Abstract
Background:
Fascioliasis is one of the most common zoonotic diseases in Iran and other parts of the world. Although the largest epidemic of this disease has occurred in northern provinces of Iran (Guilan) during the past two decades and a few cases have also been reported in Tehran and the other provinces, there has been no evidence of its’ occurrence in western provinces of Iran such as Kermanshah before the outbreak which is being reported.
Methods:
The study was conducted by teamwork of infectious disease specialist, parasitologist, general practitioner, entomologist, and laboratory technician. It is an “epidemic investigation” and a cross sectional descriptive one. Clinical data and para-clinical changes are recorded considering all of the population of a village in Kangavar, one of the suburbs of Kermanshah (western Iran).
Results:
The mean age was 21.65, (SD=12.44). Fifty three percent were female, and all of them were farmers. Eighty two percent had a history of watercress ingestion in a period of 1-2 months before the admission and 18% consumed other vegetables. The average of eosinophils was 32.35% (SD=26). The patients’ Enzyme Linked Immunosorbent Assay (ELISA) and Counter Current Immuno-electrophoresis (CCIE) serological tests were reported positive by the department of parasitology, Pasteur Institute of Iran. Treatment response to triclabendazole was excellent. A ten-year clinical and laboratory follow-up revealed no evidence of abnormality in treated patients.
Conclusions:
It was the first case of human fascioliasis in west Iran and was a real epidemic and an emerging infectious disease for this area at that time. The clinical symptoms were less severe compared with other reports. Health education to inhabitants and health care workers can lead to rapid detection of such outbreaks.
Keywords: Epidemic, eosinophilia, fascioliasis, new-emerging, watercress
INTRODUCTION
Infection with the sheep liver fluke Fasciola hepatica results from ingesting uncooked watercress or other fresh aquatic vegetables in many countries worldwide, especially in sheep-and cattle-raising areas. The global prevalence of human infection is in excess of three million with the highest rates of infection in Bolivia, Peru, Egypt, Iran, Portugal, and France. Mature worms in their natural hosts live in the common and hepatic bile ducts where they deposit their eggs.[1]
Many human and animal cases have been reported all over Iran.[2] It is present in many Iranian provinces: Kurdistan, Zandjan, Mazandaran, Tehran, Azarbaijan, and Guilan. Human cases have been reported throughout the country over a long period.[3] It has occurred several epidemics in the North of the country. Even in the epidemic of 1988 and 1989, more than ten thousand persons were infected.[4]
After shedding by snail, the infective stage of the parasite or cercariae attaches themselves to various species of aquatic plants and transform into metacercariae. Metacercariae remain on the surface of aquatic plants and grasses and when they are ingested by the final host, bore their way through the wall of the small intestine and migrate into the body cavity in which they seek out the liver and bore through its capsule and reach the bile ducts by passing through the parenchyma of the liver. The eggs reach the intestine via the bile. They can therefore be identified in the stool.[5]
The natural source of the parasite is sheep and cow. By eating the aquatic grasses which are contaminated by metacercariae, man gets infected. The infection can also be acquired by drinking water contaminated with metacercariae. Cattle, sheep, and other domesticated herbivores are the definitive hosts and reservoirs.[6]
The infection is not communicable directly from person to person. It is worthy to note that man irrespective of his age is susceptible to the disease. The period of the disease is undetermined.[2] Two types of the parasite, Fasciola hepatica and Fasciola gigantica are known in Iran. The difference in the appearance of the two parasites is trivial, but Fasciola gigantica is more dangerous than Fasciola hepatica. The most sensitive host for Fasciola hepatica is lymnaea and for Fasciola gigantica is known to be lymnaea Bergravicularia in Iran.[7]
The objectives of this report were to analyze the clinical manifestations and laboratory changes of human fascioliasis with special emphasis on investigation of the first epidemic of human fascioliasis in Kermanshah (western Iran).
METHODS
This report is an epidemic investigation and a cross sectional research based on the principles of epidemiology and statistics. It had happened during the end of the winter, 1998 and the beginning of the following spring, 1999. The whole population of a village in Kangavar, one of the suburbs of Kermanshah (western Iran), was examined. Our investigating team of this study recorded observations, clinical data, and laboratory changes.
RESULTS
The report of the epidemic
Despite the evidence of the disease in some areas such as Guilan Province at northern Iran and other areas, not even a single human case has been reported by serological or by parasitological tests in western areas of our country in the past years. The first diagnosed cases which were admitted to Sina hospital in Kermanshah, came from a family with two children - a sister and a brother 14 and 16 years old, respectively, from a village called “Cheshmeh Derazeh”. They had symptoms such as fever, weakness, fatigue, and anorexia for a month. Laboratory findings showed the leukocytosis of more than 15000 cells/mm[3] and the eosinophilia of about 60%. According to the previous concepts, the disease was considered to be toxocariasis and then thiabendazole was prescribed. At the same time, their serum samples were analyzed by Indirect Fluorescent Antibody test (IFA). Test results of the subjects were reported to be positive for toxocariasis. Not only there was no recovery, but also their general conditions, especially the digestive symptoms such as abdominal pain got worse gradually. Although, respiratory symptoms were more considerable than gastrointestinal symptoms at first, we were misleaded to diagnose the condition as toxocariasis. The severity of digestive symptoms encouraged guesses about fascioliasis, despite the fact that there has never been reported in west of the country. Thus the IFA, enzyme linked immunosorbent assay (ELISA) and Counter Current Immuno-electrophoresis (CCIE) tests were applied for the two patients and other six members of the family–who have shown the clinical symptoms and some abnormal signs and symptoms with milder intensity and had also leukocytosis and eosinophilia.
The results were positive as expected. After admission of all the family members (patients’) in the hospital and administering triclabendazole, all of them showed clinical recovery within a week. No signs of anemia up to a month and a considerable recovery of anemia within two months were observed. Since the consumption of watercress was mentioned by all of the family members from the mid-winter, state of epidemic was declared and very soon, preventive actions which will briefly be referred to were taken not only in that village, but also in many other cities such as Kermanshah, Harsin, Sahneh, Songhor, Javanroud, Paveh, and Guilan-Gharb, [Figure 1] in which a few more cases reported after our declaration. With the cooperation of the Health center of the city, the chief organization of veterinary department of the Kermanshah, Pasteur Institute of Iran, and the Health Faculty of Tehran University, the following events took place:
Figure 1.
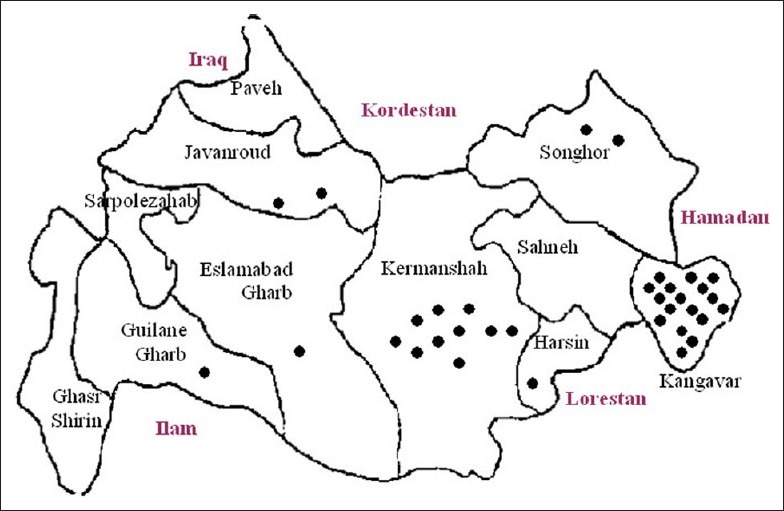
Distribution of human fascioliasis in Kangavar (N=17) and other areas o Kermanshah province (N=17), Iran, Spring 1999
Verifying the diagnosis
The serum samples which were sent to Pasteur Institute and of Health at Tehran University approved the existence of anti–Fasciola hepatica antibodies by means of IFA, ELISA, and CCIE tests. It's worth noticing that diagnosis of the disease in this stage is only possible through serological tests. In other words, the serological tests are useful during acute infection because symptoms develop one to two months before eggs are detectable in the stool,[1] but specific antibodies to Fasciola may be detectable within two to four weeks after infection which is five to seven weeks before eggs appear in stool.[8] So the first cases were recognized when it was not expected that the stool analysis being positive for eggs. We also faced with a case that was under surgery because of the initial diagnosis of cholecystitis, but a lot of Fasciola hepatica parasite was found in his gall bladder.
Establishing the existence of epidemics
Regarding the fact that human fascioliasis, had not been reported, in Kermanshah and also in other parts of western areas of Iran, until 1998, occurrence of only a single case should have been considered as an epidemic and also a new-emerging infectious disease in this area.
The susceptible population
All of the population of “Cheshmeh Derazeh”, a village in Kangavar, in Kermanshah consists of more than one hundred persons.
Medical surveys
All of the people in the village were interviewed and checked by medical and epidemiological observations. The samples of their blood and stool were collected to examine for the parasitological and serological purposes.
Epidemiological surveys
The clinical and serological survey of all of the population detected 17 positive cases by ELISA and CCIE tests out of one hundred persons. In addition to the former 17 cases in other areas of the Kermanshah province in 1998, another 17 cases were identified.
Evaluation of ecological elements
We consulted with rural officials about the sanitation of fountain water.
Excrement of the herbivorous animals of the village was tested parasitologically. One percent of contamination by the eggs of Fasciola was reported.
A serum sample of 500 sheep was tested by CCIE and 1.5 percent of contamination was reported.
Study of at risk populations and reservoirs
Medical examination, as mentioned before, this examination was directed to discover new cases in addition to former ones.
Serologic tests
They showed 17 positive cases in villages mentioned above and another 17 cases in other areas of Kermanshah province.
Stool examination
This was directed and showed no egg of Fasciola hepatica, but in 16 cases showed giardia cysts and eggs of Tenia saginata in two cases.
Study of the animals of the region
Ten percent of 500 cattle and sheep were randomly selected in the mentioned village. Stool analyses proved that 1% of the cases were positive. CCIE test of the animal samples analyzed in the Pasteur institute revealed 1.5% positive.
Study of the plants of the region
The samples that were sent to the Pasteur Institute were contaminated.
Study of the snails of the region
Samples of the snails were gathered and studied by experts and were reported to be of lymnaeidae type.
Informing the susceptible people
Urban and rural citizens of the region were informed about the disease by the local media and press. People were trained how to prevent the infection. And the associated organizations were informed about the epidemic.
Data analysis
Place
“Cheshmeh Derazeh”, a suburb village of Kangavar (western Iran).
Population
All of the people at the mentioned village.
Attack rate
17 percent.
Case fatality rate
Zero percent.
Frequency of the cases with and without clinical symptoms
15 patients (88%) with and 2 patients (12%) without clinical symptoms.
Complications
The population of the village were re-examined after one year and no sign and symptom was found.
Probable ways of spreading
Contaminated plants of the region.
Ecological causes of the epidemic
Traditional agricultural practices.
Long-term follow-ups
A ten-year clinical and laboratory follow-up (once a year) revealed no evidence of re-infection, no gastrointestinal discomfort, no eosinophilia, and no abnormality of liver function tests in treated patients, and no another outbreak in the area influenced by continuous health education in the area and screening and mass therapy of animal reservoir by triclabendazole.
Clinical and laboratory results
The youngest patient was a 4-year-old girl and the oldest one was a 48-year-old man [Figure 2]. The mean of age was 21.65 years (SD=12.44). Forty seven percent of them were male and reminders 53% were female. All the patients’ were diagnosed and admitted at spring were occupied with traditional farming and animal husbandry. Watercress was consumed by 82% of them during the winter, the remaining 18% had reported to consume un-disinfected local vegetables and unpurified water.
Figure 2.
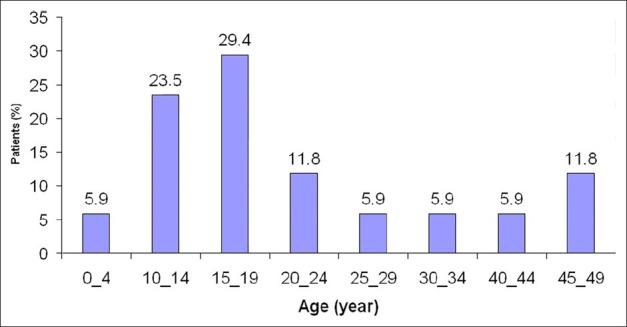
Outbreak of human fascioliasis by patients’ age (n=17) Kangavar, Kermanshah province (western Iran) Spring 1999
The major complaints of the patients consists of weight loss (47%), epigastric pain (41%), abdominal pain (29%), right upper quadrant pain (24%), fever, chills, headache, anorexia and chest pain (18%), low back pain, coughing and itching (12%), myalgia, neck pain, dyspnea, and urticaria (6%) [Figure 3]. Furthermore, clinical findings consisted of tachypnea (65%), hepato-splenomegalia (29%), right hypochondria tenderness and sweating (24%), and tachycardia (18%).
Figure 3.
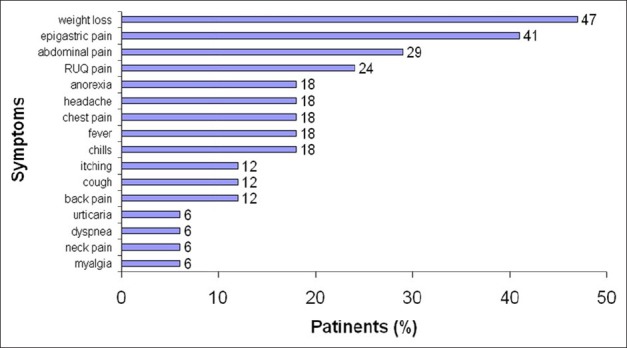
Outbreak of human fascioliasis, by symptoms (N=17) Kangavar, Kermanshah province (western Iran) Spring 1999
Abdominal sonography of seven patients showed mild ascites in four cases, mild to moderate splenomegaly in four cases, hepatomegaly with heterogeneous echo of the tissue similar to infiltration of fatty liver in two out of the five cases, diameter of the geographic hypoecho was between 1 and 1.5 cm, lymph node was larger than 1-2 cm in porta hepatis area in one case, and in another case, There were some stones in the gallbladder, which had caused distention and also a dilatation in choleduch by 7-8 mm diameter. No abnormality was found in other intra-abdominal organs in any of the cases during sonography. Subsequent sonographic evaluation reported no effect of the disease and all the patients felt quite well (till the end of 2009).
The white blood cells count indicates 6% of leukopenia, 38% leukocytosis, and 56% of normal condition. Almost half of the patients had lymphocytosis, but the number of white blood cells became quite normal, 10 months after the therapy. More than 88% of the patients had eosinophilia. Almost 42% of the patients had shown eosinophilia which was more than 30%, but it became normal again only among half of them, 10 months after the therapy. Forty one percent of the patients dealt with decreased hemoglobin concentration, which rose to normal level after 10 months. Patient's Erythrocyte Sedimentation Rate (ESR) in 44% of the cases was normal, and in 56% of the cases, it indicated a slight up to a mild rise. Furthermore, 6% of them showed high ESR which returned to normal range after 10 months.
The result of ELISA and CCIE tests for all of the cases and half of the cases IFA tests were positive. Response to triclabendzole was accompanied with feeling well within five days showing no sign of anemia within two months and severe reduction of eosinophilia within two months.
DISCUSSION
It is the first cluster of human fascioliasis which has happened in west Iran and was a real epidemic and an emerging infectious disease for this area at that time. About 59% of the patients were younger than 20 years old; from this respect, the epidemic is similar to the one occurred in the Guilan province, 1988, in which contamination rate for the age category below 20 years old was higher than other age categories. The relationship between the prevalence of fascioliasis and age differs in human endemic and non-endemic areas. In high prevalence areas, children under 15 years of age usually present the highest rates, in contrast to the current situation in human non-endemic areas.[9] So the age distribution of patients in Guilan province is not surprising in this respect, but cannot be attributed to our study.
Although there is no evidence advocating sex effect on the disease severity, 53% of the patients were female and 47% male, in comparison with Guilan epidemics, in which 78% of the patients were women (P=0.019).[10,11] It might be due to the consumption of more vegetables by females in the Guilan province. All of the patients were diagnosed and admitted to hospital during spring which is another similarity to the Guilan's epidemic.[10] The reason could be due to delay time between consumption of the plants in winter and its relevant manifestations at the next spring.[2]
The symptoms of the disease in patients from the Kermanshah were milder than the ones from the Guilan and other regions.[12] As this disease is directly influenced by parasite's load, we can conclude that the contamination was lower in Kermanshah. Thirty eight percent of our patients had leukocytosis in comparison with Guilanian ones, which was 56% (P=0.08). Eosinophilia more than 30% which was reported in 80% of persons during Guilan epidemic was presented for only 42% of patients in Kermanshah (P=0. 001).
The rise of patient's ESR in Kermanshah was slower than Guilan. Furthermore, despite the reports indicated rises of transaminases in experimental surveys,[13] no such rises was found in Kermanshah.
As it was mentioned before, in our patients who were in acute stage of fascioliasis, the diagnosis was based on the results of ELISA and CCIE tests. We failed to detect the parasite's eggs from the patient's stool which was not unexpected because the outbreak had happened too quickly, and in fact, was recognized when the parasites were migrating to the liver, but had not oviposited yet.[14,15]
Furthermore, as the symptoms were not severe, the number of the parasites must not have been too many. Most of the reliable references have not mentioned finding the parasites’ eggs necessary for diagnosis; instead, serological tests were required. For example, “during acute phase in which the parasite is migrating to the liver, the patients symptoms consist of fever, eosinophilia, and painful hepatomegaly. Even when the infection is documented, it is difficult to find the parasite's eggs in stool.[14]
The clinical symptoms of patients from Kermanshah were similar to Guilanian patients but of less severity. Another interesting point was fever which appeared only in 27% of our patients. If we consider fever as a rare symptom, the patients are expected to refer to an internist, gastro-enterologist or even to a neurologist or a surgeon. Patients with symptoms such as digestive problems or unclear pain are recommended to undergo differential diagnosis. Furthermore in the endemic areas, in patients who are suffering from fever, hepatomegaly, and eosinophilia and had aquatic vegetables recently, fascioliasis must be taken in to the consideration. At these situations, serological tests are usually helpful. At the beginning of the acute phase, when flukes are still immature, there may not be any parasite's eggs in the stool. Therefore, the immunodiagnostic tests are required to identify early infections.[15,16]
Response to triclabendazole was surprising and cure rate was 100%. According to a research in Rasht and Anzali (Northern Iran) based on patients’ recovery, triclabendazole and bitionole were considered to be the most helpful. But as triclabendazole was effective with smaller doses and would affect in a short time and it was preferred to bitionole.[16,17] The drug is referred to as a drug of choice in children and adult fascioliasis[18] and is emphasized to be used with food.[19]
It must be mentioned that using triclabendazole during the North epidemic has caused slight general and known digestive and biliary side effects that were disappeared quickly.[10] There was no such problem in Kermanshah.
It was the first case of human fascioliasis in west of Iran. In fact, we were faced with a real epidemic of human fascioliasis for the first time and an emerging infectious disease in this area. But the biggest epidemic of disease in northern Iran (1988) was a re-emerging one.[20] Because the disease had been endemic in that area but suddenly had flared up in 1998.
RECOMMENDATIONS
Effort should be made to educate the population at risk regarding the danger of eating raw watercress.
Avoiding the use of animal feces for agricultural purposes in endemic regions.
Draining the pools and destroying the snails.
The early diagnosis of the disease by means of specific and sensitive serological tests particularly for those who had eosinophilia (of any rate).
The physicians should be reminded though fascioliasis is a type of zoonoses and is expected to cause fever, but most of the time; it is with the symptoms as vague pains, colitis, cholecystitis, acute abdomen, chronic allergy, anemia, and eosinophilia of different degree. Thus, physicians must have diagnosis in mind when eosinophilia is recognized, especially in endemic areas.
Because ova are not produced until approximately three to four months after infection, diagnosis during acute stage of disease must really on a combination of epidemiological observation, clinical findings, immunological tests, and imaging studies.
ACKNOWLEDGMENTS
We express our appreciation to colleagues of Infectious diseases department, Sina Hospital, Kermanshah University of Medical Sciences.
We also thank our colleagues at the Department of Parasitology, Pasteur Institute of Iran.
This study was financially supported by educational and research deputy, Kermanshah University of Medical Sciences. We wish to thank the assistance of relevant authorities.
Footnotes
Source of Support: Supported by educational and research deputy, Kermanshah University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Maguire JH. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious diseases. 7th ed. United States: Churchill Livingstone Publishers; 2010. Trematodes; pp. 3595–605. [Google Scholar]
- 2.Hatami H, Mobedi I. Geographical Medicine of Iran. In: Hatami H, Razavi SM, Eftekhar AH, Majlesi F, Sayed Nozadi M, Parizadeh SM, editors. Textbook of Public Health. 2nd ed. Tehran: Arjmand Publishers; 2006. pp. 1038–77. [Google Scholar]
- 3.Moghaddam AS, Massoud J, Mahmoodi M, Mahvi AH, Periago MV, Artigas P, et al. Human and animal fascioliasis in Mazandaran province, Northern Iran. Parasitol Res. 2004;94:61–9. doi: 10.1007/s00436-004-1169-6. [DOI] [PubMed] [Google Scholar]
- 4.Rokni MB, Massoud J, O’Neill Sandra M, Parkinson M, Dalton John P. Diagnosis of human fasciolosis in the Guilan province of Northern Iran. Diagn Microbiol Infect Dis. 2002;44:175–9. doi: 10.1016/s0732-8893(02)00431-5. [DOI] [PubMed] [Google Scholar]
- 5.Nazari P. Epidemiology of Helminthic Diseases. In: Azizi F, Janghorbani M, Hatami H, editors. Epidemiology and Control of Common Diseases in Iran. 3rd ed. Tehran, Iran: Khosravi Publishers; 2010. pp. 541–60. [Google Scholar]
- 6.Case Records of the Massachusetts General Hospital. N Engl J Med. 2002;346:1236–9. doi: 10.1056/NEJM196105042641813. [DOI] [PubMed] [Google Scholar]
- 7.Mobedi I. An Introduction to Geographical Pathology, School of Health, Tehran University of Medical Sciences. 2005:1–132. [Google Scholar]
- 8.Hillyer GV. Serological diagnosis of Fasciola hepatica. Parasitol al Dia. 1993;17:130–6. [Google Scholar]
- 9.Asmar M. Seroepidemiology of Fascioliasis in Guilan province. The first congress of parasitic diseases in Iran. Guilan University of Medical Sciences. 1990. [Last cited on 2011 October 8]. Available from: http://www.elib.hbi.ir/persian/PERSIAN_COMPUTERIZED_BOOKS_FOR_DOS/COMPUTERIZED.htm .
- 10.Forghan Parast KD, Yadegarynia, Asmar M. A clinico-epidemiolgical study on human fascioliasis in Guilan - Iran. Journal of Guilan University of Medical Sciences. 1993;6-7:1–4. [Google Scholar]
- 11.Assmar M, Yadgarinia D. Seroepidemiological investigation of fascioliasis in northern Iran. Med J Islam Republic Iran. 1991;5:23–6. [Google Scholar]
- 12.CRC Handbook Series in Zoonoses (7, 8) Section C: Parasitic Zoonoses. In: Steele JH, editor; Zowghi D.V.M E, translator. Farsi ed Vol. 3. 1997. [Google Scholar]
- 13.Yang Q, Mao WH, Ferre I, Bayon JE, Mao XZ, Gonzalez-Gallego J. Plasma Aspartate Aminotransferase (AST) Glutamate dehydrogenase (GLDH) and Gamma-glutamyl transpeptidase (GGT) activities in water buffaloes with experimental subclinical fasciolosis. Vet Parasitol. 1998;78:129–36. doi: 10.1016/s0304-4017(98)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Baily G. Cestode and Trematode Infections. In: Armstrong D, Cohen J, editors. Infectious Diseases. 1st ed. London: Mosby; 1999. pp. 6.28.1–6.28.4. [Google Scholar]
- 15.Summer AP, Clinton White A, Fischer PR. In: Feigin and Cherry's Textbook of Pediatric Infectious Diseases. 6th ed. Philadelphia: Saunders Publishers; 2009. Trematodes; pp. 3015–22. [Google Scholar]
- 16.Yadegarynia D, Talaie H, Masoud J. Clinical Trial of triclabendazole on Human Fascioliasis: A long - term follow up. Med J Islam Republic Iran. 1999;2:89–91. [Google Scholar]
- 17.Yadgarynia D, Forghanparast K, Samar M. Survey of Praziquantel's effect on fascioliasis. Med J Islam Republic Iran. 1991;5:43–4. [Google Scholar]
- 18.Yilmaz H, Oner AF, Akdeniz H, Arsalan S. The effect of triclabendazole in Children with Fascioliasis. J Egypt Soc Parasitol. 1998;28:497–502. [PubMed] [Google Scholar]
- 19.Miranda L, Pacheco R, Mull R, Poltera AA. Effect of food on the Bioavailability of triclabendazole in patients with fascioliasis. Br J Clin Pharmacol. 1998;45:601–4. doi: 10.1046/j.1365-2125.1998.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asmar M, Hatami H. Emerging and Reemerging Fascioliasis in Islamic Republic of Iran. In: Hatami H, editor. Emerging, Reemerging Infectious diseases and Employee Health. 1st ed. Iran: Center for Diseases Management; 2003. pp. 741–53. Ministry of Health and Medical Education. [Google Scholar]


