7. doi: 10.1212/01.wnl.0000180600.09719.53. [DOI] [PubMed] [Google Scholar]
- 62.Flood A, Subar AF, Hull SG, Zimmerman TP, Jenkins DJ, Schatzkin A. Methodology for adding glycemic load values to the National Cancer Institute Diet History Questionnaire database. J Am Diet Assoc. 2006;106:393–402. doi: 10.1016/j.jada.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Millen AE, Midthune D, Thompson FE, Kipnis V, Subar AF. The National Cancer Institute diet history questionnaire: Validation of pyramid food servings. Am J Epidemiol. 2006;163:279–88. doi: 10.1093/aje/kwj031. [DOI] [PubMed] [Google Scholar]
- 64.Subar AF. Developing dietary assessment tools. J Am Diet Assoc. 2004;104:769–70. doi: 10.1016/j.jada.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, et al. Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson Heart Study. J Am Diet Assoc. 2009;109:1184–93. doi: 10.1016/j.jada.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kushi LH. Gaps in epidemiologic research methods: Design considerations for studies that use food-frequency questionnaires. Am J Clin Nutr. 1994;59(1 Suppl):180S–4S. doi: 10.1093/ajcn/59.1.180S. [DOI] [PubMed] [Google Scholar]
- 67.Pleis JR, Lucas JW. Summary Health Statistics for US Adults: National Health Interview Survey, 2007.National Center for Health Statistics. Vital Health Stat 10. 2009;240:1–159. [PubMed] [Google Scholar]
- 68.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 69.Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: A meta-analysis. Circulation. 2011;124:789–95. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mansikkaniemi K, Juonala M, Taimela S, Hirvensalo M, Telama R, Huupponen R, et al. Cross-sectional associations between physical activity and selected coronary heart disease risk factors in young adults. The Cardiovascular Risk in Young Finns Study. Ann Med. 2011 doi: 10.3109/07853890.2011.590146. [In press] [DOI] [PubMed] [Google Scholar]
- 71.Moholdt T, Wisloff U, Nilsen TI, Slordahl SA. Physical activity and mortality in men and women with coronary heart disease: A prospective population-based cohort study in Norway (the HUNT study) Eur J Cardiovasc Prev Rehabil. 2008;15:639–45. doi: 10.1097/HJR.0b013e3283101671. [DOI] [PubMed] [Google Scholar]
- 72.Church T. Exercise in obesity, metabolic syndrome, and diabetes. Prog Cardiovasc Dis. 2011;53:412–8. doi: 10.1016/j.pcad.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med. 2004;34:371–418. doi: 10.2165/00007256-200434060-00004. [DOI] [PubMed] [Google Scholar]
- 74.Long AN, Dagogo-Jack S. Comorbidities of diabetes and hypertension: Mechanisms and approach to target organ protection. J Clin Hypertens (Greenwich) 2011;13:244–51. doi: 10.1111/j.1751-7176.2011.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dollman J, Okely AD, Hardy L, Timperio A, Salmon J, Hills AP. A hitchhiker's guide to assessing young people's physical activity: Deciding what method to use. J Sci Med Sport. 2009;12:518–25. doi: 10.1016/j.jsams.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 76.Alomari MA, Keewan EF, Qhatan R, Amer A, Khabour OF, Maayah MF, et al. Blood pressure and circulatory relationships with physical activity level in young normotensive individuals: IPAQ validity and reliability considerations. Clin Exp Hypertens. 2011;33:345–53. doi: 10.3109/10641963.2010.531848. [DOI] [PubMed] [Google Scholar]
- 77.Bauman A, Ainsworth BE, Sallis JF, Hagstromer M, Craig CL, Bull FC, et al. The descriptive epidemiology of sitting a 20-country comparison using the international physical activity questionnaire (IPAQ) Am J Prev Med. 2011;41:228–35. doi: 10.1016/j.amepre.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 78.Schembre SM, Riebe DA. Non-exercise estimation of VO(2)max using the International Physical Activity Questionnaire. Meas Phys Educ Exerc Sci. 2011;15:168–81. doi: 10.1080/1091367X.2011.568369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tomioka K, Iwamoto J, Saeki K, Okamoto N. Reliability and Validity of the International Physical Activity Questionnaire (IPAQ) in Elderly Adults: The Fujiwara-kyo Study. J Epidemiol. 2011;21:459–65. doi: 10.2188/jea.JE20110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 81.Ottevaere C, Huybrechts I, De Bourdeaudhuij I, Sjostrom M, Ruiz JR, Ortega FB, et al. Comparison of the IPAQ-A and actigraph in relation to VO2max among European adolescents: the HELENA study. J Sci Med Sport. 2011;14:317–24. doi: 10.1016/j.jsams.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 82.Rangul V, Holmen TL, Kurtze N, Cuypers K, Midthjell K. Reliability and validity of two frequently used self-administered physical activity questionnaires in adolescents. BMC Med Res Methodol. 2008;8:47. doi: 10.1186/1471-2288-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hagstromer M, Bergman P, De Bourdeaudhuij I, Ortega FB, Ruiz JR, Manios Y, et al. Concurrent validity of a modified version of the International Physical Activity Questionnaire (IPAQ-A) in European adolescents: The HELENA Study. Int J Obes (Lond) 2008;32(Suppl 5):S42–8. doi: 10.1038/ijo.2008.182. [DOI] [PubMed] [Google Scholar]
- 84.Ottevaere C, Huybrechts I, De Meester F, De Bourdeaudhuij I, Cuenca-Garcia M, De Henauw S. The use of accelerometry in adolescents and its implementation with non-wear time activity diaries in free-living conditions. J Sports Sci. 2011;29:103–13. doi: 10.1080/02640414.2010.521169. [DOI] [PubMed] [Google Scholar]
- 85.Pleis JR, Lucas JW, Ward BW. Summary health statistics for US adults: National Health Interview Survey, 2008. Vital Health Stat 10. 2009;(242):1–157. [PubMed] [Google Scholar]
- 86.Willett WC, Green A, Stampfer MJ, Speizer FE, Colditz GA, Rosner B, et al. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med. 1987;317:1303–9. doi: 10.1056/NEJM198711193172102. [DOI] [PubMed] [Google Scholar]
- 87.Mamun AA, Peeters A, Barendregt J, Willekens F, Nusselder W, Bonneux L. Smoking decreases the duration of life lived with and without cardiovascular disease: A life course analysis of the Framingham Heart Study. Eur Heart J. 2004;25:409–15. doi: 10.1016/j.ehj.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 88.Conen D, Everett BM, Kurth T, Creager MA, Buring JE, Ridker PM, et al. Smoking, smoking status, and risk for symptomatic peripheral artery disease in women: A cohort study. Ann Intern Med. 2011;154:719–26. doi: 10.1059/0003-4819-154-11-201106070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20:344–53. doi: 10.1053/euhj.1998.1194. [DOI] [PubMed] [Google Scholar]
- 90.Lee YH, Shin MH, Kweon SS, Choi JS, Rhee JA, Ahn HR, et al. Cumulative smoking exposure, duration of smoking cessation, and peripheral arterial disease in middle-aged and older Korean men. BMC Public Health. 2011;11:94. doi: 10.1186/1471-2458-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang LR, Wong ND, Shi P, Zhao LC, Wu LX, Xie GQ, et al. Cross-sectional and longitudinal association of cigarette smoking with carotid atherosclerosis in Chinese adults. Prev Med. 2009;49:62–7. doi: 10.1016/j.ypmed.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 92.Tomiyama H, Hashimoto H, Tanaka H, Matsumoto C, Odaira M, Yamada J, et al. Continuous smoking and progression of arterial stiffening: A prospective study. J Am Coll Cardiol. 2010;55:1979–87. doi: 10.1016/j.jacc.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 93.Stoner L, Sabatier MJ, Black CD, McCully KK. Occasional cigarette smoking chronically affects arterial function. Ultrasound Med Biol. 2008;34:1885–92. doi: 10.1016/j.ultrasmedbio.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 94.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J Am Coll Cardiol. 2004;43:1731–7. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 95.Stoner L, Sabatier M, Edge K, McCully K. Relationship between blood velocity and conduit artery diameter and the effects of smoking on vascular responsiveness. J Appl Physiol. 2004;96:2139–45. doi: 10.1152/japplphysiol.01107.2003. [DOI] [PubMed] [Google Scholar]
- 96.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 97.Quyyumi AA. Prognostic value of endothelial function. Am J Cardiol. 2003;91:19H–24H. doi: 10.1016/s0002-9149(03)00430-2. [DOI] [PubMed] [Google Scholar]
- 98.Cohn JN. Vascular wall function as a risk marker for cardiovascular disease. J Hypertens Suppl. 1999;17:S41–4. [PubMed] [Google Scholar]
- 99.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–8. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 100.Nissen SE, Yock P. Intravascular Ultrasound: Novel Pathophysiological Insights and Current Clinical Applications. Circulation. 2001;103:604–16. doi: 10.1161/01.cir.103.4.604. [DOI] [PubMed] [Google Scholar]
- 101.Ross R. Atherosclerosis – An Inflammatory Disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 102.Luscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20:3–10. [PubMed] [Google Scholar]
- 103.Boger RH, Bode-Boger SM, Tsao PS, Lin PS, Chan JR, Cooke JP. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J Am Coll Cardiol. 2000;36:2287–95. doi: 10.1016/s0735-1097(00)01013-5. [DOI] [PubMed] [Google Scholar]
- 104.Landim MB, Casella Filho A, Chagas AC. Asymmetric dimethylarginine (ADMA) and endothelial dysfunction: Implications for atherogenesis. Clinics (Sao Paulo) 2009;64:471–8. doi: 10.1590/S1807-59322009000500015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boger RH, Maas R, Schulze F, Schwedhelm E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality–an update on patient populations with a wide range of cardiovascular risk. Pharmacol Res. 2009;60:481–7. doi: 10.1016/j.phrs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 106.Anderssohn M, Schwedhelm E, Lüneburg N, Vasan RS, Boger RH. Asymmetric dimethylarginine as a mediator of vascular dysfunction and a marker of cardiovascular disease and mortality: an intriguing interaction with diabetes mellitus. Diab Vasc Dis Res. 2010;7:105–18. doi: 10.1177/1479164110366053. [DOI] [PubMed] [Google Scholar]
- 107.Szuba A, Podgorski M. Asymmetric dimethylarginine (ADMA) a novel cardiovascular risk factor–evidence from epidemiological and prospective clinical trials. Pharmacol Rep. 2006;58(Suppl):16–20. [PubMed] [Google Scholar]
- 108.Haberka M, Mizia-Stec K, Gasior Z, Mizia M, Janowska J, Holecki M, et al. Serum ADMA concentration–an independent factor determining FMD impairment in cardiac syndrome X. Ups J Med Sci. 2009;114:221–7. doi: 10.3109/03009730903225537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Irace C, Ceravolo R, Notarangelo L, Crescenzo A, Ventura G, Tamburrini O, et al. Comparison of endothelial function evaluated by strain gauge plethysmography and brachial artery ultrasound. Atherosclerosis. 2001;158:53–9. doi: 10.1016/s0021-9150(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 110.Walker HA, Jackson G, Ritter JM, Chowienczyk PJ. Assessment of forearm vasodilator responses to acetylcholine and albuterol by strain gauge plethysmography: Reproducibility and influence of strain gauge placement. Br J Clin Pharmacol. 2001;51:225–9. doi: 10.1046/j.1365-2125.2001.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alomari MA, Solomito A, Reyes R, Khalil SM, Wood RH, Welsch MA. Measurements of vascular function using strain-gauge plethysmography: Technical considerations, standardization, and physiological findings. Am J Physiol Heart Circ Physiol. 2004;286:H99–107. doi: 10.1152/ajpheart.00529.2003. [DOI] [PubMed] [Google Scholar]
- 112.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: Methodology and clinical applications. Br J Clin Pharmacol. 2001;52:631–46. doi: 10.1046/j.1365-2125.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hayward CS, Kraidly M, Webb CM, Collins P. Assessment of endothelial function using peripheral waveform analysis: a clinical application. J Am Coll Cardiol. 2002;40:521–8. doi: 10.1016/s0735-1097(02)01991-5. [DOI] [PubMed] [Google Scholar]
- 114.Wilkinson IB, Hall IR, MacCallum H, Mackenzie IS, McEniery CM, van der Arend BJ, et al. Pulse-wave analysis: Clinical evaluation of a noninvasive, widely applicable method for assessing endothelial function. Arterioscler Thromb Vasc Biol. 2002;22:147–52. doi: 10.1161/hq0102.101770. [DOI] [PubMed] [Google Scholar]
- 115.Chowienczyk PJ, Kelly RP, MacCallum H, Millasseau SC, Andersson TL, Gosling RG, et al. Photoplethysmographic assessment of pulse wave reflection: Blunted response to endothelium-dependent beta2-adrenergic vasodilation in type II diabetes mellitus. J Am Coll Cardiol. 1999;34:2007–14. doi: 10.1016/s0735-1097(99)00441-6. [DOI] [PubMed] [Google Scholar]
- 116.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 117.Stoner L, Sabatier MJ. In: Assessments of endothelial function using ultrasound. Ainslie P, editor. Ultrasound. Rijeka, Croatia: Intech; 2012. [Google Scholar]
- 118.Schroeder S, Enderle MD, Ossen R, Meisner C, Baumbach A, Pfohl M, et al. Noninvasive determination of endothelium-mediated vasodilation as a screening test for coronary artery disease: pilot study to assess the predictive value in comparison with angina pectoris, exercise electrocardiography, and myocardial perfusion imaging. Am Heart J. 1999;138:731–9. doi: 10.1016/s0002-8703(99)70189-4. [DOI] [PubMed] [Google Scholar]
- 119.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 120.Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–10. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 121.Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest. 1994;93:50–5. doi: 10.1172/JCI116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peretz A, Leotta DF, Sullivan JH, Trenga CA, Sands FN, Aulet MR, et al. Flow mediated dilation of the brachial artery: An investigation of methods requiring further standardization. BMC Cardiovasc Disord. 2007;7:11. doi: 10.1186/1471-2261-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 124.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–85. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stoner L, Sabatier MJ. Use of ultrasound for non-invasive assessment of flow-mediated dilation. J Atheroscler Thromb. 2012 Mar 1; doi: 10.5551/jat.11395. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 127.Stephens C, Porter J, Nettleton C, Willis R. Disappearing, displaced, and undervalued: a call to action for Indigenous health worldwide. Lancet. 2006;367:2019–28. doi: 10.1016/S0140-6736(06)68892-2. [DOI] [PubMed] [Google Scholar]
- 128.King M, Smith A, Gracey M. Indigenous health part 2: The underlying causes of the health gap. Lancet. 2009;374:76–85. doi: 10.1016/S0140-6736(09)60827-8. [DOI] [PubMed] [Google Scholar]
- 129.Paul CL, Sanson-Fisher R, Stewart J, Anderson AE. Being sorry is not enough: the sorry state of the evidence base for improving the health of indigenous populations. Am J Prev Med. 2010;38:566–8. doi: 10.1016/j.amepre.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 130.Cochrane Effective Practice and Organisation of Care Review Group. Data collection checklist. [Last cited on 2011 Sept 19]. Available from: http://www.epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/datacollectionchecklist.pdf .
- 131.A guide to the development, implementation and evaluation of clinical practice guidelines, 1999. Canberra: National Health and Medical Research Council (NHMRC); 1999. National Health and Medical Research Council (NHMRC) [Google Scholar]


