Abstract
Background:
Phyllanthus amarus is commonly used for treatment such as in gastro, urogenital diseases and infection. However, it is speculated to have some toxic effects such as renal tubular damage.
Aims:
This study was to investigate the histological effects of chronic administration of the herb on kidney of adult Wistar rats.
Material and Methods:
Rats of both sexes (n = 24), with average weight of 200g were randomly assigned into two treatments (A and B) and control (C) groups of 8 rats each. Rats in treatment groups (A) and (B) respectively received daily administration of 400mg and 800mg of aqueous Phyllanthus amarus, per 70kg body weight for 30days through the orogastric tube. The control group received distilled water through the same route. All rats were fed with grower's mash and given water liberally. The rats were sacrificed by cervical dislocation on the thirty-first day of the experiment and the kidneys were carefully dissected out and quickly fixed in 10% formal saline for histological study.
Results:
The observations indicate that rats in the treated groups showed some varying degree of distortion and disruption in microanatomy of the kidney including interstitial oedema and tubular necrosis, when compared to the control section.
Conclusion:
This report provides further evidence that medicinal use of Phyllanthus amarus has a potential adverse effect. This warrants further studies to establish or rule out any untoward side-effect of chronic renal dysfunctions.
Keywords: Antioxidant toxicity, ethnomedicinal practice, histological effects, Phyllanthus amarus, renal dysfunction
Introduction
Phyllanthus amarus (P. amarus) is an ethnobotanical plant that is distributed in almost all tropical countries and regions including America, India and Nigeria. It is a common weed, which grows well in moist, shady and sunny places. It is known by several other names such as carry-me-seed, chanca piedra, and quinine weed to mention a few[1].
P. amarus is believed to possess anti-diabetic, anti-nociceptive, antioxidant, antiseptic, antiviral, contraceptive, diuretic, hypotensive and stomachic properties. It is used accordidng in many countries[2–5]. It is particularly used traditionally in the treatment of several other diseases including diabetes, diarrhea, dysentery, fevers, jaundice, ulcers, urogenital diseases and wounds[1,6–9].
Herbal medicines are widely perceived by the public as being natural, healthful and free from side effects. However, plants contain hundreds of constituents and some of them may elicit toxic side effects. Phytochemically, P. amarus contains alkaloids, antioxidants (including flavonoids, phenols and polyphenols) and lignans[1]. All of these chemical components have their useful as well as toxic effects. Hence safety of herbal medicines is still an issue worldwide[10,11].
The toxic effects of P. amarus has been suggested in literature[12,13]. However, histological perspectives are yet to be elaborated. In humans, the majority of drugs administered are eliminated by a combination of hepatic metabolism and renal excretion. Given the suggestion of potential toxicity of P. amarus, the objective of this study is to examine the effects of chronic consumption of P. amarus on the microanatomy of kidney.
Materials and Methods
Animals and ethical concerns
Twenty-four adult Wistar rats of both sexes with average weight of 200g were equally and randomly assigned into two treatment groups [A] and [B]; and untreated Control group [C] of (n = 8) per group. The School of Basic Medical Sciences, University of Benin granted approval before the work began. The animal care and use ethics was in compliance to the Animal Holdings protocol overseen by the head of department through the Animal Holding unit. The rats were obtained and maintained in the Animal Holdings of the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Edo State, Nigeria. The animals were fed with grower's mash obtained from Edo Feeds and Flour Mill Limited, Ewu, Edo State, Nigeria and given feeds liberally.
Preparation and administration of P. amarus
The P. amarus leaves were obtained in Benin City. The leaves were cleaned, oven-dried at 50°C and macerated into dry powder. The powder was extracted with distilled water using Soxhlet apparatus and concentrated by rotary evaporator at 65°C. It was then transferred into a suitable container and freeze dried ready for the experiment. All preparations were performed at the Department of Pharmacognosy, Faculty of Pharmacy, University of Benin, Benin city, Edo State, Nigeria.
Treatment of animals
Animals in group A were given the aqueous extract of Phyllanthus amarus at a single dose of 400mg/kg body weight daily for thirty days through the orogastric tube, while animals in group B received 800mg/kg body weight daily via the same route and the same period. Animals in group C received equal volume of distilled water, for the same period and through the same route of administration. The rats were sacrificed by cervical dislocation on the thirty-one day of the experiment and the kidney was quickly dissected out and fixed in 10% formal saline for routine histological techniques.
Histological study
The tissues were dehydrated in an ascending grade of alcohol (ethanol), cleared in xylene and embedded in paraffin wax. Serial sections of 7 microns thick were obtained using a rotatory microtome. The deparaffinized sections were stained routinely with haematoxyline and eosin (H & E). Photomicrographs of the results were obtained using research photographic microscope in the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Edo State, Nigeria.
Results
The photomicrograph of the control kidney section show normal histological features. The section indicated a detailed cortical parenchyma and the renal corpuscles appeared as dense rounded structures (Fig. 1).
Fig. 1.
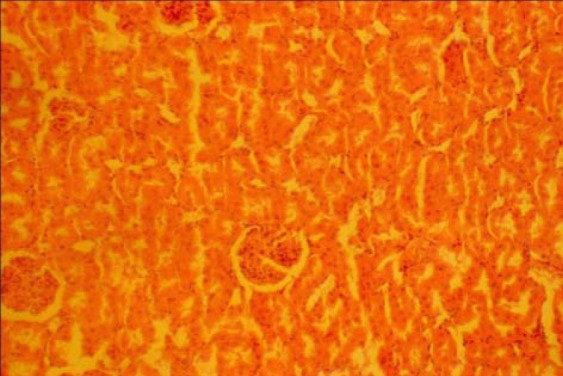
Control section of the Kidney (H & E ×100)
The kidney sections from the treated groups (A) and (B) revealed some varying degree of distortion and disruption in microanatomy of the renal cortex, including queried edema, when compared to the control group (Fig. 2). There is no remarkable difference in observed distortion between the treated groups A and B.
Fig. 2.
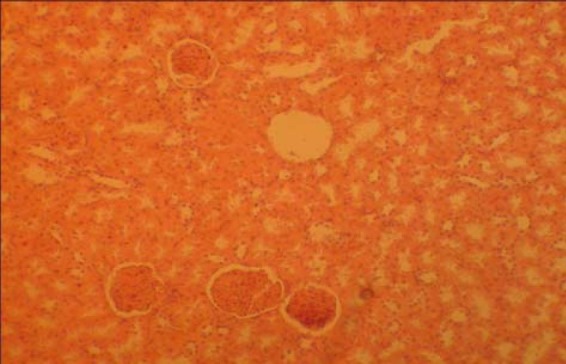
Treated section of the kidney from group B (H & E ×100)
Discussion
This report presents the toxic effects of chronic administration of P. amarus on the microanatomy of the renal cortical structure. It has been speculated that toxic effects could be independent of concentration [14]. In this study, we observed no remarkable effect with higher concentration. Further studies are necessary to establish the effects of different doses of P. amarus on different tissues and/or organs.
The histological effects observed in this experiment is in consonance with the report of Manjrekar et al. who observed that P. amarus induced deleterious changes on the renal tubules and testes of male rats[12,13]. It is also in consonance with the reported effects of damiana (Turnera diffusa) on matured Wistar rats where distortion of the renal cortical structures, reduced number and size of the renal corpuscles were observed[15].
It is noteworthy that P. amarus contains alkaloids and lots of antioxidants[1,5], which has been given to apparently healthy animals. Our observation is consistent with the notion that antioxidant is associated with toxicities especially if taken arbitrarily[16]. Although, antioxidants are essential for alleviation of oxidative stress, indiscrete intake of alkaloids and antioxidant constituents of P. amarus may present their toxic effects by inducing oxidative stress[17,18]. Therefore, we suggest that the distortion and disruption of the architecture of the kidney observed in this experiment could be a factor of its antioxidant content. That is, a histological perspective of antioxidant toxicity on renal system. The implication is that arbitrary chronic and/or excessive consumption of P. amarus may be detrimental to the health status of animals.
The importance of this report lies in the potential adverse effects of P. amarus on the microanatomy of tissues and organs. In the kidney, it has been indicated to cause necrosis and protein casts in the kidney tubules[12,13]. The observation of distortion from this study provides further evidence that medicinal use of P. amarus has adverse side effects. It calls for caution and discretion as with other medicines.
Conclusion
The study shows histological evidence that chronic administration of P. amarus has potential adverse effect on kidney. The hypothetical implication is that the function of the kidney may be adversely affected by P. amarus. It is recommended that further studies be carried out to examine this hypothesis.
References
- 1.Fernand VE. Louisiana State University; 1998. Initial characterization of crude extracts from Phyllanthus amarus schum. and thonn. and Quassia amara L. using normal phase thin layer chromatography [Thesis] pp. 6–13. [Google Scholar]
- 2.Calixto JB, Santos AR, Cechinel-Filho V, Yunes RA. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology, and therapeutic potential. Med Res Rev. 1978;18:225–258. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Faremi TY, Suru SM, Fafunso MA, Obioha UE. Hepatoprotective potentials of Phyllanthus amarus against ethanol-induced oxidative stress in rats. Food Chem Toxicol. 2008;46:2658–2664. doi: 10.1016/j.fct.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Harikumar KB, Kuttan G, Kuttan R. Inhibition of Viral Carcinogenesis by Phyllanthus amarus. Integr Cancer Ther. 2009;8:254–260. doi: 10.1177/1534735409340162. [DOI] [PubMed] [Google Scholar]
- 5.Naaz F, Javed S, Abdin MZ. Hepatoprotective effect of ethanolic extract of Phyllanthus amarus Schum. et Thonn. on aflatoxin B1-induced liver damage in mice. J Ethnopharmacol. 2007;113:503–509. doi: 10.1016/j.jep.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Adeneye AA, Amole OO, Adeneye AK. Hypoglycemic and hypocholesterolemic activities of the aqueous leaf and seed extract of Phyllanthus amarus in mice. Fitoterapia. 2006;77:511–514. doi: 10.1016/j.fitote.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Harikumar KB, Kuttan R. An extract of Phyllanthus amarus protects mouse chromosomes and intestine from radiation induced damages. J Radiat Res (Tokyo) 2007;48:469–476. doi: 10.1269/jrr.07039. [DOI] [PubMed] [Google Scholar]
- 8.Rao MV, Alice KM. Contraceptive effects of Phyllanthus amarus in female mice. Phytother Res. 2001;15:265–267. doi: 10.1002/ptr.735. [DOI] [PubMed] [Google Scholar]
- 9.Santos ARS, De Campos ROP, Miguel OG, Cechinel Filho V, Siani AC, Yunes RA, et al. Antinociceptive properties of extracts of new species of plants of the genus Phyllanthus (Euphorbiaceae) J Ethnopharmacol. 2000;72:229–238. doi: 10.1016/s0378-8741(00)00256-7. [DOI] [PubMed] [Google Scholar]
- 10.Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Braz J Med Biol Res. 2000;33:179–189. doi: 10.1590/s0100-879x2000000200004. [DOI] [PubMed] [Google Scholar]
- 11.Calapai G. European Legislation on Herbal Medicines: A Look into the Future. Drug Safety. 2008;31:428–431. doi: 10.2165/00002018-200831050-00009. [DOI] [PubMed] [Google Scholar]
- 12.Manjrekar AP, Jisha V, Bag PP, Adhikary B, Pai MM, Hegde A, et al. Effect of Phyllanthus niruri Linn.treatment on liver, kidney and testes in CCl4 induced hepatotoxic rats. Indian J Exp Biol. 2008;46:514–520. [PubMed] [Google Scholar]
- 13.Adedapo AA, Adegbayibi AY, Emikpe BO. Some clinico-pathological changes associated with the aqueous extract of the leaves of Phyllanthus amarus in rats. Phytother Res. 2005;19:971–976. doi: 10.1002/ptr.1768. [DOI] [PubMed] [Google Scholar]
- 14.Campos AH, Schor N. Phyllanthus niruri inhibits calcium oxalate endocytosis by renal tubular cells: its role in urolithiasis. Nephron. 1999;81:393–397. doi: 10.1159/000045322. [DOI] [PubMed] [Google Scholar]
- 15.Enaibe BU, Adjene JO, Eweka AO. Histological studies of the effects of oral administration of Damiana (Turnera diffusa) on the kidney of matured Wistar rats. Int J Biomed Hlth Sci. 2007;3:43–48. [Google Scholar]
- 16.Miller ER, 3rd, 3rd , Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 17.Atici S, Cinel I, Cinel L, Doruk N, Eskandari G, Oral U. Liver and kidney toxicity in chronic use of opioids: An experimental long term treatment model. J Biosci. 2005;30:245–252. doi: 10.1007/BF02703705. [DOI] [PubMed] [Google Scholar]
- 18.Galati G, O’Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]


