Abstract
Backgrounds:
Iron deficiency anemia (IDA) is one of the well recognized presentations of celiac disease (CD). According to the lack of data from our population in this regard, we determined the prevalence of CD in patients presenting with IDA to see if it is worthwhile to do a precise screening for CD in such patients.
Methods:
This cross-sectional study was conducted on patients referred with IDA to Poursina Hakim Gastroenterology Clinic, Isfahan (IRAN). All included patients underwent upper gastrointestinal endoscopy and duodenal biopsy. Histopathological changes were assessed according to the Marsh classification. Also, patients were evaluated for IgA anti-tissue transglutaminase (t-TG) antibody with enzyme-linked immunosorbent assay (ELISA) technique. CD was defined as having Marsh II or above histopathology or being seropositive with Marsh I histopathology and having a good response to gluten free diet (GFD).
Results:
During the study, 130 patients with the mean age of 35.5±13.7 (67.7% female [20.4% post-menopausal]) were undergone seropathological studies. According to histopathological study and a clinical response to GFD, 13 patients (10%) were ultimately diagnosed with CD. Nine patients (6.9%) were seropositive, from which, five patients (3.8%) were ultimately diagnosed as CD cases. IgA anti-tTG became negative in all of these patients after six months of GFD.
Conclusion:
CD should be considered in any adult patient presenting with unexplained IDA, even if not accompanied with gastrointestinal symptoms. Routine duodenal biopsy performed during diagnostic upper gastrointestinal endoscopy is worthwhile in order to investigate for CD as an underlying cause of IDA in adult patients.
Keywords: Celiac disease, diagnosis, iron deficiency anemia
INTRODUCTION
Iron deficiency is the most common cause of microcytic, hypochromic anemia worldwide[1,2] and is frequently caused by either overt or occult chronic blood loss from the gastrointestinal tract. Therefore, the standard evaluation of a patient presenting with iron deficiency anemia (IDA) includes a complete evaluation of the gastrointestinal tract to identify a source of bleeding.[3,4] Once gastrointestinal blood losses have been excluded, intestinal malabsorption syndromes should be investigated as the IDA may be the only presenting sign of malabsorption due to the presence of intestinal villous atrophy.[5]
Celiac disease (CD) is a malabsorption syndrome that was thought to be rare in the past.[6–8] Recent screening studies using valuable screening tools showed that CD is one of the most frequent genetically based diseases which occur worldwide with a prevalence ranging from 1:85 to 1:500 in different populations.[9–12] The study that performed in our area showed that its prevalence is 0.6%[13] to 0.8%[14] among healthy blood donors and 0.5%[15] to 0.9% in general population.[16]
It is known that CD has a wide clinical heterogeneity from typical malabsorption syndrome to atypical intestinal complaints or extra intestinal mono-symptomatic presentation, such as short stature or IDA.[17] CD was first described with classic symptoms of malabsorption syndrome such as diarrhea, steatorrhea, and weight loss, but nowadays, IDA is one of the well recognized presentations of CD in any age, sex, or ethnic group. Despite such a high prevalence of CD among these patients, CD is often unrecognized in IDA.[18] The prevalence of CD in patients with IDA has been estimated to be approximately 2.8% in a prospective study by Karnam et al.[19] Studies in Europe using anti-gliadin and anti-endomysial antibodies have shown a prevalence of 5%.[20–22]
According to the lack of data from our population on the prevalence of CD in patients presenting with IDA, we determined the prevalence of CD in patients presenting with IDA to see if it is worthwhile to do a routine precise screening for CD in such patients.
METHODS
Patients and setting
This study was done from 2003 to 2008 in Poursina Hakim Research Institute, a tertiary referral gastrointestinal center in Isfahan, IRAN. All patients who were referred to the endoscopic unit of the institute for evaluation of IDA were the target population of our study. Primary evaluation of anemia was done by the hematologist who referred the patients for exclusion of gastrointestinal causes of anemia. IDA was defined as hemoglobin <12g/ dl in women (normal, 12.0–16.0 g/dl) and <14g/dl in men (normal, 14.0–18.0 g/dl), and ferritin <15 μg/L (normal, 25–300 μg/L). Those with prior gastrointestinal surgery, ulcerative disease of the gastrointestinal tract, known gastrointestinal malignancy, chronic diseases, and known cases of CD were not included. The ethics committee of the Isfahan University of Medical Sciences approved the study and informed consent was obtained from all patients after explaining the aims and protocol of the study.
Serological assessment for CD
Serum level of IgA was measured in all patients to find cases with selective serum IgA deficiency. IgA anti-tissue transglutaminase (anti-tTG) antibody was determined in all patients using an enzyme-linked immunosorbent assay (ELISA) technique by a commercially available kit (ORG540 A, ORGENTEC Diagnostica GmbH). IgA anti- t-TG antibody above 10 Au/ml was considered positive, as determined by the manufacturer.
Pathological assessment for CD
All included patients underwent upper gastrointestinal endoscopy. After oropharyngeal anesthesia, a “Pentax EPM-3300” EG2940 scope was used for the endoscopy procedure. Patients with an obvious source of gastrointestinal bleeding were excluded from the study. The second part of duodenum was observed carefully in all patients and at least four spike biopsies were taken from the second part of duodenum (D2). Biopsy specimens were fixed in 10% buffered formalin, embedded in paraffin, cut in 4 μm sections, and stained with hematoxylin-eosin for histopathologic examination. Biopsy specimens were evaluated according to Marsh 1992, revised and validated in 1997 as Marsh I to III (Marsh IIIA, Marsh IIIB, and Marsh IIIC).[23]
Definition of CD
Patients with Marsh II or above histopathology or seropositive patients with Marsh I histopathology and an expected good response to gluten free diet (GFD) were considered to have CD. We classified patients with villous atrophy and a negative antibody profile as having antibody negative CD only if they had good response to GFD. IgA anti- t-TG antibody was re-tested after GFD every three months.
Statistical analyses
Data are presented as mean±SD or percentage. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software version 16.0, t-test for comparison of the means of quantitative variables and Chi-Square test for comparison of qualitative variables. P<0.05 was considered statistically significant.
RESULTS
During the study, 130 patients with the mean age of 35.5±13.7 (88 [67.7%] female [20.4% post-menopausal]) were included in our study after exclusion of patients with obvious source of IDA in endoscopy. In all 130 patients, D2 biopsies were taken. According to histopathological study and a clinical response to GFD, 13 patients (10%) were ultimately diagnosed with CD. Also, IgA anti-tTG antibody was determined in all patients and nine patients were seropositive. From these patients, five patients were ultimately diagnosed as CD cases based on histopathological study. IgA anti-tTG became negative in all of the five patients after six months of GFD [Table 1].
Table 1.
CD patients’ characteristics (n=13)
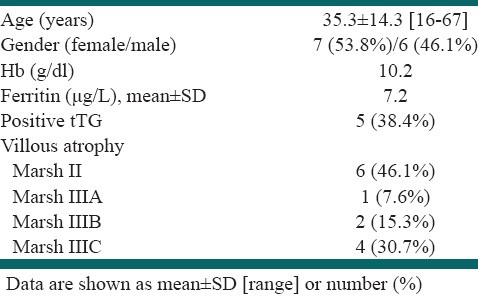
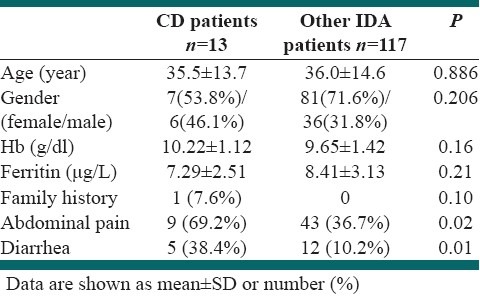
There was no significant difference between those with and without CD in age, gender, Hb or ferritin level, or family history of CD (P >0.05). However, abdominal pain and diarrhea were more prevalent in CD patients (P<0.05); [Table 2].
Table 2.
Hemoglobin and ferritin levels in CD compared with other patients
DISCUSSION
IDA, as the only presenting feature of CD, is not uncommon in adult or even in elderly patients. As the prevalence of CD is high in the community, it should therefore be considered as a potential cause in any patient presenting with IDA. Recent guidelines from the British Society of Gastroenterology recommended that duodenal biopsies should be taken during endoscopy if no obvious cause of iron deficiency could be found.[24] In our study, we evaluated the patients with IDA that were referred by hematologists after primary evaluations. Patients with an obvious origin of bleeding in upper gastrointestinal endoscopy were excluded from our study. We found CD as the cause of IDA of obscure origin in a significant proportion (10%) of the patients. In a study by Zamani et al., they also have found that there is a high prevalence (14%) of CD in patients with IDA of obscure origin. In their study, the prevalence of CD was amongst the highest rates reported. One possible reason was that they evaluated CD among highly selected patients in whom the cause of IDA could not be identified after extensive evaluations. Also, they considered patients with positive serological tests and milder degrees of duodenal mucosal lesions (e.g. Marsh I or II) as having CD.[8] Lower rates (2-3%) of the prevalence of CD in IDA patients have been reported among different studies.[19,25–27,24] This disparity could possibly be related to differences in local prevalence of CD as well as patient selection criteria. Umaprassana et al. prospectively evaluated all patients presenting with IDA without excluding other gastrointestinal (GI) disorders and found the prevalence of CD to be 2.8% in these patients. Their results comparing to our study could be due to that they did not exclude other IDA patients with an obvious site of bleeding at upper and lower endoscopy.[19]
In our study, the prevalence of abdominal pain and diarrhea was significantly higher in CD than in non-CD cases that could be a clue to case finding. However, a significant proportion of CD patients did not report any gastrointestinal symptoms which showed that CD should be considered in any patient with unexplained IDA, even if they do not have any gastrointestinal symptoms. In this study, we used a human recombinant protein based tTG test, which has a higher sensitivity and accuracy than a guinea pig protein-based tTG test. However, anti-tTG antibody test is not 100% sensitive, as 8 out of 13 CD patients (61.5%) had negative serology but high levels of villous atrophy (M II to M III). Thus, we recommend a routine duodenal biopsy among patients presenting with unexplained IDA.
CONCLUSION
According to the high prevalence of CD among adult patients presenting with IDA, routine duodenal biopsy, performed during diagnostic upper gastrointestinal endoscopy, and seropathological studies for diagnosing CD is recommended. CD should be considered in any patient with unexplained IDA, even if they do not have typical or atypical gastrointestinal symptoms suggesting the disease.
ACKNOWLEDGMENTS
Authors are thankful to the personnel of Poursina Hakim Research Institute and Iranian Celiac Association especially Mrs. Zahra Bashari, Dr. Zahra Kazemi, and Dr. Ali Gholamrezaei who helped us in conducting the study and preparing the report.
Footnotes
Source of Support: Isfahan University of Medical Sciences (Grant # 82398)
Conflict of Interest: None declared.
REFERENCES
- 1.Provan D. Mechanisms and management of iron deficiency anaemia. Br J Haematol. 1999;105:S19–26. [PubMed] [Google Scholar]
- 2.Frewin R, Henson A, Provan D. ABC of clinical haematology. Iron deficiency anaemia. BMJ. 1997;314:360–3. doi: 10.1136/bmj.314.7077.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annibale B, Capurso G, Delle Fave G. The stomach and iron deficiency anemia: A forgotten link. Dig Liver Dis. 2003;35:288–95. doi: 10.1016/s1590-8658(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 4.Annibale B, Severi C, Chistiloni A, Antonelli G, Lahner E, Marcheggiano A, et al. Efficacy of gluten-free diet alone on recovery from iron deficiency anemia in adult celiac patients. Am J Gastroenterol. 2001;96:132–7. doi: 10.1111/j.1572-0241.2001.03463.x. [DOI] [PubMed] [Google Scholar]
- 5.lonso Cotoner C, Casellas Jordá F, Chicharro Serrano ML, de Torres Ramírez I, Malagelada Benaprés JR. Iron deficiency: Not always blood losses. Ann Med Intern. 2003;20:227–31. [PubMed] [Google Scholar]
- 6.Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: An evolving spectrum. Gastroenterology. 2001;120:636–51. doi: 10.1053/gast.2001.22123. [DOI] [PubMed] [Google Scholar]
- 7.Goggins M, Kelleher D. Celiac disease and other nutrient related injuries to the gastrointestinal tract. Am J Gastroenterol. 1994;89:S2–17. [PubMed] [Google Scholar]
- 8.Zamani F, Mohamadnejad M, Shakeri R, Amiri A, Najafi S, Alimohamadi SM, et al. Gluten sensitive enteropathy in patients with iron deficiency anemia of unknown origin. World J Gastroenterol. 2008;14:7381–5. doi: 10.3748/wjg.14.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolho KL, Farkkila MA, Savilahti E. Undiagnosed coeliac disease is common in Finnish adults. Scand J Gastroenterol. 1998;33:1280–3. doi: 10.1080/00365529850172368. [DOI] [PubMed] [Google Scholar]
- 10.Catassi C, Fabiani E, Ratsch IM, Coppa GV, Giorgi PL, Pierdomenico R, et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr Suppl. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 11.Accomando S, Cataldo F. The global village of celiac disease. Dig Liver Dis. 2004;36:492–8. doi: 10.1016/j.dld.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Akbari MR, Mohammadkhani A, Fakheri H, Zahedi M Javad, Shahbazkhani B, Nouraie M, et al. Screening of the adult population in Iran for coeliac disease: Comparison of the tissue-transglutaminase antibody and anti-endomysial antibody tests. Eur J Gastroenterol Hepatol. 2006;18:1181–6. doi: 10.1097/01.meg.0000224477.51428.32. [DOI] [PubMed] [Google Scholar]
- 13.Shahbazkhani B, Malekzadeh R, Sotoudeh M, Moghadam KF, Farhadi M, Ansari R, et al. High prevalence of coeliac disease in apparently healthy Iranian blood donors. Eur J Gastroenterol Hepatol. 2003;15:475–8. doi: 10.1097/01.meg.0000059118.41030.96. [DOI] [PubMed] [Google Scholar]
- 14.Bahari A, Karimi M, Sanei-Moghaddam I, Firouzi F, Hashemi M. Prevalence of celiac disease among blood donors in Sistan and Balouchestan Province, Southeastern Iran. Arch Iran Med. 2010;13:301–5. [PubMed] [Google Scholar]
- 15.Saberi-Firouzi M, Omrani GR, Nejabat M, Mehrabani D, Khademolhosseini F. Prevalence of celiac disease in Shiraz, southern Iran. Saudi J Gastroenterol. 2008;14:135–8. doi: 10.4103/1319-3767.41732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbari MR, Mohammadkhani A, Fakheri H, Zahedi M Javad, Shahbazkhani B, Nouraie M, et al. Screening of the adult population in Iran for coeliac disease: Comparison of the tissue-transglutaminase antibody and anti-endomysial antibody tests. Eur J Gastroenterol Hepatol. 2006;18:1181–6. doi: 10.1097/01.meg.0000224477.51428.32. [DOI] [PubMed] [Google Scholar]
- 17.Doganci T, Bozkurt S. Celiac disease with various presentations. Pediatr Int. 2004;46:693–6. doi: 10.1111/j.1442-200x.2004.01986.x. [DOI] [PubMed] [Google Scholar]
- 18.Halfdanarson TR, Litzow MR, Murray JA. Hematologic manifestations of celiac disease. Blood. 2007;109:412–21. doi: 10.1182/blood-2006-07-031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karnam US, Felder LR, Raskin JB. Prevalence of Occult Celiac Disease in Patients with Iron-Deficiency Anemia: A Prospective Study. South Med J. 2004;97:30–4. doi: 10.1097/01.SMJ.0000051059.23259.56. [DOI] [PubMed] [Google Scholar]
- 20.Corazza GR, Frisoni M, Treggiari EA, Valentini RA, Filipponi C, Volta U, et al. Subclinical celiac sprue: Increasing occurrence and clues to its diagnosis. J Clin Gastroenterol. 1993;16:16–21. [PubMed] [Google Scholar]
- 21.Swinson CM, Levi AJ. Is coeliac disease underdiagnosed? BMJ. 1980;281:1258–60. doi: 10.1136/bmj.281.6250.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catassi C, Rätsch IM, Fabiani E, Rossini M, Bordicchia F, Candela F, et al. Coeliac disease in the year 2000: Exploring the iceberg. Lancet. 1994;343:200–3. doi: 10.1016/s0140-6736(94)90989-x. [DOI] [PubMed] [Google Scholar]
- 23.Rostami K, Kerckhaert J, Tiemessen R, von Blomberg BM, Meijer JW, Mulder CJ. Sensitivity of Antiendomysium and Antigliadin Antibodies in Untreated Coeliac Disease: Disappointing in Clinical Practice. Am J Gastroenterol. 1999;94:888–94. doi: 10.1111/j.1572-0241.1999.983_f.x. [DOI] [PubMed] [Google Scholar]
- 24.British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut. 2000;46:S1–5. doi: 10.1136/gut.46.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandal AK, Mehdi I, Munshi SK, Lo TC. Value of routine duodenal biopsy in diagnosing coeliac disease in patients with iron deficiency anaemia. Postgrad Med J. 2004;80:475–7. doi: 10.1136/pgmj.2003.014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grisolano SW, Oxentenko AS, Murray JA, Burgart LJ, Dierkhising RA, Alexander JA. The Usefulness of Routine Small Bowel Biopsies in Evaluation of Iron Deficiency Anemia. Clin Gastroenterol. 2004;38:756–60. doi: 10.1097/01.mcg.0000139034.38568.51. [DOI] [PubMed] [Google Scholar]
- 27.Ackerman Z, Eliakim R, Stalnikowicz R, Rachmilewitz D. Role of small bowel biopsy in the endoscopic evaluation of adults with iron deficiency anemia. Am J Gastroenterol. 1996;91:2099–102. [PubMed] [Google Scholar]


